Abstract
Background:
The “hepatic factor,” a molecule or group of molecules present in the hepatic venous blood, essential for the prevention of the development of pulmonary arteriovenous malfor-mations (PAVMs) and right-to-left shunting has been a conceptual enigma in the understanding of many related conditions.
Methods:
Patients with various forms of liver diseases including acute hepatic failure, and others with normal hepatic function like hereditary hemorrhagic telangiectasia (HHT), inflammatory and parasitic disorders, cardiogenic hepatopulmonary syndrome (cHPS) and skin disorders like Dyskeratosis con-genita are all known to cause PAVMs. Over a period of the last two decades our understanding of the pathogenesis of PAVMs has changed, but the mechanisms are still not clearly understood. The pres-ence of PAVMs once considered a contraindication for liver transplantation is now a cure for PAVMs in patients with HPS.
Results:
In this article the molecular mechanisms and the underlying pathogenesis of PAVMs are dis-cussed and the role of microRNA (miRNA) in its pathogenesis is favorably argued. Identifying and preventing or treating the underlying mechanisms will significantly influence the management of a large group of patients who at present cannot be effectively treated with a very poor prognosis. Progressive polycythemia, desaturation, stroke, and infection are serious complications of PAVMs.
Conclusion:
The clinical data and current understanding leads to the possible role of miRNA, which inhibits Vascular Endothelial Growth Factor (VEGF) synthesis as a pathogenic mechanism for the development of PAVMs.
Keywords: Endoglin, hepatic factor, hepatopulmonary syndrome, hereditary hemorrhagic telangiectasia, miRNA, pulmonary arteriovenous malformations, vascular endothelial growth factor
1. INTRODUCTION
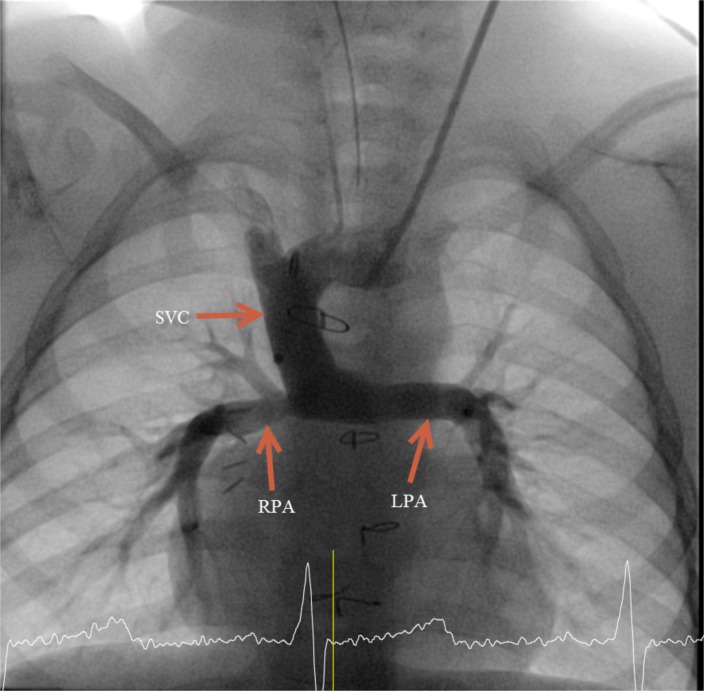
The hypothesis for the presence of a “hepatic factor” in the human circulation was first raised in 1995 as an explanation for desaturation associated with pulmonary arteriovenous malformations (PAVMs) in children with complex single ventricle palliation [1]. In this condition, one of the ventricles is too small to support the circulation, and the developed ventricle is used to pump blood into the aorta; the systemic veins are connected directly in to the pulmonary arteries (PAs). The palliation is done in stages to prepare the lung vasculature for a low pressure circulation so the veins can flow directly into the PAs. Often the superior vena cava (SVC) is connected in the first stage of surgery to the PAs followed by the inferior vena cava (IVC), resulting in a palliated state known as the Fontan circulation. The initial connection of the SVC to the PA is known as the superior cavopulmonary anastomosis (SCPA) or the Glenn procedure (Fig. 1).
Fig. (1).
Angiogram of a patient with SCPA showing injection of contrast into the SVC flowing directly into the pulmonary arteries.
In some patients with atrial isomerism, the IVC ascends above the diaphragm and connects into the SVC; the hepatic veins drain directly into the atria. When the SVC is connected to the PA, all of the venous flow except the hepatic vein is connected to the lungs. This repair is known as the Kawashima operation.
