Summary
Removal of astringency by endogenously formed acetaldehyde, achieved by postharvest anaerobic treatment, is of critical importance for many types of persimmon fruit. Although an anaerobic environment accelerates de‐astringency, it also has the deleterious effect of promoting excessive softening, reducing shelf life and marketability. Some hypoxia‐responsive ethylene response factors ( ERFs) participate in anaerobic de‐astringency, but their role in accelerated softening was unclear. Undesirable rapid softening induced by high CO 2 (95%) was ameliorated by adding the ethylene inhibitor 1‐MCP (1 μL/L), resulting in reduced astringency while maintaining firmness, suggesting that CO 2‐induced softening involves ethylene signalling. Among the hypoxia‐responsive genes, expression of eight involved in fruit cell wall metabolism (Dkβ‐gal1/4, DkEGase1, DkPE1/2, DkPG1, DkXTH9/10) and three ethylene response factor genes (DkERF8/16/19) showed significant correlations with postdeastringency fruit softening. Dual‐luciferase assay indicated that DkERF8/16/19 could trans‐activate the DkXTH9 promoter and this interaction was abolished by a mutation introduced into the C‐repeat/dehydration‐responsive element of the DkXTH9 promoter, supporting the conclusion that these DkERFs bind directly to the DkXTH9 promoter and regulate this gene, which encodes an important cell wall metabolism enzyme. Some hypoxia‐responsive ERF genes are involved in deastringency and softening, and this linkage was uncoupled by 1‐MCP. Fruit of the Japanese cultivar ‘Tonewase’ provide a model for altered anaerobic response, as they lost astringency yet maintained firmness after CO 2 treatment without 1‐MCP and changes in cell wall enzymes and ERFs did not occur.
Keywords: Astringency removal, ERF , high CO 2 , hypoxia, persimmon fruit, postharvest softening, transcriptional regulation
Introduction
Plant responses to anoxia involve a range of metabolic and morphological changes on different timescales, including rapid induction of anaerobic metabolism (Kennedy et al., 1992; Voesenek and Bailey‐Serres, 2015). For persimmon (Diospyros kaki), the anaerobic metabolite acetaldehyde, which accumulates under high CO2 treatment (95%), participates in fruit postharvest deastringency by converting the soluble tannins to insoluble products (Min et al., 2012; Pesis and Ben‐Arie, 1984; Taira et al., 1992, 2001). However, deastringency is usually also accompanied by rapid fruit softening (Arnal and Del Río, 2004; Yin et al., 2012). Thus, although useful for taste improvement, anaerobic treatment has very adverse effects on persimmon fruit storage life.
Softening in most fruit occurs naturally at the commencement of ripening and continues after harvest. It is due primarily to partial cell wall degradation and a reduction in intercellular adhesion (Li et al., 2010) catalysed by a battery of enzymes including pectin methylesterase (PME, EC 3.1.1.11), polygalacturonase (PG, endo‐type, EC 3.2.1.15; exo‐type, EC 3.2.1.67) and β‐galactosidase (β‐gal, EC 3.2.1.23) (Brummell and Harpster, 2001; Payasi et al., 2009; Vicente et al., 2007). Understanding the roles of individual genes and enzymes in changing fruit texture has continued to advance since the first experiments on PG antisense transgenic tomato (Smith et al., 1988). Some reports indicated that modulation of individual genes effectively influenced fruit texture/softening, such as PG (Carrington et al., 1993; Kramer et al., 1992), TBG4 (a β‐gal gene, Smith et al., 2002), Pmeu1 (a pectinesterase gene, Phan et al., 2007) and PL (pectate lyase, Silvia et al., 2002). Additional research has indicated that multiple genes contribute to fruit texture, and in tomato, Fir s.p. QTL2.5 (containing three PME genes) is tightly correlated with fruit firmness (Chapman et al., 2012) and double‐suppression of LePG and LeEXP1 resulted in increased firmness compared to single gene repression (Powell et al., 2003). Recently, a tomato pectate lyase has been implicated in playing a major role in reducing cell adhesion and firmness (Uluisik et al., 2016). Many of these investigations have been conducted in tomato and strawberry. Limited studies on cell wall‐related genes in persimmon have highlighted the importance for fruit softening, of genes such as DkXTH1 and DkXTH2, encoding xyloglucan endotransglycosylase/hydrolases (Nakatsuka et al., 2011; Zhu et al., 2013) and DkExp3, which encodes an expansin (Zhang et al., 2012).
Plants respond to hypoxia by the N‐end rule pathway which post‐translationally regulates levels of group VII ERFs (Gibbs et al., 2011; Licausi et al., 2011). Among other responses, this induces alcohol dehydrogenase (ADH) and pyruvate decarboxylase (PDC) genes (Hinz et al., 2010; Licausi et al., 2010; Papdi et al., 2015; Yang et al., 2011) which generate the acetaldehyde which in persimmon fruit precipitates soluble tannins. Several transcription factors have been implicated in regulating this process, including ethylene response factors (ERFs), and some may also be involved in the softening that accompanies deastringency. Some AP2/ERF transcription factors have also been associated with fruit cell degradation and softening in various other fruit (Xie et al., 2016), such as kiwifruit AdERF9, which is a transcriptional repressor acting on the AdXET5 promoter (Yin et al., 2010), and MdCBF (an AP2/ERF member), which activates MdPG1 (Tacken et al., 2010). Using introgression lines, SlERF2.2 was shown to underlie the firmness QTL, Fir s.p. QTL2.2, and its expression is tightly correlated with fruit texture (Chapman et al., 2012). Another AP2/ERF gene, SlAP2a, is also associated with retarding fruit softening, as SlAP2a antisense tomatoes are softer than wild‐type fruit (Chung et al., 2010), and LeERF1 antisense transgenic fruit had significant longer shelf life (Li et al., 2007). However, the hypoxia‐responsive ERFs have mainly been investigated for their regulation of anaerobic related genes, and their possible relationship with other target genes is largely unknown.
Twenty‐two hypoxia‐responsive DkERF genes have been isolated from persimmon (Min et al., 2012, 2014; Yin et al., 2012) and DkERF9/10/19/22 were shown to be involved directly in transcriptional regulation of anaerobic metabolism genes involved in persimmon deastringency (Min et al., 2012, 2014), but a possible role in postdeastringency fruit softening was not investigated. In the present research, a combination of high CO2 storage and 1‐MCP treatment (together with CO2 treatment) was shown to maintain fruit firmness while removing astringency, implicating some ERFs in the excessive postdeastringency softening. The roles of DkERFs in controlling cell wall metabolism‐related genes were analysed during these treatments and in a Japanese cultivar ‘Tonewase’, which appears to have an altered anaerobic response, and lacks the ethylene and softening response to high CO2.
Results
Effects of CO2 and 1‐MCP on ‘Mopanshi’ persimmon deastringency and softening
Mature ‘Mopanshi’ persimmon fruit were astringent at harvest and the soluble tannin content was maintained at approximately 1.1%, during 4‐day storage (Figure 1a). CO2 treatment (95%, 1 day) caused a decline in soluble tannins to 0.65% after 1 day and 0.47% after 2 day (Figure 1a). This was accompanied by a rapid decrease in firmness in CO2‐treated fruit, to 33.7 N at 3 day and 22.6 N at 4 day, compared to control fruit firmness of 48.7 N at 3 day and 47.9 N at 4 day (Figure 1b).
Figure 1.
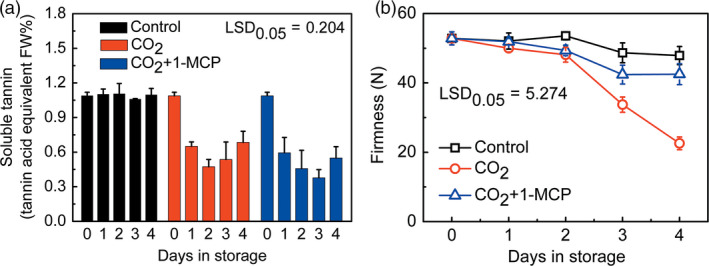
Effects of CO 2 and CO 2 + 1‐MCP treatments on soluble tannins (a) and firmness (b) in ‘Mopanshi’ persimmon fruit at 20 °C. The persimmon fruit were treated with CO 2 (95%) and CO 2 + 1‐MCP (95% CO 2 and 1 μL/L 1‐MCP) for 1 day, while control fruit were sealed in airtight containers. All treatments and subsequent storage was at 20 °C. Error bars indicate SEs from 3 (for soluble tannins) or 10 (for firmness) replicates.
