Abstract
Uterine tumors resembling ovarian sex cord tumors (UTROSCTs) are rare and commonly characterized as benign tumors, with infrequent reports of metastasis and recurrence. Treatment recommendations have not been well established, particularly for more advanced cases. We present the first reported death from a metastatic UTROSCT, summarize the available literature, and describe characteristics common to UTROSCTs with aggressive features. In this case, a 49-year-old woman presented with abdominal distension and pain; initial imaging and diagnostic workup suggested metastatic epithelial ovarian cancer to be the cause. The patient subsequently underwent neoadjuvant chemotherapy followed by optimal cytoreductive surgery and adjuvant chemotherapy. Final pathology revealed UTROSCT with omental and peritoneal metastases. She then underwent adjuvant chemotherapy with subsequent recurrence and died 15 months after her initial diagnosis. Our analysis of this case and the available literature led us to identify pathologic risk factors that may help predict aggressive UTROSCT behavior.
Keywords: Chemotherapy; Metastasis, Uterine cancer; Uterine tumor resembling ovarian sex cord tumor
Highlights
-
•
Uterine tumors resembling ovarian sex cord tumors are rare and generally considered benign.
-
•
Few aggressive/metastatic cases have been reported, and we report on the first death from this disease.
-
•
Analysis of the literature allowed for identification of potential pathologic risk factors of aggressive disease.
-
•
Better identifying these risk factors could help guide treatment recommendations for this tumor type.
1. Introduction
Type II uterine tumors resembling ovarian sex cord tumors (UTROSCTs) are rare and generally behave in a benign fashion (Liu et al., 2015). There are few reports of metastasis or recurrence and UTROSCTs are considered to be of uncertain malignant potential without formal treatment recommendations. Here, we describe a case of metastatic UTROSCT resulting in the first reported death from this tumor type. The case description is followed by a review of clinical and pathologic data from previously reported aggressive cases of UTROSCTs with the goal of identifying features that may assist in predicting high risk disease.
2. Case report
A 49-year-old woman presented to the emergency department with abdominal pain, vomiting, and dyspnea. Imaging revealed a left ovarian mass, moderate ascites, omental caking, and lymphadenopathy. Serum cancer antigen (CA) 125 level was 2210 U/mL. Paracentesis cytology suggested metastatic adenocarcinoma of epithelial origin. Image-guided omental biopsy revealed high-grade adenocarcinoma with staining suggestive of gynecologic origin (CK 7 positive, vimentin partially positive, and WT1, CK20, p53 negative). A diagnosis of metastatic epithelial ovarian carcinoma was suspected.
Given the patient's complex medical history, including immune thrombocytopenic purpura status post splenectomy, chronic portal vein thrombosis, recent pulmonary embolism, type II diabetes and poor functional status, neoadjuvant chemotherapy with intravenous carboplatin and paclitaxel was initiated. 5 cycles of platinum- and taxane-based neoadjuvant chemotherapy were completed and a clinical response was achieved. The chemotherapy course was complicated by neuropathy and groin abscesses requiring repeated hospital admission. CA-125 decreased to 32.3 U/mL and reduction in disease burden was seen on imaging. Interval optimal cytoreductive surgery was performed 7 months after diagnosis.
Final pathology revealed sex cord stromal tumor resembling a granulosa cell tumor arising from the uterus (6 cm intramural tumor, > 50% myometrial invasion, tumor present 1 mm from serosa). There was metastasis involving bilateral ovarian surfaces and omentum. Histologically, the neoplastic cells formed trabeculae, rosette-like structures, nests, and well-formed tubules (Fig. 1). Immunohistochemistry analysis revealed polyphenotypic staining (Fig. 2).
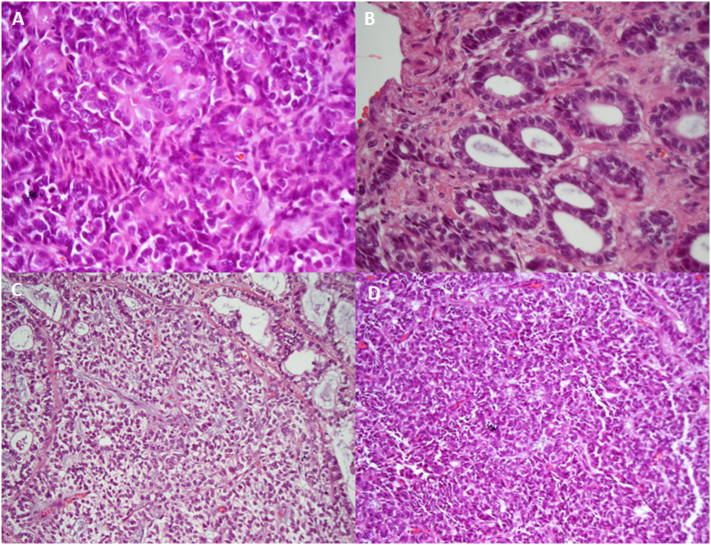
Fig. 1.