Patients with SCPA and Kawashima procedure develop progressive desaturation. This was attributed to a combination of factors including
Relative decrease in venous return due to the proportionate growth and relative decrease of the venous return from the upper part of the body;
Non pulsatile nature of the blood flow through the PAs due to the absence of the pumping action of the ventricle;
The disparity in differential flow (too much flow to the dependent areas of the lungs), and
The absence of hepatic venous effluent in the pulmonary circulation, which may contain the factor(s) necessary to maintain the integrity of the pulmonary vasculature.
It was postulated that the absence of hepatic venous effluent from the pulmonary circulation is the probable cause of intrapulmonary shunting and the development of PAVMs observed in these patients [2]. For patients with anatomic anomalies, restoration of the normal hepato-pulmonary venous relationship results in resolution of the AVMs [3]. However, children undergoing SCPA [4] to palliate single ventricle heart defects cannot have the normal hepato-pulmonary circulation restored until they are old enough to undergo the Fontan procedure. In the meantime, AVMs may cause significant morbidity in these patients. In fact, we have shown that all patients with the Glenn anatomy have pulmonary AV shunts demonstrable by radionucleotide scan even in the absence of angiographically apparent AVMs [5, 6]. A landmark paper by Knight and Mee [7] showing the resolution of PAVMs after reconnection of the hepatic veins into the pulmonary circulation in Kawashima patients led to further studies in the field. Their scientific contribution forms the basis of the concept of a factor in the hepatic venous blood that is essential to preserve the normal integrity of the pulmonary vasculature: the Hepatic Factor.
2. CURRENT UNDERSTANDING OF PAVMs
Patients with various forms of liver disease also develop intrapulmonary shunting and hypoxemia associated with PAVMs, a condition known as hepatopulmonary syndrome (HPS). The association of intrapulmonary shunting and hypoxemia in patients with liver disease was first described in 1884 [8], but the term HPS was first coined in 1977 [9]. Over a period of time our understanding and thus the definition of HPS has changed. This has led to liver transplantation as a cure for HPS, which was once a contraindication [10].
HPS is associated with fulminant hepatic failure, cirrhosis, portal hypertension, and rejection of allograft liver transplant. The histopathology studies of HPS shows diffuse or localized pre- & post capillary vasodilatation, pleural AVMs and porto-pulmonary collaterals [11]. There have been various mechanisms postulated to explain the pathogenesis of HPS, which includes the failure of the damaged liver to clear circulating vasodilators, production of vasodilators by the damaged liver, inhibition of vasoconstrictors by the damaged liver and blunted hypoxic pulmonary vasoconstriction. There was also postulation of a factor present in the portal venous blood that causes PAVMs called a Portal venous Factor or Fistulin [5, 6].
The occurrence of HPS in the presence of normal liver function has led to the concept that the factors present in the portal venous blood are responsible for the pathogenesis of PAVMs and the role of the liver is to inhibit these factors before they reach the lungs. However, if the portal blood is already processed by the liver as in patients with SCPA, then diversion of hepatic blood flow should not cause AVMS. Thus, the final substrate for the development of PAVMs reaches the lungs from the systemic veins and the role of the liver is to inhibit those factors present in the venous blood. In other words, the liver silences the vasodilatory influences present in the systemic venous blood, which is perhaps more abundant in the portal venous blood.
About 25% of the systemic venous return is from the hepato-portal system of which 75% is from the portal vein. Accessing portal venous blood to learn about its constituents and metabolites is difficult and once it traverses the liver parenchyma, the constituents are altered after metabolism in the liver. The hepatic venous effluents are significantly different from venous blood coming from other veins due to synthetic function of the liver and any foreign material entering through the gastrointestinal system including the endotoxins are processed by the liver. Hence looking in the hepatic venous effluent for a hepatic factor is extremely difficult. Hepatic Kupffer cells and macrophages act as scavengers of bacterial products and in the presence of severe liver disease or diversion of portal blood as in portal hypertension, these products can enter the circulation causing systemic vasodilatation through the inducible NO pathway.