The effects of adding the ethylene action antagonist 1‐MCP (1 μL/L) to the CO2 (95%) treatment were investigated in order to test whether this could alleviate the rapid softening that occurred during astringency removal. The results indicated that 1‐MCP‐treated fruit had higher firmness than the control fruit in CO2 alone, with 42.4 N at 3 days and 42.5 N at 4 days, slightly lower than control fruit in air (Figure 1b). CO2 + 1‐MCP also enhanced ‘Mopanshi’ persimmon astringency removal, as indicated by the decrease in soluble tannin, to 0.59% after 1 day and 0.46% after 2 day, values which were similar to those in CO2‐treated fruit without 1‐MCP (Figure 1a). Similar effects were confirmed in a subsequent replication in a different year with ‘Mopanshi’ fruit (data not shown).
Isolation and analysis of deastringency‐responsive cell wall degradation‐related and DkERF genes associated with ‘Mopanshi’ persimmon softening
Using the previously generated RNA‐seq data (Min et al., 2014), deastringency‐responsive cell wall‐related unigenes were obtained. After RACE experiments, 35 genes were cloned encoding the following cell wall degrading enzymes: α‐L‐arabinofuranosidase (DkAraf1‐2, KX259530–KX259531), endoglucanase (DkEGase1‐2, KX259532–KX259533), β‐galactosidase (Dkβ‐gal1‐8, KX259534–KX259541), Mannan endo‐1,4‐beta‐mannosidase (DkMAN1, KX259542), polygalacturonase (DkPG1, Jiang et al., 2010; DkPG2‐5, KX259551–KX259554), pectinesterase (DkPE1‐8, KX259543–KX259550), pectate lyase (DkPL1, KX259555) and xyloglucan endotransglycosylase/hydrolase (DkXTH1‐2, DkXTH4, Han et al., 2015; DkXTH8‐9, KF318888‐9; DkXTH10‐12, KX259556‐KX259558).
These genes were all expressed in fruit but responded differentially to CO2 treatment, with only DkEGase1, Dkβ‐gal1, Dkβ‐gal4, DkPG1, DkPG4, DkPE1, DkPE2, DkXTH1, DkXTH4, DkXTH9‐11 showing a strong increase, while the others were nonresponsive or were repressed by CO2 treatment (Figures 2, S1). Among the CO2 treatment‐induced genes, DkEGase1 and DkXTH10 were highly responsive, and at 2 days, their mRNA abundance increased approximately 1233‐ and 110‐fold, respectively (Figure 2). Furthermore, increases in mRNA from most of the CO2‐induced cell wall degrading genes were reduced in CO2 + 1‐MCP‐treated fruit, with the exception of DkPG4, DkXTH1, DkXTH4 and DkXTH11. For instance, DkXTH1 mRNA was enhanced 6.36‐fold at 1 day by CO2 treatment, and a similar increase was found in CO2 + 1‐MCP‐treated fruit (5.71‐fold at 1 day; Figure S1). Thus, of the 35 cell wall degradation‐related genes, DkEGase1, Dkβ‐gal1, Dkβ‐gal4, DkPG1, DkPE1, DkPE2, DkXTH9 and DkXTH10 were the most likely to be involved in major persimmon fruit softening during and after CO2 treatment (Figures 2 and S1).
Figure 2.
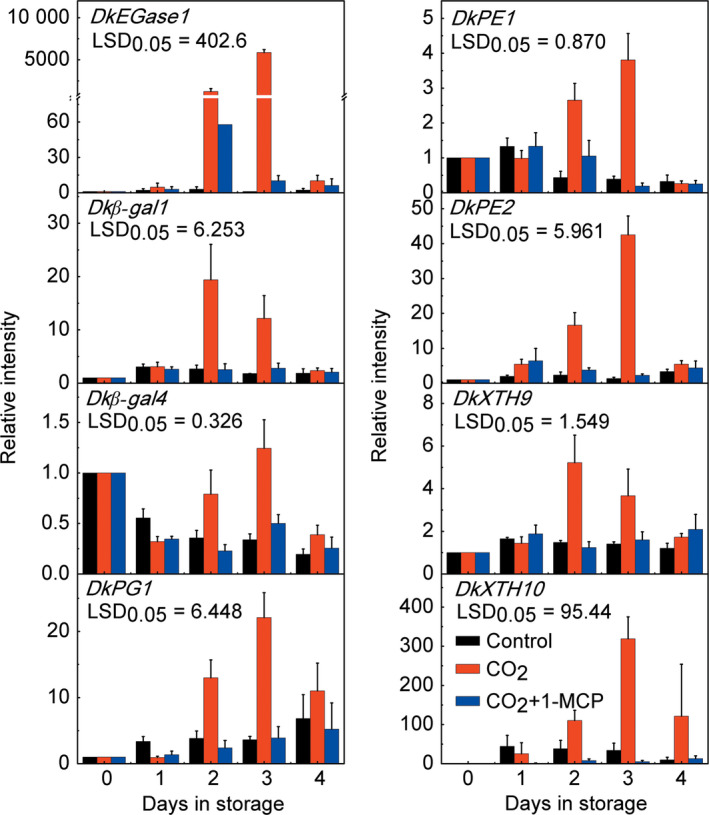
Accumulation of mRNAs from eight cell wall‐related genes in response to CO 2 and CO 2 + 1‐MCP treatments in ‘Mopanshi’ persimmon fruit at 20 °C. Gene expression was analysed by real‐time PCR. Error bars indicate SEs from three replications. DkEGase1: endoglucanase; Dkβ‐gal1/4: β‐galactosidase; DkPG1: polygalacturonase; DkPE1/2: pectinesterase; DkXTH9/10: xyloglucan endotransglycosylase/hydrolase.
mRNAs from twenty‐two DkERF genes were found to increase in abundance during deastringency treatment (high CO2); however, only four, DkERF9/10/19/22, have been shown previously to be involved in transcriptional regulation of anoxia‐related genes during persimmon fruit deastringency (Min et al., 2012, 2014). The possibility that other DkERF genes might also participate in fruit softening during astringency removal was investigated. Using the ‘Mopanshi’ persimmon, expression of twenty‐two DkERF genes was analysed and mRNAs for all of these were up‐regulated by CO2 treatment (Figure 3). Adding 1‐MCP blocked the enrichment of four DkERF genes transcripts, DkERF7, DkERF8, DkERF16 and DkERF19, suggesting they could play a role in fruit softening (Figures 1b, 3). One, DkERF7, contained an EAR motif within the coding region and is a putative transcriptional repressor (Kagale and Rozwadowski, 2011), while the accumulation of mRNAs from the other three DkERF genes was positively correlated with fruit softening.
Figure 3.