Morphologic patterns exhibited in this case of metastatic UTROSCT including (A) rosette pattern, (B) tubular pattern, (C) gross pattern resembling sertoli cell tumor, and (D) solid tumor pattern.
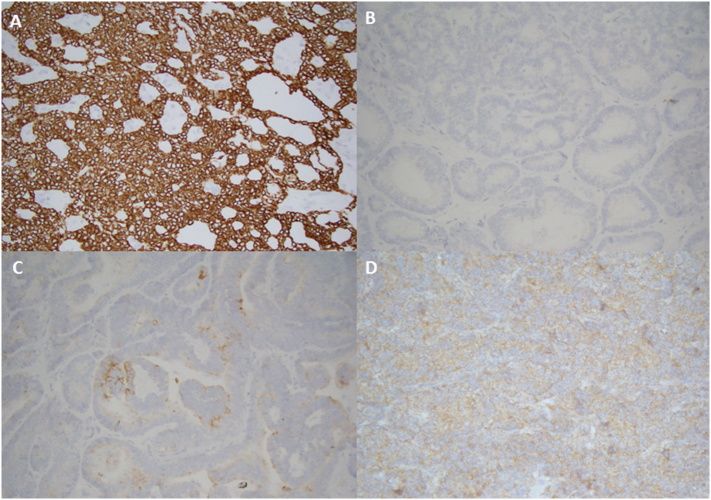
Fig. 2.
Immunohistochemistry staining showing (A) cytokeratin diffusely positive, (B) calretinin negative, (C) epithelial membrane antigen focally positive, and (D) inhibin weakly positive results.
2 cycles of adjuvant carboplatin and docetaxel were completed with further reduction of CA-125 to 18.7. There was minimal remaining disease on imaging. At the 4 month surveillance visit, CA-125 was elevated at 213. Imaging revealed moderate ascites, new hepatic and peritoneal implants and disease progression in the pelvis. Chemotherapy with pegylated liposomal doxorubicin was recommended but was postponed secondary to severe hyperglycemia. The patient decompensated over the following month and was admitted to an outside facility with recurrent ascites, hypoxia, fluid overload, and new-onset atrial fibrillation. Chemotherapy was unable to be restarted and the patient opted for hospice care. She subsequently died of her disease 15 months after initial diagnosis.
3. Discussion
UTROSCTs were initially described by Morehead and Bowman in 1945 who noted the close resemblance to ovarian granulosa cell tumors (Liu et al., 2015). Clement and Scully further classified the tumors into two groups in 1976 (Byun et al., 2015). Group I tumors (endometrial stromal tumors with sex cord-like elements (ESTSCLEs) were characterized as having focal (< 50%) sex cord-like differentiation and exhibited a significant rate of malignant behavior (approximately 15%). By comparison, type II tumors (classic UTROSCTs) displayed a predominant ovarian sex cord pattern (> 50%) and followed a benign course (Byun et al., 2015, Guntupalli et al., 2012). UTROSCTs usually occur between the fourth and sixth decades of life but may occur in younger women, however, incidence of these tumors is low (Liu et al., 2015, Pradhan and Mohanty, 2013, Blake et al., 2014). Patients often present with symptoms of abnormal uterine bleeding or pelvic pain (Liu et al., 2015).
Although benign behavior is typically described, metastatic and recurrent cases have been reported. Variations in tumor behavior complicate treatment recommendations; therefore, we asked whether certain pathologic features may identify aggressive disease. A literature review was performed on all English-language reports of UTROSCTs to identify cases with extra-uterine spread, distant metastatic disease, or recurrent disease. 8 cases were identified (Table 1) (Umeda et al., 2014, Kantelip et al., 1986, Biermann et al., 2008, O'Meara et al., 2009, Mačák et al., 2014, Gomes et al., 2016, Endo et al., 2016). A compilation and analysis of clinical and pathologic data from aggressive cases of UTROSCT has not previously been performed. Here, we review the features described.