Luo and Fallon have done extensive studies on a rat model of HPS for decades [12, 13]. Their common bile duct ligation model of HPS has led to various hypotheses to explain the pathogenesis of HPS. This includes over expression of endothelin-1 (ET-1), tumor necrosis factor alpha (TNF-α), heme oxygenase-1, nitric oxide (NO), and endothelin receptor type B (ETB). Although portal vein ligation alone does not cause elevation of ET-1 or TNF-α, administering ET-1 in them leads to HPS. This led to the belief that ET-1, a vasoconstrictor, is the cause for HPS. However, other causes of liver damage by Thioacetamide did not cause HPS. These rats showed evidence of over expression of TNF-α and ETB. One of the most significant findings from the study is that TNF-α inhibition with pentoxifylline prevents the development of HPS [14].
The term Cardiogenic HPS (cHPS) was coined to distinguish the intrapulmonary shunting resulting from SCPA or Kawashima procedure from other causes of HPS [6]. The diagnostic features of cHPS are:
Rapid transit of blood through pulmonary circulation,
Reticular appearance on angiogram,
Presence of positive bubble contrast study, which could also be due to veno-venous collaterals and
Desaturation at rest in the absence of lung disease.
If the absence of hepatic venous effluent in the pulmonary circulation as in cHPS is the cause for intrapulmonary shunting and development of PAVMs, then there may be a unifying pathologic mechanism to explain HPS in both liver disease and CHD. If we consider that the absence of hepatic venous effluent in the pulmonary circulation is the cause for cHPS, then one has to agree that the HPS and PAVMs associated with liver disease have a common pathogenesis.
Although the problem of PAVMs was described in 1897 [15], the question of the influence of hepatic venous blood in maintaining normal pulmonary vascularity and its contribution in development of PAVMs was only postulated in 1995 [1].
In a study by Srivastava looking at patients with SCPA from 1970-1993, it was found that exclusion of hepatic veins from the pulmonary circulation was the one and only cause for PAVM associated with SCPA [1]. However, PAVMs occur in only 21% of Kawashima patients with a 28% occurrence rate within the first 5 years [1].
Those palliated with SCPA developed varying degrees of arteriovenous shunting before manifesting macroscopic AVMs. This was postulated to be due to the sustained and inappropriate vasodilatation and may lead to gross structural alterations in vulnerable areas, which manifest as macroscopic AVMs. Possible hepatic factor from the competitive flow was shown to reduce the intra pulmonary shunting [5, 6].
There is now clinical evidence confirming that in those hypoxemic patients with single ventricle circulation palliated with SCPA and exclusion of hepatic venous blood from the pulmonary circulation have a fistula promoting factor(s) in the systemic venous blood which is inhibited by the “hepatic factor” from the liver effluent. By redirecting the hepatic flow to the PAs in Kawashima patients, the PAVMs resolve and prevent further development and progression similar to those after liver transplantation or resolution of the hepatic injury in HPS.
Our understanding from HPS and cHPS defines HF as “a factor produced or modified by the liver and removed by the circulation on first pass and is essential for maintaining the integrity of the pulmonary micro circulation” [5, 6].
There are other known associations with PAVMs with normal liver function. These include: Hereditary Hemorrhagic Telangiectasia (HHT) a hereditary form of diffuse AVMs [16], parasitic infections, skin disorders like Dyskeratosis congenita [17], immunogenic causes and rare anomalies like isolated abnormal drainage of the hepatic veins into the left atrium [18]. Research into their etiology will help in understanding the pathogenesis of this complex condition. In the setting of cHPS, there have been multiple animal studies performed to delineate the causative factor(s). Table 1 provides a summary of these and gives some insights into the pathogenesis of PAVMs from all animal studies conducted from 2000 to 2013 [19-30].
Table 1.
Summary of all the animal studies in to the pathogenesis of PAVMs from 2000 to 2013.