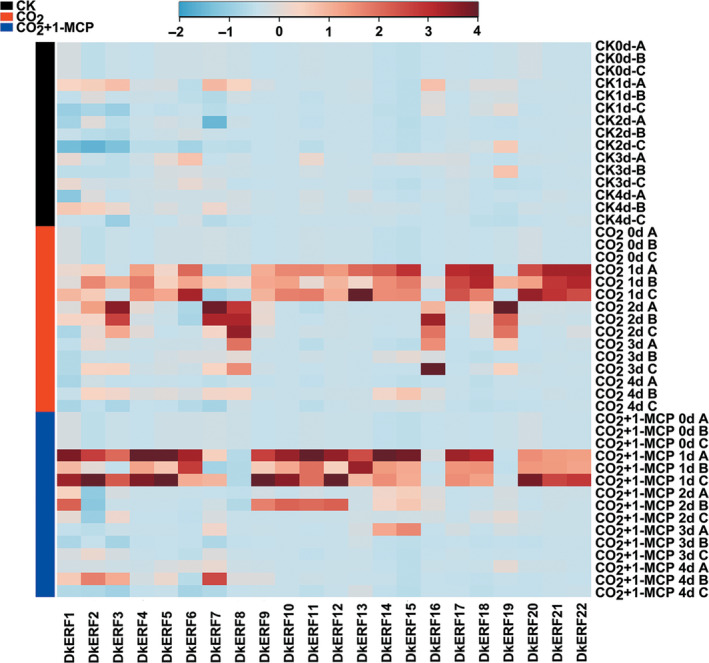
Accumulation of mRNA from hypoxia‐responsive DkERF genes in response to CO 2 and CO 2 + 1‐MCP treatments in ‘Mopanshi’ persimmon fruit at 20 °C. Gene expression was analysed by real‐time PCR. The heatmap was constructed by MetaboAnalyst 3.0 and indicates the mRNA abundance. A, B and C represent three biological replicates. DkERF1‐22: ethylene response factors.
Effect of CO2 and CO2 + 1‐MCP treatments on persimmon fruit deastringency and softening in various cultivars
In order to confirm the effect of CO2 + 1‐MCP treatment on persimmon fruit deastringency and softening, another Chinese astringent‐type cultivar, ‘Jingmianshi’, was studied. CO2 (95%, 1 day) treatment effectively accelerated deastringency as indicated by the darker staining from tannin printing of control fruit, compared with treated fruit (Figure 4). CO2 + 1‐MCP treatment had similar effects on deastringency as CO2 alone (Figure 4), but there were major differences in fruit softening (Figure 4). Whereas the firmness of ‘Jingmianshi’ fruit in CO2 decreased from 21.48 N at 0 day to 1.19 N at 6 day, fruit in CO2 + 1‐MCP retained a firmness of 16.26 N at 6 day (Figure 4).
Figure 4.
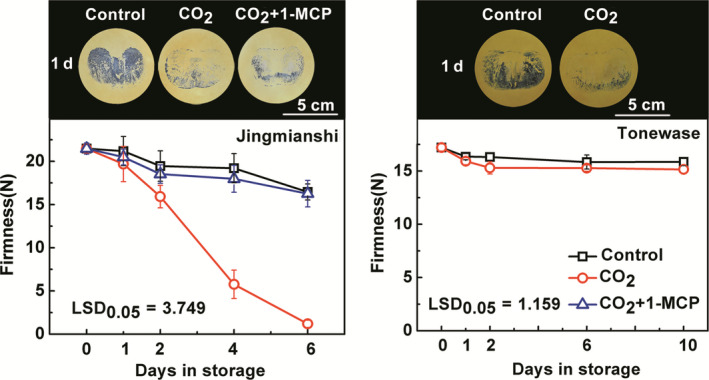
Changes in soluble tannins and firmness in two astringent cultivars, ‘Jingmianshi’ and ‘Tonewase’, in response to postharvest treatments. The Chinese cultivar ‘Jinmianshi’ fruit were treated with CO 2 (95%) or CO 2 + 1‐MCP (95% CO 2 and 1 μL/L 1‐MCP) for 1 day, separately. Japanese astringent cultivar ‘Tonewase’ fruit were only treated with CO 2 (95%) for 1 day. Control fruit was sealed in airtight containers. All treatments and subsequent storage were at 20 °C. Astringency was indicated by soluble tannin content, using the tannin printing method. The black colour indicates soluble tannins and the intensity of black reflects the soluble tannin content. Error bars indicate SEs from eight replicates.
A comparison was made with a Japanese astringent cultivar, ‘Tonewase’, which responded similarly to deastringency in CO2 (95%, 1 day) but did not exhibit the rapid softening observed in the Chinese cultivars, even in the absence of 1‐MCP (Figure 4). During the 10‐day storage, both CO2‐treated and control fruit firmness decreased only slightly from 17.2 N at 0 day to 15.86 N and 15.15 N at 10 days for control and CO2‐treated fruit, respectively.
Accumulation of mRNAs from fruit softening‐related genes in various cultivars
Using ‘Jingmianshi’ and ‘Tonewase’, the involvement of the selected eight cell wall‐related genes and three DkERF genes in fruit softening was assessed. The heatmap (Figure 5) shows that these eleven genes were all highly expressed in CO2‐treated fruit of the ‘Jingmianshi’ cultivar, which undergoes both deastringency and rapid softening, and were significantly inhibited in the CO2 + 1‐MCP treatment, where fruit underwent deastringency but remained firm. In the astringent Japanese cultivar ‘Tonewase’, however, mRNAs for these eleven genes remained at basal levels in response to CO2 treatment alone, resulting in deastringency but maintaining firmness (Figure 5). Taking the results from the three cultivars ‘Mopanshi’, ‘Jingmianshi’ and ‘Tonewase’ together, accumulation of mRNAs for these eleven genes, encoding five cell wall enzymes (DkEGase1, Dkβ‐gal1, Dkβ‐gal4, DkPG1, DkPE1, DkPE2, DkXTH9, DkXTH10) and three ERF transcription factors (DkERF8, DkERF16 and DkERF19) is highly correlated with CO2‐triggered softening associated with deastringency in Chinese cultivars of persimmon.
Figure 5.
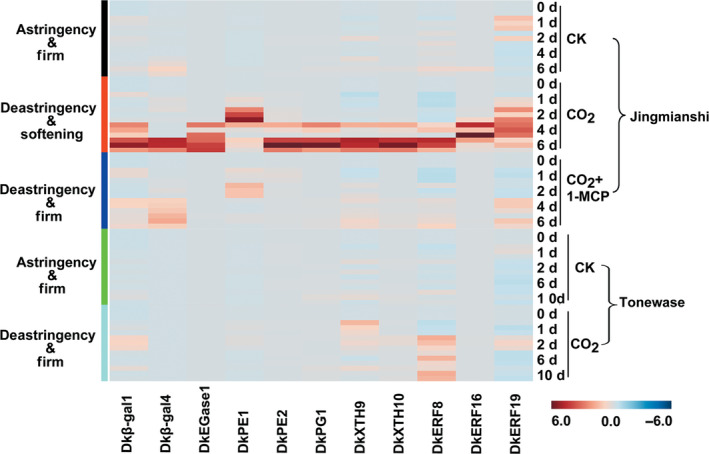
Relationship between accumulation of mRNAs from cell wall‐related genes, DkERF and persimmon fruit softening in different cultivars. The astringent Chinese cultivar ‘Jingmianshi’ was treated with CO 2 and CO 2 + 1‐MCP, while the Japanese astringent cultivar ‘Tonewase’ was only treated with CO 2. The concentration of CO 2 and 1‐MCP was 95% and 1 μL/L. All treatments and post‐treatment storage were conducted at 20 °C. Gene expression was analysed by real‐time PCR. The heatmap was constructed by MetaboAnalyst 3.0 and indicates the mRNA abundance. DkEGase1: endoglucanase; Dkβ‐gal1/4: β‐galactosidase; DkPG1: polygalacturonase; DkPE1/2: pectinesterase; DkXTH9/10: xyloglucan endotransglycosylase/hydrolase; DkERF8/16/19: ethylene response factors.
Roles of softening‐related DkERFs in controlling expression of genes encoding cell wall degrading enzymes
In order to investigate the possible regulatory linkage between DkERF genes and transcription of cell wall‐related genes, the promoters of DkEGase1, Dkβ‐gal1, Dkβ‐gal4, DkPG1, DkPE1, DkPE2, DkXTH9 and DkXTH10 were isolated (Table S5), using genome walking, due to the lack of persimmon genome information. Analysis of cis‐elements indicated that the GCC box and C‐repeat/dehydration‐responsive element (DRE), recognized by AP2/ERF transcription factors, were only distributed in some promoters, such as Dkβ‐gal4, DkPE1 and DkXTH9 (Figure 6a). Dual‐luciferase assays indicated that DkERF8, DkERF16 and DkERF19 could trans‐activate DkXTH9 promoters, with 2.13‐, 2.13‐ and 1.74‐fold enhancement, respectively (Figure 6b). However, these DkERF genes had limited effects on the promoters of the other seven putative softening‐related genes, although some in vivo regulations had the statistical differences.
