Abstract
Objective: The aim of this study was to evaluate the safety and efficiency of enucleation (EU) for proximal pancreatic non-invasive neoplasms. Methods: Patients with solitary non-invasive neoplasms in the proximal pancreas from January 1998 to April 2014 at the Second Affiliated Hospital of Zhejiang University, Hangzhou, China were included. Different operations and outcomes were analyzed. Results: A total of 123 patients were enrolled. Forty patients (32.5%) underwent EU including 18 patients who had tumors close to the main pancreatic duct (MPD). Sixty-one patients (49.6%) had pancreaticoduodenectomy (PD) performed and 22 (17.9%) underwent central pancreatectomy (CP). Pathological outcomes included neuroendocrine tumors, cystic lesions, and solid pseudopapillary tumors. Operation time, intra-operative blood loss, and duration of hospital stay were significantly reduced in the EU group. PD was associated with the greatest complication rate (55.7%), followed by EU (50%) and CP (40.9%), though the pancreatic fistula rate after EU was the highest (50%), especially in patients with tumors larger than 3 cm and tumors close to the MPD. EU had advantages in the preservation of pancreatic parenchyma and endocrine and exocrine function. Conclusions: EU can be carried out safely and effectively for tumors in the proximal pancreas with improved outcomes compared with standard resections, even if the tumor is larger than 3 cm and close to the MPD.
Keywords: Enucleation, Pancreatic fistula, Pancreaticoduodenectomy, Central pancreatectomy
1. Introduction
Benign and low-grade malignant tumors of the pancreas, including most cystic pancreatic lesions, neuro-endocrine tumors (NETs), and tumors with other various pathologies, are now being discovered more and more in pancreatic centers. When located in the proximal pancreas, these tumors have been traditionally treated with standard resections such as pancreaticoduodenectomies (PDs). Though low mortality at high-volume medical centers has been achieved (Ho and Heslin, 2003), PDs are still associated with high postoperative morbidity and exo-and endocrine dysfunction (Ghaneh and Neoptolemos, 1999; Grobmyer et al., 2007; Tran et al., 2009). Long-term biliary and pancreatic anastomotic complications following PDs have also been noted (Reid-Lombardo et al., 2007). The amount of resected pancreatic parenchyma largely affects postoperative quality of life of the patients, especially for young patients with a long life expectancy. In the last decade, with its parenchyma-preserving nature and reduced operative invasion, tumor enucleation (EU) serves as an alternative approach to standard resections. Favorable results on surgical outcomes and postoperative pancreatic function have been reported in patients with benign and low-grade malignant tumors after EU (Talamini et al., 1998; Pitt et al., 2009; Hackert et al., 2011). EU has proved to be a safe and adequate procedure for small low-grade neuroendocrine tumors (NETs) and serous cystadenomas (SCAs) (Talamini et al., 1998; Crippa et al., 2010; Hackert et al., 2011; Zhang et al., 2013). EUs of mucinous cystic neoplasms (MCNs), solid pseudopapillary tumors (SPTs), and branch-duct intraductal papillary mucinous neoplasms (IPMNs) have also been reported (Sciaudone et al., 2000; Papavramidis and Papavramidis, 2005; Hackert et al., 2011). However, several studies have reported a high incidence of pancreatic fistula (PF) after EU (Crippa et al., 2007; Hackert et al., 2011; Zhang et al., 2013). The most important complication was damage to the main pancreatic duct (MPD). Considering the potential risk of fistula, large tumors (tumor size larger than 3 cm) and proximity to MPD were generally accepted as contraindications for EUs. Nevertheless, nearly one third of pancreatic benign and borderline tumors arise from the proximal pancreas and may have a close relationship with MPD and the conjunction area of MPD and common bile duct (CBD). Additionally, small tumors are usually asymptomatic, and the mean sizes of benign and low-grade malignant tumors are larger than 3 cm when discovered (Valsangkar et al., 2012). In this study, we focused on the possibility of EU for patients with proximal pancreatic tumors larger than 3 cm and close to the MPD, by evaluating the indications, surgical outcomes, and long-term complications of patients undergoing proximal pancreatic tumor EUs and comparing this approach with standard resections including PDs and central pancreatectomies (CPs).
2. Materials and methods
2.1. Patients and data collection
Data of patients undergoing proximal pancreatic surgery for benign and low-grade malignant neoplasm between January 1998 and June 2014 were collected from an electronic database of the Second Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou, China. Enrolled benign and low-grade malignant tumors included cystic adenomas, SPTs, NETs, and other pathologically proved non-invasive lesions. Only solitary tumor and lesions arising from the pancreatic parenchyma were included. A total of 126 patients were identified; two patients whose tumors arose from extrapancreatic tissue and one IPMN patient who underwent total pancreatectomy were excluded. Consequently, 123 patients were included.
Demographics, clinical presentation, preoperative evaluation, and intraoperative details such as the duration of the procedure and blood loss were collected. Postoperative complications and length of hospital stay as well as pathological data were also recorded.
