Abstract
Objectives
The aim of this study is to compare the cost and benefit of four different cervical cancer screening strategies involving primary HPV 16/18 genotyping, hrHPV testing alone and cytology for detecting CIN2 +.
Methods
Economical analysis using Markov modeling approach to combine the epidemiological data from current population-based study of The National Cancer Institute of Thailand. A cohort of 100,000 hypothetical female population age 30–65 years was simulated in each strategy. The compared strategies are HPV 16/18 genotyping with reflexed cytology, hrHPV testing alone followed by colposcopy, Papanicolaou standard cytology and liquid based cytology followed by colposcopy. The interval of screening was 5 years' interval. The main outcomes were defined as a number of CIN2 + cases and cost per 100,000 women screening over 35 years.
Results
Model predictions indicated that, the most cost-effectiveness strategy is hrHPV testing alone by reducing cost and also increase CIN2 + detection rate. It identify an additional 130 cases and decrease cost by 46,950,840 THB (1,394,441 USD) per 100,000 women screened when compared to HPV 16/18 genotyping. Compared with cytology, hrHPV testing decrease cost by 51,279,781 THB (1,523,011 USD) and detected more 506 cases of CIN2 +. From sensitivity analysis, the cost of HPV testing, cost of colposcopy, incidence of HPV infection and sensitivity of cytology may affect the results. (1 USD = 33.67 Baht).
Conclusion
The results of this cost-effectiveness analysis support the full scale implementation of HPV testing as a primary cervical cancer screening in Thailand.
Keywords: Cervical cancer, Screening, Human papillomavirus testing, Liquid based cytology, Markov model, Cost effectiveness analysis
Highlights
-
•
This is the first economic study of HPV testing as a screening method in Thailand.
-
•
The primary HPV testing is more effective than cytology method.
-
•
The most cost-effectiveness strategy is HR-HPV testing alone.
1. Introduction
Cervical cancer is a significant public health concern in Thailand. Each year approximately 4000 Thai women die from this cancer (Bruni et al., 2015). In 2005, the National Health Security Office and Ministry of Public Health (MoPH) of Thailand initiated a comprehensive cervical cancer screening program. Thai women at the ages of 30–60 years are encouraged to undergo a cytology based screening program once every 5 years. We manage the women with abnormal cytology result as ASCCP guideline. Although we have implemented a cytology-based screening program for a long time, the mortality rate is still high. There are many limitations of this program, including lack of women's awareness, the low sensitivity of Pap test, limited cytological services, poor compliance of women and ineffective diagnostic and treatment services. To reduce the mortality rate, we focus on an effective program using a new strategy that can improve women's compliance, increase sensitivity for precancerous lesion and appropriate for our country's settings. Meta-analyses and pooled analyses have established that HPV tests have higher sensitivity than cytology for detecting high-grade cervical intraepithelial neoplasia (Cuzick et al., 2006).
Many studies recommend the introduction of HPV DNA primary testing for cervical cancer screening strategy (Huh et al., 2015a; Jin et al., 2016; CK et al., 2014). However, most of these occur in high resource setting countries and there is the discrepancy between guidelines and model-based evaluations among several studies. In order to consider the HPV testing as the primary screening of cervical cancer in the national program, a detailed economic analysis using Thai data is needed.
Economic evaluation of policy options for prevention of cervical cancer in Thailand found that combination of Visual Inspection with Acetic acid (VIA) and sequential PAP smear is the most cost effective policy compared with conventional cytology screening and HPV vaccination. (Praditsitthikorn et al., 2011) Campos, et al. developed a framework for examining health and economic tradeoffs between screening test (HPV test, VIA) sensitivity population coverage and follow-up of screen-positive women. (Campos et al., 2015) Two visit HPV testing was more effective and more cost effective than one visit VIA, an even sensitivity of VIA increase to 60% and of HPV test decline to 70%.
From the review literature, there have been no studies in Thailand that evaluate the use of HPV testing in cervical cancer screening. The objective of this study is to determine cost – effectiveness of HPV primary screening compare with the current practice (cytology method).