Figure 6.
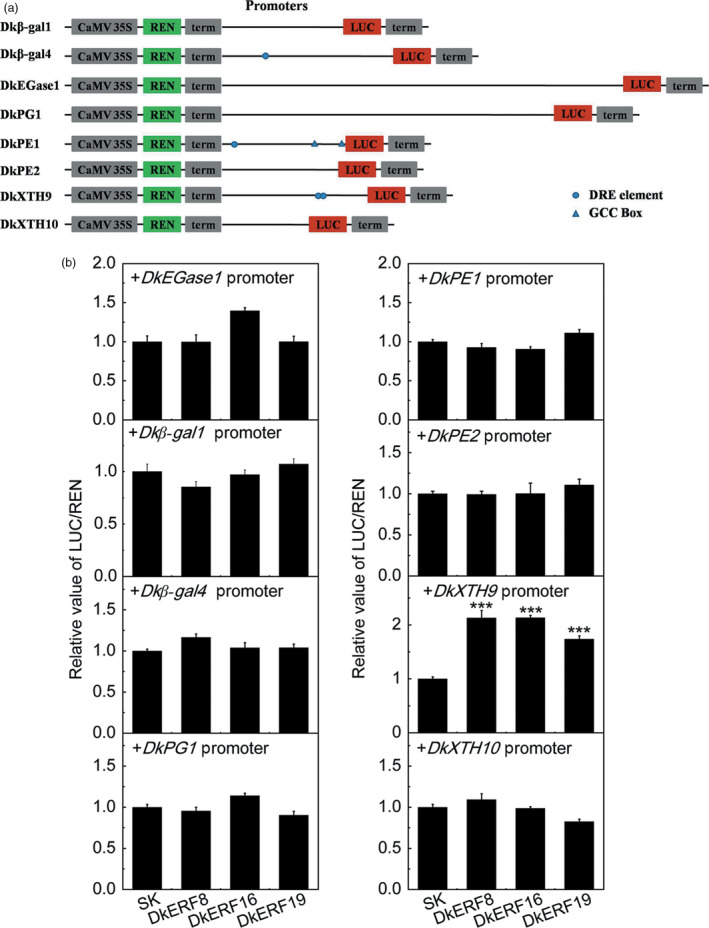
Effect of DkERF on transcription from the promoters of cell wall metabolism‐related genes. (a) Schematics of promoters: lines indicate promoter length, triangles show GCC box, circles represent DRE motifs, and triangles indicate GCC boxes. (b) In vivo interactions between DkERF and promoters were measured by dual‐luciferase assay. Error bars indicate SE from five replicates (***P < 0.001).
DkERF interaction with the DkXTH9 promoter
DkERF trans‐activated the DkXTH9 promoter, but in a yeast one‐hybrid system, the DkXTH9 promoter exhibited auto‐activation (Figure S2), so an alternative approach was used to test the interaction. When the two DRE elements (CCGAC) within the DkXTH9 promoter were mutated to CTGAG (mDkXTH9, Figure 7a), dual‐luciferase assay indicated that all three DkERFs had lower trans‐activation activity on the mutated DkXTH9 promoter, compared to DkXTH9 promoter (Figure 7b).
Figure 7.
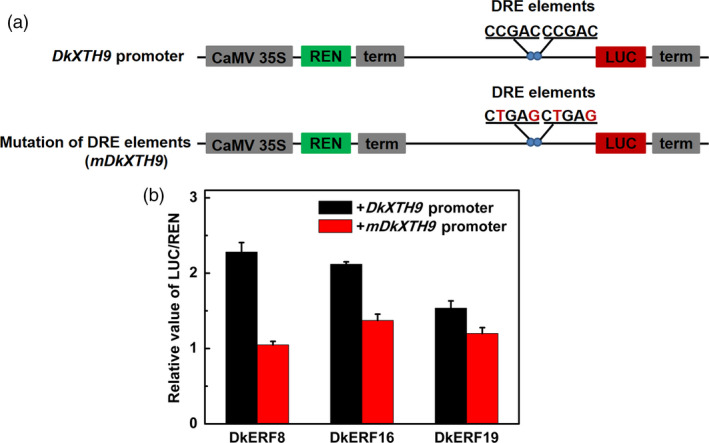
Effect of mutation of DRE elements in the DkXTH9 promoter on transcription by DkERF. (a) Mutation of DRE elements. (b) Activity of DkERF on transcription from normal and mutated DkXTH9 promoters. Error bars indicate SE from five replicates.
Discussion
Regulation of anaerobic treatment on persimmon fruit deastringency and softening
For persimmon, postharvest softening not only depends on ripening stage, but also occurs rapidly during astringency removal (Arnal and Del Río, 2004; Nakatsuka et al., 2011; Ortiz et al., 2005; Taira et al., 1992), which is essential because the main persimmon cultivars are of the astringent type (Luo et al., 2014; Yamada et al., 2002). Thus, although deastringency treatments (including the most widely used high CO2 treatment, and also ethylene treatment (Min et al., 2012)) remove soluble tannins successfully, they significantly shorten persimmon fruit shelf life. Here, fruit of two Chinese persimmon cultivars, ‘Mopanshi’ and ‘Jingmianshi’, of the astringent type exhibited rapid softening after high CO2 (95%) treatment, which is similar to the results from other persimmon cultivars, such as ‘Rojo Brillante’ (Arnal and Del Río, 2004) and ‘Saijo’ (Xu et al., 2003). ‘Tonewase’, a Japanese persimmon cultivar, maintained firmness after CO2‐driven deastringency (Figure 4). It should note that ‘Tonewase’ obtained from different orchards (Itamura et al., 1995) or environments (e.g. humidity, Nakano et al., 2002) has been reported to have varied softening rates. The responses of ‘Tonewase’, the Japanese cultivar, to high CO2 treatment (same treatment as for the two Chinese cultivars) show that in this cultivar, deastringency and the accompanying softening are effectively uncoupled.
In the Chinese cultivars ‘Mopanshi’ and ‘Jingmianshi’, the softening that accompanies the anoxia‐induced deastringency could be prevented by application of the ethylene antagonist 1‐MCP and softening was retarded while astringency was reduced or removed (Figures 1 and 4), indicating that ethylene signalling was probably responsible for the softening process during deastringency without 1‐MCP. While 1‐MCP had little effect on the removal of astringency, it was extremely effective at preventing fruit softening in the Chinese cultivars, which is to be expected for an ethylene response.
Characterization of genes involved in persimmon fruit softening during deastringency
Fruit texture is generally considered as a quantitative trait, which is regulated by multiple genes (Li et al., 2010), such as PG (Atkinson et al., 2002; Smith et al., 1988), XTH (Miedes et al., 2010), β‐Gal (Kitagawa et al., 1995; Nakamura et al., 2003; Smith et al., 2002) and pectate lyase (Uluisik et al., 2016). Here, eight cell wall‐related genes (Dkβ‐gal1/4, DkEGase1, DkPE1/2, DkPG1, DkXTH9/10) were identified after a preliminary search and their expression correlated with fruit firmness in CO2‐ and CO2 + 1‐MCP‐treated ‘Mopanshi’ and ‘Jingmianshi’ fruit and CO2‐treated ‘Tonewase’ fruit. The expression of DkEGase1 in particular was significantly correlated with fruit firmness (Figure 2), indicating the possible involvement of cellulose metabolism during persimmon fruit softening. Furthermore, mRNAs for five other genes (Dkβ‐gal1/4, DkPE1/2 and DkPG1) related to modifications to pectin, one of the main cell wall components, accumulated during softening (Figure 2), which is consistent with previous demonstrations of the large decrease in pectin content during persimmon fruit softening (Cutillas‐Iturralde et al., 1993; Luo, 2007; Taira et al., 1997). None of these genes, however, has been previously reported in persimmon fruit.