2.2. Surgical procedures
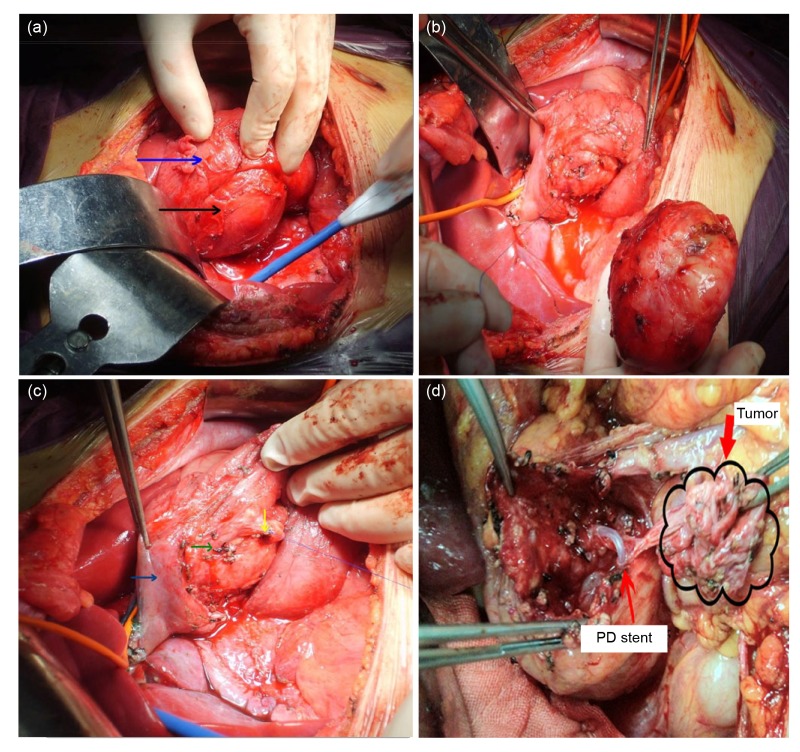
Preoperative diagnostic and staging studies including trans-abdominal ultrasound, contrast-enhanced computed tomography (CT) scan, or magnetic resonance imaging (MRI) were performed for all patients. The surgical procedures of EU have been described in a previous publication (Lu et al., 2012). For tumors in the head and uncinate process, the surgeon would palpate the tumor with Kocher’s maneuver to evaluate the risk and possibility of EU, followed by careful dissection of proximal pancreas, gastroduodenal artery and superior mesenteric vein. In some patients, intraoperative ultrasonography was performed to confirm the location of the tumor, exclude synchronous multifocal tumors, and clarify possible tumor adherence to the pancreatic duct. A precise EU of the mass along the border of the tumor was carried out after mobilization of the distal pancreas. A Foley catheter was inserted into CBD to avoid injuries if the mass was adjacent to the confluence region. Cholangiopancreatography with methylene blue would be performed to confirm the integrity of the MPD and CBD. For patients proving to have injury to the MPD or CBD, the duct would be repaired or reconstructed with polyprolene sutures. For severe MPD injury, a fine silicon tube was inserted into MPD as a stent, with the other side passing across the papilla into the duodenal cavity, and was fixed by soluble suture. Two or more drain tubes were placed near the wound surface of the proximal pancreas. A frozen section of the tumor lesion was performed in all patients to exclude invasive tumors. Invasive tumor, massive disruption of proximal pancreas and unrepairable MPD injury are considered to be contraindications of EU and a standard procedure such as PD or middle segment resection was performed alternatively. Surgical techniques are demonstrated in Fig. 1.
Fig. 1.
Surgical techniques of enucleation (EU)
(a) Dissect pancreatic head to evaluate the risk and possibility of EU and reconstruction (black arrow: tumor; blue arrow: duodenum). (b) Precise EU of the mass along the border of the tumor. (c) Check whether there is injury to common bile duct (green arrow) by methylene blue cholangiopancreatography and if there is injury to the common pancreatic duct with visiable pancreatic juice leakage, repair or reconstruct it with polyprolene suture (yellow arrow). Blue arrow indicates duodenum. (d) For severe main pancreatic duct injury, a fine silicon tube is inserted into main pancreatic duct (MPD) as a stent, with the other side into duodenum through papilla, fixed with soluble suture (Note: for interpretation of the references to color in this figure legend, the reader is referred to the web version of this article)
PDs were performed with standardized resection and reconstruction of end-to-side two-layer duct-to-mucosa pancreaticojejunostomy, end-to-side one-layer hepaticojejunostomy, and antecolic one-layer end-to-side gastrojejunostomy. For patients who performed CPs, the proximal edge of pancreatic neck was sutured up with polyprolene, and a Roux-Y anastomosis for the distal edge with the jejunum was performed. Resection margin was also examined by frozen section to ensure a tumor-free cut surface. One laparoscopic EU and two laparoscopic PDs were included and all other surgeries carried out in an open way. All surgical procedures were performed by, or under the supervision of, senior surgeons.
2.3. Postoperative management
Empirical antibiotics and octreotide were administrated postoperatively, depending on patients’ condition, laboratory results, and drainage volume. Enteral nutrition routinely began on postoperative day (POD) 3. The volume and the amylase level of the drainage were monitored and recorded. For patients with postoperative PF and signs of intra-abdominal infection, in situ continuous lavage using a fine long silicon tube through the drain tube was performed (Dong et al., 2008). The drains would be removed when the drainage volume was less than 20 ml per 24 h, with no or little intra-abdomen fluid accumulation confirmed by ultrasonography or CT scan.
Postoperative complications were recorded and evaluated. PF was defined as any volume of drainage on or after the third POD with amylase content greater than three times the upper normal limit of serum level. Grades of fistula severity were identified according to the International Study Group of Pancreatic Fistula (ISGPF) (Bassi et al., 2005). Delayed gastric emptying (DGE) was defined as nasogastric tube being required for longer than 4 d, or reinsertion after POD 3, combined with at least one of the following: unable to tolerate solid oral intake by POD 7, vomiting, gastric distension, and use of prokinetics (Wente et al., 2007b). Postpancreatectomy hemorrhage was identified and classified according to ISGPF definition (Wente et al., 2007a). Perioperative mortality was defined as death in hospital or within 30 d.
2.4. Follow-up
Follow-up was achieved through outpatient medical records and telephone contact, and consisted of clinical, radiological, and laboratory assessments. Tumor recurrence and long-term exocrine and endocrine impairments were evaluated. The presence of new-onset or worsening diabetes was confirmed by measuring serum glucose levels and oral glucose tolerance testing. New-onset exocrine insufficiency was defined by the development of steatorrhoea and weight loss, requiring oral pancreatic enzyme supplementation in the absence of tumor recurrence. CT or MRI was routinely applied during follow-up to rule out tumor recurrence and nodal metastasis.
2.5. Statistical analysis
Results were reported as mean±standard deviation (SD) or median (range) as appropriate. Continuous variables such as tumor size, operation time, and age at the time of diagnosis, were compared with Student’s t-test or the Mann-Whitney U test. χ 2 and Fisher’s exact tests were used to compare categorical data. P<0.05 was considered statistically significant. SPSS statistical software Version 13.0 (Chicago, IL, USA) was used for statistical analysis.