Table 1.
Summary of UTROSCT cases with metastasis, recurrence, or extrauterine spread of primary tumor.
| Source | Age, y | Surgical management | Mitotic activity | Myometrial invasion | Serosal involvement | LVSI | Location of metastasis or extrauterine extension | Adjuvant therapy | Follow-up, mo | Treatment for recurrence and follow-up |
|---|---|---|---|---|---|---|---|---|---|---|
| Kantelip, 1985 | 86 | TAH, BSO, epiploic mass resection | Scarce mitotic figures | … | + (Cyst ruptured through serosa) | − | Left ovary and epiploica; | N/A | 60 | N/A |
| LNs not evaluated | ||||||||||
| Umeda, 2014 | 38 | 1) Transvaginal myomectomy; | MIB-1, 3% | + | − | + | Left internal iliac lymph node | High-dose progesterone therapy | 11 | N/A |
| 2) TAH, BSO, PLND | ||||||||||
| Umeda, 2014 | 57 | TAH, BSO, appendectomy | MIB-1, 1% | − | − | + | Epiploic appendix; | N/A | 96 | N/A |
| LNs not evaluated | ||||||||||
| Macak, 2014 | 53 | 1) Hysteroscopic polypectomy | Ki-67, 5% | … | − | − | Right internal iliac LN | N/A | 10 | N/A |
| 2) Hysterectomy, BSO, PPLND | ||||||||||
| Gomes, 2016 | 53 | 1) SCH; | … | + | Myometrial involvement adjacent to serosa | + | Extension to cervix, right parametrium, and right ovarian hilum; pelvic LN negative | 4 cycles of modified BEP, EBRT, and brachytherapy | 60 | N/A |
| 2) BSO, OMX, PLND, and cervix resection | ||||||||||
| Biermann, 2007 | 68 | Hysterectomy | Ki-67, < 5% | + | − | − | No metastasis of primary tumor; | N/A | 48 (intestinal obstruction, 10 cm bowel tumor) | Surgical resection; no follow-up noted |
| LNs not evaluated | ||||||||||
| O'meara, 2009 | 35 | TAH | Ki-67, 5% | … | + | … | No metastasis of primary tumor; | N/A | 36 (recurrent galactorrhea, pelvic mass infiltrating the bladder, abdominal wall, and intestine) | Extensive tumor debulking followed by BEP; patient doing well after 12 months |
| LNs not evaluated | ||||||||||
| Endo, 2015 | 39 | Hysterectomy | … | + | … | − | No metastasis of primary tumor; | N/A | 276 (14.0 × 10.2 cm solid tumor invading the left pelvic wall) | 1) 3 months of letrozole and medroxyprogesterone acetate with no response; |
| 2) Arterial embolization of tumor; | ||||||||||
| 3) Cytoreductive surgery; patient doing well after 20 months | ||||||||||
| LNs not evaluated | ||||||||||
| Present case | 49 | 5 cycles of NACT followed by optimal cytoreductive surgery | Mitotic activity high | + | 1 mm from serosa | + | Bilateral ovarian surfaces, omentum, Morrison's pouch nodule; | 2 cycles of carboplatin and docetaxel | Death from disease 2017 | |
| LNs not evaluated |
Abbreviations: UTROSCT, uterine tumors resembling ovarian sex cord tumor; N/A, not applicable; TAH, total abdominal hysterectomy; SCH, supracervical hysterectomy; BSO, bilateral salpingo-oophorectomy; PLND, pelvic lymph node dissection; OMX, omentectomy; LN, lymph nodes; PLND, pelvic lymph node dissection; BEP, bleomycin, etoposide, cisplatin; EBRT, external beam radiation therapy; LVSI, lymphovascular space invasion; NACT, neoadjuvant chemotherapy.
… Not mentioned in article.
+ Present/− absent.
The histologic findings of a UTROSCT are those of a sex cord morphology, including cords, trabeculae, nests, and tubules (Pradhan and Mohanty, 2013). Immunohistochemical expression is polyphenotypic and a panel of 4 stains (calretinin, inhibin, CD99, and melan A) has been described to assist in diagnosis (Irving et al., 2006). Positivity for calretinin plus 1 of the other 3 stains is considered diagnostic (Pradhan and Mohanty, 2013, Irving et al., 2006). The staining profiles from the reviewed cases are summarized in Table 2.
