Abstract
Background:
Contemporary management of coronary disease focuses on the treatment of stenoses in the major epicardial vessels. However, myocardial blood flow is known to be contingent on a range of factors in addition to the patency of the epicardial vessels. These include anatomical and physiological factors such as the extent of myocardium supplied by the vessel, systemic blood pres-sure, the natural variation in vascular tone in response to physiological needs which allows for coro-nary autoregulation and pathological factors such as the presence of downstream obstruction to flow due to disease of the small coronary vessels or myocardium. The assessment of clinical effectiveness and adequacy of coronary revascularisation requires the ability to comprehensively and accurately as-sess and measure myocardial perfusion.
Conclusion:
In this article, we review the current methods of evaluating coronary blood flow and my-ocardial perfusion in the cardiac catheterisation laboratory.
Keywords: Coronary physiology, microvascular resistance, coronary blood flow measurement, myocardial blood flow, autoregulation, stenoses, epicardial vessels
1. Introduction
Coronary artery disease (CAD) is a major cause of mortality and morbidity world-wide, despite significant advances in management over the past three decades [1-3]. Invasive X-ray Coronary angiography can easily demonstrate luminal narrowing of the major epicardial coronary arteries and current management of CAD is focused around the treatment of obstructive epicardial coronary stenoses. However, except when a stenosis is very severe or very mild, angiography alone is not adequate for assessing the ‘functional’ significance of a coronary stenosis; that is, the degree to which it is the cause of myocardial ischemia [4]. Furthermore, coronary angiography is unable to evaluate the coronary microcirculation, as these vessels are too small to be visualized. Clinicians, therefore, rely on tests such as myocardial perfusion studies employing nuclear medicine techniques, stress echocardiography and stress cardiac magnetic resonance imaging studies to non-invasively assess for ischemia caused by intermediate coronary lesions. These techniques are not suitable for use in the catheterization laboratory. Further many of these techniques cannot distinguish between ischemia caused by epicardial coronary artery disease and microvascular dysfunction. Nevertheless, revascularization decisions often need to be made rapidly, so the ability to measure myocardial blood flow in the catheter laboratory would be of great help.
A clinically useful functional test of myocardial perfusion should be able to rapidly measure ‘hyperemic’ (that is, in the presence of maximum vasodilatation) blood flow, normalized to the associated myocardial mass or vascular volume. Although there are currently no methods that easily and reliably measure absolute myocardial blood flow or resistance to flow, several techniques exist which provide surrogate measures to guide clinical decision making in situations of coronary artery stenosis of intermediate degree. For example, Fractional Flow Reserve (FFR) has a robust evidence base for clinical effectiveness and is in widespread use. However, FFR does not evaluate the coronary microcirculation. In this article, we attempt to review the current methods of assessing coronary physiology in the catheter laboratory and evaluate their merits and limitations.
2. Coronary blood flow physiology and regulation
The coronary arteries are the first branches of the aorta which divide like a tree into small arteries and arterioles. The coronary arterial system can be divided into three compartments with different functions [5]. The anatomical borders of these compartments cannot be clearly defined. The proximal compartment includes the epicardial coronary arteries, (500 μm – 5 mm diameter) which have a capacitance function and in the absence of disease, offer little resistance to coronary blood flow. The intermediate compartment includes the pre-arterioles (100- 500 μm diameter), which are characterized by a measurable pressure drop along their length. They are extra-myocardial and are not under direct vasomotor control by myocardial metabolites. These vessels maintain the pressure at the origin of arterioles within a narrow range and compensate for the changes in coronary perfusion pressure or flow. The more distal compartment is represented by ‘intramural arterioles’, (diameter <100 μm). There is considerable drop in pressure along these vessels. One of their functions is the matching of myocardial blood supply and oxygen consumption (Fig. 1).
Fig. (1).
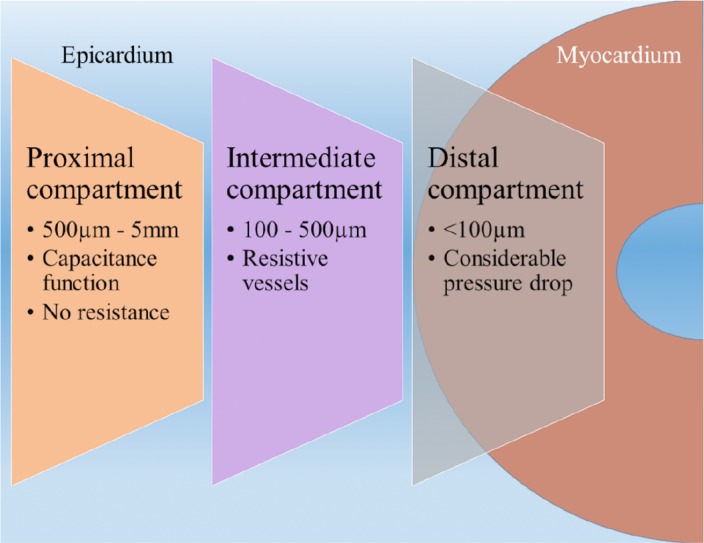
Compartments of coronary circulation.
It has been shown that in normal feline coronary arteries, resistance to coronary blood flow (CBF) is minimal in arteries larger than 400 μmin diameter [6]. Resistive vessels, smaller than 400 μm can vasodilate to modulate CBF according to physiological needs or in response to stress. CBF is kept fairly constant over a large range of perfusion pressure by adjusting coronary microvascular resistance to changes in perfusion pressures.
Coronary blood flow is unique in that the flow is impeded during left ventricular systole because of the contracting myocardial fibres. Unlike systemic circulation, in the coronary arteries, pressure is determined from both the proximal and distal ends of the artery – the aortic pressure and the pressure generated by the contraction and relaxation of the myocardium on the microvasculature [7]. Because the increase in oxygen demand of the left ventricle is largely met by increasing the CBF, it is very finely regulated. During ventricular contraction, wall tension increases and compresses the intra-myocardial microvessels impeding the coronary arterial inflow. At the same time the venous outflow is increased. Due to the differential distribution of the transmural gradient, there is re-distribution of blood from sub-endocardial to sub-epicardial layers. Conversely, during diastole, coronary arterial inflow increases and the transmural gradient favours perfusion of sub-endocardial layers [8, 9].
The two major determinants of coronary flow are coronary arterial pressure and myocardial oxygen consumption. At constant oxygen consumption, coronary flow is relatively independent of arterial pressure which is referred to as coronary autoregulation. At a given coronary arterial pressure, coronary flow increases with oxygen consumption, which is defined as metabolic adaptation [10]. These processes are vital for close regulation of CBF.
Atherosclerotic CAD causes increase in resistance in the epicardial arteries, and impairment of micro-vascular function through endothelial dysfunction. Coronary arterioles dilate and compensate for the increase in resistance to maintain the myocardial perfusion. But once the vasodilatory capacity has been exhausted, myocardial ischemia sets in.
3. Microvascular disease
In the absence of epicardial coronary stenosis, microvascular disease can lead to angina symptoms and more importantly adverse clinical outcomes [11, 12]. Our understanding of microvascular disease is limited by the difficulties in measuring coronary flow and microvascular resistance. Microvascular disease may occur without significant epicardial coronary artery stenosis or in combination with it.
Camici and Crea classified microvascular dysfunction into four categories [5] as shown in Table 1.
Table 1.
Types of microvascular disease (from [5]).
| Type | Description |
|---|---|
| 1 | Coronary microvascular dysfunction in the absence of obstructive CAD and myocardial diseases. The traditional cardiovascular risk factors of smoking, dyslipidemia and diabetes mellitus have been linked to this type. |
| 2 | Coronary microvascular dysfunction in the presence of myocardial diseases. This is thought to be due to adverse remodeling of intramural coronary arterioles. |
| 3 | Coronary microvascular dysfunction in the presence of obstructive CAD which may be in the context of stable CAD or acute coronary syndromes. |
| 4 | Iatrogenic coronary microvascular dysfunction. This includes vasoconstriction and distal embolization during procedures |
where APVbaseline and APVhyperemia are the averaged peak velocity at rest and stress respectively.
Microvascular dysfunction also is associated with poor long-term outcomes and increased mortality in the context of myocardial infarction as well as in stable CAD [11, 13]. Therefore, assessing microvascular dysfunction in the setting of Primary PCI or Acute MI offers the chance to risk stratify patients and target aggressive therapies.
4. Current methods of assessing coronary physiology
Human coronary microcirculation cannot be directly visualized in vivo. Measuring coronary blood flow is difficult and normalizing the flow to the myocardial mass of the coronary artery supplies is even more challenging. Therefore, often surrogate measures are often used.
