Abstract
Objectives
Human zonulin is a protein that increases permeability in the epithelial layer of the small intestine by reversibly modulating the intercellular tight junctions. There is not sufficient information available about zonulin's participation in inflammatory bowel diseases (IBD). The aim of this study was therefore to investigate fecal and serum zonulin in IBD patients and its relation to the disease localization, behavior and smoking status.
Design and methods
Forty IBD patients and forty healthy persons were examined for fecal and serum zonulin concentrations by competitive ELISA (DRG International Inc). Values were correlated to IBD type, localization and behavior, and smoking.
Results
Serum and fecal zonulin were significantly higher in patients with Crohn’s disease compared to ulcerative colitis (p = 0.038 for fecal zonulin, and p = 0.041 for serum zonulin concentrations). No association of serum or fecal zonulin was found with respect to IBD localization and behavior. The only difference was found with respect to smoking. Both the IBD cohort and healthy smokers showed significantly higher fecal zonulin levels (median 203 ng/mL) compared to non-smokers (median 35.8 ng/mL), p < 0.001.
Conclusions
Fecal and serum zonulin levels are elevated in patients with active Crohn’s disease but not with ulcerative colitis. High fecal zonulin levels in smokers irrespective of IBD point to the significant and undesirable up-regulation of gut permeability in cigarette smokers.
Keywords: Zonulin, Inflammatory bowel disease, Crohn's disease, Ulcerative colitis, Smoking
1. Introduction
Human zonulin is a 47-kDa human protein that increases permeability in the epithelial layer of the small intestine [1]. It is the only physiological mediator known that increases gut permeability by reversibly modulating the intercellular tight junctions, whereas proper functioning of the tight junctions is crucial for maintaining normal physiologic processes in the intestinal tract [2].
Increased serum/plasma zonulin levels have been described in celiac disease [3], type 1 and 2 diabetes [4], [5] or in obesity-associated insulin resistance [6], [7]; and circulating plasma zonulin has been suggested as a potential marker of intestinal permeability [8], [9], [10]. However, there is insufficient information about zonulin's participation in some important states of intestinal inflammation (e.g., inflammatory bowel diseases, IBD), and there is ambiguous information about serum and fecal zonulin in IBD [11].
The aim of this study was to investigate fecal and serum zonulin in IBD patients and its relation to the disease localization and behavior. Moreover, the relationship between fecal and serum zonulin and smoking status was examined.
2. Materials and methods
2.1. Specimen characteristics
Forty consecutive IBD patients – 32 with Crohn’s disease (CD) and 8 with ulcerative colitis (UC) in whom fecal and blood samples were available – were enrolled from a tertiary IBD clinical center. All IBD subjects were patients on induction infliximab therapy, and were examined before the second infliximab dose. Therefore, it was a relatively homogenous cohort of patients with severe acute gut inflammation or chronic disease resistant to corticosteroids and/or immunosuppressants. Patients´ baseline demographic and clinical characteristics are presented in Table 1. Disease behavior and localization was classified according to the Montreal classification [12].
Table 1.
Descriptive characteristics of examined IBD patients.
| Diagnosis | Crohn's disease | 32 |
| Ulcerative colitis | 8 | |
| Gender | Male | 19 |
| Female | 21 | |
| Disease duration, years | Mean | 6 |
| Range | 2 –15 | |
| Behavior of CD | B1, non-stricturing non-penetrating Crohn’s disease | 16 |
| B2, stricturing Crohn’s disease | 12 | |
| B3, penetrating Crohn’s disease | 4 | |
| Localization | L1, ileal Crohn’s disease | 8 |
| L2, colonic Crohn’s disease | 6 | |
| L3, ileocolonic Crohn’s disease | 14 | |
| L4, upper digestive Crohn’s disease | 4 | |
| E1, ulcerative proctitis | 1 | |
| E2, left-sided ulcerative colitis | 3 | |
| E3, extensive ulcerative colitis | 4 | |
| Concomitant medication | Systemic corticosteroids | 14 |
| Local corticosteroids | 6 | |
| Azathioprine | 20 | |
| Previous bowel surgery | No | 31 |
| Yes | 9 | |
| Smoking | No | 31 |
| Yes | 9 |
Forty healthy persons (laboratory technicians and their family members) without personal or family history of IBD were matched by age and gender and examined as a control (CTRL) group. There were 27 active smokers and 13 non-smokers in the CTRL group.
None of 80 examined persons (IBD patients or healthy controls) had been noted to have celiac disease or diabetes during previous clinical examinations.