Table 2.
Immunohistochemistry profiles of aggressive uterine tumor resembling ovarian sex cord tumors cases.a
| Umeda, 2014 (1) | Umeda, 2014 (2) | Maczak, 2014 | Gomes, 2015 | Biermann, 2008 | O'Meara, 2009 | Endo, 2016 | Present case | |
|---|---|---|---|---|---|---|---|---|
| Calretinin | + | + | − | + | + | + | − | |
| EMA | − | − | − | + | ||||
| CK20 | − | |||||||
| CK19 | + | − | ||||||
| CK7 | − | − | − | |||||
| S100 | − | + | − | + | ||||
| Melan A | − | + | + | − | − | − | ||
| CK18 | + | |||||||
| AE1/AE3 | + | + | − | + | + | − | + | |
| Vimentin | + | + | + | + | + | + | ||
| Chromogranin | − | |||||||
| Synaptophysin | − | − | ||||||
| NSE | − | |||||||
| HMB45 | − | − | − | − | ||||
| PAX8 | + | |||||||
| WT1 | + | + | + | + | ||||
| FOXL2 | + | − | ||||||
| CD99 | + | + | − | + | + | + | − | + |
| CD56 | + | + | + | + | + | + | ||
| CD34 | − | − | − | |||||
| Inhibin | + | − | + | + | + | + | ||
| CD10 | − | + | − | + | + | |||
| aSMA | + | + | − | − | − | + | + | |
| Desmin | + | + | + | + | + | + | − | |
| h-Caldesmon | + | + | + | |||||
| Bcl2 | + | − | + | |||||
| HHF35 | + | + | − | − | ||||
| Calponin | + | |||||||
| ER | + | + | − | + | + | + | + | + |
| PR | + | + | − | + | + | + | + | − |
Abbreviations: aSMA, alpha-smooth muscle actin; bcl2, b-cell lymphoma #2; CK, cytokeratin; EMA, epithelial membrane antigen; ER, estrogen receptor; HMB, human melanoma black; NSE, neuron specific enolase; PR, progesterone receptor.
+ Present.
− Absent.
Blank fields denote unavailable data.
Type II UTROSCTs share histologic features with different tumors (Pradhan and Mohanty, 2013). Cellular and cytogenetic markers are described to assist with diagnosis. For example, unlike UTROSCTs, ESTSCLEs express only 1 sex cord marker per tumor (Pradhan and Mohanty, 2013, Blake et al., 2014). Additionally, ESTSCLEs and endometrial stromal sarcoma (ESS) exhibit a genetic translocation resulting in the fusion of JazF1 and SUZ12 (JJaz1). This chimeric gene is absent in type II UTROSCTs suggesting different malignant driving factors (Liu et al., 2015). ESTSCLE and ESS also extensively express CD10 and epithelial membrane antigen whereas UTROSCTs have only patchy expression of both (Umeda et al., 2014). UTROSCTs can be differentiated from perivascular epithelioid cell tumors by the absence of SYT-SSX gene fusion and scant expression of HMB45. Lastly, epithelioid leiomyosarcoma exhibits extensive staining for alpha-smooth muscle actin and h-caldesmon, both of which are minimally expressed in UTROSCT (Umeda et al., 2014).
Although the diagnosis of UTROSCT is becoming more standardized, we are still unable to predict for aggressive disease. Traditional high risk features of uterine cancer are of unclear value in UTROSCT. Lymphovascular space invasion (LVSI) is a consistent predictor of lymph node metastasis, recurrence, and overall survival in endometrial cancer (Guntupalli et al., 2012). In UTROSCT, LVSI is rare (Umeda et al., 2014). However, LVSI seems more prevalent here and was present in 4 of 8 cases in Table 1. In a combined review of type I and II tumors by Blake et al., LVSI was significantly associated with decreased survival (Blake et al., 2014). These data suggest that the presence of LVSI should raise suspicion for aggressive disease. Interestingly, of the 4 reviewed cases without LVSI, 2 were metastatic suggesting the absence of LVSI is not necessarily protective.