XTH is involved in hemicellulose metabolism and is considered one of the most important enzymes that contribute to persimmon fruit softening (Cutillas‐Iturralde et al., 1994). Previously, DkXTH1 and DkXTH2 have been shown to be associated with persimmon softening (Nakatsuka et al., 2011; Zhu et al., 2013), but these two DkXTH genes were different genes with same names. Based on the similarity of nucleotide acid sequences, the present DkXTH1 and DkXTH2 were similar to DkXTH1 and DkXTH2 from ‘Fupingjianshi’ (Zhu et al., 2013), while the present DkXTH8 and DkXTH9 were similar to DkXTH1 and DkXTH2 from ‘Saijo’ (Nakatsuka et al., 2011). Here, two DkXTH genes (DkXTH9 and DkXTH10) were characterized, which indicated potential overlap between natural postharvest softening and anoxia‐induced softening on DkXTH9 (which was named as DkXTH2 in ‘Saijo’, Nakatsuka et al., 2011).
Involvement of hypoxia‐responsive ERFs in regulating postdeastringency softening‐related genes
Twenty‐two DkERF genes were previously characterized as responsive to high CO2 treatment (Figure 3; Min et al., 2012, 2014), but only four (DkERF9/10/19/22) are transcriptional regulators of persimmon fruit astringency removal by regulating genes encoding PDC and ADH (Min et al., 2012, 2014). Thus, only a minority of DkERF genes are involved in deastringency, and others, including three DkERF genes (DkERF8/16/19) were identified as candidates for regulation of postdeastringency softening.
DkERF8/16/19 were shown to trans‐activate the DkXTH9 promoter, but not the promoters of the other seven cell wall‐related genes that show increased mRNA accumulation in response to anoxia. In apple, MdCBF2 can activate the MdPG promoter (Tacken et al., 2010), and in kiwi fruit, AdERF9 represses the AdXET5 (Yin et al., 2010). In most reported cases, however (including SlERF.B3, ERF2.2, MdCBF2 and AdERF9), the binding of these transcription factors to target promoters was not addressed. Our results indicate a direct in vivo activation by DkERF8/16/19 of the DkXTH9 promoter, as the effect was greatly reduced by targeted mutations on DRE (a known binding motif for ERF) in the DkXTH9 promoter. Direct binding of transcription factors on targets promoters could be analysed by various methods, such as EMSA. Here, dual‐luciferase assay and motif mutagenesis were also widely used methods to indicate the potential direct binding. Moreover, motif mutagenesis also provided the self‐explanations for in vivo effects of DkERF8/16/19 were through the recognization on DRE motif, but not the side effects of genes themselves or infiltrations. These results indicated hypoxia‐responsive DkERF8/16/19 contributed to the softening that accompanied de‐astringency. Previously, four hypoxia‐responsive ERFs, DkERF9/10/19/22, were shown to be regulators of ADH and PDC and contribute to fruit deastringency (Min et al., 2012, 2014). Here, except for DkERF19, the other three deastringency‐related DkERF9/10/22 were also response to deastringency treatment in ‘Tonewase’ persimmon (Figure S3), which were similar to previous findings. A model incorporating these findings is shown in Figure 8. The action of some ERFs could explain the linkage between deastringency and softening, processes which are generally regarded as quite distinct (Figure 8). Furthermore, DkERF19 has regulatory roles in both deastringency (DkPDC2, Min et al., 2014) and softening (DkXTH9; Figures 6 and 7), and could genuinely be considered to have dual‐functions (Figure 8). Our previous findings (Min et al., 2012, 2014) and the present research indicated that DkERF genes appear to be involved in both astringency removal and softening of persimmon fruit. The difficulties in generating transgenic persimmon fruit can be circumvented by population screening and other approaches, to identify variants in expression of DkERF genes which can be further developed as markers, enabling breeding of persimmon varieties that rapidly lose astringency while maintaining firmness. Meanwhile, ectopic overexpression of the DkERF to tomato (a model fruit) is another alternative, but different genetic background might give the different results. The mechanism for this difference in the anaerobic response between the Chinese and Japanese cultivars requires further investigation. Furthermore, although eight cell wall‐related genes were shown to be closely associated with persimmon fruit softening during deastringency, only DkXTH9 was responsive to the DkERF transcription factors and it seems likely that further investigation will reveal other factors involving different signalling pathways, which also participate in softening regulation.
Figure 8.
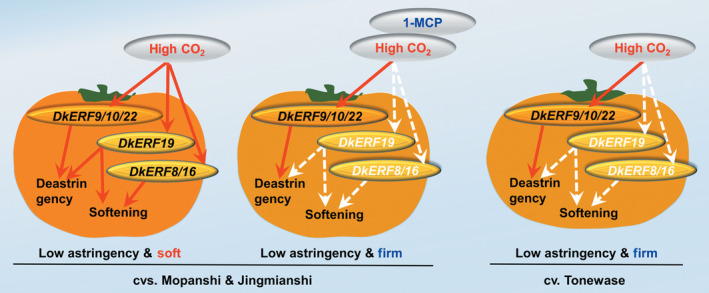
Proposed regulatory model for ethylene response factors in persimmon fruit deastringency and softening. High CO 2 treatment is an effective treatment for postharvest deastringency and widely used for industry. Six DkERF genes were characterized to be responsive to high CO 2 treatment: DkERF9/10/22 trans‐activate the DkADH/DkPDC and are involved in deastringency; DkERF8/16 are involved in regulation of cell wall metabolism‐related DkXTH9 and associated with fruit softening; DkERF19 has a dual regulatory role in deastringency and softening. Red arrows indicated significant activations; white arrows indicated the absence of activations.
Experimental procedures
Plant material and treatments
Three astringent‐type persimmon (Diospyros kaki) fruit were selected for this study, including two Chinese cultivars ‘Mopanshi’ (previously named ‘Mopan’, Min et al., 2012, 2014) and ‘Jingmianshi’ and one Japanese cultivar ‘Tonewase’.
‘Mopanshi’ persimmon fruit were harvested from a commercial orchard at Fangshan (Beijing, China) in 2012. Fruit without disease or mechanical wounding were selected. Three different treatments were conducted: (i) the first batch of fruit was treated with 95% CO2 for 1 day to remove astringency and the post‐treatment fruit exhibited rapid softening, (ii) the second batch was treated with a combination of 95% CO2 and 1 μL/L 1‐MCP for 1 day (CO2 and 1‐MCP treatments were performed at the same time), which removed the astringency and maintained fruit firmness, and (iii) the third batch of fruit was sealed in containers similar to those in the above treatments for 1 day, as control. The fruit after treatment were transferred to storage in air. All treatments and post‐treatment storage were at 20 °C.
In order to verify the combined effects of 95% CO2 and 1 μL/L 1‐MCP treatment (CO2 + 1‐MCP), ‘Mopanshi’ and another astringent‐type cultivar (‘Jingmianshi’) were collected from a commercial orchard at Qingdao (Shandong, China) in 2014. Treatments and the conditions were the same as in 2012 and the effect of CO2 + 1‐MCP on fruit deastringency and softening was similar to that in 2012 (data not shown).
In the 2014 season, an additional sample collection was conducted with a Japanese astringent‐type cultivar, ‘Tonewase’. ‘Tonewase’ fruit maintain firmness after astringency removal; thus, only CO2 treatment and control treatment were performed. The fruit were obtained from a commercial orchard at Qingdao (Shandong, China).
All of the above treatments were designed with three biological replicates (150 fruit in each). At each sampling time, flesh samples (three replicates, three fruit in each) were bulked and frozen in liquid nitrogen and stored at −80 °C until further use (soluble tannin measurements and RNA extraction).