4.1. Coronary Flow (Velocity) Reserve
Coronary flow reserve (CFR) is defined as the ratio of maximal flow to flow at rest at the same perfusion pressure. In clinical practice, cross-sectional peak velocity is measured rather than volume flow, yielding Coronary Flow Velocity Reserve (CFVR). CFVR was one of the earliest flow based techniques employed to ascertain the functional significance of coronary stenosis [14]. CFVR is determined as the ratio of mean (time-averaged) velocity during hyperemia to mean velocity at rest, defined as the ratio of the mean flow velocity during hyperemia (maximal flow) to the mean flow velocity during baseline (normal flow) conditions. There are several ways of assessing CFVR, of which two ways of invasively measuring coronary flow velocity are more widely used – using a Doppler wire and using thermodilution method. The Doppler method involves passing a Doppler wire (Cardiometrics, Inc) into the coronary artery and manipulating the wire until satisfactory velocity Doppler signals are obtained. This is dependent on the skills and experience of the operator [15, 16]. The process is then repeated to measure the flow velocity during hyperemia induced by adenosine. The ratio of the hyperemic velocity to resting velocity gives the CFVR:
where APVbaselinev and APVhyperemia are the averaged peak velocity at rest and stress respectively.
The thermodilution method of assessing CFR uses a temperature sensitive wire in the coronary artery. Boluses of room-temperature saline are injected briskly; the temperature drop is measured and a thermodilution curve is obtained. The process is repeated with hyperemia induced by adenosine. The mean transit times during baseline and hyperemia are calculated from the thermodilution curves. The ratio of the baseline mean transit time to the hyperemic mean transit time gives the CFR [16].
where QM and QR are the maximal and resting flow, V is the blood volume, and Tmn,X is the mean transit time at rest and stress.
Stress echocardiographic techniques can non-invasively assess CFVR and there is evidence to show that it adds prognostic information [17]. However, this technique has many limitations and is unlikely to be used widely outside research setting.
CFVR has been shown to be prognostically valuable in several scenarios, such as predicting left ventricular function recovery and peri-procedural outcomes after PCI, predicting major adverse cardiac events (MACE) in women with risk factors and in patients with chest pain and microvascular disease [18-21].
While an approximate threshold for recognition of inducible ischemia has been established (CFVR<2.0), the technique is inherently dependent on hemodynamic conditions that affect both baseline as well as hyperemic state measurements [22]. Factors that influence baseline flow (e.g. workload, heart rate, gender) and age were identified as major determinants of CFVR, whereas coronary risk factors or cardiomyopathies affecting the functional capacity of the small resistance vessels tend to reduce CFVR by impairing maximal flow [23-26]. To minimize these baseline flow influences, relative CFVR has been proposed which is the ratio of CFVR in a target vessel to another (ideally healthy) vessel [27]. However, in patients with known atherosclerosis, flow in angiographically healthy contralateral arteries is abnormal, and for this reason, relative CFVR has not had widespread adoption [28]. CFVR interrogates the flow status of both the epicardial artery and the microcirculation but does not allow discrimination between these two components [29]. In addition, the technique of using Doppler wire to measure the velocity is considered relatively difficult to measure accurately by all but experienced operators and for these reasons, it has currently been superseded by pressure based physiological measurements.
4.2. Fractional Flow Reserve
Early detailed studies using a simplified model of the hyperemic coronary pressure–flow relation led to the development of Fractional Flow Reserve (FFR). Pjils et al. defined FFR of the coronary artery as the ratio of maximum possible flow in the artery in the presence of a stenosis (QS) to the maximum flow expected in the same artery in the absence of that stenosis (QN) [30], and hence
A direct relation between coronary pressure and flow or flow reserve can be assumed only if coronary resistances remain constant and minimal. If that is the case, pressure measurements alone can be used to predict blood flow. Letting Pd, Pa and Pv be the blood pressure in the distal coronary artery (beyond the stenosis), in the aorta, and in the venous system respectively then
where Rs and RN are the resistance of the myocardium in the presence of the stenosis, and in myocardium if the vessel were normal. When hyperemia is induced, the resistance is assumed to be the same and negligible (i.e. Rs = RN) and Pv is also assumed to be negligible. So, the FFR equation is simplified to
Originally, FFR was classed as myocardial FFR (FFRmyo) and coronary FFR (FFRcor). This was to take into consideration the collateral flow by measuring the coronary wedge pressure (Pw). However, in practice this is rarely performed.
The functional significance of a lesion can be assessed invasively by measuring the FFR [31]. In clinical practice, this is performed by using a pressure sensitive coronary guide wire advanced into the coronary artery beyond the lesion in question. The pressure distal to the lesion and the aortic pressure are measured after the induction of hyperemia, with adenosine or similar pharmacological agents. The ratio of Pd/Pa during hyperemia is the FFR. An FFR cut-off value of 0.75 was used in earlier studies, but more recent studies have used 0.80 as the cut-off value for attributing hemodynamic significance to a lesion. An FFR of 0.75-0.80 is considered the ‘grey zone’. Over the past decade, an increasing and substantial body of evidence showing that FFR guided PCI improves outcomes has served to cement FFR as the gold standard index for assessing coronary physiology in the catheterization laboratory, most notably through the DEFER, FAME and FAME 2 trials, which are summarized in Table 2 [32-34].
Table 2.
Landmark trials that established FFR as the most widely used invasive physiologic test.
| Trial | DEFER [ 34 , 35 ] | FAME [ 32 ] | FAME 2 [ 33 ] |
|---|---|---|---|
| Design | RCT, multi-centric | RCT, multi-centric | RCT, multi-centric |
| Number of patients | 325 | 1005 | 1220 |
| Clinical syndrome | Stable CAD | Stable multi-vessel CAD | Stable CAD |
| Primary endpoint | Absence of all-cause mortality, MI, CABG, PCI, and any procedure-related complication necessitating major intervention or prolonged hospital stay at 2 years | Death, nonfatal MI, and repeat revascularization at 1 year | Death from any cause, nonfatal MI, unplanned hospitalization leading to urgent revascularization during the first 2 years |
| Key finding | Deferring PCI in lesions with FFR >0.75 is safe | FFR-guided PCI outperformed angiography guided PCI | FFR-guided PCI plus optimal medical therapy outperformed medical therapy alone |
4.3. Pitfalls of FFR
FFR measurement requires several assumptions about coronary physiology such as the coronary pressure is proportional-linear to coronary flow when coronary resistance is minimal and constant. However, experts argue that the relationship between coronary pressure and flow has a non-zero pressure intercept and is incremental linear in the physiological range of perfusion pressures, the slope of which is variable [36, 37].
Ideally, right atrial pressure (Pv) should be measured as patients may have a variety of conditions that cause this to be high. However, often in practice, additional catheterization to measure the Pv is not performed, and it is assumed to be negligible. In theory, this can lead to error in the calculation of FFR [38], as Pv may not be negligible in individual patients. However, a recent study by Toth et al. found that the impact of the Pv is in fact negligible even in patients with high venous pressure [39]. The coronary wedge pressure is also not measured in clinical practice and therefore collateral flow and venous flow is not considered, which can be other sources of error. The accuracy of FFR depends on induction of steady state hyperemia. There are several clinical scenarios where this may not be achieved and FFR assessment may therefore underestimate the significance of a lesion. These range from errors in constitution of the drug and drug delivery to drug interactions (e.g. caffeine).
It is often seen (in up to 30-40% of patients) in clinical practice that FFR and CFVR are discordant [36]. A low coronary flow state can co-exist with a normal FFR and vice versa. Echavarria-Pinto et al. demonstrated that more than half of the coronary arteries with intermediate stenoses and normal FFR can have abnormal hemodynamics if CFVR and Index of micro-circulatory resistance are taken into account [40]. Patients with normal FFR but an abnormal CFVR have been noted to be associated with significantly increased major adverse cardiac events rates during long-term follow-up [41] as opposed to those with abnormal FFR and normal CFVR. The reason behind this discordance may be that FFR does not interrogate microvasculature whereas CFVR does. The discordance must not be seen as a failure of FFR or CFR, but as reflecting different pathophysiological processes at play [42].
FFR assumes that microvascular resistance is the same in stenosed and healthy vessels, but there is evidence to suggest that this is not the case [37]. If microvascular resistance is high, the FFR could be falsely elevated or normalized. Despite good evidence for improved clinical outcomes from PCI guided by FFR in general, the evidence for using cut-off values of FFR to guide the treatment in truly intermediate lesions is still limited [36]. This may be because the beneficial effects from PCI in landmark trials were driven by intervention to lesions that were truly significant (FFR<0.65) [43].
In clinical practice an FFR value of 0.80 is widely used as the cut off above which no intervention is advocated. But this cut off is arbitrary and FFR is in fact a continuum with lower values representing more significant lesions [43]. In the early clinical validation studies comparing exercise ECG with invasive FFR, an FFR value of 0.66 had the highest diagnostic accuracy at predicting an abnormal exercise ECG [44]. The sensitivity and specificity of exercise ECG can vary widely, and this could be a limitation of this study [45].