Myometrial invasion is another strong predictor of recurrence in endometrial cancer (Tejerizo-García et al., 2013). Myometrial invasion was present in 4 of 9 reviewed cases (Umeda et al., 2014, Kantelip et al., 1986, Biermann et al., 2008, O'Meara et al., 2009, Mačák et al., 2014, Gomes et al., 2016, Endo et al., 2016). O'Meara et al. reported a recurrent UTROSCT in which the primary tumor lacked LVSI and myometrial invasion, however it did exhibit serosal involvement (O'Meara et al., 2009). In this review, 4 of the 9 cases described tumor infiltration within 2 mm of the serosa (Umeda et al., 2014, Kantelip et al., 1986, Biermann et al., 2008, O'Meara et al., 2009, Mačák et al., 2014, Gomes et al., 2016, Endo et al., 2016). Although myometrial invasion and serosal involvement are known risk factors in other uterine cancer types, data are lacking in the UTROSCT population. However, when present these findings may suggest more aggressive behavior.
Lastly, abnormal cellular proliferation is associated with poor prognosis in many malignancies. A proliferation index is an indicator of mitotic degree measured by staining for proliferation-associated antigens such as Ki67 (Kriegsmann and Warth, 2016). Of the cases reviewed, only the present case, resulting in the patient's death, was noted to have high mitotic activity (Umeda et al., 2014, Kantelip et al., 1986, Biermann et al., 2008, O'Meara et al., 2009, Mačák et al., 2014, Gomes et al., 2016, Endo et al., 2016). Overall, a high proliferation index should raise concern. However, a low proliferation index does not necessarily seem to be protective.
Optimal treatment strategies for UTROSCTs are poorly defined. Primary surgical resection is curative in the majority of cases (Liu et al., 2015, Pradhan and Mohanty, 2013, Gomes et al., 2016). In a previous review, there was no significant survival benefit to hysterectomy with salpingo-oophorectomy over hysterectomy alone (Blake et al., 2014). Hysteroscopic resection and hormonal therapy have also been described with varying results (Liu et al., 2015). More extensive surgical management is reported in cases with higher disease burden (Umeda et al., 2014, Kantelip et al., 1986, Biermann et al., 2008, O'Meara et al., 2009, Mačák et al., 2014, Gomes et al., 2016, Endo et al., 2016). Based on available data, the value of lymphadenectomy in these cases remains unclear. Of note, on our review, pelvic lymph nodes were not surgically evaluated in any of the 3 recurrences (Table 1). This invites questions of whether microscopic disease would have been identified and whether lymphadenectomy would be of benefit in select patients. Pertaining to chemotherapy; bleomycin, etoposide, and cisplatin is a common regimen of choice, given the similarities to ovarian sex cord malignancy (O'Meara et al., 2009). In our case, a carboplatin- and taxane-based regimen was administered; due to suspected ovarian carcinoma. To date, a standard chemotherapy regimen for UTROSCT has not been defined. Given the potential for less invasive treatment, it would be advantageous to identify patients at risk for recurrence and limit more extensive treatment strategies to this patient population. However, adequately identifying high-risk disease remains a challenge.
4. Conclusion
UTROSCTs are rare tumors, and prospective studies to define optimal management are lacking. We present an unusual case of an aggressive UTROSCT resulting in the first reported death from this disease. Currently, predictive features of aggressive UTROSCTs are poorly understood, and there are few recommendations to guide management. Aggressive disease forms have not been previously characterized compared to their benign counterpart. Here, we have presented a review of all reported aggressive cases of UTROSCT. In this review, we found that factors predictive of aggressive behavior in other uterine tumors, including myometrial invasion, serosal involvement, LVSI, and high mitotic activity are present in these aggressive cases of UTROSCTs and may provide guidance for clinical decision making. Further studies are needed to validate these findings.
Conflict of interest
No conflict of interest relevant to this article was reported.
Editorial assistance
We thank Sonya J. Smyk (Moffitt Cancer Center) for editorial assistance. She did not receive any compensation beyond her usual salary.