Fruit firmness
Firmness was measured with a TA‐XT2i texture analyser (Stable Micro Systems, UK), and the penetration indices were calculated according to Yin et al. (2012). Over two different seasons, the firmness was measured using two different texture analyser probes, a 7.5‐mm‐diameter probe for ‘Mopanshi’ fruit in 2012 and a 5‐mm‐diameter probe used for ‘Jingmianshi’ and ‘Tonewase’ fruit in 2014. For each fruit, firmness was measured twice at the equator region at 90° intervals, after removal of 1‐mm peel. Fruit firmness was measured with 10 fruit replicates.
Soluble tannin content
Soluble tannins are the main source of persimmon fruit astringency. Here, measurements of soluble tannin content were made using two different methods. For ‘Mopanshi’ fruit, soluble tannins were measured with the Folin‐Ciocalteu reagent, using frozen samples, according to the method described by Yin et al. (2012). The results were calculated using the standard curve of tannin acids equivalents g−1 fresh weight. Soluble condensed tannin content was measured with three biological replicates. For ‘Jingmianshi’ and ‘Tonewase’, soluble tannins were visualized by the tannin printing method, according to Min et al. (2015). Fruit after treatment were immediately cut lengthwise and then printed onto 5% FeCl2‐soaked filter paper for 5 s. After removal of fruit, the soluble tannin contents were observed by the intensity of black colour and the filter paper was photographed.
RNA extraction and cDNA synthesis
Total RNA extractions were conducted by the methods described by Yin et al. (2012). Each extraction was conducted with 2.0 g frozen persimmon fruit flesh. The total RNA was treated with TURBO DNAse (Ambion) to remove the contaminated gDNA, and then used for cDNA synthesis, using an iScript™ cDNA Synthesis Kit (Bio‐Rad). Three biological replicates were used for RNA extraction.
Gene/promoter isolation
Twenty‐two high CO2‐responsive DkERF genes were previously isolated using RNA‐seq and RACE technologies (Min et al., 2012, 2014; Yin et al., 2012). Using the same RNA‐seq data, differentially expressed unigenes potentially related to cell wall degradation were obtained. The full‐length unigenes were obtained, using a SMART RACE cDNA amplification Kit (Clontech). Promoters of cell wall‐related genes were obtained using the Genome Walker Universal Kit (Clontech). Two DRE motifs of DkXTH9 promoter were mutated using the Fast Mutagenesis System Kit (Transgen). All primers used for gene and promoter isolation are described in Table S1.
Real‐time PCR analysis
For real‐time PCR, gene‐specific oligonucleotide primers were designed and are described in Table S2. Gene specificity of each pair of primers was double‐checked by melting curve and PCR product re‐sequencing. The DkACT was chosen as a housekeeping gene to monitor the abundance of mRNA (Min et al., 2014).
Real‐time PCR reactions were performed on a CFX96 instrument (Bio‐Rad). The PCR program comprised an initial step at 95 °C 3 min, 45 cycles of 95 °C 10 s and 60 °C 30 s, ending with a melting curve analysis programme. The PCR mixture (20 μL total volume) consisted of 10 μL Ssofast EvaGreen Supermix (Bio‐Rad), 1 μL of each primer (10 μm), 2 μL of 10‐fold diluted cDNA and 6 μL H2O. No‐template controls and melting curve analysis were included for each gene during each run. 2−ΔΔCt method was used to calculate the relative expression levels of genes (Livak and Schmitten, 2001).
The heatmap was used to present the genes expression results and was constructed by MetaboAnalyst 3.0. The different colours indicated up (red) or down (blue) regulations. mRNA abundance was indicated with the intensity of colours. The A, B and C represent three biological replicates.
Dual‐luciferase assay
Dual‐luciferase assay was used as an efficient and rapid method to detect in vivo trans‐activation or trans‐repression effects of transcription factors (Yin et al., 2010; Zeng et al., 2015). Full‐length DkERF genes were fused to pGreen II 0029 62‐SK vector (SK) by Min et al. (2012, 2014). Due to the lack of availability of a persimmon genome, promoters of Dkβ‐gal1/4, DkEGase1, DkPE1/2, DkPG1 and DkXTH9/10 were obtained with GenomeWalker™ Universal Kit (Clontech), using the primers described in Table S3. The promoters were amplified with the primers described in Table S4 and were cloned to pGreen II 0800‐LUC vector (LUC). Details of vector information are described in Hellens et al. (2005).
All of the constructs were electroporated into Agrobacterium tumefaciens GV3101. The dual‐luciferase assays were performed with Nicotiana benthamiana leaves. Agrobacterium cultures were prepared with infiltration buffer (10 mm MES, 10 mm MgCl2, 150 μm acetosyringone, pH 5.6) to an OD600 from 0.7 to 1.0. Agrobacterium culture mixtures of transcription factor and promoter (v/v, 10 : 1) were infiltrated into tobacco leaves using needleless syringes. Tobacco plants were grown in a growth chamber, with light : dark cycles of 16 : 8 h. Three days after infiltration, firefly luciferase and renilla luciferase were assayed using the dual‐luciferase assay reagents (Promega). The results were calculated from at least five replicates.
Statistical analysis
Statistical significance of differences was calculated using Student's t‐test or least significant difference (LSD) using DPS7.05 (Zhejiang University, Hangzhou, China). Figures were drawn using Origin 8.0 (Microcal Software Inc., Northampton, MA).
Conflict of interest
Authors have no conflict of interest to declare.
Supporting information
Figure S1 Expression of thirty‐five cell wall‐related genes in response to CO2 (95%) and CO2+1 ‐ MCP (1 μL L−1) treatments in ‘Mopanshi’ persimmon fruit at 20 °C.
Figure S2 Auto‐activation test for DkXTH9 promoter.
Figure S3 Expression of DkERF9/10/22 genes in response to CO2 (95%) treatment in ‘Tonewase’ persimmon fruit at 20 °C.
Table S1 Sequences of the primers used for gene isolation.
Table S2 Sequences of the primers used for gene expression analysis.
Table S3 Sequences of the primers used for genome walking.
Table S4 Sequences of the primers used for promoter amplification.
Table S5 Sequences of the promoters of cell wall‐related genes from ‘Mopanshi’ persimmon.
Acknowledgements
We wish to thank Dr. Shaolan Yang (Qingdao Agriculture University) for help with the collection of persimmon fruit. This research was supported by the National Key Research and Development Program (2016YFD0400100), the Special Fund for Agro‐scientific Research in the Public Interest (201203047), National Natural Science Foundation of China (31372114; 31672204) and the Natural Science Foundation of Zhejiang Province, China (LR16C150001).