2. Methods
2.1. Decision model
We constructed Markov cohort model of women who undergo cervical cancer screening in each program to estimate the number of accumulated cases of high-grade Cervical Intraepithelial Neoplasia 2 or worse (CIN 2 +). We model the natural history of CIN2, including healthy women, high-risk HPV infection, abnormal PAP smear (low grade, high grade), CIN2 + and death. All cost and clinical parameter were discounted at an annual rate of 3%.
2.2. Screening strategies
We compare 4 screening strategies, two of them are cytology-based programs which are current screening recommendation in Thailand. Other strategies were HPV-based screening methods, HPV 16/18 genotyping and HR-HPV testing alone. We selected primary method and modeled decision tree in each strategy based on evidence of cost-effectiveness data from previous studies and feasibility in the current setting of our country. We did not include visual inspection with acetic acid (VIA) because this method is used only in some provinces.
New technologies such as mRNA testing, coding for E6 or E7, or immunostaining is candidate marker which could triage HPV-positive women, but all of these still has limited data and not widely used in Thailand.
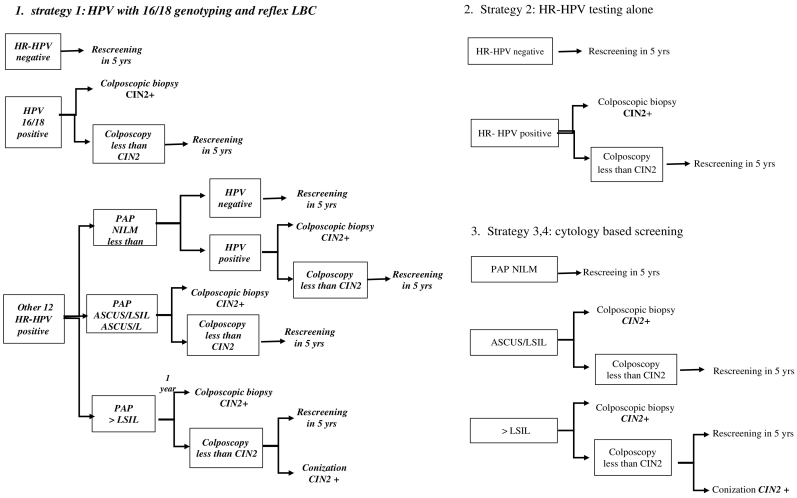
The decision tree models of all strategies were shown in Fig. 1.
Fig. 1.
Screening strategies for detection of CIN2 +.
Strategy 1: HPV 16/18 genotyping is used as primary screening then refer to colposcopy if the result is positive for HPV 16 or 18. Liquid-based cytology was performed in a case of other 12 high risks HPV positive. A cytology of ASCUS or worse leads to immediate colposcopy. Women who are negative result return to routine screening in 5 years.
Strategy 2: Using HR-HPV testing alone every 5 years followed by colposcopy for women with high-risk HPV positive result.
Strategy 3: Screening by a cytology-based program using conventional cytology (Papanicolaou standard cytology) followed by colposcopy if the result is ASCUS or worse. This is the most common method currently used in Thailand.
Strategy 4: The algorithm is the same as strategy 3 but we used liquid-based cytology (LBC) replacing the conventional (Pap) method. This strategy is more commonly used in the private hospitals than the public hospitals in Thailand.
Diagnostic conization was considered if there was a discrepancy between the result of cervical cytology and colposcopic biopsy in strategy 1, 3, and 4.
A cohort of 100,000 hypothetical healthy female population age 30–65 years was simulated in each strategy. The interval of screening in all strategies was 5 years based on the clinical guideline of National cancer institute of Thailand. The simulation model continued until women had died or CIN2 + was diagnosed.
2.3. Model assumptions
Colposcopies were assumed to be 100% sensitive and specific for CIN2 + and compliance with periodic screening was held constant at 100% (Table 1).
Table 1.