| Study | Animal model | SVC blood | IVC blood | Analysis | Candidate molecule | Results | Mechanism of PAVM |
|---|---|---|---|---|---|---|---|
| Malhotra 2001 | Lamb N=24 |
RPA | RA-RV-LPA | Tissue mRNA expression Blood from RPA |
ACE Angiotensin II |
Reversible decreased ACE activity in lung | Enzyme involved in angiogenesis inhibition |
| Malhotra 2002 | Sheep | Tissue | Angiotensin receptors | Increased type 1, 2 angiotensin receptors | Pathological vascular remodeling | ||
| Malhotra 2002 | Lamb N=6 |
HIF | Upregulation of HO1, GLUT1 | Oxidative stress | |||
| Starnes 2002 | Rat N=8 |
Tissue microscopy Immunohistochemistry |
Nil | Time dependent increase in micro-vessel density in the shunted lung | Angiogenesis | ||
| Ikai 2004 | Lamb N=6 |
RA-RV-LPA LPA band |
Tissue-lung Western blot, Immunostaining |
HGF Cmet Bcl2 |
C-met expression increased in lung | Growth factor in angiogenesis anti-apoptotic |
|
| Ikai 2004 | Rabbit | RA-RV-LPA | Tissue | HIF1α | Lack of hypoxic pulmonary vasoconstriction | Oxidative stress | |
| Mumtaz 2004 | Rat N-=3 |
RA-RV-LPA | Tissue mRNA expression | VEGF | Progressive increase in VEGF mRNA | Final pathway VEGF | |
| Ikai 2005 | Rabbit | RPA/ PA band | RA-RV-LPA | Tissue | Nil | Even partial maintenance of right lung blood supply (from hepatic vein) maintains hypoxic pulmonary vasoconstriction | Oxidative stress |
| McMullan 2008 | Lamb N=23 |
RPA | RA-RV-LPA | Tissue morphology | Nil | Morphology of PAVMs | |
| Kavarana 2013 | Porcine N=5 |
RPA | RA-RV-LPA | Tissue gene expression | Angiopoietin1 TIE2 (Angiopoietin receptor) Angiostatin |
Conversion of PAEC to a proangiogenic phenotype Increased proliferation and tubule formation |
Angiogenesis HIF Gene expression not different |
| Henaine 2013 | Porcine N=10 |
RPA/PA band | RA-RV-LPA | Tissue | Pulsatile flow | PAVMs, Increased PAP, PVR in non-pulsatile > micro-pulsatile > pulsatile | Antegrade pulsatile flow prevents PAVMs |
Note. 11 studies using Rabbit, Lamb, pigs and rats. SVC blood connected to RPA and IVC blood from IVC to RA to LPA after banding or disconnecting the RPA. Looked for blood or tissue for ACE, Angiotensin, HGF, HIF, VEGF, Angiopoietin, angiopoietin receptor or angiostatin or to define the pulsatility of pulmonary blood flow. There is evidence of angiogenesis in the lungs, ACE is involved in inhibition of angiogenesis, and the final pathway for PAVMs is through VEGF evidenced by progressive increase in VEGF mRNA [19-30].
3. PAVMS and HHT
There are 3 different types of HHTs known. Type 1 is related to mutation of Endoglin (ENG) which has the highest incidence of PAVMs. Type 2 is related to Activin-like receptor kinase (ACVRL1); PAVMs are the most common cause of hemorrhagic stroke in young adults. Both ENG and ACVRL1 are involved in the vascular development. Mutations of ENG and ACVRL1gene are known to be associated with PAVMs. These are auxiliary receptors for the TGF family of ligands which are expressed in vascular endothelial cells and are involved in vascular development (Table 2). Mouse models with conditional mutation of ENG gene on angiogenic stimulation developed delayed remodeling of the capillary plexus and increased proliferation of endothelial cells with localized AVMs in the retina. Muscularization of the blood vessels occurred due to increased blood flow. Most conditions associated with PAVMs have generalized AMVs in their skin, brain, liver and other organs. In these mouse models ENG loss and angiogenesis (VEGF) lead to AVMs.
Table 2.
TGF beta family of ligands, identified receptors and inhibitors.
| TGF Beta Superfamily Ligand | Type II Receptor | Type I Receptor | R-SMADs | coSMAD | Ligand Inhibitors |
|---|---|---|---|---|---|
| Activin A | ACVR2A | ACVR1B (ALK4) | SMAD2, SMAD3 | SMAD4 | Follistatin |
| GDF1 | ACVR2A | ACVR1B (ALK4) | SMAD2, SMAD3 | SMAD4 | |
| GDF11 | ACVR2B | ACVR1B (ALK4), TGFβRI (ALK5) |
SMAD2, SMAD3 | SMAD4 | |
| Bone morphogenetic proteins | BMPR2 | BMPR1A (ALK3), BMPR1B (ALK6) |
SMAD1 SMAD5, SMAD8 |
SMAD4 | Noggin, Chordin, DAN |
| Nodal | ACVR2B | ACVR1B (ALK4), ACVR1C (ALK7) |
SMAD2, SMAD3 | SMAD4 | Lefty |
| TGFβs | TGFβRII | TGFβRI (ALK5) | SMAD2, SMAD3 | SMAD4 | LTBP1, THBS, Decorin |
It has been shown that TNF-α causes local ENG depletion leading to transient ENG null phenotype. The inflammatory loss of ENG combined with angiogenic stimuli mediated through VEGF leads to local AVM formation. Persistent hypoxemia in cHPS is a potent angiogenic stimuli mediated through VEGF. Our studies (unpublished data) have shown that VEGF levels are significantly increased in patients with cHPS with macroscopic PAVMs. Increase in TNF-α occurs in cHPS, liver diseases, DKC and other known causes of PAVMs. These correspond to spider nevi or capillary dilations in hepatopulmonary syndrome. PAVMS, without evidence of hemangiomas elsewhere, is unique for cHPS [31].
