Abstract
The aim of this study was to optimize the conditions for the extraction of low-abundance proteins (LAPs) and the removal of abundant proteins (APs; β-conglycinin and glycinin) from soybean meal. Single factor and orthogonal experiments were designed to determine the effects of four factors (isopropanol concentration, total extraction time, ultrasonic power, and ultrasonic time) on protein concentration in isopropanol extracts. Proteins in the isopropanol supernatant and the cold acetone precipitate of isopropanol were identified by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and matrix-assisted laser desorption/ionization-time of flight mass spectrometry (MALDI-TOF-MS). The results showed that the optimal conditions were 50% isopropanol, ultrasonic pretreatment for 15 min at 350 W, and a total extraction time of 1 h. Under these conditions, the protein concentration in the isopropanol extracts reached 0.8081 g/L. Many LAPs were detected, including β-amylase, soybean agglutinin, soybean trypsin inhibitor, fumarylacetoacetase-like, phospholipase D alpha 1-like, oleosin, and even some unknown soybean proteins. The soybean APs (β-conglycinin and glycinin) were not found. The method may be useful for discovering new soybean proteins and extracting enough LAPs of soybean to allow further studies of their physiological effects on animals without the influence of APs.
Keywords: Protein extraction, Abundant protein, Low-abundance protein, Defatted soybean meal, MALDI-TOF-MS
1. Introduction
Soybean proteins have complex compositions, which result in the diversity of their classification and function. According to their abundance in soybeans, they can be classified as abundant proteins (APs) or low-abundance proteins (LAPs). As soybeans are the main source of plant protein, the extraction and physiological functions of soybean proteins have been widely studied. Most such studies have focused on soybean meal (Tibaldi et al., 2006; Wang et al., 2006; Saki et al., 2012), soybean protein isolates (SPIs) (Jankowski et al., 2009; Cornish et al., 2011), and the soybean APs (β-conglycinin and glycinin) (Deak et al., 2006; Adams et al., 2008; Sun et al., 2008). The extraction of most LAPs is beyond the scope of traditional techniques (Deak et al., 2006), and they are very difficult to separate from APs. New methods are needed to facilitate studies of their extraction and physiological functions on animals and humans.
With the rapid development of proteomics techniques, the separation and identification of soybean LAPs have received increasing attention. Treatment with trichloroacetic acid (TCA)/acetone, verified as an efficient and reliable method for the separation and identification of soybean proteins, has been used in the characterization of soybean storage proteins (Natarajan et al., 2006a) and for the analysis of allergen and anti-nutritional proteins in wild and cultivated soybean seeds (Natarajan et al., 2006b). Natarajan et al. (2009) found that isopropanol treatment resulted in the depletion of APs and was better than TCA for preferentially enriching soybean LAPs. On the other hand, many studies have confirmed that ultrasound treatment facilitates the disintegration of particles. High-power ultrasound was reported as a powerful method to enhance the extraction of intracellular compounds from plant materials, including the extraction of oil, sugar, and protein from soy flakes (Li et al., 2004; Karki et al., 2009a; 2009b; Bu et al., 2012).
To obtain enough LAPs for further studies of their physiological functions on animals without the influence of APs, we carried out single factor (isopropanol concentration, ultrasonic power, ultrasonic time, and the total extraction time) and orthogonal experiments to optimize the conditions for LAP extraction and AP removal. The concentration and identity of the proteins in the isopropanol extracts were determined by Coomassie brilliant blue R-250 (CBB) kit, sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and mass spectrometry (MS).
2. Materials and methods
2.1. Materials and chemicals
Defatted soybean meal (crude protein, 55%) was obtained from Jilin Fengzheng Soybean Food Limited Company (China). A Coomassie brilliant blue kit was purchased from the Nanjing Jiancheng Bioengineering Institute (China). Ammonium bicarbonate, acetonitrile, and trifluoroacetic acid (TFA) were all of chromatographic grade (Sigma, USA). Trypsin (Promega, USA) was of sequencing grade. A 170-kD protein molecular mass marker (Thermo, USA) was used in electrophoretic analyses. Isopropanol and other reagents were of analytical grade (Sinopharm, China). Ultrapure water was self-made (Thermo, USA) and used for making all solutions.