Model parameters (prevalence).
| Rate | Range | Ref | |
|---|---|---|---|
| Strategy 1 | |||
| HPV16/18 | 0.0093 | 0.0084–0.0279 | NCI |
| Other 12HR positive | 0.025 | 0.0025–0.075 | NCI |
| HPV16/18 + → Colpo CIN2 + | 0.191 | – | NCI |
| Other 12 HR + ve → LG cyto | 0.296 | – | NCI |
| Other 12 HR + ve → LG cyto → Colpo CIN2 | 0.078 | – | NCI |
| Other 12 HR + ve → NILM → (wait 1y) HPV + ve | 0.25 | – | a |
| Other 12 HR + ve → NILM → (wait 1y) HPV + ve → Colpo CIN2 + | 0.2 | – | a |
| Other 12 HR + ve → HG cyto | 0.148 | – | NCI |
| Other 12 HR + ve → HG cyto → Colpo –ve → Conization CIN2 + | 0.06 | – | NCI |
| Other 12 HR + ve → HG cyto → Colpo CIN2 + | 0.15 | – | NCI |
| Strategy 2 | |||
| HR-HPV + ve | 0.0346 | 0.0311–0.1038 | NCI |
| HR HPV + ve → Colpo CIN2 + | 0.114 | – | NCI |
| Strategy 3 | |||
| LG cyto + ve | 0.008 | 0.0072–0.024 | NCI |
| LG cyto + ve → Colpo CIN2 + | 0.11 | – | NCI |
| HG cyto + ve | 0.0067 | 0.00603–0.201 | NCI |
| HG cyto → Colpo CIN2 + | 0.2 | – | NCI |
| HG cyto → Colpo − ve → Conization CIN2 + | 0.074 | – | NCI |
| Strategy 4 | |||
| LG cyto + ve | 0.00336 | – | NCI |
| LG cyto + ve → Colpo CIN2 + | 0.058 | – | NCI |
| HG cyto + ve | 0.00059 | – | NCI |
| HG cyto → Colpo CIN2 + | 0.2 | – | NCI |
| HG cyto → Colpo − ve → Conization CIN2 + | 0.07 | – | NCI |
LG cyto: low grade cytology ASCUS, LSIL HG cyto: high grade cytology > LSIL.
NCI: National Cancer Institute of Thailand.
Reference form Expert's opinion.
2.4. Model parameters
Epidemiological existing data were taken from the current population-based study of National Cancer Institute of Thailand (unpublished data, 2016), which is the first and the largest trial to evaluate HPV testing compared with cytology method as primary cervical cancer screening program in Thailand. Age-adjusted annual probabilities of death for women without cervical cancer were derived from the general population estimates reported in Estimated Generation Life Tables for Thailand of Five-Year Birth Cohorts: 1900–2000 (Prasartkul, 2002).
2.5. Cost data (Table 2)
Table 2.
Cost of screening (Baht).
| Procedure | Base case | Values for sensitivity analysis |
Reference | |
|---|---|---|---|---|
| Low value | High value | |||
| Cytology, conventional method | 273.3 | – | – | KCMH |
| Cytology, liquid based cytology | 361.3 | 325.17 | 1083.9 | KCMH |
| HPV genotyping | 417.5 | 375.75 | 1252.5 | NCI |
| HR HPV testing | 417.5 | 375.75 | 1252.5 | NCI |
| Colposcopy | 357 | 321.3 | 3573 | KCMH |
| Conization | 33,805 | 30,424.5 | 101,415 | KCMH |
KCMH: King Chulalongkorn Memorial Hospital.
NCI: National Cancer Institute of Thailand.
All of the directed medical costs provided by Center of Health Assurance at King Chulalongkorn Memorial Hospital (KCMH) except for the cost of HPV testing. The cost of HR-HPV testing derived from National Cancer Institute of Thailand which is the potential costs for screening purposes in Thailand from the manufacturer. Indirect costs such as loss of productivity and transportation costs were assumed to be the same among patients. Treatment costs for diseases were not included because of equal occurrence in both groups.
2.6. Cost effective analysis
The main outcomes were defined as an accumulation of CIN2 + cases and cost per 100,000 women screening over 35 years. Incremental cost-effectiveness ratios (ICERs) were calculated to compare the cost and effectiveness of each screening strategy. We used the gross domestic product (GDP) per capita, which is suggested by the WHO as the threshold for the most cost-effective strategy.