3. Results
3.1. Demographic characteristics
From January 1998 to June 2014, 123 patients underwent surgical resections of a benign or low-grade malignant tumor in the proximal pancreas. Surgical procedures performed included EU, PD, and CP. The procedures performed on the patients were decided by the surgeon according to preoperative examinations and explorations during the operation. Evolving patterns of surgical procedures are shown in Fig. 2. Nearly one-third of patients (n=40, 32.5%) underwent EU. PD and CP were performed in 61 (49.6%) and 22 (17.9%) patients, respectively. The median follow-up time was 46 months, ranging from 2 to 192 months.
Fig. 2.
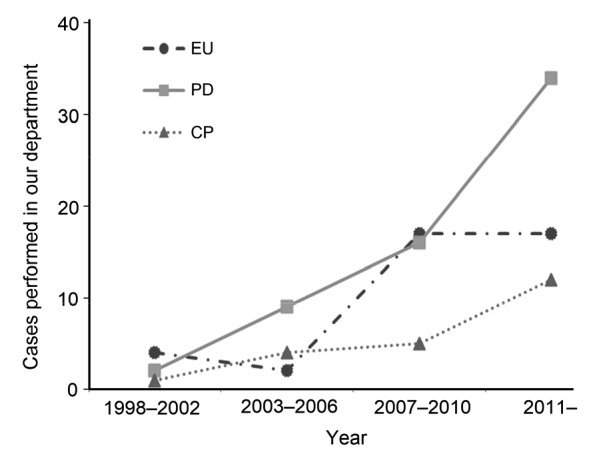
Evolving patterns of surgical procedures
EU: enucleation; PD: pancreaticoduodenectomy; CP: central pancreatectomy
For patients undergoing EU, the mean age at time of operation was 46 years (range, 14 to 75 years). Twenty-one (52.5%) patients were female and 25 (62.5%) patients presented with symptoms. Average diameter of tumor was (31.1±25.0) mm. Large tumors, defined as tumor diameter >3 cm, were found in 14 patients (35.0%). Pathological outcomes for EU included NETs, SCAs, SPTs, and MCNs. Other histopathology found included two lipomas and one hemangioma. Nearly two thirds of tumors (65.0%) were located in the head or the uncinate process of the pancreas. Tumor in the neck and junction parts of the head and neck constituted 27.5% and 7.5% of the cases, respectively. No significant differences regarding gender, age at the time of diagnosis, American Society of Anesthesiologists (ASA) classification, mean tumor size, or sites involved between patients who accepted different operations were found. Data on patients’ demographic characteristics are shown in Table 1.
Table 1.
Patients’ characteristics
| Parameter | EU | PD | CP |
| Number of patients | 40 | 61 | 22 |
| Gender, female (%) | 52.5 | 47.5 | 86.4 |
| Mean age (range) (year) | 46 (14‒75) | 54 (15‒81) | 46 (20‒76) |
| Symptomatic (%) | 62.5 | 52.4 | 40.9 |
| ASA classification (%) | |||
| I | 42.5 | 31.1 | 59.1 |
| II | 55.0 | 59.0 | 40.9 |
| III | 2.5 | 9.8 | 0 |
| Mean size, ±SD (mm) | 31.1±25.0 | 39.9±22.0 | 34.1±18.6 |
| Histology, n (%) | |||
| NETs | 25 (62.5%) | 15 (26.2%)** | 7 (31.8%)* |
| SCAs | 5 (12.5%) | 12 (19.7%) | 5 (22.7%) |
| SPTs | 5 (12.5%) | 12 (19.7%) | 9 (40.9%) |
| MCNs | 2 (5.0%) | 5 (9.8%) | 1 (4.5%) |
| IPMNs | 0 | 10 (16.4%)* | 0 |
| Others | 3 (7.5%) | 7 (11.5%) | 0 |
| Site involved, n (%) | |||
| Head/ucinate process | 26 (65.0%) | 48 (78.7%) | 4 (18.2%)** |
| Neck | 11 (27.5%) | 4 (6.6%)** | 18 (81.8%)** |
| Junction of head and neck | 3 (7.5%) | 9 (14.8%) | 0 |
| Median follow-up (range) (month) | 50 (3‒192) | 40 (2‒167) | 39 (2‒168) |
EU, enucleation; PD, pancreaticoduodenectomy; CP, central pancreatectomy; ASA, American Society of Anesthesiologists; NET, neuroendocrine tumors; SCA, serous cystadenoma; SPT, solid pseudopapillary tumor; MCN, mucinous cystic neoplasm; IPMN, intraductal papillarry mucinous neoplasm. Data are expressed as number, percent, number (percent), mean±SD, or median (range).
P<0.05,
P<0.01 vs. EU
3.2. Surgical outcomes and postoperative complications
Operation time, intraoperative blood loss, and duration of hospital stay were significantly reduced in the EU group compared with the PD and CP groups. One patient had a second surgery due to choleperitonitis after tumor EU. The reoperation rates were 11.5% and 9.1% in the PD and CP groups, respectively. The reoperation reasons included fistula and sequent infection, intra-abdominal bleeding, and bowel obstruction. There was no mortality after EU or CP, but following PD, one patient died due to multiple organ failure. Relevant data are demonstrated in Table 2.
Table 2.
Operative outcomes
| Group | n | Operation timea (min) | Blood lossa (ml) | Complication (%) | Hospital mortality (%) | Reoperation rate (%) | Median hospital staya (d) |
| EU | 40 | 155±50 | 183±156 | 50.0 | 0 | 2.5 | 15±13 |
| PD | 61 | 365±109** | 543±413** | 55.7 | 1.6 | 11.5 | 24±26** |
| CP | 22 | 270±55** | 291±135** | 40.9 | 0 | 9.1 | 21±26* |
EU, enucleation; PD, pancreaticoduodenectomy; CP, central pancreatectomy; n, number of patients.