2.2. Single factor and orthogonal experimental design
First, without ultrasound treatment, the effects of isopropanol concentration and total extraction time on the extraction of soybean proteins were determined. Then, under the conditions of 40% isopropanol, a 1-h total extraction time, and ultrasound treatment for 20 min, the effect of ultrasonic power was investigated. The fourth experiment was conducted using 40% isopropanol, a 1-h total extraction time and ultrasound pretreatment at 400 W, to confirm the influence of ultrasonic time on soybean protein extraction.
To further determine the optimal conditions for LAP extraction and AP removal from defatted soybean meal, orthogonal experiments were designed based on the results of the above experiments (Table 1), and the proteins in extracts were identified by SDS-PAGE and MS.
Table 1.
L9(34) orthogonal design
| Level | Factor |
|||
| A (%) | B (W) | C (min) | D (min) | |
| 1 | 40 | 350 | 5 | 40 |
| 2 | 45 | 400 | 10 | 60 |
| 3 | 50 | 450 | 15 | 90 |
A: isopropanol concentration; B: ultrasonic power; C: ultrasonic time; D: total extraction time
2.3. Protein extraction from defatted soybean meal
The defatted soybean meal powder (500 mg) was homogenized with 5 ml of isopropanol solutions of known concentrations (35%, 40%, 45%, 50%, and 55% (v/v)), and then extractions were carried out for certain total time (0.5, 1, 2, and 3 h; ultrasound treatment and vibrating water-bath in sequence). The ultrasound treatment included ultrasonication at variable power (0, 200, 300, 400, or 500 W) for 10 min, or for a variable time (0, 5, 10, 15, or 20 min) at 400 W. Finally, the supernatant of the extracts was obtained after centrifugation at 12 000 r/min for 15 min at 4 °C, and the protein concentration in the supernatants was determined immediately using the Coomassie brilliant blue kit.
The supernatant was isolated and placed in a clean 20-ml tube, and then double the volume of cold acetone was added to the supernatant before incubation at −20 °C overnight. Next day, the samples were centrifuged for 10 min at 12 000 r/min at 4 °C and the pellet was dried using a freeze dryer.
2.4. Identification of proteins in isopropanol extracts
2.4.1. SDS-PAGE
According to Natarajan et al. (2009) and Krishnan et al. (2009b), the proteins in isopropanol extracts and cold acetone precipitates can be separated by SDS-PAGE on a 12% resolving gel at 100 V using a Mini-PROTEAN Tetra System (Bio-Bad, USA) and visualized by staining for about 1 h with CBB and fading overnight.
2.4.2. Trypsin digestion and mass spectrometry
Trypsin digestion and MS were performed using the method of Liu et al. (2016). After being excised and washed twice with ultrapure water and three times with 25 mmol/L ammonium bicarbonate in 50% acetonitrile for destaining, the protein bands of the CBB-stained gel were dehydrated with 200 μl of acetonitrile. The protein band pieces were then re-swollen in 10.0 ng/ml trypsin for trypsin digestion. The digestion was performed overnight at 37 °C after incubation at 4 °C for 30 min. Next day, the supernatant was extracted three times in 50% acetonitrile containing 0.1% trifluoracetic acid (TFA) and dried using a freeze dryer.
All samples were analyzed using a 5800 matrix-assisted laser desorption/ionization-time of flight (MALDI TOF)/TOF analyzer (Applied Biosystems, Framingham, MA, USA). Mass spectra (m/z 800–4000) were acquired in positive ion reflector mode, and the 20 most intense ions were selected for subsequent MS/MS sequencing analysis in 2 kV modes. Protein identification was performed by searching MS/MS spectra, and the peptide mass was obtained from National Center for Biotechnology Information (NCBI) protein databases using the search engine Matrix Science (http://www.matrixscience.com).
2.5. Statistical analyses
SPSS 16.0 statistical software was used for data analysis. Data were analyzed by one-way analysis of variance (ANOVA).