FFR measurements as described already are influenced by conditions that increase microvascular resistance. Acute myocardial infarction is such a situation where FFR measurements are unreliable and could be mis-interpreted. In patients with Diabetes Mellitus, deferring revascularization based on an FFR above 0.80 led to higher rates of target lesion failure as compared to non-diabetic patients [46]. Age and gender also have influence on FFR measurements with older age and female gender associated with higher FFR values [47, 48].
4.4. Instantaneous Wave-free Ratio
The instantaneous wave-free ratio (iFR) was proposed as a surrogate for FFR removing the need to induce hyperemia, saving time, cost, and reducing side effects related to adenosine and other pharmacological agents [49]. The index iFR uses a ‘wave-free period’ during diastole identified from coronary wave intensity analysis, during which resistance is considered to be minimal and stable, within which time distal and proximal pressures to a stenosis are recorded. The diastolic resting myocardial resistance is assumed to be equal to mean hyperemic resistance. Measuring iFR also requires passage of a pressure sensitive wire connected to a recording unit with analysis software capable of identifying the wave free period (Philips Volcano). In a core-laboratory based analysis, iFR showed an overall accuracy of 80% compared with FFR, with the potential to avoid hyperemia in 65% of cases [50]. Results of comparative studies to FFR had been mixed, showing that the two indices were well matched for extreme values of FFR, but produced poor correspondence for the clinically important intermediate range [51, 52].
Recent publication of two large studies have been game changers for iFR as a clinical tool. In the Functional Lesion Assessment of Intermediate Stenosis to Guide Revascularization (DEFINE-FLAIR) study published in March 2017, 2492 patients undergoing PCI for stable CAD or ACS were randomized to undergo evaluation of all stenosis of questionable severity by iFR or FFR. The 1 year outcomes revealed that iFR was non-inferior to revascularization guided by FFR with respect to the risk of major adverse cardiac events [53]. Unsurprisingly, the incidence of adverse events associated with adenosine such as bronchospasm, arrhythmias, dyspnea and chest pain were all significantly lower in the iFR group as it does not need induction of hyperemia.
These results are similar the findings of the Instantaneous Wave-free Ratio versus Fractional Flow Reserve in Patients with Stable Angina Pectoris or Acute Coronary Syndrome (iFR-SWEDEHEART) study also published in the same journal issue [54]. In this trial, 2037 patients with stable CAD or NSTEMI were enrolled and randomized into FFR guided revascularization and iFR guided revascularization groups. The study found that an iFR-guided revascularization strategy was non-inferior to an FFR-guided revascularization strategy with respect to the rate of major adverse cardiac events at 12 months, and a low reports of adenosine related side effects in the iFR group. Both these studies used an iFR cut-off at 0.89 below which the lesion was considered significant and classed as needing revascularization.
The avoidance of adenosine may have other advantages in cost and time of the procedure as well. Interestingly FFR trials recruited stable CAD patients whereas iFR trials recruited ACS patients as well, however only non-culprit lesions were studied in ACS patients. It remains to be seen whether iFR will replace FFR as the standard of care in PCI. This may well happen as longer term results from these trials become available and more and more physicians get used to the technique of measuring iFR.
4.5. Index of Microcirculatory Resistance
Fearon et al. described this new measure of assessing the microcirculatory resistance [55]. Index of microcirculatory resistance (IMR) is defined as the distal coronary pressure divided by the inverse of the hyperemic mean transit time [55]. It employs a thermodilution based technique and assumes that at peak hyperemia the variability of resting vascular tone will be eliminated, and the minimum microvascular resistance will be achieved. The method employs the use of a temperature and pressure sensitive guide wire (PressureWireTM, St Jude Medical) within the coronary artery. A small bolus (usually 3 ml) of sterile normal saline at room temperature is then injected through the guiding catheter to obtain an indicator-dilution curve. From this the mean transit time (Tmn) can be calculated which is inversely proportional to the flow.
is the pressure difference across the myocardium, i.e. Pd - Pv, and Q the blood flow. Remember, Q is related to the mean transit time as Q = V/Tmn and IMR uses the reciprocal of the mean transit time as an index of flow.
Therefore, IMR can be calculated as follows
Assuming Pv << Pd
IMR has also been found to be more reproducible and less dependent on hemodynamics as compared to CFVR [29]. IMR measured at the time of primary PCI was found to predict adverse outcomes including death and repeat hospitalizations in patients with STEMI [56]. It has also been shown to predict adverse outcomes assessed by cardiac MRI [57-60]. An IMR greater than 40 has been associated with poorer outcomes post myocardial infarction, independently of infarct size [60].
Measuring IMR requires instrumenting the coronary arteries with a coronary guide wire. Although it is largely independent of epicardial coronary stenosis it is affected by the presence of collateral flow [61, 62]. To accurately calculate IMR, collateral blood flow must be considered by incorporating coronary wedge pressure into the equation.
where Pw is the coronary wedge pressure. Pw is calculated by inflating a balloon passed over the pressure wire to occlude the coronary artery. The residual pressure (Pw) recorded represents collateral blood flow
4.6. Hyperemic Stenosis Resistance and Hyperemic Microvascular Resistance
The independent measurement of the resistances of a stenotic lesion and myocardium is desirable, and either can be done by calculating the ratio pressure difference across the stenosis (or myocardium) to the blood flow. However, in practice, as a reliable in vivo measure of flow is unavailable, indices of resistances are used. Hyperemic Stenosis Resistance (HSR) Index is defined as the ratio of hyperemic stenosis pressure gradient and hyperemic average peak flow velocity (APV), obtained by means of a Doppler wire [63].
where APV is the average peak flow velocity.
Hyperemic microvascular resistance index (HMR) is measured as the ratio of mean distal coronary pressure to mean distal coronary flow velocity [64, 65].
This derivation again assumes that Pv is negligible.
Both these indices use a Doppler wire and therefore suffer from lack of reproducibility and operator dependence. Although incorporating such indices could provide a better understanding of coronary physiology, there are no outcome studies to support their routine clinical use.
5. Importance of absolute coronary blood flow measurements
The potential applications of being able to assess coronary flow and in turn the resistance to flow are many. The benefit of revascularization in reducing the future adverse events is dependent on the improvement in coronary flow. Smalling et al. demonstrated in an animal model that myocardial function is dependent on coronary flow rather than pressure [66]. A flow based decision-making process may allow optimal patient selection, the key benefit is the ability to evaluate flow impairment not due to obstructive CAD [36]. Such an approach may be able to better evaluate patients with Cardiac Syndrome X, who present with angina symptoms and positive stress test, but then turn out to have normal coronary arteries on coronary angiography.
Situations where epicardial coronary disease co-exists with microvascular disease are common. It is difficult to assess the relative contribution of each to the limitation of coronary flow. Methods to assess coronary flow, when combined with FFR can assess the differential contribution of epicardial coronary stenoses and microvascular disease towards causing myocardial ischemia and angina.
Despite advances in treatment, more than 50% of patients treated with PCI for acute myocardial infarction experience further myocardial damage and increase in infarct size from microvascular impairment commonly due to distal embolization of atherothrombotic debris [67]. The most extreme form of microvascular impairment has been termed ‘no-reflow’ phenomenon and is often evident on coronary angiogram images [68]. No-reflow is an independent predictor of mortality [69]. It is not possible to accurately identify less severe forms of microvascular impairment from angiography alone. Out of the many factors that cause microvascular impairment, microvascular obstruction (MVO), as identified by cardiac MRI (CMR) has been associated with increased cardiovascular complications, larger infarct size (IS) and predicts long term adverse prognosis [70]. Assessing coronary flow may help to identify less obvious MVO and help immediately target specific vasodilator and antithrombotic therapy.
Atherosclerosis is a gradual progressive process and often micro-circulatory dysfunction precedes the development of angiographic stenosis, perfusion defects or regional wall motion abnormalities (RWMA) on functional imaging [71, 72]. Murthy et al. studied over 2700 patients referred for rest/stress positron emission tomography for a median of 1.4 years and found that coronary microvascular dysfunction, as measured by coronary flow reserve (CFR), was an independent predictor of cardiac mortality [73]. Being able to measure coronary flow without additional instrumentation of coronary arteries offers the opportunity for better risk stratification in the catheterization laboratory.
Coronary flow measurements are useful not only for more comprehensively assessing coronary artery disease and microvascular disease, but also in noncoronary cardiac diseases [74]. Coronary blood flow measurements may help in assessing the prognosis and for monitoring the effectiveness of risk reduction strategies [36, 75, 76].
6. Non- invasive methods of coronary flow quantification
6.1. Positron Emission Tomography
Positron Emission Tomographic (PET) assessment of regional myocardial blood flow involves the dynamic acquisition of images during the intravenous injection of a positron-emitting perfusion tracer, such as 13N-ammonia, 15O-water etc. Tracer kinetic models are then used to quantify MBF. It can yield regional MBF per unit of myocardial mass. This has been shown to be highly correlated with MBF from microspheres in animal models over a wide range of flows [77]. 15O-water is considered as the most accurate PET flow tracer, because of its free diffusibility across capillary and cell membranes and 100% extraction, independent of flow [78]. The ratio of MBF at stress to MBF at rest can be calculated which is called myocardial perfusion reserve (MPR). PET quantification of MBF & MPR, although a promising technique, is limited by several factors such as lack of availability of PET scanning, use of radiation exposure, need for an on-site cyclotron to generate tracers and the high costs involved. Furthermore, the results are not available in real time to enable clinical decision making in the catheterization laboratory.
