Dear Editor,
The CRISPR/Cas9‐based genome‐editing tool has been used in diverse applications related to plant research, including for crop improvement (Liu et al., 2017; Sun et al., 2016). Mutant plants may be generated via transient transformations or DNA‐free editing (Liang et al., 2017). However, plant genomes are often edited during the production of transgenic plants, in a process that involves the identification of targeted edits in regenerated T0 plants and the subsequent elimination of transgenes in T1 plants (Sun et al., 2016). Because T‐DNA (e.g. CRISPR/Cas9) is randomly inserted into plant genomes, it may be silent (e.g. when incorporated in heterochromatin) or is actively silenced; hence, theoretically only a portion of the T0 plants carry active transgenes, although high genome‐editing rates have been observed (Xie et al., 2015). Therefore, selecting transgene‐free plants with an edited genome may be a time‐consuming and costly part of genome‐editing projects. This is especially true for practical breeding programmes when many plants need to be produced and screened, considering that successful application is often genotype dependent (Zhu et al., 2017).
The RNA interference (RNAi) technique has already been used to decrease the abundance of unwanted grain ingredients or increase resistance to viral pathogens (Kamthan et al., 2015). Functional RNAi elements (e.g. hairpin RNAi, hpRNAi) can be detected in T0 plants because of their dominant nature. Therefore, we hypothesized that when an RNAi expression element is incorporated into a CRISPR/Cas9 vector, the activity and presence of the T‐DNA in the transgenic plants can be monitored based on RNAi. This would enable a PCR‐free, phenotype‐based identification of genome‐edited T0 plants, and a subsequent selection of transgene‐free T1 plants.
In rice, CYP81A6 encodes a P450 cytochrome protein that confers resistance to bentazon. Silencing CYP81A6 may render rice plants susceptible to the herbicide (Pan et al., 2006). We speculated that a CYP81A6‐hpRNAi may be a suitable marker for CRISPR/Cas9 expression. To produce a CYP81A6‐hpRNAi element, we first amplified a 300‐bp fragment from CYP81A6 using forward (5′AGCTTAGCCATGGATAACGCCTAC3′) and reverse (5′AAGGTCACGTCGTGCTCGGTGAAGCACTC3′) primers. The fragment was then inserted into the pBSSK‐IN vector to form a hairpin structure, which was then introduced into a pCAMBIA‐1300 vector between the double 35S (d35S) promoter and the Nos terminator (nos). We inserted the d35S‐hpRNAi‐nos element into the HpaI site near the left border of a CRISPR/Cas9 vector, pHun4c12, to construct a new CRISPR/Cas9 vector, pHun4c12s (Figure 1a). To test the utility of pHun4c12s, we constructed another CRISPR/Cas9 expression vector, pHun4c12s‐lct1, to target OsLCT1, which encodes a Cd transporter (Uraguchi et al., 2011). Ninety‐six independent transgenic T0 plants were generated via Agrobacterium tumefaciens‐mediated transformation of japonica rice genotype Xidao #1 cells with pHun4c12s‐lct1 and analysed further.
Figure 1.