2.7. Sensitivity analysis
A one-way sensitivity analysis was performed to estimate the impact of uncertainty in different parameters. Besides the colposcopic cost, a range of sensitivity analysis was 10% below and three times above of parameters. Colposcopy had variable costs among hospitals and the cost used in this study was quite low compared with other hospitals. Therefore, we used ten times above for upper value of this cost in sensitivity analysis.
3. Results
3.1. Base case analysis
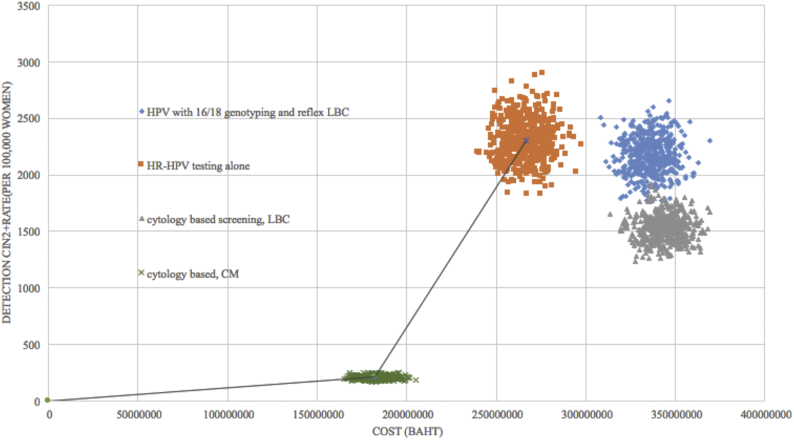
The effectiveness for detection of CIN2 + cases and cost of four strategies are presented in Table 3. Regarding the effectiveness, the result showed that primary HPV screening (strategy 1,2) was preferred over primary cytology screening (strategy 3,4). HPV testing alone (strategy 2) was the most effective method. Its detection rate of CIN2 + cases was 1520 women per 100,000 women, whereas the detected cases of HPV 16/18 genotyping, LBC method and Conventional Pap smear were 1389 1013 and 138 cases per 100,000 women respectively. Conventional cytology method (strategy 4) had the least effectiveness and the least cost among all strategies.
Table 3.
Base case results of cost, outcome and ICER.
| Strategy | Cost (Baht) | Outcomea (detected cases) | Incremental cost | Incremental effectiveness | ICERb (Baht/detected case) |
|---|---|---|---|---|---|
| Cytology based screening, CM (strategy 4) | 121,990,372 | 138.48 | – | – | – |
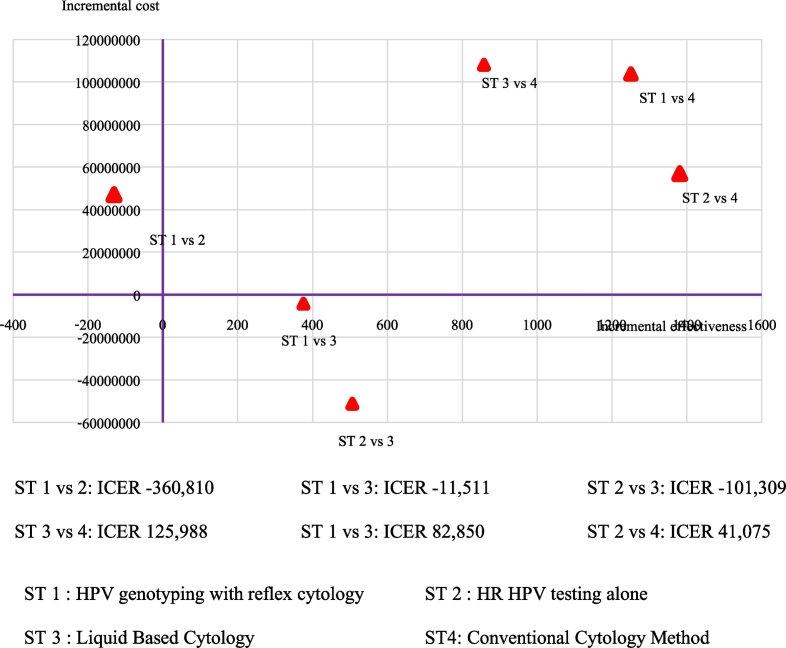
| HR-HPV testing alone (strategy 2) | 178,735,576.7 | 1520.10 | 56,745,205 | 1381.5 | 41,075.1 |
| HPV with 16/18 genotyping (strategy 1) | 225,686,417.2 | 1389.98 | 46,950,840.5 | − 130.1 | − 360,810 (dominated)c |
| Cytology based screening, LBC (strategy 3) | 230,015,358.0 | 1013.94 | 4,328,940.8 | − 130.1 | − 11,511.8 (dominated)c |
Outcome was defined as the number of detected cases of CIN2 CIN3 or Cervical cancer per 100,000 women.