6.2. Cardiac Magnetic Resonance Imaging
Improvements in magnetic resonance scanners, techniques and image analysis software have enabled CMR imaging to assess myocardial blood flow [79, 80]. CMR has the advantages of high spatial and temporal resolution and avoiding use of ionizing radiation. CMR quantification of myocardial blood flow, however, involves non-volumetric ventricular coverage and relatively complex post-processing steps which are known disadvantages [75]. CMR can be used to estimate MBF from time–intensity curves (TICs) for the LV tissue and LV cavity using extraction fraction models [81, 82]. CMR can also be used to calculated MPR. Patients with pacemakers or other metal implants cannot undergo CMR studies and in some patients, claustrophobia can be an issue as well. However, more and more patients are receiving MRI compatible pacemaker implants and more development is anticipated with MRI compatible implantable cardioverter defibrillators on the horizon.
6.3. Dynamic Computed Tomography Imaging
Computed tomography (CT) coronary angiography is gaining popularity as a non-invasive anatomic test of choice, due to its ability to provide information on coronary anatomy relatively quickly and with limited radiation exposure [83]. Dual-source CT imaging protocols have been developed to assess myocardial perfusion along with anatomic assessment [84]. The ability to non-invasively and simultaneously assess coronary anatomy and myocardial perfusion using the same modality is certainly an interesting concept. Computational flow dynamics (CFD) have been employed to calculate FFR from CT coronary angiogram images (FFRCT). FFRCT has been shown to be good at stratifying lesions to those producing ischemia and those that do not [85, 86]. Dynamic CT myocardial perfusion imaging produces serial datasets that allow the evaluation of contrast kinetics in the myocardium. This allows for absolute MBF quantification, at the expense of increased radiation exposure [75, 87]. Dynamic CT MBF has been shown to have excellent correlation with 15O-H2O PET MBF [88]. Dynamic CT acquisition techniques may allow for the identification of more subtle perfusion changes produced by moderate coronary stenosis [89].
7. Invasive methods of coronary flow measurement
7.1. TIMI Myocardial Perfusion Grade
The TIMI myocardial perfusion grade is not a true measure of coronary or myocardial blood flow. Nevertheless, it is a useful qualitative clinical tool. It describes the “blush,” or intensity, of the radio-opacity of the myocardium achieved with an intra-coronary injection of contrast medium, and the time taken for the blush to clear [90]. An intense myocardial blush that is fast to clear corresponds to go microvascular function. The TIMI myocardial perfusion grade (TMPG) is scored on a scale of 0 to 3, with higher scores indicating better perfusion [91, 92]. Higher TIMI perfusion grades have been noted to have better outcomes. Although TMP grade is most commonly employed in STEMI patients, there is emerging evidence that assessing the blush grade may have prognostic utility in NSTEMI patients as well [93].
7.2. Doppler Wire Method of Coronary Flow Calculation
Doucette et al. used a Doppler-tipped guide wire to assess coronary hemodynamics [15] and described a method of quantitative flow calculation. A guidewire equipped with a Doppler transducer is advanced to the proximal segment of the coronary artery and Doppler signals obtained. A time-averaged parabolic velocity profile was assumed across the vessel with a mean velocity calculated as 0.5 x average peak velocity (APV) measured by the Doppler wire. Flow is calculated as follows
QD = πD2/4 (0.5 x APV)
where QD is the Doppler derived time-average flow, D is the vessel diameter, and APV is the average peak velocity calculated from the Doppler traces. The vessel diameter was measured by quantitative coronary angiography (QCA) 5 mm distal to the wire tip. This technique has not been widely adopted in clinical use due to several limitations. The assumption that coronary flow has a parabolic velocity profile is not correct. The technique of obtaining good Doppler signals from the coronary arteries is dependent on the operator’s experience. QCA is also prone to errors, not least due to the fact that any disease will often mean the arterial lumen is not circular in cross section.
7.3. Thermodilution Method of Volumetric Coronary Flow Measurement
Aarnoudse et al. first described a method for measuring direct volumetric blood flow in selective coronary arteries during cardiac catheterization by using the principles of thermodilution method first described by Ganz et al. in 1971 [94, 95]. The method relied on the continuous infusion of saline at room temperature through a 2.8 F infusion catheter, advanced over a pressure and temperature sensitive coronary wire. This technique is different to the assessment of CFVR by thermodilution described earlier.
Theoretically, during steady-state hyperemia, the absolute coronary blood flow during saline infusion () can be calculated as follows:
where Qi is the volumetric infusion rate of the saline, Tb is the temperature of the blood before the start of saline infusion, Ti is the temperature of the saline infusate at the tip of the infusion catheter, and T is the temperature at the sensor in the distal coronary artery during steady-state infusion (i.e., the temperature of the blood after complete mixing with the infused saline). A correction factor 1.08 is applied to compensate for the difference in specific heat between saline and blood.
When Tb is set to zero and Ti and T are expressed as the deviation of the respective temperatures from Tb, the equation can be rewritten as:
The technique has been validated in an animal model and human patients [94]. In the animal study, excellent correlation was seen between the blood flow measured using thermodilution method and directly measured flow using a perivascular ring-mounted volumetric flow meter placed around the coronary artery surgically. In the human model, the validation was indirect by comparing the improvement in coronary flow measured by thermodilution to the ratio of coronary FFR before and after stenting. The main advantage of measuring the flow using this method is that the FFR and distal coronary pressure data are also simultaneously obtained from which coronary artery resistance can be calculated using an absolute measure of flow rather than the indices used in HSR and HMR.
This method, although promising, is not used widely in clinical practice, most likely due to practical reasons. The setup and methodology is cumbersome requiring additional specially designed infusion catheter and infusion lines for saline. Further absolute coronary blood flow cannot be interpreted without knowledge of the amount of myocardium the coronary artery supplies. For this reason, there are no normal values of volumetric coronary blood flow available yet.
8. X-ray based techniques
Applications of X-ray angiographic methods of assessing coronary arterial blood flow have been investigated previously [96]. After contrast injection, blood flow is determined with the contrast pass curve data derived from the epicardial arteries or the myocardial vascular bed. These techniques were limited by need to accurately quantify arterial dimensions and need for high frame rate acquisitions [96]. Methods for CBF measurement with three-dimensional reconstruction of the arterial tree have been reported [97]. This technique is based on 3D modelling of coronary artery from two planes of coronary angiography and therefore prone to errors, which limit its utility. Three dimensional models of coronary arteries generated from X-ray angiography have enabled the application of CFD techniques to calculate FFR without the need pressure wires [98].
Molloi et al. described a method of quantifying volumetric coronary blood flow using dual energy digital subtraction angiography in a swine model [96, 99]. The method was based on a first-pass distribution theory where the volume of the vascular bed supplied by a major coronary artery is modelled as a reservoir with a single input. Contrast agent was injected into the coronary artery during image acquisition with a motion-immune dual-energy digital subtraction angiography system. Tissue-suppressed energy-subtracted images were used to generate time-density curves. Blood flow was measured in the Left anterior descending artery (LAD) vascular bed using the time-density curve. They have also reported using the same principle to quantify the myocardial resistance by using the pressure data and found that myocardial resistance provided the most accurate method of assessing microcirculation [100]. This technique is promising, but thus far has not been tested in human subjects.
9. Need for newer techniques
Measurement of coronary or myocardial blood flow and resistance will provide a better comprehensive understanding of the pathological processes at play. Although non-invasive methods such as PET scanning can be useful in select patients, it is not practical to be applied to most patients. CFVR assessments by thermodilution or Doppler wire provides velocity measurements which are often used as surrogates for coronary flow. Both methods are cumbersome and involve instrumentation of coronary arteries. The thermodilution method of measuring volumetric coronary flow is similarly cumbersome and time consuming with the need to special catheters, pumps and connectors. Thrombolysis in myocardial infarction (TIMI) frame count and myocardial perfusion grade (TMPG) are of limited use as they are subjective and do not detect the full spectrum of microvascular impairment [92]. A large proportion of coronary angiography is undertaken in centres without the capacity for instrumentation of the coronary artery. Therefore, a technique that can be applied widely without the requirement to instrument the coronary artery would be a welcome addition to the cardiologists’ armamentarium.