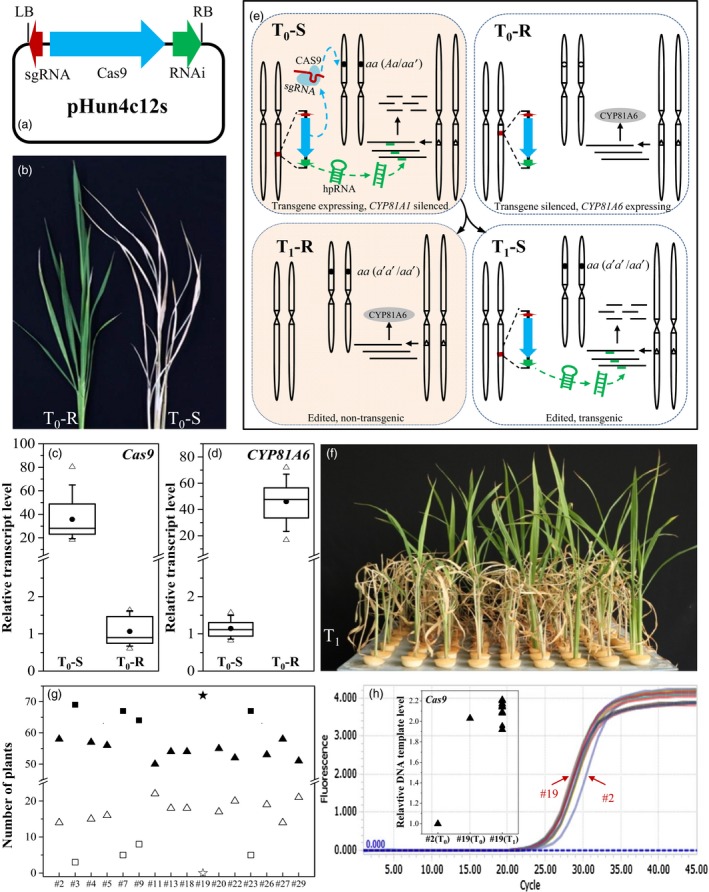
Production of genome‐edited, transgene‐free rice plants. (a) Diagram of a CRISPR‐S vector, pHun4c12s, derived from pHun4c12. (b) Bentazon‐resistant (left, T0‐R) and bentazon‐susceptible (right, T0‐S) tillers of transgenic T0 plants. (c) and (d) Box plot representations of Cas9 (c) and CYP81A6 (d) relative transcript levels of T0‐S and T0‐R plants. The values of one arbitrarily chosen T0‐R plant (c) and one T0‐S plant (d) were set as 1. For each box plot, the 10th, 25th, median, 75th and 90th percentiles of relative transcript levels were represented by horizontal lines (bottom to top), the average as filled circles, and the minimum and maximum indicated by empty triangles. (e) Illustration of the genetics of genome editing, bentazon susceptibility, and segregation of transgene and genome edits. In transgene‐expressing T0 plants, the target gene (empty circle) is edited by the CAS9 and sgRNA complex to introduce mutations (filled circle). Meanwhile, CYP81A6‐hpRNAi degrades CYP81A6 transcripts, rendering plants susceptible to bentazon. In contrast, the genomes of T0 plants with a silenced transgene are not edited, and these plants remain resistant to bentazon. Transgene‐free (i.e. resistant to bentazon), genome‐edited T1‐R plants are produced by bentazon treatment, which kills transgenic T1‐S plants. (f) Symptoms of Line #2 T1 seedlings 1 week after a bentazon foliar spray (1000 mg/L). (g) Segregation of bentazon‐susceptible (filled) and bentazon‐resistant (empty) plants in 16 T1 lines. Lines fit the 3:1 or 16:1 ratios based on a chi‐square test are indicated with triangles or squares, respectively. Line #19 T1 plants are indicated with a filled star. (h) T‐DNA copy number in Line #19 plants assessed by a qRT‐PCR analysis of Cas9, with Actin as the internal standard, and the value of Line #2 T0 plant set as 1. Actin was used as the internal standard in qRT‐PCR analysis.
First, we tested the susceptibility of the T0 plants to bentazon using a foliar spray. Tillers of T0 plants were separated and grown as two subplants. One normally growing subplant of each T0 plant was treated with a bentazon solution (2000 mg/L) by spraying until droplets were visible on leaves. About 1 week later, 29 T0 subplants appeared to be susceptible and eventually died (T0‐S plants; Figure 1b). The other 67 T0 plants were not visibly affected (T0‐R plants).
Second, we sequenced the OsLCT1 target region of the T0 plants. All 29 T0‐S plants were affected by homozygous (aa), biallelic (aa′) or heterozygous (Aa) mutations in the target region. Mutations were not detected in the remaining plants. Thus, bentazon susceptibility was 100% correlated with the targeted mutations in T0 plants.