The study was approved by the Institutional Ethical Committee. The purpose and procedures of the study were explained to participants, who signed informed consent forms.
2.2. Fecal samples
Raw stool samples from the IBD and CTRL groups were frozen and stored at −80 °C within 12 h after the sampling. Before the laboratory analysis, stool samples were thawed, and mechanical homogenization was performed using an inoculation loop. The Fecal Sample Preparation kit (Roche Diagnostics, Germany) for the preparation of fecal eluates was used. In this system, 100 mg of stool sample is suspended in 5 mL of appropriate extraction buffer using a vortex and subsequently centrifuged for 5 min at 2000g using a refrigerated centrifuge. For subsequent ELISA analysis, stool sample supernatants (eluates) were used immediately after their preparation.
2.3. Serum samples
Blood samples were collected into commercially available serum-separating tubes (Vacutainer, Becton Dickinson, USA). After collection, the blood was allowed to clot at room temperature for 30 min. The clot was removed by centrifugation for 10 min at 2000 g using a refrigerated centrifuge. Serum samples were stored at −80 °C immediately after their preparation.
2.4. Laboratory analysis
Fecal and serum zonulin concentrations were measured by competitive ELISA assays: Zonulin (Serum) EIA and Zonulin (Stool) EIA (both DRG International Inc, USA, catalog numbers EIA-5515 and EIA-5418, respectively). Briefly, as a first preparation step, a biotinylated zonulin tracer was added to the samples, standards and calibrators. Afterwards, aliquots of the treated preparations were transferred and incubated in microtiter plate wells coated with polyclonal anti-zonulin antibodies. During the incubation, the free target antigen in the samples competed with the biotinylated zonulin tracer for the binding of the polyclonal anti-zonulin antibodies immobilized on the microtiter plate wells. The unbound components were removed by a washing step. During a second incubation step, a streptavidin-labeled-peroxidase antibody, which binds to the biotinylated zonulin tracer, was added into each microtiter well. After a washing step to remove the unbound components, the peroxidase substrate tetramethylbenzidine (TMB) was added. Finally, the enzymatic reaction was terminated by an acidic stop solution.
Fecal calprotectin as a marker of gut inflammation was measured by the Calprotectin Alegria ELISA kit from OrgenTec, Germany.
According to the manufacturer, a median concentration of 61 ng/mL (± 46 ng/mL) fecal zonulin was estimated as ‘normal’ for healthy persons. For serum zonulin, a median value of 34 ng/mL (± 14 ng/mL) was declared as normal for healthy persons. A reference value of 50 µg/g for normal fecal calprotectin values was taken as normal.
2.5. Statistical analysis
Statistical analysis was performed using Statisctica CZ 12.0 (StatSoft, USA). Standard descriptive statistical analyses were performed, including frequency distributions for categorical data and calculation of median and interquartile range (IQR) for continuous variables. The non-parametric Kruskal–Wallis (KW) test was used for the comparison of zonulin serum and fecal concentrations in different subgroups of patients. Spearman’s rank correlation coefficient was used as a nonparametric measure of dependence between the variables examined. A p-value less than 0.05 was considered significant.
3. Results
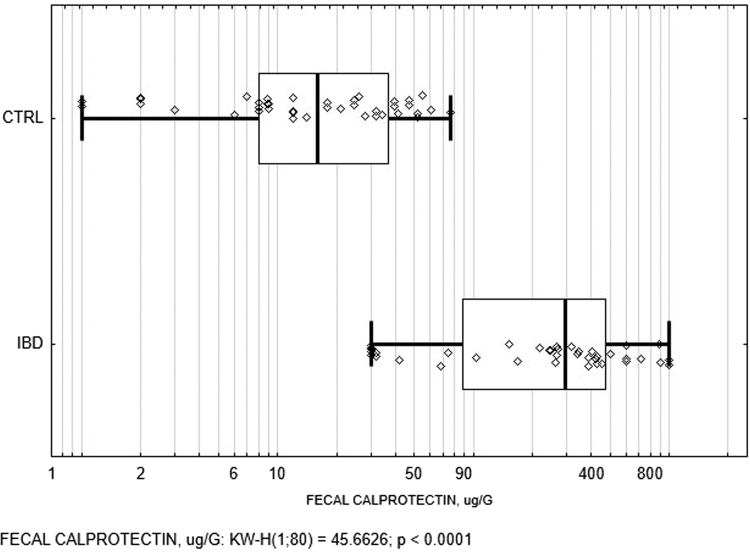
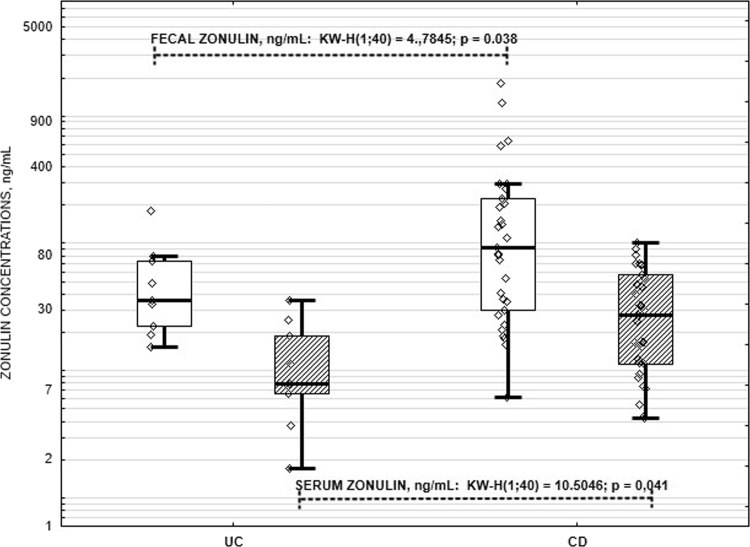