Conclusion
There are currently a number of different techniques which allow physicians to assess epicardial and microvascular disease, and the development of these and new methods will continue. However, the ability to quantify coronary blood flow and microvascular resistance in real time remains highly desirable in the assessment of coronary physiology. Although employing surrogate indices such as FFR has improved clinical outcomes, a simplistic approach of just utilizing FFR (or iFR based on recent evidence) as the gold standard of coronary physiology assessment is incomplete. To evaluate the microvasculature, the best invasive method seems to be to apply IMR and/or CFR. Currently the best approach for a comprehensive physiologic assessment is to apply a combination of these indices tailored to individual patients for clinical decision making. There is a need for newer methods for a comprehensive evaluation of coronary circulation that can be widely used.
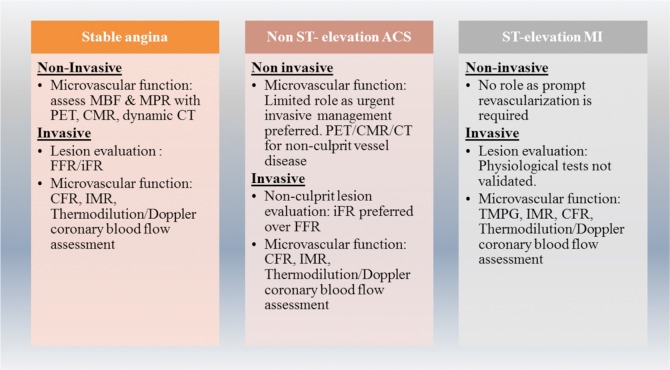
Fig. (2).
Non-invasive and invasive tests that can be employed for coronary physiology assessment in different clinical scenarios.
Table 3.
TIMI myocardial perfusion grades.
| TMP Grade | Description |
|---|---|
| 0 | No apparent tissue level perfusion or no blush |
| 1 | Blush present but no clearance from microvasculature |
| 2 | Blush clears slowly (blush is strongly persistent and diminishes minimally or not at all during 3 cardiac cycles of the washout phase |
| 3 | Blush begins to clear during washout (blush is minimally persistent after 3 cardiac cycles of washout |
Table 4.
Summary of various techniques used for assessing coronary physiology.
| Technique | Invasive /Non-invasive & Method | Advantages | Disadvantages | ||
|---|---|---|---|---|---|
| Surrogate indices of coronary/myocardial blood flow in the catheterization laboratory | |||||
| Coronary flow velocity reserve (CFVR) |
Thermodilution/Doppler coronary wire | Can assess coronary stenosis and microcirculation | Cannot distinguish between microvascular disease and coronary stenosis Affected by hemodynamic conditions |
||
| Fractional Flow reserve (FFR) |
Pressure sensitive coronary wire | Can assess significance of coronary stenosis. Robust evidence base for FFR guided revascularization. Reproducible. |
Cannot assess microcirculation. Influenced by conditions causing high microvascular resistance | ||
| Instantaneous wave_Free ratio (iFR) |
Pressure sensitive coronary wire | Good correlation with FFR at extreme values of FFR at assessing coronary stenoses. Avoids the use of adenosine. Recent large trials prove non-inferiority with FFR |
Cannot assess microcirculation. Not very accurate at intermediate values of FFR. | ||
| Index of microcirculatory resistance (IMR) | Thermodilution | Can assess microcirculation. | Cannot assess coronary stenoses. | ||
| Non-invasive methods of myocardial flow quantification | |||||
| Positron emission tomography (PET) | Non-invasive, uses radioactive tracer | Able to quantify regional myocardial blood flow accurately | Limited clinical utility as not available for decision making in the catheterization laboratory. Not widely available |
||
| Cardiac magnetic resonance (CMR) | Non-invasive, no ionizing radiation | High spatial resolution. CMR sequences have been shown to be able to calculate myocardial blood flow. |
Non-volumetric ventricular imaging, relatively complex post processing steps. Limited clinical utility as not available in the catheterization laboratory. Still a research tool. |
||
| Invasive methods of coronary flow quantification in the catheterization laboratory | |||||
| TIMI myocardial perfusion grade (TMPG) | Invasive but no need for coronary wire | Easily available, semi-quantitative tool for assessing myocardial perfusion. | Can be subjective. It is a crude method | ||
| Doppler wire method of coronary flow calculation | Doppler coronary wire | Coronary flow calculation using flow velocity from Doppler wire | Operator dependent. Lacks reproducibility |
||
| Thermodilution method of volumetric coronary flow measurement | Continuous thermodilution | Volumetric coronary blood flow measurements | Cannot distinguish between coronary stenosis and micro circulation. Need for specially designed infusion catheter | ||
ACKNOWLEDGEMENTS
Declared none.
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Go A.S., Mozaffarian D., Roger V.L., et al. Executive summary: heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation. 2014;129(3):399–410. doi: 10.1161/01.cir.0000442015.53336.12. [DOI] [PubMed] [Google Scholar]
- 2.Townsend N.W., Bhatnagar P., Smolina K., Nichols M.L., Luengo-Fernandez R., Rayner M. Coronary heart disease statistics. 2012 edition. London: British Heart Foundation; 2012. [Google Scholar]
- 3.Moran A.E., Forouzanfar M.H., Roth G.A., et al. The global burden of ischemic heart disease in 1990 and 2010: the Global Burden of Disease 2010 study. Circulation. 2014;129(14):1493–1501. doi: 10.1161/CIRCULATIONAHA.113.004046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.White C.W., Wright C.B., Doty D.B., et al. Does visual interpretation of the coronary arteriogram predict the physiologic importance of a coronary stenosis? N. Engl. J. Med. 1984;310(13):819–824. doi: 10.1056/NEJM198403293101304. [DOI] [PubMed] [Google Scholar]
- 5.Camici P.G., Crea F. Coronary microvascular dysfunction. N. Engl. J. Med. 2007;356(8):830–840. doi: 10.1056/NEJMra061889. [DOI] [PubMed] [Google Scholar]
- 6.Chilian W.M., Layne S.M., Klausner E.C., Eastham C.L., Marcus M.L. Redistribution of coronary microvascular resistance produced by dipyridamole. Am. J. Physiol. 1989;256(2 Pt 2):H383–H390. doi: 10.1152/ajpheart.1989.256.2.H383. [DOI] [PubMed] [Google Scholar]
- 7.Sen S., Petraco R., Mayet J., Davies J. Wave intensity analysis in the human coronary circulation in health and disease. Curr. Cardiol. Rev. 2014;10(1):17–23. doi: 10.2174/1573403X10999140226121300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoffman J.I., Spaan J.A. Pressure-flow relations in coronary circulation. Physiol. Rev. 1990;70(2):331–390. doi: 10.1152/physrev.1990.70.2.331. [DOI] [PubMed] [Google Scholar]
- 9.Duncker D.J., Koller A., Merkus D., Canty J.M., Jr Regulation of coronary blood flow in health and ischemic heart disease. Prog. Cardiovasc. Dis. 2015;57(5):409–422. doi: 10.1016/j.pcad.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van de Hoef T.P., Nolte F., Rolandi M.C., et al. Coronary pressure-flow relations as basis for the understanding of coronary physiology. J. Mol. Cell. Cardiol. 2012;52(4):786–793. doi: 10.1016/j.yjmcc.2011.07.025. [DOI] [PubMed] [Google Scholar]
- 11.van de Hoef T.P., Bax M., Damman P., et al. Impaired coronary autoregulation is associated with long-term fatal events in patients with stable coronary artery disease. Circ. Cardiovasc. Interv. 2013;6(4):329–335. doi: 10.1161/CIRCINTERVENTIONS.113.000378. [DOI] [PubMed] [Google Scholar]
- 12.Jespersen L., Hvelplund A., Abildstrom S.Z., et al. Stable angina pectoris with no obstructive coronary artery disease is associated with increased risks of major adverse cardiovascular events. Eur. Heart J. 2012;33(6):734–744. doi: 10.1093/eurheartj/ehr331. [DOI] [PubMed] [Google Scholar]
- 13.van de Hoef T.P., Bax M., Meuwissen M., et al. Impact of coronary microvascular function on long-term cardiac mortality in patients with acute ST-segment-elevation myocardial infarction. Circ. Cardiovasc. Interv. 2013;6(3):207–215. doi: 10.1161/CIRCINTERVENTIONS.112.000168. [DOI] [PubMed] [Google Scholar]
- 14.Gould K.L., Lipscomb K. Effects of coronary stenoses on coronary flow reserve and resistance. Am. J. Cardiol. 1974;34(1):48–55. doi: 10.1016/0002-9149(74)90092-7. [DOI] [PubMed] [Google Scholar]