Data are expressed as mean±SD.
P<0.05,
P<0.01 vs. EU
At least one complication was experienced by 40%‒55% of the patients, depending on the surgical procedure performed. PD was associated with the greatest complication rate (55.7%), followed by EU (50.0%) and CP (40.9%). The complication pattern varies with different procedures. PF was more frequently seen in patients after EU (50.0%), compared with 27.9% (P<0.05 vs. EU) and 31.8% in the PD and CP groups, respectively. Most fistulas after EU were symptomatic (42.5%), and all recovered after in situ continuous abdominal lavage with uneventful courses. In contrast, though ISGPF type B fistulas less commonly occurred after PD (16.4%, P<0.05 vs. EU) and CP (22.7%), surgical reoperations were indicated in two patients after PD due to an ISGPF type C fistula. Postoperative bleeding (including early and delayed hemorrhages) was more frequent, but not significantly so, in the PD group than in the other two procedure groups. Five patients in the PD group and two patients in the CP group had laparotomy hemostasis. Almost one fifth of patients experienced DGE after PD. EU and CP had DGE rates of 7.5% (P<0.05 vs. PD) and 9.1%, respectively. In terms of long-term complications, no patient developed exo-or endocrine dysfunction after tumor EU. In comparison, new-onset diabetes or worsening diabetes occurred in 14.8% (P<0.05 vs. EU) and 9.1% (not significant, vs. EU) of patients after PD and CP, respectively. Exocrine deficiency was a more prevalent long-term consequence after PD. Of the 61 patients, a total of 19 (31.1%, P<0.01 vs. EU) patients required oral pancreatic enzyme supplementation. The rates of bile leakage, wound infection, abdominal abscess, pneumonia, cardiac events were similar among the three groups. Table 3 summarizes the data on postoperative complications.
Table 3.
Postoperative complications
| Group | n | PF (%) |
Postoperative bleeding (%) | DGE (%) | GI anastomosis fistula (%) | ||||||
| Overall | A | B | C | ||||||||
| EU | 40 | 50.0 | 7.5 | 42.5 | 0 | 2.5 | 7.5 | 0 | |||
| PD | 61 | 27.9* | 9.8 | 14.8** | 3.3 | 14.8 | 19.7 | 8.2 | |||
| CP | 22 | 31.8 | 9.1 | 22.7 | 0 | 18.2 | 9.1 | 0 | |||
|
| |||||||||||
|
| |||||||||||
| Group | Bile leakage (%) | Wound infection (%) | Abdominal abscess (%) | Pneumonia (%) | Cardiac dysfunction (%) | Endocrine dysfunction (%) | Exocrine dysfunction (%) | ||||
|
| |||||||||||
| EU | 2.5 | 2.5 | 5.0 | 0 | 0 | 0 | 0 | ||||
| PD | 8.2 | 0 | 14.8 | 9.8* | 3.3 | 14.8* | 31.1** | ||||
| CP | 0 | 4.5 | 9.1 | 4.5 | 0 | 18.4 | 4.5 | ||||
EU, enucleation; PD, pancreaticoduodenectomy; CP, central pancreatectomy; n, number of patients; PF, pancreatic fistula; DGE, delay gastric empty; GI, gastrointestinal.
P<0.05,
P<0.01 vs. EU
3.3. Enucleation of tumor adjacent to main pancreatic duct
Among 40 patients undergoing EU, the tumors of 18 patients (18/40, 45.0%) were close to the MPD. The term “close” we used was defined as direct contact of the tumor with the MPD on preoperative scans, or exposure of the MPD after EU of the tumor. The median diameter of the neoplasms was 40 (10‒100) mm.About 61.1% (11/18) patients presented with a tumor large than 3 cm. As shown in Table 4, of the 18 patients, six cases with tumors close to CBD simultaneously underwent cholecystectomies. Six patients had MPD injuries repaired with polypropylene intraoperatively. CBD injury was found in two patients, and for those CBD neoplasty and T-tube drainage were performed.
Table 4.
Patients’ characteristics and complications regarding tumors adjacent to main pancreatic duct
| No. | Sex | Age (year) | Follow-up (month) | Size (cm) | His. | CC | CBD injury | MPD injury | DT (d) | PF | PKD (ml/24 h) | DGE | RL |
| 1 | M | 57 | 162 | 1.5 | NET | 39 | B | 1500 | Y | ||||
| 2 | M | 67 | 159 | 3.0 | NET | Y | Y | 10 | A | 430 | |||
| 3 | F | 29 | 117 | 10.0 | SPT | 45 | B | 1060 | Y | ||||
| 4 | M | 30 | 94 | 1.5 | NET | 25 | B | 250 | |||||
| 5 | M | 56 | 89 | 2.0 | NET | 5 | |||||||
| 6 | F | 40 | 79 | 5.0 | SCA | Y | Y | 62 | B | 1200 | Y | ||
| 7 | M | 37 | 67 | 6.0 | SCA | Y | 165 | B | 300 | ||||
| 8 | M | 28 | 66 | 2.7 | NET | 72 | B | 300 | |||||
| 9 | F | 16 | 61 | 5.0 | SPT | 23 | B | 300 | |||||
| 10 | F | 46 | 61 | 3.0 | NET | 11 | |||||||
| 11 | M | 54 | 60 | 1.0 | NET | 10 | |||||||
| 12 | F | 14 | 59 | 8.0 | SPT | Y | Y | Y | 281 | B | 1000 | Y | |
| 13 | F | 49 | 50 | 4.0 | SCA | Y | 51 | B | 600 | ||||
| 14 | F | 30 | 28 | 6.0 | SCA | Y | 31 | B | 1120 | ||||
| 15 | M | 54 | 17 | 8.0 | HA | Y | Y | 13 | |||||
| 16 | F | 53 | 6 | 3.9 | NET | 10 | |||||||
| 17 | M | 57 | 3 | 8.0 | Lipoma | Y | 17 | B | 800 | ||||
| 18 | F | 54 | 3 | 4.0 | NET | Y | 9 |
M, male; F, female; His., histology; NET, neuroendocrine tumors; SPT, solid pseudopapillary tumor; SCA, serous cystadenoma; HA, hemangioma; CC, cholecystectomy; Y, yes; CBD, common bile duct; MPD, main pancreatic duct; DT, drainage time; PF, pancreatic fistula; PKD, peak drainage; DGE, delayed gastric empty; RL, relaparotomy
Postoperative complications of these 18 patients are listed in Table 4. Average operation time and estimated blood loss were (152±50) min and (189±197) ml, respectively. A PF developed in 12 (12/18, 66.7%) patients, including all the 6 cases which had MPDs injured and repaired, but none had signs of severe infection or sepsis. Median peak drainage volume was 800 ml/24 h, ranging from 250 to 1500 ml/24 h. The majority are classified as ISGPF type B fistula because of persistent and high-output drainage. Clinical conditions of these 12 patients are extremely well except for prolonged drainage time ((48.83±69.26) d). DGE was also found in three patients (3/18, 16.7%). Reoperation was required in one patient due to choleperitonitis caused by an accidental drop of the T-tube on POD 6.