References
- Arnal, L. and Del Río, M.A. (2004) Effect of cold storage and removal astringency on quality of persimmon fruit (Diospyros kaki, L.) cv. Rojo Brillante. Food Sci. Technol. Int. 10, 179–185. [Google Scholar]
- Atkinson, R.G. , Schroder, R. , Hallett, I.C. , Cohen, D. and MacRae, E.A. (2002) Overexpression of POLYGALACTURONASE in transgenic apple trees leads to a range of novel phenotypes involving changes in cell adhesion. Plant Physiol. 129, 122–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummell, D.A. and Harpster, M.H. (2001) Cell wall metabolism in fruit softening and quality and its manipulation in transgenic plants. Plant Mol. Biol. 47, 311–340. [PubMed] [Google Scholar]
- Carrington, C.M.S. , Greve, L.C. and Labavitch, J.M. (1993) Cell wall metabolism in ripening fruit. VI. Effect of the antisense polygalacturonase gene on cell wall changes accompanying ripening in transgenic tomatoes. Plant Physiol. 103, 429–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman, N.H. , Bonnet, J. , Grivet, L. , Lynn, J. , Graham, N. , Smith, R. , Sun, G.P. et al. (2012) High‐resolution mapping of a fruit firmness‐related quantitative trait locus in tomato reveals epistatic interactions associated with a complex combinatorial locus. Plant Physiol. 159, 1644–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung, M.Y. , Vrebalov, J. , Alba, R. , Lee, J. , McQuinn, R. , Chung, J.D. , Klein, P. et al. (2010) A tomato (Solanum lycopersicum) APETALA2/ERF gene, SlAP2a, is a negative regulator of fruit ripening. Plant J. 64, 936–947. [DOI] [PubMed] [Google Scholar]
- Cutillas‐Iturralde, A. , Zarra, I. and Lorences, E.P. (1993) Metabolism of cell wall polysaccharides from persimmon fruit. Pectin solubilization during fruit ripening occurs in apparent absence of polygalacturonase activity. Physiol. Plantarum, 89, 369–375. [Google Scholar]
- Cutillas‐Iturralde, A. , Zarra, I. , Fry, S.C. and Lorences, E.P. (1994) Implication of persimmon fruit hemicellulose metabolism in the softening process. Importance of xyloglucan endotransglycosylase. Physiol. Plantarum, 91, 169–176. [Google Scholar]
- Gibbs, D.J. , Lee, S.C. , Isa, N.M. , Gramuglia, S. , Fukao, T. , Bassel, G.W. , Correia, C.S. et al. (2011) Homeostatic response to hypoxia is regulated by the N‐end rule pathway in plants. Nature, 479, 415–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, Y. , Zhu, Q.G. , Zhang, Z.K. , Meng, K. , Hou, Y.L. , Ban, Q.Y. , Suo, J.T. et al. (2015) Analysis of xyloglucan endotransglycosylase/hydrolase (XTH) genes and diverse roles of isoenzymes during persimmon fruit development and postharvest softening. PLoS ONE, 10, e0123668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellens, R.P. , Allan, A.C. , Friel, E.N. , Bolitho, K. , Grafton, K. , Templeton, M.D. , Karunairetnam, S. et al. (2005) Transient expression vectors for functional genomics, quantification of promoter activity and RNA silencing in plants. Plant Methods, 1, e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz, M. , Wilson, I.W. , Yang, J. , Buerstenbinder, K. , Llewellyn, D. , Dennis, E.S. , Sauter, M. et al. (2010) Arabidopsis RAP2.2: an ethylene response transcription factor that is important for hypoxia survival. Plant Physiol. 153, 757–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itamura, H. , Tanigawa, T. and Yamamura, H. (1995) Composition of cell‐wall polysaccharides during fruit softening in ‘Tonewase’ Japanese persimmon. Acta Hortic. 398, 131–138. [Google Scholar]
- Jiang, N.N. , Rao, J.P. , Fu, R.S. and Suo, J.T. (2010) Effects of propylene and 1‐methylcyclopropene on PG activities and expression of DkPG1 gene during persimmon softening process. Acta Hortic. Sin. 37, 1507–1512. (In Chinese, with English abstract) [Google Scholar]
- Kagale, S. and Rozwadowski, K. (2011) EAR motif‐mediated transcriptional repression in plants: an underlying mechanism for epigenetic regulation of gene expression. Epigenetics, 6, 141–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy, R.A. , Rumpho, M.E. and Fox, T.C. (1992) Anaerobic metabolism in plants. Plant Physiol. 100, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa, Y. , Kanayama, Y. and Yamaki, S. (1995) Isolation of β‐galactosidase fractions from Japanese pear: activity against native cell wall polysaccharides. Physiol. Plantarum, 93, 545–550. [Google Scholar]
- Kramer, M. , Sanders, R. , Bolkan, H. , Waters, C. , Sheeny, R.E. and Hiatt, W.R. (1992) Postharvest evaluation of transgenic tomatoes with reduced levels of polygalacturonase: processing, firmness and disease resistance. Postharvest Biol. Technol. 1, 241–255. [Google Scholar]
- Li, Y.C. , Zhu, B.Z. , Xu, W.T. , Zhu, H.L. , Chen, A.J. , Xie, Y.H. , Shao, Y. et al. (2007) LeERF1 positively modulated ethylene triple response on etiolated seedling, plant development and fruit ripening and softening in tomato. Plant Cell Rep. 26, 1999–2008. [DOI] [PubMed] [Google Scholar]
- Li, X. , Xu, C.J. , Korban, S.S. and Chen, K.S. (2010) Regulatory mechanisms of textural changes in ripening fruits. Crit. Rev. Plant Sci. 29, 222–243. [Google Scholar]
- Licausi, F. , van Dongen, J.T. , Giuntoli, B. , Novi, G. , Santaniello, A. , Geigenberger, P. and Perata, P. (2010) HRE1 and HRE2, two hypoxia‐inducible ethylene response factors, affect anaerobic responses in Arabidopsis thaliana . Plant J. 62, 302–315. [DOI] [PubMed] [Google Scholar]
- Licausi, F. , Kosmacz, M. , Weits, D.A. , Giuntoli, B. , Giorgi, F.M. , Voesenek, L.A.C.J. , Perata, P. et al. (2011) Oxygen sensing in plants is mediated by an N‐end rule pathway for protein destabilization. Nature, 479, 419–422. [DOI] [PubMed] [Google Scholar]
- Livak, J. and Schmitten, T.D. (2001) Analysis of relative gene expression data using real‐time quantitative PCR and the 2−ΔΔCT method. Methods, 25, 402–408. [DOI] [PubMed] [Google Scholar]
- Luo, Z.S. (2007) Effect of 1‐methylcyclopropene on ripening of postharvest persimmon (Diospyros kaki L.) fruit. LWT Food Sci. Technol. 40, 285–291. [Google Scholar]
- Luo, C. , Zhang, Q.L. and Luo, Z.R. (2014) Genome‐wide transcriptome analysis of Chinese pollination‐constant nonastringent persimmon fruit treated with ethanol. BMC Genomics. 15, e112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miedes, E. , Herbers, K. , Sonnewald, U. and Lorences, E.P. (2010) Overexpression of a cell wall enzyme reduces xyloglucan depolymerization and softening of transgenic tomato fruits. J. Agric. Food Chem. 58, 5708–5713. [DOI] [PubMed] [Google Scholar]
- Min, T. , Yin, X.R. , Shi, Y.N. , Luo, Z.R. , Yao, Y.C. , Grierson, D. , Ferguson, I.B. et al. (2012) Ethylene‐responsive transcription factors interact with promoters of ADH and PDC involved in persimmon (Diospyros kaki) fruit de‐astringency. J. Exp. Bot. 63, 6393–6405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min, T. , Fang, F. , Ge, H. , Shi, Y.N. , Luo, Z.R. , Yao, Y.C. , Grierson, D. et al. (2014) Two novel anoxia‐induced ethylene response factors that interact with promoters of deastringency‐related genes from persimmon. PLoS ONE, 9, e97043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min, T. , Wang, M.M. , Wang, H.X. , Liu, X.F. , Fang, F. , Grierson, D. , Yin, X.R. et al. (2015) Isolation and expression of NAC genes during persimmon fruit postharvest astringency removal. Int. J. Mol. Sci. 16, 1894–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura, A. , Maeda, H. , Mizuno, M. , Koshi, Y. and Nagamatsu, Y. (2003) β‐Galactosidase and its significance in ripening of “Saijyo” Japanese persimmon fruit. Biosci. Biotechnol. Biochem. 67, 68–76. [DOI] [PubMed] [Google Scholar]