4. THE HYPOTHESIS: ROLE OF MIRNA IN THE PATHOGENESIS OF HPS
The discovery of miRNA and further delineation of its role in post transcriptional gene regulation have revolutionized our understanding of gene expression in humans. miR
NAs are small non-coding RNA molecules (containing about 22 nucleotides) that function predominantly by silencing translation of messenger RNA (mRNA) and targeting mRNA for degradation [32]. Thus far, more than 1400 miRNA sequences have been identified [33]. Due to the inhibitory nature of the miRNA and our current understanding of hepatic factor as an inhibitor of the formation of PAVMs, we hypothesize that the liver may secrete miRNAs into circulation that play a key regulatory role in vascular homeostasis in the lungs. A recently proposed molecular pathway suggests that secretion of TGF-β by endothelial cells stimulates the transfer of miRNA 143/145 from vascular smooth muscle to endothelial cells. miRNA 155 is also implicated in vascular neogenesis [34].
Currently, a prospective study is underway to define and characterize the miRNA in the hepatic veins in comparison to systemic and pulmonary venous circulation.
5. PATHOGENESIS OF PAVMs
There has been extensive research into the pathogenesis of PAVMs and associated disorders. HPS and PAVMs associated with liver disorders were initially thought to be the result of acute hepatic decompensation. Subsequently various liver disorders including acute and chronic liver disease have been shown to cause hepatopulmonary syndrome and PAVMs. The association of various other conditions with normal liver function discussed in this review points to a common pathogenic mechanism for the development of PAVMs. Thus a unifying hypothesis of an inhibitory factor(s) produced from the liver that silences the vasodilatory and vasoneogenic influences in the venous blood has emerged. Recent studies into the pathogenesis of PAVMs points to the role of VEGF and conditional loss of ENGs which modulates the effect of HHT and TNF on TGF beta family of ligands. This is further supported by the significantly high expressions of VEGF mRNA in the liver, spleen and intestine in post-partial hepatectomised rats compared with pre-hepatectomised rats [35] signifying the elevated levels of VEGF in the portal system and the role of the liver in its regulation. It is also now established that blocking VEGF activity reverses the hyperdynamic circulation and vascular neogenesis induced by portal hypertension [36].
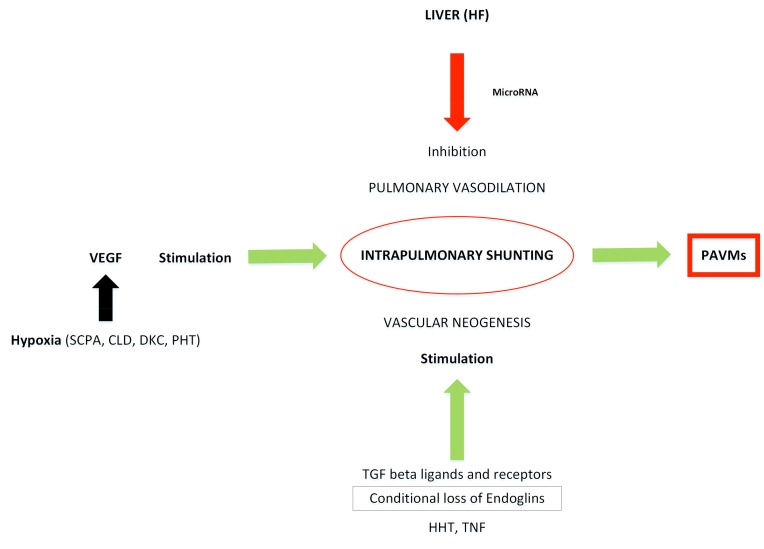
If one takes into consideration all the known causes and association of PAVMs and assuming that the pathogenesis is interrelated, then the following facts emerge (Fig. 2):
Fig. (2).
Schematic diagram illustrating the molecular basis for the development of PAVMs.