3.4. Effects of tumor size on surgical outcomes and strategies
Of the 40 patients undergoing EU, patients with large tumors (larger than 3 cm) showed higher possibilities of MPD injury and fistula occurrence than those with small ones (35.7% vs. 3.8%, P<0.05; 71.4% vs. 38.5%, P<0.05). Also, large tumor patients kept drainage tubes for a longer period of time. However, no significant difference regarding operation time or blood loss was noticed between these two subgroups (Table 5).
Table 5.
Postoperative complications regarding tumor size
| Tumor size (cm) | n | Operation time (min) | Blood loss (ml) | Hospital Stay (d) | MPD injury (%) | PF (%) |
DC with drains (%) | DGE (%) | ||
| Overall | A | B | ||||||||
| ≤3 | 26 | 151±52 | 177±144 | 15±8 | 1 (3.8%) | 10 (38.5%) | 2 (11.5%) | 8 (30.8%) | 6 (23.1%) | 1 (3.8%) |
| >3 | 14 | 163±47 | 193±183 | 24±20 | 5 (35.7%)* | 10 (71.4%)* | 1 (7.1%) | 9 (64.3%)* | 9 (64.3%)* | 3 (21.4%) |
n, number of patients; MPD, main pancreatic duct; PF, pancreatic fistula; DC, discharge; DGE, delayed gastric empty.
P<0.05 vs. tumor size ≤3 cm
There was a step-wise increase in the number of patients who underwent PDs and a parallel decrease in the proportion of patients who had EUs in line with the increase of tumor size. When tumor sizes were no larger than 1 cm, predilection for EU was most obvious. A total of 75.0% (9/12) of patients had EUs. Standard resections were done in the other three patients. Of 52 patients with tumor diameter between 1 and 3 cm, the spectrum of surgical strategies was significant. PDs were performed in nearly 50.0% of patients. Tumor EUs were carried out in 17 (32.6%) patients, followed by CPs in 12 (20.7%) patients. For 59 patients whose tumors were larger than 3 cm, 23.7% of patients underwent EUs, and 36 (61.0%) and 9 (15.2%) patients underwent PDs and CPs, respectively (Fig. 3).
Fig. 3.
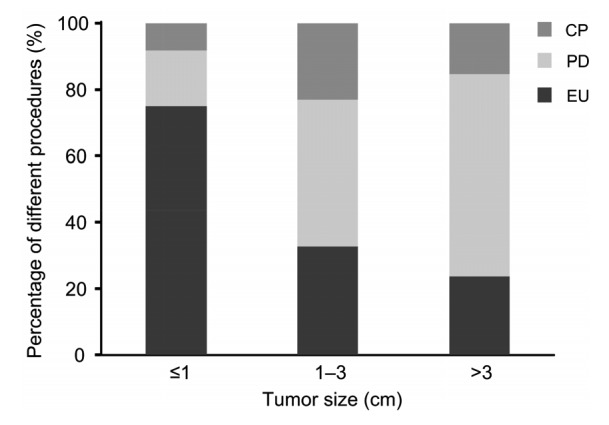
Percentages of surgical procedures varied with tumor size
EU, enucleation; PD, pancreaticoduodenectomy; CP, central pancreatectomy
3.5. Follow-up
The median follow-up time for the EU and standard resection groups was 50 and 40 months, ranging from 3 to 192 and 2 to 168 months, respectively. Of the 47 patients with NETs, two were lost during follow-up. Of the remaining 45 patients, the median follow-up was 43 months (range, 3 to 192 months). No recurrences or metastases were observed in these patients. Regarding follow-up of the 44 patients with IPMN, MCN, and SPT, the median follow-up time was 39 months (range, 2 to 121 months); again no recurrence was discovered.
4. Discussion
PDs and CPs, as standard procedures for proximal pancreatic tumors, have been performed on patients for decades. Considering the good prognosis of most benign and low-grade malignant tumors and their predilection for the young or middle-aged population (Valsangkar et al., 2012), patients could benefit more from parenchyma sparing and “no gastrointestinal tract disturbance” surgical procedures such as tumor EU. EU, first reported in 1898 by Ernesto Tricomi (Howard and Hess, 2002), has been widely accepted as an alternative procedure for benign and low-grade malignant pancreatic tumors including endocrine tumors (Ramage et al., 2005; Norton, 2006; Crippa et al., 2007; Falconi et al., 2010; Hackert et al., 2011; Zhang et al., 2013), SCAs, MCNs, SPTs, branch-duct IPMNs, pseudocysts, and so forth (Talamini et al., 1998; Kiely et al., 2003; Madan et al., 2004; Papavramidis and Papavramidis, 2005; Crippa et al., 2007; Ge et al., 2010; Hackert et al., 2011; Turrini et al., 2011; Zhang et al., 2013). EU has proved to be a procedure characterized by less operation time and blood loss, faster recovery, and preserving more of the pancreas parenchyma, compared with standard procedures (Casadei et al., 2010; Falconi et al., 2010; Hackert et al., 2011; Yan et al., 2015). Yet there are some problems for the application of EUs, including PF afterwards and indications and contraindications for EUs. In this study, we analyzed patients who had proximal pancreatic operations over the last 16 years to reveal the indications and outcomes of tumor EUs, and compared these results with standard resections. This series, to our knowledge, represents the first and largest series regarding EU of large tumors adjacent to MPD.