3. Results and discussion
3.1. Effects of single factors on soybean protein extraction
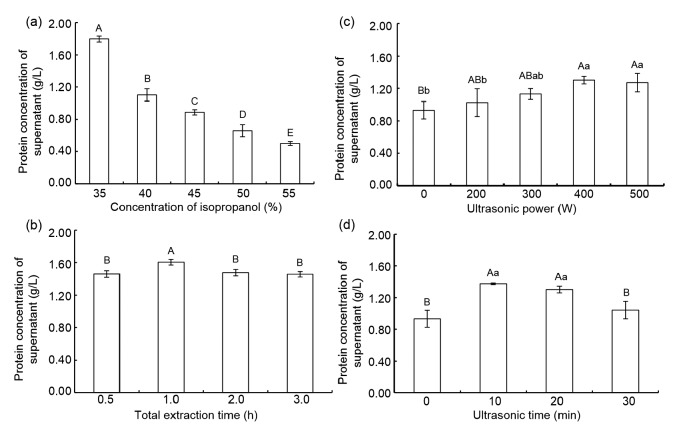
There were significant differences in the protein concentrations of extracts obtained using five different isopropanol concentrations (35%, 40%, 45%, 50%, and 55%) (P<0.01; Fig. 1a). Krishnan (2004) found that isopropanol could facilitate the enrichment of LAPs and the removal of APs in soybean, but Natarajan et al. (2009) found that high isopropanol concentrations (exceeding 50%) resulted in a sharp decrease in both APs and LAPs identified by SDS-PAGE. This may be why in our study we found a highly significant (P<0.01) decrease in protein concentrations with increasing isopropanol concentrations.
Fig. 1.
Effects of isopropanol concentration (a), total extraction time (b), ultrasonic power (c), and ultrasonic time (d) on protein extraction from defatted soybean meal
Data are expressed as mean±standard deviation (n=6). Columns labeled with different capital letters indicate a significant difference at the 0.01 level (P<0.01), and columns labeled with different lowercase letters indicate a significant difference at the 0.05 level (P<0.05)
In a kinetic study, the expression of dissolution was found to be analogous to the surface diffusion control of crystal growth (Tang and Nancollas, 2002), implying that dissolution continues until equilibrium is reached. Before reaching equilibrium, the soybean protein dissolved in the extraction solution increased with increasing extraction time. In this study, the protein concentration after 1 h of extraction was significantly higher than that after 0.5, 2, or 3 h (P<0.01). There were no significant differences between protein concentrations after 0.5, 2, and 3 h of extraction (P>0.05). So, we considered 1 h as the optimal total extraction time for protein extraction from defatted soybean meal (Fig. 1b).
It has been reported that while LAPs have been observed in 30%, 40%, and 50% isopropanol extracts by 1D-PAGE analysis, there was a decrease in both APs and LAPs at higher isopropanol concentrations (Natarajan et al., 2009). So, it is impossible to separate the maximum number of LAPs (without APs) in defatted soybean meal by changing only the isopropanol concentration. Karki et al. (2009b) found that ultrasound did not modify the peptide profile, regardless of the ultrasound conditions used, and after ultrasound for 120 s the SPI yield was increased by 13% at low power and 34% at high power. The particle size decreased nearly 10-fold following ultrasonic treatment at high amplitude for 120 s, resulting in the highest increase in total protein yield (46%), compared to samples with no ultrasonic treatment (Karki et al., 2009a). In our study, ultrasound treatment improved the protein concentration in the isopropanol extracts, which showed a parabolic tendency in response to increasing ultrasonic power or duration (Figs. 1c and 1d). When the ultrasonic power exceeded 400 W, the protein concentration was higher in isopropanol extracts than in those without ultrasonic pretreatment (P<0.01), but there was no difference between 400 and 500 W (P>0.05). The protein concentration was higher in extracts with ultrasound treatment for 10 or 20 min than in those without ultrasonic pretreatment (P<0.01).
3.2. Orthogonal experiment for the optimization of LAP extraction and AP removal
We confirmed that the concentration of proteins extracted by isopropanol from defatted soybean meal was higher at low isopropanol concentrations (35%, 40%, and 45%) than at the higher isopropanol concentration (50%) (Fig. 1a). This was confirmed by the lower protein concentration under conditions 3 (5 min (ultrasonic time), 450 W (ultrasonic power), 50% (isopropanol concentration), 90 min (total extraction time)), 5 (10 min, 400 W, 50%, 40 min), and 7 (15 min, 350 W, 50%, 60 min) compared to those under the other conditions (Fig. 2). In addition, the protein concentration under condition 7 increased by 9.54% and 8.93% compared with those under conditions 3 and 5, respectively, indicating that ultrasonic power, ultrasonic time, and total extraction time play important roles in soybean protein extraction. In our study, we also found that with 45% isopropanol the protein concentration increased by 12.98% under the conditions of ultrasound treatment for 15 min at 450 W with 40 min of total extraction time compared to that of ultrasound treatment for 10 min at 350 W with 90 min of total extraction time (Fig. 2). This not only confirmed that the four factors we investigated all affect soybean protein extraction, but also showed that ultrasound treatment had a greater influence on protein extraction than the total extraction time.