There is a unique role of hepatic venous effluent in the development of PAVMs. This is closely associated with interrupted inferior vena cava and hepatic venous diversion from the pulmonary circulation as in cHPS.
Molecular mechanism of hepatopulmonary syndrome includes TGF-β signaling pathway with significant contribution from the ligands of molecules that influence these receptors (like ENG, ACVRL1 as in HHT).
Mediators of inflammation especially tumor necrosis factor (TNFα), may have a role in the development of PAVMs by causing conditional loss of ENG in the pathogenesis of PAVMs in infections and inflammation.
High levels of VEGF in the portal venous blood and hepatic vein as in liver disease or partial hepatectomised animals and high levels of VEGF in patients with PAVMs suggest its causative role in the pathogenesis of PAVMs.
The primary inhibitory role of miRNA in the post transcriptional gene regulation and recent studies indicating significantly elevated levels of VEGF in the hepatic venous blood points to the role of miRNA which inhibits VEGF synthesis as the possible pathogenic mechanism.
CONCLUSION
Understanding the molecular mechanism and defining the hepatic factor(s) involved in the pathogenesis of PAVMs will significantly influence the management of a large group of patients who at present cannot be effectively treated for a condition with very poor prognosis. Progressive polycythemia, desaturation, stroke, and infection are serious complications of PAVMs.
Identification of miRNA which inhibits VEGF synthesis with its presumed role in the pathogenesis of PAVMs may lead to further studies aimed at delineating the mechanisms and prevention of PAVMs. Currently, a study is underway to characterize these miRNAs in human circulation.
ACKNOWLEDGEMENTS
Declared none.
LIST OF ABBREVIATIONS
- AVM
Arteriovenous malformation
- cHPS
Cardiogenic hepatopulmonary syndrome
- ENG
Endoglin
- ET-1
Endothelin-1
- ETB
Endothelin receptor type B
- HHT
Hereditary hemorrhagic telangiectasia
- HPS
Hepatopulmonary syndrome
- IVC
Inferior vena cava
- NO
Nitric oxide
- PA
Pulmonary artery
- PAVM
Pulmonary arteriovenous malformation
- SCPA
Superior cavopulmonary anastomosis
- SVC
Superior vena cava
- TNF-α
Tumor necrosis factor alpha
- VEGF
Vascular endothelial growth factor
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The author confirms that this article content has no conflict of interest.
REFERENCES
- 1.Srivastava D., Preminger T., Lock J.E., et al. Hepatic venous blood and the development of pulmonary arteriovenous malformations in congenital heart disease. Circulation. 1995;92(5):1217–1222. doi: 10.1161/01.cir.92.5.1217. [DOI] [PubMed] [Google Scholar]
- 2.Stoller J.K., Hoffman R.M., White R.D., Mee R.B. Anomalous hepatic venous drainage into the left atrium: an unusual cause of hypoxemia. Respir. Care. 2003;48(1):58–62. [PubMed] [Google Scholar]
- 3.Shah M.J., Rychik J., Fogel M.A., Murphy J.D., Jacobs M.L. Pulmonary AV malformations after superior cavopulmonary connection: resolution after inclusion of hepatic veins in the pulmonary circulation. Ann. Thorac. Surg. 1997;63(4):960–963. doi: 10.1016/s0003-4975(96)00961-7. [DOI] [PubMed] [Google Scholar]
- 4.Glenn W.W. Circulatory bypass of the right side of the heart. IV. Shunt between superior vena cava and distal right pulmonary artery; report of clinical application. N. Engl. J. Med. 1958;259(3):117–120. doi: 10.1056/NEJM195807172590304. [DOI] [PubMed] [Google Scholar]
- 5.Vettukattil J.J., Slavik Z., Lamb R.K., et al. Intrapulmonary arteriovenous shunting may be a universal phenomenon in patients with the superior cavopulmonary anastomosis: a radionuclide study. Heart. 2000;83(4):425–428. doi: 10.1136/heart.83.4.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vettukattil J.J. Pathogenesis of pulmonary arteriovenous malformations: role of hepatopulmonary interactions. Heart. 2002;88(6):561–563. doi: 10.1136/heart.88.6.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Knight W.B., Mee R.B. A cure for pulmonary arteriovenous fistulas? Ann. Thorac. Surg. 1995;59(4):999–1001. doi: 10.1016/0003-4975(94)00735-p. [DOI] [PubMed] [Google Scholar]