References
- Biermann K., Heukamp L.C., Büttner R., Zhou H. Uterine tumor resembling an ovarian sex cord tumor associated with metastasis. Int. J. Gynecol. Pathol. 2008;27(1):58–60. doi: 10.1097/pgp.0b013e318057faf5. [DOI] [PubMed] [Google Scholar]
- Blake E.A., Sheridan T.B., Wang K.L., Takiuchi T., Kodama M., Sawada K., Matsuo K. Clinical characteristics and outcomes of uterine tumors resembling ovarian sex-cord tumors (UTROSCT): a systematic review of literature. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014;181:163–170. doi: 10.1016/j.ejogrb.2014.07.050. [DOI] [PubMed] [Google Scholar]
- Byun J.M., Kim K.T., Yoon H.K., Jeong D.H., Kim Y.N., Lee K.B., Sung M.S. Uterine tumors resembling ovarian sex cord tumor in postmenopausal woman. J. Obstet. Gynaecol. India. 2015;65(4):273–277. doi: 10.1007/s13224-014-0545-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo D., Todo Y., Okamoto K., Suzuki H. A case of recurrent group II uterine tumor resembling ovarian sex-cord tumors, against which two hormonal agents were ineffective. Taiwan J. Obstet. Gynecol. 2016;55(5):751–753. doi: 10.1016/j.tjog.2015.04.008. [DOI] [PubMed] [Google Scholar]
- Gomes J.R., Carvalho F.M., Abrão M., Maluf F.C. Uterine tumors resembling ovarian sex-cord tumor: a case-report and a review of literature. Gynecol. Oncol. Rep. 2016;15:22–24. doi: 10.1016/j.gore.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guntupalli S.R., Zighelboim I., Kizer N.T., Zhang Q., Powell M.A., Thaker P.H., Goodfellow P.J., Mutch D.G. Lymphovascular space invasion is an independent risk factor for nodal disease and poor outcomes in endometrioid endometrial cancer. Gynecol. Oncol. 2012;124(1):31–35. doi: 10.1016/j.ygyno.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irving J.A., Carinelli S., Prat J. Uterine tumors resembling ovarian sex cord tumors are polyphenotypic neoplasms with true sex cord differentiation. Mod. Pathol. 2006;19:17–24. doi: 10.1038/modpathol.3800475. [DOI] [PubMed] [Google Scholar]
- Kantelip B., Cloup N., Dechelotte P. Uterine tumor resembling ovarian sex cord tumors: report of a case with ultrastructural study. Hum. Pathol. 1986;17:91–94. doi: 10.1016/s0046-8177(86)80161-7. [DOI] [PubMed] [Google Scholar]
- Kriegsmann M., Warth A. What is better/reliable, mitosis counting or Ki67/MIB1 staining? Transl. Lung Cancer Res. 2016;5(5):543–546. doi: 10.21037/tlcr.2016.10.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C.Y., Shen Y., Zhao J.G., Qu P.P. Clinical experience of uterine tumors resembling ovarian sex cord tumors: a clinicopathological analysis of 6 cases. Int. J. Clin. Exp. Pathol. 2015;8:4158–4164. [PMC free article] [PubMed] [Google Scholar]
- Mačák J., Dundr P., Dvořáčková J., Klát J. Uterine tumors resembling ovarian sex cord tumors (UTROSCT): report of a case with lymph node metastasis. Cesk. Patol. 2014;50(1):46–49. [PubMed] [Google Scholar]
- O'Meara A.C., Giger O.T., Kurrer M., Schaer G. Case report: recurrence of a uterine tumor resembling ovarian sex-cord tumor. Gynecol. Oncol. 2009;114:140–142. doi: 10.1016/j.ygyno.2009.03.021. [DOI] [PubMed] [Google Scholar]
- Pradhan D., Mohanty S.K. Uterine tumors resembling ovarian sex cord tumors. Arch. Pathol. Lab. Med. 2013;137:1832–1836. doi: 10.5858/arpa.2012-0634-RS. [DOI] [PubMed] [Google Scholar]
- Tejerizo-García Á., Jiménez-López J.S., Muñoz-González J.L., Bartolomé-Sotillos S., Marqueta-Marqués L., López-González G., Gómez J.F. Overall survival and disease-free survival in endometrial cancer: prognostic factors in 276 patients. Onco. Targets Ther. 2013;6:1305–1313. doi: 10.2147/OTT.S51532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umeda S., Tateno M., Miyagi E., Sakurai K., Tanaka R., Tateishi Y., Tokinaga A., Ohashi K., Furuya M. Uterine tumors resembling ovarian sex cord tumors (UTROSCT) with metastasis: clinicopathological study of two cases. Int. J. Clin. Exp. Pathol. 2014;7(3):1051–1059. [PMC free article] [PubMed] [Google Scholar]