For EU of pancreatic tumors, PF is still considered to be the most frequent complication that contributes substantially to surgical morbidity rate and prolongs hospital stay. An overall PF rate of 50% after tumor EU was observed in this series, in comparison with 20% to 60% in other studies (Crippa et al., 2007; Pitt et al., 2009; Falconi et al., 2010; Hackert et al., 2011; Zhang et al., 2013). Normal underlying pancreatic texture partly contributes to more frequent PF occurrence. In addition, common risk factors identified for EU associated with PF in other literature included: soft texture of remnant pancreas, MPD diameter less than 3 mm, distance between tumor and MPD, body mass index, New York Heart Association (NYHA) class II or III, operation time ≥180 min, certain inherited genetic diseases, and deep EU (Goasguen et al., 2009; Brient et al., 2012; Inchauste et al., 2012; Zhang et al., 2013; Heeger et al., 2014; Atema et al., 2015).
Given the fact that post-operative PF is related to tumor size and distance to the MPD, for most previous reports, pancreatic EUs were mainly performed in small or outwardly-growing tumors. In a review of 709 patients who had EU between 1991 and 2012, the mean tumor size was 2.4 cm (Beger et al., 2014). Upper limitation of 2 or 3 cm is a well-accepted size criterion for application of the EU technique. In our series, 40 patients had an average tumor diameter of (31.1±25.0) mm under EU. Tumors with diameters over 3 cm were found in 14 patients and 78.6% (11/14) of them were close to the MPD. We noted that overall postoperative PF and ISGPF grade B fistula rates correlate well with tumor size and its relationship with the MPD. Higher fistula rates were observed in the large tumor subgroup (10/14, 71.4%) and tumors adjacent to the MPD (11/18, 61.1%). Thus the higher fistula rate and the fact that there were more ISGPF grade B cases in this cohort are presumably attributable to the inclusion of more patients with a large tumor with proximity to the pancreatic duct. Although EUs were related to a higher incidence of fistulas compared to standard resections, all fistulas developed after EU were defined as ISGPF grade A/B fistulas. Symptomatic fistulas were all successfully treated by in situ high-volume modified continuous lavage (Dong et al., 2008). No further surgical treatment was indicated. Therefore, despite a longer drainage time, a more important issue here is that PF following EU was less complicated than other standard resections, because the pancreatic juice is kept out of contact with jejunal fluid in patients after EUs.
Distance to the MPD is also a factor to be considered in performing EU with a concern of duct injuries. A distance of 2‒3 mm between tumor and the MPD was proven by several reports to be the criterion for a safe EU (Pitt et al., 2009; Casadei et al., 2010). Heeger et al. (2014) recently published a study on deep EU with tumor proximity to MPD, showing that compared with standard EU, deep EU was associated with a high rate of fistulas and morbidities. In addition, the distance between tumor and MPD negatively influenced the occurrence of ISGPF grades B and C fistulas. We went a step further in our practice. The application of EU was expanded to tumors with direct involvement with the MPD, as long as duct injuries could be repaired. For patients with irreparable MPD injury or massive disruption of the proximal pancreas, standard resections were applied. Similar results for PFs were observed in our studies. Among the 18 patients with a tumor adjacent to the MPD, six patients also underwent MPD repair and reconstruction during the surgery, and CBD neoplasty and T-tube drainage were performed in two patients due to CBD injuries. A total of 12 patients (66.7%), including all the 6 patients who had the MPD injured and repaired, developed symptomatic and high-output fistulas after surgery. This is comparable with ISGPF grade B/C rate of 70% in other research (Heeger et al., 2014). All 12 patients recovered after in situ high-volume modified continuous lavage and simple drainage. Thus, repairable damage to MPD should not be considered as contraindications for EU.
Nevertheless, another issue that needs to be considered is the possibility of malignancy in a large tumor group. Oncological adequacy of EU for NETs is still debated. According to the National Comprehensive Cancer Network (NCCN) guidelines, a NET larger than 2 cm should be treated with standard resections, considering the potential possibility of malignancy (Kulke et al., 2012). Some other authors argued that tumor size was not associated with the probability of nodal metastases (Ferrone et al., 2007; Falconi et al., 2010). In this sense, the necessity for lymph node sampling needs to be further investigated. In our practice, EUs were administered in 25 NET patients with a mean tumor size of 18.3 mm. Tumors with diameters larger than 2 cm were found in seven patients. None had proper lymphadenectomy. Final histological grading of the 25 patients gave all G1. Up to now, no evidence of tumor recurrence or nodal metastasis was found. Even so, strict follow-up of these patients is strongly recommended. Patients with a low malignancy tumor, such as MCNs and SPTs, also had a high likelihood of malignant transformation. In situ carcinoma was reported in 19.2% and 17.3%‒36.0% of resected SPTs and MCNs, respectively (Beger et al., 2014), and tumor size at presentation was related to malignant disease (Butte et al., 2011). Thus some articles proposed that EU might be indicated for small low malignancy tumors localized in the periphery of the pancreas (Zhang et al., 2013). In our study, in follow-up of two patients with MCNs (mean size: 60.0 mm) and five with SPTs (mean size: 53.4 mm), subsequent EU did not show signs of tumor recurrence. These results were supported by a few other studies regarding treating MCNs and SPTs with EU (le Borgne et al., 1999; Kiely et al., 2003; Cauley et al., 2012). Reoperation was warranted if a positive margin or a malignant tumor was found on final pathology. With an IPMN lesion up to 2‒3 cm, 25%‒30% showed premalignant or malignant features, and EUs were not adequate for these patients (Schmidt et al., 2007).