- 8.Fluckinger M. Vorkommen von Trommelschagelformigen fingerend Phalagen ohne chronische Veranderungen an der Lungen oder am Herzen. Wien. Med. Wochenschr. 1884;49:1457. [Google Scholar]
- 9.Kennedy T.C., Knudson R.J. Exercise-aggravated hypoxemia and orthodeoxia in cirrhosis. Chest. 1977;72(3):305–309. doi: 10.1378/chest.72.3.305. [DOI] [PubMed] [Google Scholar]
- 10.Gupta S., Castel H., Rao R.V., et al. Improved survival after liver transplantation in patients with hepatopulmonary syndrome. Am. J. Transplant. 2010;10(2):354–363. doi: 10.1111/j.1600-6143.2009.02822.x. [DOI] [PubMed] [Google Scholar]
- 11.Williams A., Trewby P., Williams R., Reid L. Structural alterations to the pulmonary circulation in fulminant hepatic failure. Thorax. 1979;34(4):447–453. doi: 10.1136/thx.34.4.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luo B., Abrams G.A., Fallon M.B. Endothelin-1 in the rat bile duct ligation model of hepatopulmonary syndrome: correlation with pulmonary dysfunction. J. Hepatol. 1998;29(4):571–578. doi: 10.1016/s0168-8278(98)80152-9. [DOI] [PubMed] [Google Scholar]
- 13.Luo B., Liu L., Tang L., Zhang J., Ling Y., Fallon M.B. ET-1 and TNF-alpha in HPS: analysis in prehepatic portal hypertension and biliary and nonbiliary cirrhosis in rats. Am. J. Physiol. Gastrointest. Liver Physiol. 2004;286(2):G294–G303. doi: 10.1152/ajpgi.00298.2003. [DOI] [PubMed] [Google Scholar]
- 14.Zhang J., Ling Y., Tang L., et al. Pentoxifylline attenuation of experimental hepatopulmonary syndrome. J. Appl. Physiol. 2007;102(3):949–955. doi: 10.1152/japplphysiol.01048.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Churton T. Multiple aneurysm of pulmonary artery. BMJ. 1897;1:1223. [Google Scholar]
- 16.Garrido-Martin E.M., Nguyen H.L., Cunningham T.A., et al. Common and distinctive pathogenetic features of arteriovenous malformations in hereditary hemorrhagic telangiectasia 1 and hereditary hemorrhagic telangiectasia 2 animal models--brief report. Arterioscler. Thromb. Vasc. Biol. 2014;34(10):2232–2236. doi: 10.1161/ATVBAHA.114.303984. [DOI] [PubMed] [Google Scholar]
- 17.Samuel B.P., Duffner U.A., Abdel-Mageed A.S., Vettukattil J.J. Pulmonary arteriovenous malformations in dyskeratosis congenita. Pediatr. Dermatol. 2015;32(4):e165–e166. doi: 10.1111/pde.12589. [DOI] [PubMed] [Google Scholar]
- 18.Lee J., Menkis A.H., Rosenberg H.C. Reversal of pulmonary arteriovenous malformation after diversion of anomalous hepatic drainage. Ann. Thorac. Surg. 1998;65(3):848–849. doi: 10.1016/s0003-4975(98)00011-3. [DOI] [PubMed] [Google Scholar]
- 19.Malhotra S.P., Riemer R.K., Thelitz S., He Y.P., Hanley F.L., Reddy V.M. Superior cavopulmonary anastomosis suppresses the activity and expression of pulmonary angiotensin-converting enzyme. J. Thorac. Cardiovasc. Surg. 2001;122(3):464–469. doi: 10.1067/mtc.2001.115698. [DOI] [PubMed] [Google Scholar]
- 20.Malhotra S.P., Reddy V.M., Thelitz S., et al. Cavopulmonary anastomosis induces pulmonary expression of the angiotensin II receptor family. J. Thorac. Cardiovasc. Surg. 2002;123(4):655–660. doi: 10.1067/mtc.2002.119699. [DOI] [PubMed] [Google Scholar]
- 21.Malhotra S.P., Reddy V.M., Thelitz S., et al. The role of oxidative stress in the development of pulmonary arteriovenous malformations after cavopulmonary anastomosis. J. Thorac. Cardiovasc. Surg. 2002;124(3):479–485. doi: 10.1067/mtc.2002.120346. [DOI] [PubMed] [Google Scholar]