- 15.Doucette J.W., Corl P.D., Payne H.M., et al. Validation of a Doppler guide wire for intravascular measurement of coronary artery flow velocity. Circulation. 1992;85(5):1899–1911. doi: 10.1161/01.cir.85.5.1899. [DOI] [PubMed] [Google Scholar]
- 16.Barbato E., Aarnoudse W., Aengevaeren W.R., et al. Validation of coronary flow reserve measurements by thermodilution in clinical practice. Eur. Heart J. 2004;25(3):219–223. doi: 10.1016/j.ehj.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 17.Rigo F., Sicari R., Gherardi S., Djordjevic-Dikic A., Cortigiani L., Picano E. The additive prognostic value of wall motion abnormalities and coronary flow reserve during dipyridamole stress echo. Eur. Heart J. 2008;29(1):79–88. doi: 10.1093/eurheartj/ehm527. [DOI] [PubMed] [Google Scholar]
- 18.Beleslin B., Ostojic M., Djordjevic-Dikic A., et al. The value of fractional and coronary flow reserve in predicting myocardial recovery in patients with previous myocardial infarction. Eur. Heart J. 2008;29(21):2617–2624. doi: 10.1093/eurheartj/ehn418. [DOI] [PubMed] [Google Scholar]
- 19.Pepine C.J., Anderson R.D., Sharaf B.L., et al. Coronary microvascular reactivity to adenosine predicts adverse outcome in women evaluated for suspected ischemia results from the National Heart, Lung and Blood Institute WISE (Women’s Ischemia Syndrome Evaluation) study. J. Am. Coll. Cardiol. 2010;55(25):2825–2832. doi: 10.1016/j.jacc.2010.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Albertal M., Voskuil M., Piek J.J., et al. Coronary flow velocity reserve after percutaneous interventions is predictive of periprocedural outcome. Circulation. 2002;105(13):1573–1578. doi: 10.1161/01.cir.0000012514.15806.dd. [DOI] [PubMed] [Google Scholar]
- 21.Marks D.S., Gudapati S., Prisant L.M., et al. Mortality in patients with microvascular disease. J. Clin. Hypertens. 2004;6(6):304–309. doi: 10.1111/j.1524-6175.2004.03254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoffman J.I. Problems of coronary flow reserve. Ann. Biomed. Eng. 2000;28(8):884–896. doi: 10.1114/1.1308503. [DOI] [PubMed] [Google Scholar]
- 23.Rossen J.D., Winniford M.D. Effect of increases in heart rate and arterial pressure on coronary flow reserve in humans. J. Am. Coll. Cardiol. 1993;21(2):343–348. doi: 10.1016/0735-1097(93)90673-o. [DOI] [PubMed] [Google Scholar]
- 24.Antony I., Nitenberg A., Foult J.M., Aptecar E. Coronary vasodilator reserve in untreated and treated hypertensive patients with and without left ventricular hypertrophy. J. Am. Coll. Cardiol. 1993;22(2):514–520. doi: 10.1016/0735-1097(93)90058-9. [DOI] [PubMed] [Google Scholar]
- 25.Czernin J., Muller P., Chan S., et al. Influence of age and hemodynamics on myocardial blood flow and flow reserve. Circulation. 1993;88(1):62–69. doi: 10.1161/01.cir.88.1.62. [DOI] [PubMed] [Google Scholar]
- 26.Marcus M.L., Doty D.B., Hiratzka L.F., Wright C.B., Eastham C.L. Decreased coronary reserve: a mechanism for angina pectoris in patients with aortic stenosis and normal coronary arteries. N. Engl. J. Med. 1982;307(22):1362–1366. doi: 10.1056/NEJM198211253072202. [DOI] [PubMed] [Google Scholar]
- 27.Baumgart D., Haude M., Goerge G., et al. Improved assessment of coronary stenosis severity using the relative flow velocity reserve. Circulation. 1998;98(1):40–46. doi: 10.1161/01.cir.98.1.40. [DOI] [PubMed] [Google Scholar]
- 28.De Bruyne B., Hersbach F., Pijls N.H., et al. Abnormal epicardial coronary resistance in patients with diffuse atherosclerosis but “Normal” coronary angiography. Circulation. 2001;104(20):2401–2406. doi: 10.1161/hc4501.099316. [DOI] [PubMed] [Google Scholar]
- 29.Ng M.K., Yeung A.C., Fearon W.F. Invasive assessment of the coronary microcirculation: superior reproducibility and less hemodynamic dependence of index of microcirculatory resistance compared with coronary flow reserve. Circulation. 2006;113(17):2054–2061. doi: 10.1161/CIRCULATIONAHA.105.603522. [DOI] [PubMed] [Google Scholar]
- 30.Pijls N.H., van Son J.A., Kirkeeide R.L., De Bruyne B., Gould K.L. Experimental basis of determining maximum coronary, myocardial, and collateral blood flow by pressure measurements for assessing functional stenosis severity before and after percutaneous transluminal coronary angioplasty. Circulation. 1993;87(4):1354–1367. doi: 10.1161/01.cir.87.4.1354. [DOI] [PubMed] [Google Scholar]
- 31.Pijls N.H., De Bruyne B., Peels K., et al. Measurement of fractional flow reserve to assess the functional severity of coronary-artery stenoses. N. Engl. J. Med. 1996;334(26):1703–1708. doi: 10.1056/NEJM199606273342604. [DOI] [PubMed] [Google Scholar]
- 32.Tonino P.A., De Bruyne B., Pijls N.H., et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N. Engl. J. Med. 2009;360(3):213–224. doi: 10.1056/NEJMoa0807611. [DOI] [PubMed] [Google Scholar]
- 33.De Bruyne B., Fearon W.F., Pijls N.H., et al. Fractional flow reserve-guided PCI for stable coronary artery disease. N. Engl. J. Med. 2014;371(13):1208–1217. doi: 10.1056/NEJMoa1408758. [DOI] [PubMed] [Google Scholar]
- 34.Pijls N.H., van Schaardenburgh P., Manoharan G., et al. Percutaneous Coronary Intervention of Functionally Nonsignificant Stenosis: 5-Year Follow-Up of the DEFER Study. J. Am. Coll. Cardiol. 2007;49(21):2105–2111. doi: 10.1016/j.jacc.2007.01.087. [DOI] [PubMed] [Google Scholar]
- 35.Bech G.J., De Bruyne B., Pijls N.H., et al. Fractional flow reserve to determine the appropriateness of angioplasty in moderate coronary stenosis: a randomized trial. Circulation. 2001;103(24):2928–2934. doi: 10.1161/01.cir.103.24.2928. [DOI] [PubMed] [Google Scholar]
- 36.van de Hoef T.P., Siebes M., Spaan J.A., Piek J.J. Fundamentals in clinical coronary physiology: why coronary flow is more important than coronary pressure. Eur. Heart J. 2015:ehv235. doi: 10.1093/eurheartj/ehv235. [DOI] [PubMed] [Google Scholar]
- 37.van de Hoef T.P., Meuwissen M., Escaned J., et al. Fractional flow reserve as a surrogate for inducible myocardial ischaemia. Nat. Rev. Cardiol. 2013;10(8):439–452. doi: 10.1038/nrcardio.2013.86. [DOI] [PubMed] [Google Scholar]
- 38.Perera D., Biggart S., Postema P., et al. Right atrial pressure: can it be ignored when calculating fractional flow reserve and collateral flow index? J. Am. Coll. Cardiol. 2004;44(10):2089–2091. doi: 10.1016/j.jacc.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 39.Toth G.G., De Bruyne B., Rusinaru D., et al. Impact of right atrial pressure on fractional flow reserve measurements: comparison of fractional flow reserve and myocardial fractional flow reserve in 1,600 coronary stenoses. JACC Cardiovasc. Interv. 2016;9(5):453–459. doi: 10.1016/j.jcin.2015.11.021. [DOI] [PubMed] [Google Scholar]
- 40.Echavarria-Pinto M., Escaned J., Macias E., et al. Disturbed coronary hemodynamics in vessels with intermediate stenoses evaluated with fractional flow reserve: a combined analysis of epicardial and microcirculatory involvement in ischemic heart disease. Circulation. 2013;128(24):2557–2566. doi: 10.1161/CIRCULATIONAHA.112.001345. [DOI] [PubMed] [Google Scholar]
- 41.van de Hoef T.P., van Lavieren M.A., Damman P., et al. Physiological basis and long-term clinical outcome of discordance between fractional flow reserve and coronary flow velocity reserve in coronary stenoses of intermediate severity. Circ. Cardiovasc. Interv. 2014;7(3):301–311. doi: 10.1161/CIRCINTERVENTIONS.113.001049. [DOI] [PubMed] [Google Scholar]
- 42.Johnson N.P., Kirkeeide R.L., Gould K.L. Is discordance of coronary flow reserve and fractional flow reserve due to methodology or clinically relevant coronary pathophysiology? JACC Cardiovasc. Imaging. 2012;5(2):193–202. doi: 10.1016/j.jcmg.2011.09.020. [DOI] [PubMed] [Google Scholar]
- 43.Johnson N.P., Toth G.G., Lai D., et al. Prognostic value of fractional flow reserve: linking physiologic severity to clinical outcomes. J. Am. Coll. Cardiol. 2014;64(16):1641–1654. doi: 10.1016/j.jacc.2014.07.973. [DOI] [PubMed] [Google Scholar]