Fig. 2.

Effects of different conditions on the soybean protein concentration of isopropanol extracts
1: 5 min, 350 W, 40%, 40 min; 2: 5 min, 400 W, 45%, 60 min; 3: 5 min, 450 W, 50%, 90 min; 4: 10 min, 350 W, 45%, 90 min; 5: 10 min, 400 W, 50%, 40 min; 6: 10 min, 450 W, 40%, 60 min; 7: 15 min, 350 W, 50%, 60 min; 8: 15 min, 400 W, 40%, 90 min; 9: 15 min, 450 W, 45%, 40 min. Data are expressed as mean±standard deviation (n=6). Columns labeled with different capital and lowercase letters indicate a significant difference at P<0.01 and P<0.05, respectively
To confirm the optimal conditions for LAP extraction and AP removal, the proteins in the isopropanol extracts under nine different conditions were identified by SDS-PAGE (Fig. 3) and MS (Table 2). There were more protein bands or the staining intensity of protein bands was stronger at lower isopropanol concentrations irrespective of the ultrasonic time, ultrasonic power, and total extraction time (Fig. 3). The staining intensity of protein bands in lane 7 (condition 7) was stronger than those in lanes 3 and 5, which was in accord with their protein content in isopropanol extracts (Fig. 2).
Fig. 3.

1-D electrophoretogram of isopropanol extracts under different conditions
M, marker, indicates 170, 130, 100, 70, 55, 40, 35, 25, 15, and 10 kD; Lanes 1–9 were the conditions 1–9 as shown in Fig. 2
Table 2.
Proteins in the isopropanol extracts identified by MS
| Band No. | Protein accession | Protein mass | Protein score | Protein description [species] |
| 1 | gi|62122635 | 56 406 | 1280 | β-Amylase [Glycine max] |
| 2 | gi|356555724 | 46 192 | 141 | Fumarylacetoacetase-like [Glycine max] |
| 3 | gi|6729836 | 27 555 | 231 | Chain A, soybean agglutinin complexed with 2,6-pentasaccharide |
| 4 | gi|543177317 | 27 512 | 110 | Iron-superoxide dismutase [Phaseolus vulgaris] |
| 5 | gi|37495455 | 23 720 | 217 | Dehydrin [Glycine max] |
| 6 | gi|9967357 | 63 184 | 144 | α Subunit of β-conglycinin [Glycine max] |
| 7 | gi|9967357 | 63 184 | 121 | α Subunit of β-conglycinin [Glycine max] |
| 8 | gi|157838208 | 19 853 | 516 | Chain B, complex porcine pancreatic trypsin soybean trypsin inhibitor, orthorhombic crystal form |
| 9 | gi|356515553 | 17 534 | 80 | Oleosin 16 kDa-like [Glycine max] |
| 10 | gi|13375351 | 16 124 | 74 | Truncated kunitz trypsin inhibitor [Glycine max] |
| 11 | gi|145495350 | 17 407 | 143 | Hypothetical protein [Paramecium tetraurelia] |
| 12 | gi|157838208 | 19 853 | 179 | Chain B, complex porcine pancreatic trypsin soybean trypsin inhibitor, orthorhombic crystal form |
β-Amylase, the major enzyme of starch breakdown in leaves (Fulton et al., 2008), soybean trypsin inhibitor (STI) and soybean agglutinin (SBA), accounting for 6% and 5%–7% of soybean proteins, respectively (Rackis et al., 1986; Bajpai et al., 2005), have been shown to be the important anti-nutritional factors of soybean (Fasina et al., 2006; Zang et al., 2006; Hart et al., 2010). β-Conglycinin is composed of three subunits, α', α, and β, which are potential food allergens (Krishnan et al., 2009a). In our study, the proteins in SDS-PAGE gels were excised and identified by MS (Table 2). β-Amylase, kunitz trypsin inhibitor, and SBA were the top three proteins in the nine isopropanol extracts. The α subunit of β-conglycinin and many other LAPs, such as fumarylacetoacetase-like, dehydrin, oleosin 16 kDa-like, and iron-superoxide dismutase, were also detected in defatted soybean meal under some conditions (Table 2). The α subunit of β-conglycinin was not detected under conditions 3, 5, or 7.