5. Conclusions
Proximal pancreatic tumor EU can be carried out with results better than those with standard resection, in terms of operation time, blood loss, hospital stay, and long-term pancreatic function. Small tumor size correlates well with the possibility for EU and low postoperative fistula rate. Large tumor size and a close relationship to MPD are not absolute contraindications for local EU, though subsequent fistula rate would be high. EU of tumors in the proximal pancreas should be recommended in patien
Footnotes
Project supported by the National Natural Science Foundation of China (Nos. 30672072 and 30872531), the Foundation of Science and Technology Department of Zhejiang Province, China (Nos. 2014C 33187), and the National High-Tech R&D Program (863) of China (No. 2007AA02Z476)
Compliance with ethics guidelines: Wen-jie LU, Hao-lei CAI, Ma-dong YE, Yu-lian WU, and Bin XU declare that they have no conflict of interest.
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008 (5). Informed consent was obtained from all patients for being included in the study.
References
- 1.Atema JJ, Jilesen AP, Busch OR, et al. Pancreatic fistulae after pancreatic resections for neuroendocrine tumours compared with resections for other lesions. HPB. 2015;17(1):38–45. doi: 10.1111/hpb.12319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bassi C, Dervenis C, Butturini G, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138(1):8–13. doi: 10.1016/j.surg.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 3.Beger HG, Poch B, Vasilescu C. Benign cystic neoplasm and endocrine tumours of the pancreas–when and how to operate–an overview. Int J Surg. 2014;12(6):606–614. doi: 10.1016/j.ijsu.2014.03.020. [DOI] [PubMed] [Google Scholar]
- 4.Brient C, Regenet N, Sulpice L, et al. Risk factors for postoperative pancreatic fistulization subsequent to enucleation. J Gastrointest Surg. 2012;16(10):1883–1887. doi: 10.1007/s11605-012-1971-x. [DOI] [PubMed] [Google Scholar]
- 5.Butte JM, Brennan MF, Gönen M, et al. Solid pseudopapillary tumors of the pancreas. Clinical features, surgical outcomes, and long-term survival in 45 consecutive patients from a single center. J Gastrointest Surg. 2011;15(2):350–357. doi: 10.1007/s11605-010-1337-1. [DOI] [PubMed] [Google Scholar]
- 6.Casadei R, Ricci C, Rega D, et al. Pancreatic endocrine tumors less than 4 cm in diameter: resect or enucleate? A single-center experience. Pancreas. 2010;39(6):825–828. doi: 10.1097/MPA.0b013e3181cf155c. [DOI] [PubMed] [Google Scholar]
- 7.Cauley CE, Pitt HA, Ziegler KM, et al. Pancreatic enucleation: improved outcomes compared to resection. J Gastrointest Surg. 2012;16(7):1347–1353. doi: 10.1007/s11605-012-1893-7. [DOI] [PubMed] [Google Scholar]
- 8.Crippa S, Bassi C, Salvia R, et al. Enucleation of pancreatic neoplasms. Br J Surg. 2007;94(10):1254–1259. doi: 10.1002/bjs.5833. [DOI] [PubMed] [Google Scholar]
- 9.Crippa S, Boninsegna L, Partelli S, et al. Parenchyma-sparing resections for pancreatic neoplasms. J Hepatobiliary Pancreat Sci. 2010;17(6):782–787. doi: 10.1007/s00534-009-0224-1. [DOI] [PubMed] [Google Scholar]
- 10.Dong X, Gao SL, Xie QP, et al. In situ high-volume modified continuous closed and/or open lavage for infected necrotizing pancreatitis. Pancreas. 2008;36(1):44–49. doi: 10.1097/mpa.0b013e31812e9688. [DOI] [PubMed] [Google Scholar]
- 11.Falconi M, Zerbi A, Crippa S, et al. Parenchyma-preserving resections for small nonfunctioning pancreatic endocrine tumors. Ann Surg Oncol. 2010;17(6):1621–1627. doi: 10.1245/s10434-010-0949-8. [DOI] [PubMed] [Google Scholar]
- 12.Ferrone CR, Tang LH, Tomlinson J, et al. Determining prognosis in patients with pancreatic endocrine neoplasms: can the WHO classification system be simplified? J Clin Oncol. 2007;25(35):5609–5615. doi: 10.1200/JCO.2007.12.9809. [DOI] [PubMed] [Google Scholar]
- 13.Ge C, Luo X, Chen X, et al. Enucleation of pancreatic cystadenomas. J Gastrointest Surg. 2010;14(1):141–147. doi: 10.1007/s11605-009-1023-3. [DOI] [PubMed] [Google Scholar]
- 14.Ghaneh P, Neoptolemos JP. Exocrine pancreatic function following pancreatectomy. Ann N Y Acad Sci. 1999;880:308–318. doi: 10.1111/j.1749-6632.1999.tb09534.x. [DOI] [PubMed] [Google Scholar]
- 15.Goasguen N, Bourrier A, Ponsot P, et al. Endoscopic management of pancreatic fistula after distal pancreatectomy and enucleation. Am J Surg. 2009;197(6):715–720. doi: 10.1016/j.amjsurg.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 16.Grobmyer SR, Pieracci FM, Allen PJ, et al. Defining morbidity after pancreaticoduodenectomy: use of a prospective complication grading system. J Am Coll Surg. 2007;204(3):356–364. doi: 10.1016/j.jamcollsurg.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 17.Hackert T, Hinz U, Fritz S, et al. Enucleation in pancreatic surgery: indications, technique, and outcome compared to standard pancreatic resections. Langenbeck’s Arch Surg. 2011;396(8):1197–1203. doi: 10.1007/s00423-011-0801-z. [DOI] [PubMed] [Google Scholar]
- 18.Heeger K, Falconi M, Partelli S, et al. Increased rate of clinically relevant pancreatic fistula after deep enucleation of small pancreatic tumors. Langenbeck’s Arch Surg. 2014;399(3):315–321. doi: 10.1007/s00423-014-1171-0. [DOI] [PubMed] [Google Scholar]