Third, we analysed the abundance of Cas9 and CYP81A6 transcripts in T0‐R and T0‐S plants using a quantitative real‐time PCR (qRT‐PCR). Overall, the Cas9 transcript was significantly more abundant in T0‐S plants than in T0‐R plants (Figure 1c). In contrast, the abundance of the CYP81A6 transcript was lowest in the T0‐S plants (Figure 1d), suggesting that CYP81A6‐hpRNAi was more highly expressed in these plants. The expression of CYP81A6‐hpRNAi resulted in the degradation of CYP81A6 transcripts via RNAi in transgene‐expressing, T0‐S plants. In contrast, the transgene‐silenced T0 plants remained resistant to bentazon (Figure 1e). These observations imply that CYP81A6‐hpRNAi enables the efficient selection of genome‐edited T0 plants.
Our system was also designed to simplify the selection of transgene‐free T1 plants. We grew 72 seedlings for each of 16 T1 lines derived from homozygous or biallelic OsLCT1 mutant T0 plants treated them with 1000 mg/L bentazon in a foliar spray (approximately 100 mL/m2) at around the four‐leaf stage. About 4 days later, we observed that all Xidao #1 seedlings were growing normally, but some of the T1 seedlings started dehydrating and eventually died (Figure 1f). Furthermore, we proved that all bentazon‐susceptible plants were transgenic, while all bentazon‐resistant plants lacked T‐DNA. The segregation of T1 plants derived from a homozygous or biallelic genome‐edited T0 plant is presented in Figure 1e, in which the genome‐edited, transgene‐free plants are resistant to bentazon because the CYP81A6‐hpRNAi no longer exists. Meanwhile, transgenic plants are susceptible because the CYP81A6 transcripts are degraded.
Transgenic plants produced by A. tumefaciens‐mediated transformation often carry one or two copies of T‐DNA (Collier et al., 2017). The segregation ratio observed for bentazon susceptibility was consistent with this fact in all T1 lines except for Line #19 (Figure 1g). All Line #19 T1 seedlings were susceptible to bentazon and died. Based on the 3:1 segregation ratio of its T1 population (Figure 1g), the Line #2 T0 plant was expected to have a single T‐DNA insertion and was therefore used as the control for the qRT‐PCR analysis. The Line #19 T0 plant and its T1 progenies appeared to have the same T‐DNA copy number, and they all had double the number of insertions of the Line #2 T0 plant (Figure 1h). This implies that T‐DNA copies were incorporated into sister chromosomes in Line #19 T0 plant. Otherwise, variability in the T‐DNA copy numbers among the T1 plants would have been detected.
To test the utility of CRISPR‐S for other genes, we constructed a pHun4c12s‐frg vector to target OsBADH2 (Bradbury et al., 2005). We transformed nine rice genotypes and obtained 4–22 T0 plants for each genotype. All T0 plants susceptible to bentazon were confirmed to carry mutations. Similarly, transgene‐free, OsBADH2‐edited T1 plants were identified following a bentazon treatment of T1 seedlings.
The results of our proof‐of‐concept study revealed that the Cas9 expression level could be indirectly estimated using a marker trait generated by an RNAi element incorporated into the CRISPR/Cas9 expression vector. This new CRISPR/Cas9 system provides a relatively simple way of identifying and eliminating T0 plants in which the genome has not been edited. More importantly, our method enables the selection of transgene‐free T1 plants, with almost no cost, confirming the value of this system.
Antibiotic or herbicide resistance genes in binary vectors are important for selecting transgenic plants. However, they cannot be directly used to select transgene‐free T1 plants because they would kill the plants. Seed‐localized fluorescent reporters have been used to discriminate between transgenic and nontransgenic seeds in a few plant species. Unfortunately, they are unsuitable for species that produce seeds with hulls or glumes such as rice. Although our system has been demonstrated in rice, it may be possible to develop similar systems in other plant species. Highly conserved homologs of CYP81A6 have been detected in monocots. Thus, the bentazon‐susceptibility trait may also be used in these plant species. Furthermore, other suitable marker traits can be generated by down‐regulating the expression of other genes to introduce visible morphological changes to leaf shape and colour, among other characteristics.