The difference in cost divided by the difference in detected case for each strategy compared with the next best strategy.
Strategies shown cost more but were less effective than the next most expensive strategy and were therefore dominated.
HR-HPV testing alone would turn an additional 130 cases and decrease cost (cost saving) by 46,950,840 Baht (1,394,441.34 USD) per 100,000 women screened when compared to HPV 16/18 genotyping. Compare with liquid-based cytology, HR-HPV testing decreased cost (cost saving) by 51,279,781 Baht (1,523,011.02 USD) and detected more 506 cases of CIN2 +.
Fig. 2 shows the cost-effectiveness frontier between lifetime cost and detection rate of CIN2 + of each strategy. Any strategy that was placed on the frontier is reasonably efficient, whereas one falling to the right required further justification for reimbursement at that price. Incremental cost-effectiveness ratio (ICER) was calculated as the additional Baht divided by the additional CIN2 + detected cases gained from a strategy in relation to the next costly strategy. Comparing HR-HPV testing alone with the conventional method, HR-HPV testing alone is a non-dominated strategy with ICER 41,075 Baht (1219.92 USD) per detected case.
Fig. 2.
Cost effectiveness frontier: detected CIN2 + case (per 100,000 women) versus life time costs.
The ICER plane in Fig. 3 and cost-effectiveness analysis in Table 3 show that HPV 16/18 genotyping (strategy 1) and liquid-based cytology method (strategy 3) were dominated by HPV testing alone (strategy 2). Compare strategy 3 and 4; Conventional Pap smear was less costly than Liquid base cytology, whereas LBC method provided a more effective option at an incremental cost-effectiveness ratio (ICER) of 125,988 Baht (3741.84 USD) per detected case.
Fig. 3.
The ICER plane of difference strategies.
3.2. Sensitivity analysis
A one-way sensitivity analysis was performed by changing the prevalence of HPV infection in strategy 1and 2, abnormal cytology in strategy 3, discount rate and prices of all screening tools in strategy 1 to 3. The incidence of HPV infection, the sensitivity of cytology, cost of HPV testing and cost of colposcopy may affect the results. HPV 16/18 genotyping would be the most cost effective method if the cost of colposcopy increase to 3573 Baht (106.11 USD) or the incidence of HPV infection increase 3 times or more. Liquid base cytology would be more effective if the sensitivity of cytology was increased.
4. Discussion
In a developed country, primary HPV testing is widely accepted as being more effective than cytology for screening cervical cancer. (Huh et al., 2015a; Arbyn et al., 2006) We also found that screening with HPV- based strategy detected more CIN2 + cases than cytology-based especially conventional method cytology. Initial screening from NCI of Thailand study showed that conventional Pap smear had the lowest sensitivity and highest number of missed CIN2 + cases. Accumulation of CIN2 + cases over 35 years was lower than others strategy almost 10 times. The total cost of this strategy was cheapest due to the lowest number of women to refer to the next step. Although it had the lowest cost, using this method as primary screening was unacceptable. The HPV-based screening was more cost-effective than liquid-based cytology. It is consistent with previous several studies conducted in both high and low-income countries (UK, Netherlands, France, Italy, US, Spain, Germany, Mexico, Iran and Sub-Saharan Africa) (Jin et al., 2016; CK et al., 2014; Arbyn et al., 2006; Beal et al., 2014; Nahvijou et al., 2016; Holmes et al., 2005; de Kok et al., 2012). Comparing between HR-HPV testing alone and HPV16/18 genotyping, HR-HPV testing alone was more effective and less expensive. This result is different from large study of Huh which prefer HPV 16/18 genotyping. (Huh et al., 2015b) Huh's study compared four strategies, cytology, co-testing, HPV testing with reflex cytology and HPV16/18 genotyping with reflex cytology as primary screening. The decision tree of HPV 16/18 strategy was the same as our study but Huh did not mention about the next step for women with HR-HPV non 16/18 positive and negative cytology after 1 year follow-up. From threshold analysis of Huh's study revealed that HPV 16/18 genotyping was less cost effective when the sensitivity was reduced by > 50% of the current estimate. The number of women who positive for HPV16/18 of NCI of Thailand study which was used in model parameter of our study was low and < 50% of ATHENA trial which used in parameter of Huh's study. This may explain why the result was different. The result from sensitivity analysis of our study also supported that if the incidence of HPV infection increased 3 times or more, HPV 16/18 genotyping would be more cost effective strategy.