3.3. Determination of LAP extraction and AP removal
The preferable orthogonal experimental conditions (conditions 3, 5, and 7) were inconsistent with those identified in our previous study (Liu et al., 2016) of the effects of ultrasonic treatment on LAP extraction (10 min, 400 W, 50%, 60 min). So, to determine the optimal conditions for LAP extraction and AP removal, a comparative analysis was carried out between the orthogonal experiment (under conditions 3, 5, and 7) and the ultrasonic treatment experiment (under optimal conditions). The results are shown in Figs. 4 and 5, and Table 3. The protein concentration of up to 0.8081 g/L under condition 7 was higher than that under the optimal conditions from the ultrasonic treatment experiment (10 min, 400 W, 50%, 60 min) (P<0.05), and condition 5 (P<0.05) and condition 3 (P<0.01) from the orthogonal experiment. This result confirmed the important role of the ultrasonic treatment and total extraction time in LAP extraction (Fig. 4).
Fig. 4.

Effects of various conditions on the soybean protein concentration of extracts
1: 10 min, 400 W, 50%, 60 min; 2: 15 min, 350 W, 50%, 60 min (condition 7); 3: 10 min, 400 W, 50%, 40 min (condition 5); 4: 5 min, 450 W, 50%, 90 min (condition 3). Data are expressed as mean±standard deviation (n=6). Columns labeled with different capital and lowercase letters indicate a significant difference at P<0.01 and P<0.05, respectively
Fig. 5.

1-D electrophoretogram of cold acetone precipitation under four different conditions
M, marker, indicates 170, 130, 100, 70, 55, 40, 35, 25, 15, and 10 kD; Lanes 1–4 were the conditions as shown in Fig. 4
Table 3.
Proteins in cold acetone precipitates identified by MS
| Band No. | Protein accession | Protein mass | Protein score | Best protein description [species] |
| 1 | gi|571503799 | 92 477 | 144 | Phospholipase D alpha 1-like isoform X2 [Glycine max] |
| 2 | gi|62122633 | 56 391 | 267 | β-Amylase [Glycine max] |
| 3 | gi|62122635 | 56 406 | 725 | β-Amylase [Glycine max] |
| 4 | gi|63259123 | 56 457 | 353 | β-Amylase [Glycine max] |
| 5 | gi|363806904 | 29 349 | 153 | Uncharacterized protein LOC100799849 [Glycine max] |
| 6 | gi|255638626 | 17 463 | 131 | 16.5 kDa oleosin [Glycine max] |
| 7 | gi|255638626 | 36 632 | 147 | Unknown [Glycine max] |
| 8 | gi|351720944 | 22 718 | 290 | Uncharacterized protein LOC100305855 precursor [Glycine max] |
| 9 | gi|157838209 | 19 853 | 450 | Chain B, complex porcine pancreatic soybean trypsin inhibitor, tetragonal crystal form |
| 10 | gi|571459448 | 22 698 | 285 | Oleosin 1-like [Glycine max] |
| 11 | gi|571459448 | 22 698 | 132 | Oleosin 1-like [Glycine max] |
| 12 | gi|356556690 | 15 180 | 98 | Uncharacterized protein LOC100800510 [Glycine max] |
| 13 | gi|351726299 | 17 463 | 176 | 16.5 kDa oleosin [Glycine max] |
Cold acetone precipitation of isopropanol extracts was performed by SDS-PAGE and the proteins were identified by MS (Fig. 5, Table 3). There were no differences in the number of protein bands but some differences in the intensity of their staining among the four conditions, indicating different protein contents but the presence of the same protein varieties. This result was consistent with the protein concentrations of isopropanol extracts (Fig. 4). Some LAPs were detected in the cold acetone precipitates, such as phospholipase D, β-amylase, oleosin 1-like, uncharacterized and even an unknown protein (Table 3).