As anticipated, fecal calprotectin concentrations were significantly higher in IBD patients compared to healthy controls (Fig. 1). Calprotectin is a strong and potent biomarker of gut inflammation, so this fact confirms organic inflammatory gut involvement in IBD patients. Serum and fecal zonulin was significantly higher in persons with CD compared to UC (Fig. 2).
Fig. 1.
Box and whisker plots of the fecal calprotectin in IBD patients and healthy controls. CTRL, healthy controls; IBD, inflammatory bowel disease; KW-H, Kruskal-Wallis test. The vertical line within the box indicates the median, boundaries of the box indicate the 25th- and 75th percentile, and the whiskers indicate the highest and lowest values of the results. Each individual fecal calprotectin value is depicted as a point.
Fig. 2.
Box and whisker plots of the fecal and serum zonulin in IBD patients. UC, ulcerative colitis; CD, Crohn’s disease; KW-H, Kruskal-Wallis test. The horizontal line within the box indicates the median, boundaries of the box indicate the 25th- and 75th percentile, and the whiskers indicate the highest and lowest values without outliers. Each individual zonulin value is depicted as a point. Transparent boxes represent fecal zonulin values, and striped boxes represent serum zonulin values.
In the IBD group, no association of serum or fecal zonulin was found with respect to the disease localization and behavior (Table 2). In upper digestive tract Crohn’s disease, fecal zonulin seems to tend towards higher values compared to other CD groups; however, the p-value did not reach significance in the KW non-parametric test. The only difference was found with respect to smoking, while IBD smokers showed significantly higher fecal zonulin levels than non-smokers (Table 2).
Table 2.
Fecal and serum zonulin concentrations in IBD patients with respect to disease characteristics.
| Fecal zonulin ng/Ml median [IQR] | Serum zonulin ng/mL median [IQR] | |
|---|---|---|
| Behavior of Crohn's disease | ||
| B1, non-stricturing non-penetrating Crohn’s disease | 92.6 [34.0;168.4] | 39.5 [16.9; 63.1] |
| B2, stricturing Crohn’s disease | 65.8 [21.8; 87.3] | 22.2 [10.3; 51.0] |
| B3, penetrating Crohn’s disease | 130.8 [67.1; 203.1] | 18.1 [10.5; 31.7] |
| P - value | 0.355, NS | 0.0769, NS |
| Localization of Crohn's disease | ||
| L1, ileal Crohn’s disease | 48.6 [27.9; 76.9] | 35.1 [16.4; 70.8] |
| L2, colonic Crohn’s disease | 57.2 [22.5; 103.8] | 21.5 [9.0; 38.1] |
| L3, ileocolonic Crohn’s disease | 89.8 [37,8; 204.5] | 31.1 [16.9; 64.8] |
| L4, upper digestive Crohn’s disease | 120.3 [55.2; 206.9] | 18.0 [6.3; 20.3] |
| P - value | 0.053, NS | 0.166, NS |
| Localization of ulcerative colitis | ||
| E1, ulcerative proctitis | – | – |
| E2, left-sided ulcerative colitis | 72.5 [52.9; 76.2] | 7.9 [4.5; 29.8] |
| E3, extensive ulcerative colitis | 59.1 [20.6; 78.9] | 11.2 [ 3.3; 23.3] |
| P - value | 0.354, NS | 0.245, NS |
| Previous bowel surgery | ||
| no | 72.5 [28.5; 121.4] | 18.8 [9.1; 47.1] |
| yes | 53.2 [22.9; 87.0] | 32.5 [7.5; 47.3] |
| P - value | 0.974, NS | 0.987, NS |
| Smoking | ||
| no | 36.8 [21.6; 71.3] | 18.8 [7.8; 41.0] |
| yes | 207.9 [178.6; 226.7] | 27.3 [11.6; 56.9] |
| P-value | < 0.0001 | 0.237, NS |
Fecal calprotectin, as a biomarker of intestinal inflammation, was measured in both the IBD and CTRL groups. In the IBD group, a positive correlation of fecal calprotectin with fecal zonulin was proven (r = 0.430, p = 0.006). In the CTRL group, which showed low fecal calprotectin levels (median of concentrations 20.5 µg/g), no association of calprotectin and zonulin levels was observed in stool samples (r = 0.201, p = 0.216).