Funding
This work was supported by the Zhejiang Provincial S & T Project on Breeding of Agricultural (Food) Crops (grant no. 2016C02050‐2).
Author Contributions
Q.‐Y. S., H.‐P. L. and J.‐Z. H designed the experiments, analysed the data and wrote the manuscript; H.‐P. L. conducted the experiments with the assistance of S.‐M. L., S.‐L. X., W.‐Y. C., X. Z. and Y.‐Y. T.
Acknowledgments
We thank Dr. Pengcheng Wei for providing the original CRISPR/Cas9 vector (pHun4c12). The authors have no conflicts of interest to declare.
References
- Bradbury, L. , Fitzgerald, T. , Henry, R. , Jin, Q. and Waters, D. (2005) The gene for fragrance in rice. Plant Biot. J. 3, 363–370. [DOI] [PubMed] [Google Scholar]
- Collier, R. , Dasgupta, K. , Xing, Y.P. , Hernandez, B.T. , Shao, M. , Rohozinski, D. , Kovak, E. et al (2017) Accurate measurement of transgene copy number in crop plants using droplet digital PCR. Plant J. 90, 1014–1025 https://doi.org/10.1111/tpj.13517. [DOI] [PubMed] [Google Scholar]
- Kamthan, A. , Chaudhuri, A. , Kamthan, M. and Datta, A. (2015) Small RNAs in plants: recent development and application for crop improvement. Front. Plant Sci. 6, 208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang, Z. , Chen, K.L. , Li, T.D. , Zhang, Y. , Wang, Y.P. , Zhao, Q. , Liu, J.X. et al (2017) Efficient DNA‐free genome editing of bread wheat using CRISPR/Cas9 ribonucleoprotein complexes. Nat. Comm. 8, 14261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, H. , Ding, Y.D. , Zhou, Y.Q. , Jin, W.Q. , Xie, K.B. and Chen, L.L. (2017) CRISPR‐P 2.0: an improved CRISPR‐Cas9 tool for genome editing in plants. Mol. Plant, 10, 530–532. [DOI] [PubMed] [Google Scholar]
- Pan, G. , Zhang, X.Y. , Liu, K.D. , Zhang, J.W. , Wu, X.Z. , Zhu, J. and Tu, J.M. (2006) Map‐based cloning of a novel rice cytochrome P450 gene CYP81A6 that confers resistance to two different classes of herbicides. Plant Mol. Biol. 61, 933–943. [DOI] [PubMed] [Google Scholar]
- Sun, Y.W. , Li, J.Y. and Xia, L.Q. (2016) Precise genome modification via sequence‐specific nucleases‐mediated gene targeting for crop improvement. Front. Plant Sci. 7, 1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uraguchi, S. , Kamiya, T. , Sakamoto, T. , Kasai, K. , Sato, Y. , Nagamura, Y. , Yoshida, A. et al (2011) Low‐affinity cation transporter (OsLCT1) regulates cadmium transport into rice grains. Proc. Natl. Acad. Sci. USA, 108, 20959–20964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie, K.B. , Minkenberg, B. and Yang, Y.N. (2015) Boosting CRISPR/Cas9 multiplex editing capability with the endogenous tRNA‐processing system. Proc. Natl. Acad. Sci. USA, 112, 3570–3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, C. , Bortesi, L. , Baysal, C. , Twyman, R.M. , Fischer, R. , Capell, T. , Schillberg, S. et al (2017) Characteristics of genome editing mutations in cereal crops. Trend Plant Sci. 22, 38–52. [DOI] [PubMed] [Google Scholar]