HR-HPV testing alone had the highest number of colposcopic rates so that it may affect the total cost of screening. But the result showed that this strategy was less expensive than strategy 1 and 3. Although it had more colposcopic rates, it had less frequency of triages and follow-up tests and there was no cost of conization involved in the strategy. Moreover, it still be the most effective strategy, even increase cost of colposcopy or decrease cost of conization. Liquid-based cytology had less effective and costlier than HPV-based strategies (strategy 1 and 2). The main reason may be the low sensitivity of cytology compared with HPV testing. If the sensitivity of cytology increased, it would be a more cost-effective strategy. Another important factor that may affect the result is the price of HPV testing. If the HPV testing's price increased 3 times or more, HPV-based strategies would not be cost effective strategy whether it be strategy 1 or strategy 2 compared with liquid-based cytology. This point should be considered if we plan to implement HPV testing as primary testing for National Program.
A specific strength of our study is the first economic evaluation of cervical screening with HPV testing in Thailand. We used data input from the most recent prospective study in our country. It was the study from single trial, contrary to other models. So there is no bias from difference study designs, disease prevalence and statistical method. Data from the NCI of Thailand study facilitated comparison of all strategies within the same cohort, thereby reducing variability.
There are some limitations of our study. First, we have limited data about incidence rate of HPV infection and sensitivity of HPV testing in Thailand. We use data of one province, which has relatively low incidence of HPV infection. This conclusion cannot be generalized to all Thai women. Second, we used surrogate outcome over a short time period, it may be unable to estimate the life expectancy or quality-adjusted life years (QALYs). Third, only direct medical costs from the perspective of healthcare were considered. Last, we assumed that all women had completed a follow-up program. It may affect the total number of detected cases.
Future research is needed to evaluate the cost-effectiveness of these strategies based on data from a larger trial which conducted in several regions of Thailand. Long term effectiveness such as lifetime risk cervical cancer or impact of quality of life should be assessed. This research should include the new molecular biomarkers integrated along HPV testing or cytology in screening algorithms. In addition, introduction of the HPV vaccine, with was shown to be cost effective in Thailand (Huh et al., 2015b; Termrungruanglert et al., 2012) and most of the countries, will lead to a reduction of HPV infection and precancerous lesion, further reduction in the efficacy of screening modalities. The impact of HPV vaccination should be evaluated accordingly.
In conclusion, primary HPV testing is an option as being more effective than cytology for cervical cancer screening. This result may encourage policy makers to plan for implementation of HPV DNA testing in Thailand.
Funding source
None.
Conflict of interest
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.gore.2017.09.007.