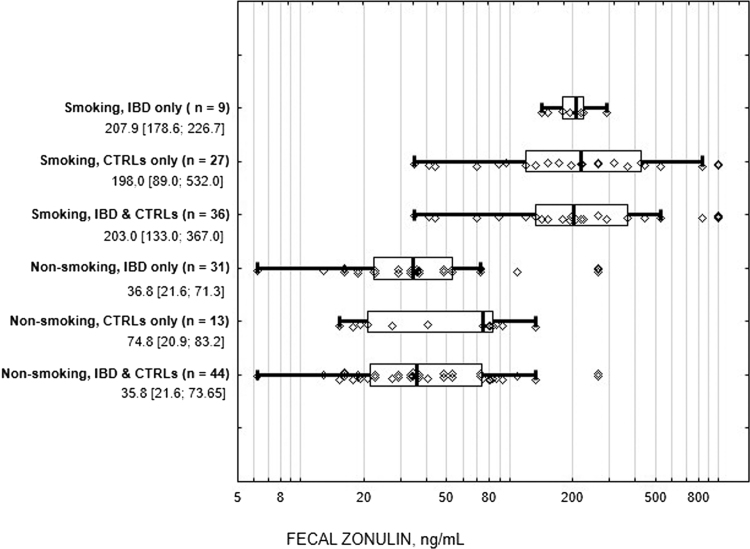
Interestingly, the only strong association in both groups examined was found between active cigarette smoking and fecal zonulin levels: smokers showed conclusively higher fecal zonulin values, irrespective of whether IBD diagnosis was present or not (see Fig. 3). There were no significant differences in fecal zonulin concentrations in IBD and healthy smokers (medians 207.9 and 198.0 ng/mL, respectively, p = 0.324). No association with smoking was found either for fecal calprotectin or serum zonulin levels.
Fig. 3.
Box and whisker plots of the fecal zonulin in active smokers and non-smokers in both groups (IBD and CTRLs). IBD, inflammatory bowel disease; CTRLs, healthy controls. The vertical line within the box indicates the median, boundaries of the box indicate the 25th- and 75th percentile, and the whiskers indicate the highest and lowest values without outliers, each individual fecal zonulin value is depicted as a point. Median values and interquartile ranges of fecal zonulin concentrations are given for all groups.
4. Discussion
As far as we know, this is the first work that describes fecal and serum zonulin levels in IBD patients with respect to IBD localization and behavior. Our results showed higher fecal and serum zonulin levels only in patients with Crohn’s disease and not in patients with ulcerative colitis, and there was a tendency towards higher fecal zonulin concentrations in Crohn’s disease with upper digestive tract involvement. Moreover, our work surprisingly noted high fecal zonulin levels in active smokers regardless of the gut inflammatory involvement.
Zonulin was firstly described by Fasano as a human protein analogue to the Vibrio cholerae -derived Zonula occludens toxin, which induces the disassembly of tight junctions and subsequently increases intestinal permeability [13]. According to Fasano’s workgroup, zonulin expression is raised in intestinal tissues during the acute phase of celiac disease [14]. Fasano had foretold, in 2000 [15], that dysregulation of zonulin might contribute to the perturbation of the intestinal barrier functions, leading to the passage of antigens involved in the pathogenesis of different immunopathological diseases such as food allergies, infections of the gastrointestinal tract, systemic autoimmune diseases and inflammatory bowel diseases. Bacteria were identified as powerful triggers of zonulin release [16].