- 22.Starnes S.L., Duncan B.W., Fraga C.H., et al. Rat model of pulmonary arteriovenous malformations after right superior cavopulmonary anastomosis. Am. J. Physiol. Heart Circ. Physiol. 2002;283(5):H2151–H2156. doi: 10.1152/ajpheart.00368.2002. [DOI] [PubMed] [Google Scholar]
- 23.Ikai A., Riemer R.K., Ma X., Reinhartz O., Hanley F.L., Reddy V.M. Pulmonary expression of the hepatocyte growth factor receptor c-Met shifts from medial to intimal layer after cavopulmonary anastomosis. J. Thorac. Cardiovasc. Surg. 2004;127(5):1442–1449. doi: 10.1016/j.jtcvs.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 24.Ikai A., Shirai M., Nishimura K., et al. Hypoxic pulmonary vasoconstriction disappears in a rabbit model of cavopulmonary shunt. J. Thorac. Cardiovasc. Surg. 2004;127(5):1450–1457. doi: 10.1016/s0022-5223(03)01191-7. [DOI] [PubMed] [Google Scholar]
- 25.Mumtaz M.A., Fraga C.H., Nicholls C.M., et al. Increased expression of vascular endothelial growth factor messenger RNA in lungs of rats after cavopulmonary anastomosis. J. Thorac. Cardiovasc. Surg. 2005;129(1):209–210. doi: 10.1016/j.jtcvs.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 26.Ikai A., Shirai M., Nishimura K., et al. Maintenance of pulmonary vasculature tone by blood derived from the inferior vena cava in a rabbit model of cavopulmonary shunt. J. Thorac. Cardiovasc. Surg. 2005;129(1):199–206. doi: 10.1016/j.jtcvs.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 27.McMullan D.M., Reddy V.M., Gottliebson W.M., et al. Morphological studies of pulmonary arteriovenous shunting in a lamb model of superior cavopulmonary anastomosis. Pediatr. Cardiol. 2008;29(4):706–712. doi: 10.1007/s00246-007-9152-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kavarana M.N., Mukherjee R., Eckhouse S.R., et al. Pulmonary artery endothelial cell phenotypic alterations in a large animal model of pulmonary arteriovenous malformations after the Glenn shunt. Ann. Thorac. Surg. 2013;96(4):1442–1449. doi: 10.1016/j.athoracsur.2013.05.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Henaine R., Vergnat M., Bacha E.A., et al. Effects of lack of pulsatility on pulmonary endothelial function in the Fontan circulation. J. Thorac. Cardiovasc. Surg. 2013;146(3):522–529. doi: 10.1016/j.jtcvs.2012.11.031. [DOI] [PubMed] [Google Scholar]
- 30.Henaine R., Vergnat M., Mercier O., et al. Hemodynamics and arteriovenous malformations in cavopulmonary anastomosis: the case for residual antegrade pulsatile flow. J. Thorac. Cardiovasc. Surg. 2013;146(6):1359–1365. doi: 10.1016/j.jtcvs.2013.02.036. [DOI] [PubMed] [Google Scholar]
- 31.Mahmoud M., Allinson K.R., Zhai Z., et al. Pathogenesis of arteriovenous malformations in the absence of endoglin. Circ. Res. 2010;106(8):1425–1433. doi: 10.1161/CIRCRESAHA.109.211037. [DOI] [PubMed] [Google Scholar]
- 32.Bagnyukova T.V., Pogribny I.P., Chekhun V.F. MicroRNAs in normal and cancer cells: A new class of gene expression regulators. Exp. Oncol. 2006;28(4):263–269. [PubMed] [Google Scholar]
- 33.Kozomara A., Griffiths-Jones S. miRBase: Integrating microRNA annotation and deep-sequencing data. Nucleic Acids Res. 2011;39(Database issue):D152–D157. doi: 10.1093/nar/gkq1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Santulli G. MicroRNAs and endothelial (Dys) function. J. Cell. Physiol. 2016;231(8):1638–1644. doi: 10.1002/jcp.25276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamamoto C., Yagi S., Hori T., et al. Significance of portal venous VEGF during liver regeneration after hepatectomy. J. Surg. Res. 2010;159(2):e37–e43. doi: 10.1016/j.jss.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 36.Fernandez M., Mejias M., Garcia-Pras E., Mendez R., Garcia-Pagan J.C., Bosch J. Reversal of portal hypertension and hyperdynamic splanchnic circulation by combined vascular endothelial growth factor and platelet-derived growth factor blockade in rats. Hepatology. 2007;46(4):1208–1217. doi: 10.1002/hep.21785. [DOI] [PubMed] [Google Scholar]