- 44.De Bruyne B., Bartunek J., Sys S.U., Heyndrickx G.R. Relation between myocardial fractional flow reserve calculated from coronary pressure measurements and exercise-induced myocardial ischemia. Circulation. 1995;92(1):39–46. doi: 10.1161/01.cir.92.1.39. [DOI] [PubMed] [Google Scholar]
- 45.Gianrossi R., Detrano R., Mulvihill D., et al. Exercise-induced ST depression in the diagnosis of coronary artery disease. A meta-analysis. Circulation. 1989;80(1):87–98. doi: 10.1161/01.cir.80.1.87. [DOI] [PubMed] [Google Scholar]
- 46.Kennedy M.W., Kaplan E., Hermanides R.S., et al. Clinical outcomes of deferred revascularisation using fractional flow reserve in patients with and without diabetes mellitus. Cardiovasc. Diabetol. 2016;15:100. doi: 10.1186/s12933-016-0417-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lim H.S., Tonino P.A., De Bruyne B., et al. The impact of age on fractional flow reserve-guided percutaneous coronary intervention: a FAME (Fractional Flow Reserve versus Angiography for Multivessel Evaluation) trial substudy. Int. J. Cardiol. 2014;177(1):66–70. doi: 10.1016/j.ijcard.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 48.Kang S.J., Ahn J.M., Han S., et al. Sex differences in the visual-functional mismatch between coronary angiography or intravascular ultrasound versus fractional flow reserve. JACC Cardiovasc. Interv. 2013;6(6):562–568. doi: 10.1016/j.jcin.2013.02.016. [DOI] [PubMed] [Google Scholar]
- 49.Sen S., Escaned J., Malik I.S., et al. Development and validation of a new adenosine-independent index of stenosis severity from coronary wave-intensity analysis: results of the ADVISE (ADenosine Vasodilator Independent Stenosis Evaluation) study. J. Am. Coll. Cardiol. 2012;59(15):1392–1402. doi: 10.1016/j.jacc.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 50.Sen S., Asrress K.N., Nijjer S., et al. Diagnostic classification of the instantaneous wave-free ratio is equivalent to fractional flow reserve and is not improved with adenosine administration. Results of CLARIFY (Classification Accuracy of Pressure-Only Ratios Against Indices Using Flow Study). J. Am. Coll. Cardiol. 2013;61(13):1409–1420. doi: 10.1016/j.jacc.2013.01.034. [DOI] [PubMed] [Google Scholar]
- 51.Johnson N.P., Kirkeeide R.L., Asrress K.N., et al. Does the instantaneous wave-free ratio approximate the fractional flow reserve? J. Am. Coll. Cardiol. 2013;61(13):1428–1435. doi: 10.1016/j.jacc.2012.09.064. [DOI] [PubMed] [Google Scholar]
- 52.Berry C., van ’t Veer M., Witt N., et al. VERIFY (VERification of Instantaneous Wave-Free Ratio and Fractional Flow Reserve for the Assessment of Coronary Artery Stenosis Severity in EverydaY Practice): a multicenter study in consecutive patients. J. Am. Coll. Cardiol. 2013;61(13):1421–1427. doi: 10.1016/j.jacc.2012.09.065. [DOI] [PubMed] [Google Scholar]
- 53.Davies J.E., Sen S., Dehbi H.M., et al. Use of the instantaneous wave-free ratio or fractional flow reserve in PCI. N. Engl. J. Med. 2017;376(19):1824–1834. doi: 10.1056/NEJMoa1700445. [DOI] [PubMed] [Google Scholar]
- 54.Gotberg M., Christiansen E.H., Gudmundsdottir I.J., et al. Instantaneous wave-free ratio versus fractional flow reserve to guide PCI. N. Engl. J. Med. 2017;376(19):1813–1823. doi: 10.1056/NEJMoa1616540. [DOI] [PubMed] [Google Scholar]
- 55.Fearon W.F., Balsam L.B., Farouque H.M., et al. Novel Index for Invasively Assessing the Coronary Microcirculation. Circulation. 2003;107(25):3129–3132. doi: 10.1161/01.CIR.0000080700.98607.D1. [DOI] [PubMed] [Google Scholar]
- 56.Fearon W.F., Low A.F., Yong A.S., et al. Prognostic value of the index of microcirculatory resistance measured after primary percutaneous coronary intervention. Circulation. 2013;127(24):2436–2441. doi: 10.1161/CIRCULATIONAHA.112.000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McGeoch R., Watkins S., Berry C., et al. The index of microcirculatory resistance measured acutely predicts the extent and severity of myocardial infarction in patients with ST-segment elevation myocardial infarction. JACC Cardiovasc. Interv. 2010;3(7):715–722. doi: 10.1016/j.jcin.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 58.Payne A.R., Berry C., Doolin O., et al. Microvascular resistance predicts myocardial salvage and infarct characteristics in ST-elevation myocardial infarction. J. Am. Heart Assoc. 2012;1(4):24. doi: 10.1161/JAHA.112.002246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yoo S.H., Yoo T.K., Lim H.S., Kim M.Y., Koh J.H. Index of microcirculatory resistance as predictor for microvascular functional recovery in patients with anterior myocardial infarction. J. Korean Med. Sci. 2012;27(9):1044–1050. doi: 10.3346/jkms.2012.27.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Carrick D., Haig C., Ahmed N., et al. Comparative prognostic utility of indexes of microvascular function alone or in combination in patients with an acute ST-segment-elevation myocardial infarction. Circulation. 2016;134(23):1833–1847. doi: 10.1161/CIRCULATIONAHA.116.022603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aarnoudse W., van den Berg P., van de Vosse F., et al. Myocardial resistance assessed by guidewire-based pressure-temperature measurement: In vitro validation. C Catheter Cardiovasc Interv. 2004;62(1):56–63. doi: 10.1002/ccd.10793. [DOI] [PubMed] [Google Scholar]
- 62.Aarnoudse W., Fearon W.F., Manoharan G., et al. Epicardial stenosis severity does not affect minimal microcirculatory resistance. Circulation. 2004;110(15):2137–2142. doi: 10.1161/01.CIR.0000143893.18451.0E. [DOI] [PubMed] [Google Scholar]
- 63.Meuwissen M., Siebes M., Chamuleau S.A., et al. Hyperemic stenosis resistance index for evaluation of functional coronary lesion severity. Circulation. 2002;106(4):441–446. doi: 10.1161/01.cir.0000023041.26199.29. [DOI] [PubMed] [Google Scholar]
- 64.Nolte F., van de Hoef T.P., Meuwissen M., et al. Increased hyperaemic coronary microvascular resistance adds to the presence of myocardial ischaemia. EuroIntervention. 2014;9(12):1423–1431. doi: 10.4244/EIJV9I12A240. [DOI] [PubMed] [Google Scholar]
- 65.Meuwissen M., Chamuleau S.A., Siebes M., et al. Role of variability in microvascular resistance on fractional flow reserve and coronary blood flow velocity reserve in intermediate coronary lesions. Circulation. 2001;103(2):184–187. doi: 10.1161/01.cir.103.2.184. [DOI] [PubMed] [Google Scholar]
- 66.Smalling R.W., Kelley K., Kirkeeide R.L., Fisher D.J. Regional myocardial function is not affected by severe coronary depressurization provided coronary blood flow is maintained. J. Am. Coll. Cardiol. 1985;5(4):948–955. doi: 10.1016/s0735-1097(85)80438-1. [DOI] [PubMed] [Google Scholar]
- 67.Niccoli G., Burzotta F., Galiuto L., Crea F. Myocardial no-reflow in humans. J. Am. Coll. Cardiol. 2009;54(4):281–292. doi: 10.1016/j.jacc.2009.03.054. [DOI] [PubMed] [Google Scholar]
- 68.Eeckhout E., Kern M.J. The coronary no-reflow phenomenon: a review of mechanisms and therapies. Eur. Heart J. 2001;22(9):729–739. doi: 10.1053/euhj.2000.2172. [DOI] [PubMed] [Google Scholar]
- 69.Brosh D., Assali A.R., Mager A., et al. Effect of no-reflow during primary percutaneous coronary intervention for acute myocardial infarction on six-month mortality. Am. J. Cardiol. 2007;99(4):442–445. doi: 10.1016/j.amjcard.2006.08.054. [DOI] [PubMed] [Google Scholar]
- 70.Wu K.C., Zerhouni E.A., Judd R.M., et al. Prognostic significance of microvascular obstruction by magnetic resonance imaging in patients with acute myocardial infarction. Circulation. 1998;97(8):765–772. doi: 10.1161/01.cir.97.8.765. [DOI] [PubMed] [Google Scholar]
- 71.Quyyumi A.A., Dakak N., Andrews N.P., et al. Nitric oxide activity in the human coronary circulation. Impact of risk factors for coronary atherosclerosis. J. Clin. Invest. 1995;95(4):1747–1755. doi: 10.1172/JCI117852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cox D.A., Vita J.A., Treasure C.B., et al. Atherosclerosis impairs flow-mediated dilation of coronary arteries in humans. Circulation. 1989;80(3):458–465. doi: 10.1161/01.cir.80.3.458. [DOI] [PubMed] [Google Scholar]