Appendix A. Supplementary data
Reference
- Arbyn M., Sasieni P., Meijer C.J., Clavel C., Koliopoulos G., Dillner J. Chapter 9: clinical applications of HPV testing: a summary of meta-analyses. Vaccine. 2006;24(Suppl. 3):S3/78–89. doi: 10.1016/j.vaccine.2006.05.117. [DOI] [PubMed] [Google Scholar]
- Beal C.M., Salmeron J., Flores Y.N., Torres L., Granados-Garcia V., Dugan E. Cost analysis of different cervical cancer screening strategies in Mexico. Salud Publica Mex. 2014;56(5):429–501. [PubMed] [Google Scholar]
- Bruni L.B.-R.L., Albero G., Aldea M., Serrano B., Valencia S. Human Papillomavirus and Related Diseases in Thailand Summary Report. 2015. ICO Information Centre on HPV and Cancer (HPV Information Centre) pp. 12–23. [Google Scholar]
- Campos N.G., Castle P.E., Wright T.C., Jr., Kim J.J. Cervical cancer screening in low-resource settings: a cost-effectiveness framework for valuing tradeoffs between test performance and program coverage. Int. J. Cancer. 2015;137(9):2208–2219. doi: 10.1002/ijc.29594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CK H., Canfell K., Gilham C., Sargent A., Roberts C., Desai M. The clinical effectiveness and cost-effectiveness of primary human papillomavirus cervical screening in England: extended follow-up of the ARTISTIC randomised trial cohort through three screening rounds. Health Technol. Assess. (Winch. Eng.) 2014;18(23):1–196. doi: 10.3310/hta18230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuzick J., Clavel C., Petry K.U., Meijer C.J., Hoyer H., Ratnam S. Overview of the European and North American studies on HPV testing in primary cervical cancer screening. Int. J. Cancer. 2006;119(5):1095–1101. doi: 10.1002/ijc.21955. [DOI] [PubMed] [Google Scholar]
- Holmes J., Hemmett L., Garfield S. The cost-effectiveness of human papillomavirus screening for cervical cancer. A review of recent modelling studies. Eur. J. Health Econ. 2005;6(1):30–37. doi: 10.1007/s10198-004-0254-1. [DOI] [PubMed] [Google Scholar]
- Huh W.K., Ault K.A., Chelmow D., Davey D.D., Goulart R.A., Garcia F.A. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Gynecol. Oncol. 2015;136(2):178–182. doi: 10.1016/j.ygyno.2014.12.022. [DOI] [PubMed] [Google Scholar]
- Huh W.K., Williams E., Huang J., Bramley T., Poulios N. Cost effectiveness of human papillomavirus-16/18 genotyping in cervical cancer screening. Appl. Health Econ. Health Policy. 2015;13(1):95–107. doi: 10.1007/s40258-014-0135-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X.W., Lipold L., Foucher J., Sikon A., Brainard J., Belinson J. Cost-effectiveness of primary HPV testing, cytology and co-testing as cervical cancer screening for women above age 30 years. J. Gen. Intern. Med. 2016 doi: 10.1007/s11606-016-3772-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kok I.M., van Rosmalen J., Dillner J., Arbyn M., Sasieni P., Iftner T. Primary screening for human papillomavirus compared with cytology screening for cervical cancer in European settings: cost effectiveness analysis based on a Dutch microsimulation model. BMJ (Clin. Res. Ed). 2012;e670:344. doi: 10.1136/bmj.e670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahvijou A., Daroudi R., Tahmasebi M., Amouzegar Hashemi F., Rezaei Hemami M., Akbari Sari A. Cost-effectiveness of different cervical screening strategies in Islamic Republic of Iran: a middle-income country with a low incidence rate of cervical cancer. PLoS One. 2016;11(6) doi: 10.1371/journal.pone.0156705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praditsitthikorn N., Teerawattananon Y., Tantivess S., Limwattananon S., Riewpaiboon A., Chichareon S. Economic evaluation of policy options for prevention and control of cervical cancer in Thailand. PharmacoEconomics. 2011;29(9):781–806. doi: 10.2165/11586560-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Prasartkul P.R.U. Vols. 1900–2000. Institute For Population and Social Research, Mahidol University; Bangkok: 2002. Estimated Generation Life Tables for Thailand of Five-Year Birth Cohorts. [Google Scholar]
- Termrungruanglert W., Havanond P., Khemapech N., Lertmaharit S., Pongpanich S., Khorprasert C. Cost and effectiveness evaluation of prophylactic HPV vaccine in developing countries. Value Health. 2012;15(1 Suppl):S29–34. doi: 10.1016/j.jval.2011.11.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.