- Nakano, R. , Inoue, S. , Kubo, Y. and Inaba, A. (2002) Water stress‐induced ethylene in the calyx triggers autocatalytic ethylene production and fruit softening in ‘Tonewase’ persimmon grown in a heated plastic‐house. Postharvest Biol. Technol. 25, 293–300. [Google Scholar]
- Nakatsuka, A. , Maruo, T. , Ishibashi, C. , Ueda, Y. , Kobayashi, N. , Yamagishi, M. and Itamura, H. (2011) Expression of genes encoding xyloglucan endotransglycosylase/hydrolase in ‘Saijo’ persimmon fruit during softening after deastringency treatment. Postharvest Biol. Technol. 62, 89–92. [Google Scholar]
- Ortiz, G.I. , Sugaya, S. , Sekozawa, Y. , Ito, H. , Wada, K. and Gemma, H. (2005) Efficacy of 1‐Methylcyclopropene (1‐MCP) in prolonging the shelf‐life of ‘Rendaiji’ persimmon fruits previously subjected to astringency removal treatment. J. Jpn. Soc. Hortic. Sci. 74, 248–254. [Google Scholar]
- Papdi, C. , Pérez‐Salamó, I. , Joseph, M.P. , Giuntoli, B. , Bögre, L. , Koncz, C. and Szabados, L. (2015) The low oxygen, oxidative and osmotic stress responses synergistically act through the ethylene response factor VII genes RAP2.12, RAP2.2 and RAP2.3 . Plant J. 82, 772–784. [DOI] [PubMed] [Google Scholar]
- Payasi, A. , Mishra, N.N. , Chaves, A.L.S. and Singh, R. (2009) Biochemistry of fruit softening: an overview. Physiol. Mol. Biol. Plants, 15, 103–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesis, E. and Ben‐Arie, R. (1984) Involvement of acetaldehyde and ethanol accumulation during induced deastringency of persimmon fruits. J. Food Sci. 49, 896–899. [Google Scholar]
- Phan, T.D. , Bo, W. , West, G. , Lycett, G.W. and Tucker, G.A. (2007) Silencing of the major salt‐dependent isoform of pectinesterase in tomato alters fruit softening. Plant Physiol. 144, 1960–1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell, A.L.T. , Kalamaki, M.S. , Kurien, P.A. , Gurrieri, S. and Bennett, A.B. (2003) Simultaneous transgenic suppression of LePG and LeExp1 influences fruit texture and juice viscosity in a fresh market tomato variety. J. Agric. Food Chem. 51, 7450–7455. [DOI] [PubMed] [Google Scholar]
- Silvia, J.B. , José, R.N. , Juan, M.B. , Caballero, J.L. , López‐Aranda, J.M. , Victoriano, V. , Pliego‐Alfaro, F. et al. (2002) Manipulation of strawberry fruit softening by antisense expression of a pectate lyase gene. Plant Physiol. 128, 751–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, C.J.S. , Watson, C.F. , Ray, J. , Bird, C.R. , Morris, P.C. , Schuch, W. and Grierson, D. (1988) Antisense RNA inhibition of polygalacturonase gene expression in transgenic tomatoes. Nature, 334, 724–726. [Google Scholar]
- Smith, D.L. , Abbott, J.A. and Gross, K.C. (2002) Down‐regulation of tomato β‐galactosidase 4 results in decreased fruit softening. Plant Physiol. 129, 1755–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tacken, E. , Ireland, H. , Gunaseelan, K. , Karunairetnam, S. , Wang, D. , Schultz, K. , Bowen, J. et al. (2010) The role of ethylene and cold temperature in the regulation of the apple POLYGALACTURONASE1 gene and fruit softening. Plant Physiol. 153, 294–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taira, S. , Oba, S. and Watanabe, S. (1992) Removal of astringency from ‘Hiratanenashi’ persimmon fruit with a mixture of ethanol and carbon dioxide. J. Jpn. Soc. Hortic. Sci. 61, 437–443. [Google Scholar]
- Taira, S. , Ono, M. and Matsumoto, N. (1997) Reduction of persimmon astringency by complex formation between pectin and tannins. Postharvest Biol. Technol. 12, 265–271. [Google Scholar]
- Taira, S. , Ikeda, K. and Ohkawa, K. (2001) Comparison of insolubility of tannins induced by acetaldehyde vapor in fruit of three types of astringent persimmon. J. Jpn. Soc. Hortic. Sci. 48, 684–687. [Google Scholar]
- Uluisik, S. , Chapman, N. , Smith, R. , Poole, M. , Adams, G. , Gillis, R.B. , Besong, T.M.D. et al. (2016) Genetic improvement of tomato by targeted control of fruit softening. Nat. Biotechnol. 34, 950–952. [DOI] [PubMed] [Google Scholar]
- Vicente, A.R. , Saladié, M. , Rose, J.K. and Labavitch, J.M. (2007) The linkage between cell wall metabolism and fruit softening: looking to the future. J. Sci. Food Agric. 87, 1435–1448. [Google Scholar]
- Voesenek, L.A.C.J. and Bailey‐Serres, J. (2015) Flood adaptive traits and processes: an overview. New Phytol. 206, 57–73. [DOI] [PubMed] [Google Scholar]
- Xie, X.L. , Yin, X.R. and Chen, K.S. (2016) Roles of APETALA2/ethylene responsive factors in regulation of fruit quality. Crit. Rev. Plant Sci. 35, 120–130. [Google Scholar]
- Xu, C. , Nakatani, Y. , Nakatsuka, A. and Itamura, H. (2003) Effects of different methods of deastringency and storage on the shelf life of ‘Saijo’ persimmon fruit. Food Preserv. Sci. 29, 191–196. [Google Scholar]
- Yamada, M. , Taira, S. , Ohtsuki, M. , Sato, A. , Iwanami, H. , Yakushiji, H. , Wang, R.Z. et al. (2002) Varietal differences in the ease of astringency removal by carbon dioxide gas and ethanol vapor treatments among Oriental astringent persimmons of Japanese and Chinese origin. Sci. Hortic. 94, 63–72. [Google Scholar]
- Yang, C.Y. , Hsu, F.C. , Li, J.P. , Wang, N.N. and Shih, M.C. (2011) The AP2/ERF transcription factor AtERF73/HRE1 modulates ethylene responses during hypoxia in Arabidopsis. Plant Physiol. 156, 202–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin, X.R. , Allan, A.C. , Chen, K.S. and Ferguson, I.B. (2010) Kiwifruit EIL and ERF genes involved in regulating fruit ripening. Plant Physiol. 153, 1280–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin, X.R. , Shi, Y.N. , Min, T. , Luo, Z.R. , Yao, Y.C. , Xu, Q. , Ferguson, I. et al. (2012) Expression of ethylene response genes during persimmon fruit astringency removal. Planta, 235, 895–906. [DOI] [PubMed] [Google Scholar]
- Zeng, J.K. , Li, X. , Xu, Q. , Chen, J.Y. , Yin, X.R. , Ferguson, I.B. and Chen, K.S. (2015) EjAP2‐1, an AP2/ERF gene, is a novel regulator of fruit lignification induced by chilling injury, via interaction with EjMYB transcription factors. Plant Biotechnol. J. 13, 1325–1334. [DOI] [PubMed] [Google Scholar]
- Zhang, Z.K. , Fu, R.S. , Huber, D.J. , Rao, J.P. , Chang, X.X. , Hu, M.J. , Zhang, Y. et al. (2012) Expression of expansin gene (CDK‐Exp3) and its modulation by exogenous gibberellic acid during ripening and softening of persimmon fruit. HortScience, 47, 378–381. [Google Scholar]
- Zhu, Q.G. , Zhang, Z.K. , Rao, J.P. , Huber, D.J. , Lv, J.Y. , Hou, Y.L. and Song, K.H. (2013) Identification of xyloglucan endotransglucosylase/hydrolase genes (XTHs) and their expression in persimmon fruit as influenced by 1‐methylcyclopropene and gibberellic acid during storage at ambient temperature. Food Chem. 138, 471–477. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Expression of thirty‐five cell wall‐related genes in response to CO2 (95%) and CO2+1 ‐ MCP (1 μL L−1) treatments in ‘Mopanshi’ persimmon fruit at 20 °C.
Figure S2 Auto‐activation test for DkXTH9 promoter.
Figure S3 Expression of DkERF9/10/22 genes in response to CO2 (95%) treatment in ‘Tonewase’ persimmon fruit at 20 °C.
Table S1 Sequences of the primers used for gene isolation.
Table S2 Sequences of the primers used for gene expression analysis.
Table S3 Sequences of the primers used for genome walking.
Table S4 Sequences of the primers used for promoter amplification.
Table S5 Sequences of the promoters of cell wall‐related genes from ‘Mopanshi’ persimmon.