- 73.Murthy V.L., Naya M., Foster C.R., et al. Improved cardiac risk assessment with noninvasive measures of coronary flow reserveclinical perspective. Circulation. 2011;124(20):2215–2224. doi: 10.1161/CIRCULATIONAHA.111.050427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Camici P.G., Gropler R.J., Jones T., et al. The impact of myocardial blood flow quantitation with PET on the understanding of cardiac diseases. Eur. Heart J. 1996;17(1):25–34. doi: 10.1093/oxfordjournals.eurheartj.a014687. [DOI] [PubMed] [Google Scholar]
- 75.Waller AH, Blankstein R, Kwong RY, Di Carli MF. Myocardial blood flow quantification for evaluation of coronary artery disease by positron emission tomography, cardiac magnetic resonance imaging, and computed tomography. Curr Cardiol Rep. 2014;16(5):014–0483. doi: 10.1007/s11886-014-0483-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Camici P.G., Rimoldi O.E. The clinical value of myocardial blood flow measurement. J. Nucl. Med. 2009;50(7):1076–1087. doi: 10.2967/jnumed.108.054478. [DOI] [PubMed] [Google Scholar]
- 77.Kuhle W.G., Porenta G., Huang S.C., et al. Quantification of regional myocardial blood flow using 13N-ammonia and reoriented dynamic positron emission tomographic imaging. Circulation. 1992;86(3):1004–1017. doi: 10.1161/01.cir.86.3.1004. [DOI] [PubMed] [Google Scholar]
- 78.Klein R., Beanlands R.S., deKemp R.A. Quantification of myocardial blood flow and flow reserve: Technical aspects. J. Nucl. Cardiol. 2010;17(4):555–570. doi: 10.1007/s12350-010-9256-9. [DOI] [PubMed] [Google Scholar]
- 79.Kellman P., Arai A.E. Imaging sequences for first pass perfusion --a review. J. Cardiovasc. Magn. Reson. 2007;9(3):525–537. doi: 10.1080/10976640601187604. [DOI] [PubMed] [Google Scholar]
- 80.Radjenovic A., Biglands J.D., Larghat A., et al. Estimates of systolic and diastolic myocardial blood flow by dynamic contrast-enhanced MRI. Magn. Reson. Med. 2010;64(6):1696–1703. doi: 10.1002/mrm.22538. [DOI] [PubMed] [Google Scholar]
- 81.Tomiyama Y., Manabe O., Oyama-Manabe N., et al. Quantification of myocardial blood flow with dynamic perfusion 3.0 Tesla MRI: Validation with (15) O-water PET. J. Magn. Reson. Imaging. 2015;42(3):754–762. doi: 10.1002/jmri.24834. [DOI] [PubMed] [Google Scholar]
- 82.Morton G., Chiribiri A., Ishida M., et al. Quantification of absolute myocardial perfusion in patients with coronary artery disease: comparison between cardiovascular magnetic resonance and positron emission tomography. J. Am. Coll. Cardiol. 2012;60(16):1546–1555. doi: 10.1016/j.jacc.2012.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Beigel R., Oieru D., Goitein O., et al. Usefulness of routine use of multidetector coronary computed tomography in the “fast track” evaluation of patients with acute chest pain. Am. J. Cardiol. 2009;103(11):1481–1486. doi: 10.1016/j.amjcard.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 84.Blankstein R., Shturman L.D., Rogers I.S., et al. Adenosine-induced stress myocardial perfusion imaging using dual-source cardiac computed tomography. J. Am. Coll. Cardiol. 2009;54(12):1072–1084. doi: 10.1016/j.jacc.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 85.Koo B.K., Erglis A., Doh J.H., et al. Diagnosis of ischemia-causing coronary stenoses by noninvasive fractional flow reserve computed from coronary computed tomographic angiograms. Results from the prospective multicenter DISCOVER-FLOW (Diagnosis of Ischemia-Causing Stenoses Obtained Via Noninvasive Fractional Flow Reserve) study. J. Am. Coll. Cardiol. 2011;58(19):1989–1997. doi: 10.1016/j.jacc.2011.06.066. [DOI] [PubMed] [Google Scholar]
- 86.Wu W., Pan D.R., Foin N., et al. Noninvasive fractional flow reserve derived from coronary computed tomography angiography for identification of ischemic lesions: a systematic review and meta-analysis. Sci. Rep. 2016;6:29409. doi: 10.1038/srep29409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ho K.T., Chua K.C., Klotz E., Panknin C. Stress and rest dynamic myocardial perfusion imaging by evaluation of complete time-attenuation curves with dual-source CT. JACC Cardiovasc. Imaging. 2010;3(8):811–820. doi: 10.1016/j.jcmg.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 88.Kikuchi Y., Oyama-Manabe N., Naya M., et al. Quantification of myocardial blood flow using dynamic 320-row multi-detector CT as compared with (1)(5)O-H(2)O PET. Eur. Radiol. 2014;24(7):1547–1556. doi: 10.1007/s00330-014-3164-3. [DOI] [PubMed] [Google Scholar]
- 89.Schwarz F., Hinkel R., Baloch E., et al. Myocardial CT perfusion imaging in a large animal model: comparison of dynamic versus single-phase acquisitions. JACC Cardiovasc. Imaging. 2013;6(12):1229–1238. doi: 10.1016/j.jcmg.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 90.Group* TTS. The thrombolysis in myocardial infarction (TIMI) trial. N. Engl. J. Med. 1985;312(14):932–936. doi: 10.1056/NEJM198504043121437. [DOI] [PubMed] [Google Scholar]
- 91.Gibson C.M., Cannon C.P., Murphy S.A., et al. Relationship of TIMI myocardial perfusion grade to mortality after administration of thrombolytic drugs. Circulation. 2000;101(2):125–130. doi: 10.1161/01.cir.101.2.125. [DOI] [PubMed] [Google Scholar]
- 92.Gibson C.M., Cannon C.P., Murphy S.A., Marble S.J., Barron H.V., Braunwald E. Relationship of the TIMI myocardial perfusion grades, flow grades, frame count, and percutaneous coronary intervention to long-term outcomes after thrombolytic administration in acute myocardial infarction. Circulation. 2002;105(16):1909–1913. doi: 10.1161/01.cir.0000014683.52177.b5. [DOI] [PubMed] [Google Scholar]
- 93.Ng V.G., Lansky A.J., Toro S., et al. Prognostic utility of myocardial blush grade after PCI in patients with NSTE-ACS: Analysis from the ACUITY trial. Catheter. Cardiovasc. Interv. 2016;88(2):215–224. doi: 10.1002/ccd.25865. [DOI] [PubMed] [Google Scholar]
- 94.Aarnoudse W., Van’t Veer M., Pijls N.H., et al. Direct volumetric blood flow measurement in coronary arteries by thermodilution. J. Am. Coll. Cardiol. 2007;50(24):2294–2304. doi: 10.1016/j.jacc.2007.08.047. [DOI] [PubMed] [Google Scholar]
- 95.Ganz W., Tamura K., Marcus H.S., Donoso R., Yoshida S., Swan H.J. Measurement of coronary sinus blood flow by continuous thermodilution in man. Circulation. 1971;44(2):181–195. doi: 10.1161/01.cir.44.2.181. [DOI] [PubMed] [Google Scholar]
- 96.Molloi S., Ersahin A., Tang J., Hicks J., Leung C.Y. Quantification of volumetric coronary blood flow with dual-energy digital subtraction angiography. Circulation. 1996;93(10):1919–1927. doi: 10.1161/01.cir.93.10.1919. [DOI] [PubMed] [Google Scholar]
- 97.Guggenheim N., Dorsaz P.A., Doriot P.A., Suilen C., Chappuis F., Rutishauser W. 3D determination of the intravascular volume and flow of coronary arteries. Int. J. Biomed. Comput. 1994;35(1):13–23. doi: 10.1016/0020-7101(94)90045-0. [DOI] [PubMed] [Google Scholar]
- 98.Morris P.D., Ryan D., Morton A.C., et al. Virtual fractional flow reserve from coronary angiography: modeling the significance of coronary lesions: results from the VIRTU-1 (VIRTUal Fractional Flow Reserve From Coronary Angiography) study. JACC Cardiovasc. Interv. 2013;6(2):149–157. doi: 10.1016/j.jcin.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 99.Molloi S., Bednarz G., Tang J., Zhou Y., Mathur T. Absolute volumetric coronary blood flow measurement with digital subtraction angiography. Int. J. Card. Imaging. 1998;14(3):137–145. doi: 10.1023/a:1006059709539. [DOI] [PubMed] [Google Scholar]
- 100.Zhang Z., Takarada S., Molloi S. Assessment of coronary microcirculation in a swine animal model. Am. J. Physiol. Heart Circ. Physiol. 2011;301(2):H402–H408. doi: 10.1152/ajpheart.00213.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]