4. Conclusions
The results of this study show that: (1) isopropanol contributed to the removal of APs, and ultrasonic treatment and total extraction time played an important role in enriching the LAPs of soybean; (2) the effect of ultrasonic treatment on LAP enrichment was higher than that of total extraction time; (3) the optimal conditions were 50% isopropanol, ultrasonic treatment for 15 min at 350 W for a total extraction time of 1 h. This method may be useful for preparing sufficient soybean LAPs to study their physiological effects on animals without the influence of APs.
Acknowledgments
We are very grateful to the Testing Center of Yangzhou University for providing the 5800 MALDI-TOF/TOF analyzer and Yu-yang WANG (Testing Center of Yangzhou University) for the guidance in its operation.
Footnotes
Project supported by the China Agriculture Research System (No. CARS-36) and the National Natural Science Foundation of China (No. 31572430)
Compliance with ethics guidelines: Ming-mei LIU, Bin QI, Zheng-xu LIU, Jin-shun ZHAN, Kang ZHAN, and Guo-qi ZHAO declare that they have no conflicts of interest.
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Adams MR, Anthony MS, Chen H, et al. Replacement of dietary soy protein isolate with concentrates of soy 7S or 11S globulin has minimal or no effects on plasma lipoprotein profiles and biomarkers of coronary risk in monkeys. Atherosclerosis. 2008;196(1):76–80. doi: 10.1016/j.atherosclerosis.2007.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bajpai S, Sharma A, Gupta MN. Removal and recovery of antinutritional factors from soybean flour. Food Chem. 2005;89(4):497–501. doi: 10.1016/j.foodchem.2004.02.055. [DOI] [Google Scholar]
- 3.Bu G, Liu H, Chen F, et al. Effects of different factors on the forward extraction of soy protein in reverse micelle systems. Afr J Biotechnol. 2012;11(28):7247–7257. [Google Scholar]
- 4.Cornish SM, Wood CM, L'Abbe MR, et al. Sex-and age-specific immunomodulatory effects of dietary soya protein isolate and isoflavones in rats. Brit J Nutr. 2011;106(5):683–687. doi: 10.1017/S0007114511000766. [DOI] [PubMed] [Google Scholar]
- 5.Deak NA, Murphy PA, Johnson LA, et al. Fractionating soybean storage proteins using Ca2+ and NaHSO3 . J Food Sci. 2006;71(7):C413–C424. doi: 10.1111/j.1750-3841.2006.00132.x. [DOI] [Google Scholar]
- 6.Fasina YO, Classen HL, Garlich JD, et al. Response of turkey pouty to soybean lectin levels typically encountered in commercial diets. 2. Effect on intestinal development and lymphoid organs. Poult Sci. 2006;85:870–877. doi: 10.1093/ps/85.5.870. [DOI] [PubMed] [Google Scholar]
- 7.Fulton DC, Stettler M, Mettler T, et al. Β-AMYLASE4, a noncatalytic protein required for starch breakdown, acts upstream of three active β-amylases in Arabidopsis chloroplasts. Plant Cell. 2008;20(4):1040–1058. doi: 10.1105/tpc.107.056507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hart SD, Bharadwaj AS, Brown PB. Soybean lectins and trypsin inhibitors, but not oligosaccharides or the interactions of factors, impact weight gain of rainbow trout (Oncorhynchus mykiss) Aquaculture. 2010;306(1-4):310–314. doi: 10.1016/j.aquaculture.2010.03.027. [DOI] [Google Scholar]
- 9.Jankowski J, Juskiewicz J, Gulewicz K, et al. The effect of diets containing soybean meal, soybean protein concentrate, and soybean protein isolate of different oligosaccharide content on growth performance and gut function of young turkeys. Poult Sci. 2009;88(10):2132–2140. doi: 10.3382/ps.2009-00066. [DOI] [PubMed] [Google Scholar]
- 10.Karki B, Lamsal BP, Jung S, et al. Enhancing protein and sugar release from defatted soy flakes using ultrasound technology. J Food Eng. 2009;96(2):270–278. doi: 10.1016/j.jfoodeng.2009.07.023. [DOI] [Google Scholar]