- 19.Ho V, Heslin MJ. Effect of hospital volume and experience on in-hospital mortality for pancreaticoduodenectomy. Ann Surg. 2003;237(4):509–514. doi: 10.1097/01.SLA.0000059981.13160.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Howard JM, Hess W. History of the Pancreas: Mystery of a Hidden Organ. Kluwer Academic/Plenum Publisher, New York; 2002. [Google Scholar]
- 21.Inchauste SM, Lanier BJ, Libutti SK, et al. Rate of clinically significant postoperative pancreatic fistula in pancreatic neuroendocrine tumors. World J Surg. 2012;36(7):1517–1526. doi: 10.1007/s00268-012-1598-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kiely JM, Nakeeb A, Komorowski RA, et al. Cystic pancreatic neoplasms: enucleate or resect? J Gastrointest Surg. 2003;7(7):890–897. doi: 10.1007/s11605-003-0035-7. [DOI] [PubMed] [Google Scholar]
- 23.Kulke MH, Benson AB3rd, Bergsland E, et al. Neuroendocrine tumors. J Natl Compr Canc Netw. 2012;10(6):724–764. doi: 10.6004/jnccn.2012.0075. [DOI] [PubMed] [Google Scholar]
- 24.le Borgne J, de Calan L, Partensky C. Cystadenomas and cystadenocarcinomas of the pancreas: a multiinstitutional retrospective study of 398 cases. French Surgical Association. Ann Surg. 1999;230(2):152–161. doi: 10.1097/00000658-199908000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu WJ, Xu B, Gao SL, et al. Enucleation of benign or borderline pancreatic head tumors adjacent to the common pancreatic duct. Pancreas. 2012;41(2):336–337. doi: 10.1097/MPA.0b013e318229b891. [DOI] [PubMed] [Google Scholar]
- 26.Madan AK, Weldon CB, Long WP, et al. Solid and papillary epithelial neoplasm of the pancreas. J Surg Oncol. 2004;85(4):193–198. doi: 10.1002/jso.20019. [DOI] [PubMed] [Google Scholar]
- 27.Norton JA. Surgery for primary pancreatic neuroendocrine tumors. J Gastrointest Surg. 2006;10(3):327–331. doi: 10.1016/j.gassur.2005.08.023. [DOI] [PubMed] [Google Scholar]
- 28.Papavramidis T, Papavramidis S. Solid pseudopapillary tumors of the pancreas: review of 718 patients reported in English literature. J Am Coll Surg. 2005;200(6):965–972. doi: 10.1016/j.jamcollsurg.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 29.Pitt SC, Pitt HA, Baker MS, et al. Small pancreatic and periampullary neuroendocrine tumors: resect or enucleate? J Gastrointest Surg. 2009;13(9):1692–1698. doi: 10.1007/s11605-009-0946-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramage JK, Davies AH, Ardill J, et al. Guidelines for the management of gastroenteropancreatic neuroendocrine (including carcinoid) tumours. Gut. 2005;54:iv1–iv16. doi: 10.1136/gut.2004.053314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reid-Lombardo KM, Ramos-de la Medin A, Thomsen K, et al. Long-term anastomotic complications after pancreaticoduodenectomy for benign diseases. J Gastrointest Surg. 2007;11(12):1704–1711. doi: 10.1007/s11605-007-0369-7. [DOI] [PubMed] [Google Scholar]
- 32.Schmidt CM, White PB, Waters JA, et al. Intraductal papillary mucinous neoplasms: predictors of malignant and invasive pathology. Ann Surg. 2007;246(4):644–654. doi: 10.1097/SLA.0b013e318155a9e5. [DOI] [PubMed] [Google Scholar]
- 33.Sciaudone G, Perniceni T, Lévy P, et al. Enucleation of intraductal papillary-mucinous tumor of the head of the pancreas. Report of 2 cases. Gastroenterol Clin Biol. 2000;24(1):121–124. (in French) [PubMed] [Google Scholar]
- 34.Talamini MA, Moesinger R, Yeo CJ, et al. Cystadenomas of the pancreas: is enucleation an adequate operation? Ann Surg. 1998;227(6):896–903. doi: 10.1097/00000658-199806000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tran TC, van Lanschot JJ, Bruno MJ, et al. Functional changes after pancreatoduodenectomy: diagnosis and treatment. Pancreatology. 2009;9(6):729–737. doi: 10.1159/000264638. [DOI] [PubMed] [Google Scholar]
- 36.Turrini O, Schmidt CM, Pitt HA, et al. Side-branch intraductal papillary mucinous neoplasms of the pancreatic head/uncinate: resection or enucleation? HPB. 2011;13(2):126–131. doi: 10.1111/j.1477-2574.2010.00256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Valsangkar NP, Morales-Oyarvide V, Thayer SP, et al. 851 resected cystic tumors of the pancreas: a 33-year experience at the Massachusetts General Hospital. Surgery. 2012;152(3 Suppl.):S4–S12. doi: 10.1016/j.surg.2012.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wente MN, Veit JA, Bassi C, et al. Postpancreatectomy hemorrhage (PPH)–an International Study Group of Pancreatic Surgery (ISGPS) definition. Surgery. 2007;142(1):20–25. doi: 10.1016/j.surg.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 39.Wente MN, Bassi C, Dervenis C, et al. Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS) Surgery. 2007;142(5):761–768. doi: 10.1016/j.surg.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 40.Yan JF, Kuang TT, Ji DY, et al. Laparoscopic versus open distal pancreatectomy for benign or premalignant pancreatic neoplasms: a two-center comparative study. J Zhejiang Univ-Sci B (Biomed & Biotechnol) 2015;16(7):573–579. doi: 10.1631/jzus.B1400257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang T, Xu J, Wang T, et al. Enucleation of pancreatic lesions: indications, outcomes, and risk factors for clinical pancreatic fistula. J Gastrointest Surg. 2013;17(12):2099–2104. doi: 10.1007/s11605-013-2355-6. [DOI] [PubMed] [Google Scholar]