- 11.Karki B, Lamsal BP, Grewell D, et al. Functional properties of soy protein isolates produced from ultrasonicated defatted soy flakes. J Am Oil Chem Soc. 2009;86(10):1021–1028. doi: 10.1007/s11746-009-1433-0. [DOI] [Google Scholar]
- 12.Krishnan BB. A simple and rapid method to isolate low molecular weight proteinase inhibitors from soybean. Korean J Crop Sci. 2004;49:342–348. [Google Scholar]
- 13.Krishnan HB, Kim WS, Jang S, et al. All three subunits of soybean β-conglycinin are potential food allergens. J Agric Food Chem. 2009;57(3):938–943. doi: 10.1021/jf802451g. [DOI] [PubMed] [Google Scholar]
- 14.Krishnan HB, Oehrle NW, Natarajan SS. A rapid and simple procedure for the depletion of abundant storage proteins from legume seeds to advance proteome analysis: a case study using Glycine max . Proteomics. 2009;9(11):3174–3188. doi: 10.1002/pmic.200800875. [DOI] [PubMed] [Google Scholar]
- 15.Li HZ, Pordesimo L, Weiss J. High intensity ultrasound-assisted extraction of oil from soybeans. J Food Res Intern. 2004;37(7):731–738. doi: 10.1016/j.foodres.2004.02.016. [DOI] [Google Scholar]
- 16.Liu MM, Zhao GQ, Qi B, et al. Effects of ultrasonic treatment on removal of abundant proteins and enrichment of low-abundance proteins in defatted soybean meal by isopropanol. Biotechnol Eq. 2016;30(3):521–528. doi: 10.1080/13102818.2016.1149518. [DOI] [Google Scholar]
- 17.Natarajan SS, Xu CP, Bae H, et al. Characterization of storage proteins in wild (Glycine soja) and cultivated (Glycine max) soybean seeds using proteomics analysis. J Agric Food Chem. 2006;54(8):3114–3120. doi: 10.1021/jf052954k. [DOI] [PubMed] [Google Scholar]
- 18.Natarajan SS, Xu CP, Bae H, et al. Proteomic analysis of allergen and antinutritional proteins in wild and cultivated soybean seeds. J Plant Biochem Biotechnol. 2006;15(2):103–108. doi: 10.1007/BF03321912. [DOI] [Google Scholar]
- 19.Natarajan SS, Krishnan HB, Sukla L. An efficient extraction method to enhance analysis of low abundant proteins from soybean seed. Anal Biochem. 2009;394(2):259–268. doi: 10.1016/j.ab.2009.07.048. [DOI] [PubMed] [Google Scholar]
- 20.Rackis JJ, Wolf WJ, Baker EC. Protease Inhibitors in Plant Foods: Content and Inactivation. Nutritional and Toxicological Significance of Enzyme Inhibitors in Foods. Plenum Press, New York; 1986. pp. 299–349. [DOI] [PubMed] [Google Scholar]
- 21.Saki AA, Abbasinezhad M, Ghazi S, et al. Intestinal characteristics, alkaline phosphatase and broilers performance in response to extracted and mechanical soybean meal replaced by fish meal. Agric Sci Technol. 2012;14(1):105–114. [Google Scholar]
- 22.Sun P, Li DF, Li ZJ. Effects of glycinin on IgE-mediated increase of mast cell numbers and histamine release in the small intestine. J Nutr Biochem. 2008;19(9):627–633. doi: 10.1016/j.jnutbio.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 23.Tang R, Nancollas GH. New mechanism for the dissolution of sparingly soluble minerals. Pure Appl Chem. 2002;74(10):1851–1857. doi: 10.1351/pac200274101851. [DOI] [Google Scholar]
- 24.Tibaldi E, Hakim Y, Uni Z, et al. Effects of the partial substitution of dietary fish meal by differently processed soybean meals on growth performance, nutrient digestibility and activity of intestinal brush border enzymes in the European sea bass (Dicentrarchus labrax) Aquaculture. 2006;261(1):182–193. doi: 10.1016/j.aquaculture.2006.06.026. [DOI] [Google Scholar]
- 25.Wang Y, Kong LJ, Li C, et al. Effect of replacing fish meal with soybean meal on growth, feed utilization and carcass composition of cuneate drum (Nibea miichthioides) Aquaculture. 2006;261(4):1307–1313. doi: 10.1016/j.aquaculture.2006.08.045. [DOI] [Google Scholar]
- 26.Zang JJ, Li DF, Piao XS. Effects of soybean agglutinin on body composition and organ weight in rats. Arch Anim Nutr. 2006;60(3):245–253. doi: 10.1080/17450390600679082. [DOI] [PubMed] [Google Scholar]