Although the pathogenesis of IBD remains unknown, an important role can be attributed to increased intestinal permeability. It was proven that zonulin up-regulation is detected in acute phase IBD [11]. Dysmicrobia has been implicated in the pathogenesis of IBD, and small intestines exposed to different enteric bacteria secrete zonulin to the intestinal lumen and/or to the blood circulation. Our finding that zonulin levels are elevated in stool and serum only in Crohn’s disease may be in accordance with this theory. We were not able to prove any association between zonulin levels and IBD extent, fistulizing and/or penetrating behavior or previous gut surgery.
Zonulin is the fecal protein that best reflects the intestinal permeability. Its increased fecal levels are considered to be a marker of an impaired intestinal barrier, and reflect reactions secondary to inflammation. Elevated calprotectin levels in our IBD cohort reflect an inflammatory state. However, elevated fecal zonulin is suspected to reflect not only the presence of inflammation, but an increased intestinal permeability caused by other factors.
Fecal zonulin was significantly raised in Crohn’s disease, but not in ulcerative colitis patients. It could be hypothesized, that there is a different permeability abnormality in Crohn's disease compared to ulcerative colitis, and that this abnormality could be important in the genesis of disease. At least some of the reported genetic associations of Crohn's disease appear to involve these pathways [17].
Environmental components such as food may have an important role in regulating intestinal permeability. In addition, gut microbiota have also been shown to influence intestinal permeability. Our finding of significantly higher fecal zonulin levels in IBD and healthy smokers points to the important influence of gut homeostasis induced by the cigarette smoking. Smoking is considered to be one of the most important environmental risk factors in IBD pathogenesis and is to be included in the list of relevant factors that influence the composition of intestinal microbiota [18], [19], [20]. The proper mechanisms by which smoking interferes with the pathogenesis of IBD are, as yet, only poorly understood. While several potential mechanisms have been proposed - for example, modulation of mucosal immune responses, alterations in intestinal cytokine and eicosanoid levels or modifications in gut permeability - none of these hypotheses give a satisfying explanation [21]. As the importance of zonulin in gut inflammation is well established, high fecal zonulin levels in smokers may explain a potential pathogenetic link of cigarette smoking, intestinal dysmicrobia, leaky gut and the course of disease in IBD. We consider that the results of our study, which demonstrate high fecal zonulin levels even in apparently healthy persons without any signs of intestinal inflammation, point to a large complex interplay between the gut microenvironment and environmental factors such as smoking, for example, concerning possible weight gain after stopping smoking [22].
The design of our study produces potential limitations. Firstly, this is a pilot study, and as such serves as a first step in exploring a novel laboratory marker. The cohort sample size is small and based on the pragmatics of recruitment and the necessities for examining feasibility. Further research may be advisable in a larger population. Secondly, as we hypothesized that elevated zonulin levels serve as a mirror of gut dysmicrobia, we do not have sufficient information regarding the fecal microbiology to exclude infection or small intestinal bacterial overgrowth, and the antibiotic or other immunomodulatory treatments in our examined cohorts, which is an important factor that may potentially influence gut microbiota. Thirdly, as the clinical utility of stool and serum zonulin was not evaluated during the initial decision to perform endoscopic examination in IBD patients, we were unable to determine whether or not these laboratory examinations could contribute to the gastroenterologist's choice to perform an endoscopy.
In conclusion, zonulin as a marker of intestinal permeability is increased in stool and serum samples of patients with Crohn’s disease. However, zonulin levels in IBD patients are not influenced by disease location or behavior. High fecal zonulin levels in smokers with and without intestinal disease point to the significant and undesirable up-regulation of gut permeability in cigarette smokers.
Acknowledgement
This work was supported by the RVO VFN 64165 project provided by the Ministry of Health, Czech Republic.
References
- 1.Fasano A. Intestinal permeability and its regulation by zonulin: diagnostic and therapeutic implications. Clin. Gastroenterol. Hepatol. 2012;10(10):1096–1100. doi: 10.1016/j.cgh.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fasano A. Zonulin, regulation of tight junctions, and autoimmune diseases. Ann. N. Y. Acad. Sci. 2012;1258:25–33. doi: 10.1111/j.1749-6632.2012.06538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fasano A., Not T., Wang W. Zonulin, a newly discovered modulator of intestinal permeability, and its expression in coeliac disease. Lancet. 2000;29(9214):1518–1519. doi: 10.1016/S0140-6736(00)02169-3. (355) [DOI] [PubMed] [Google Scholar]
- 4.de Kort S., Keszthelyi D., Masclee A.A. Leaky gut and diabetes mellitus: what is the link? Obes. Rev. 2011;12(6):449–458. doi: 10.1111/j.1467-789X.2010.00845.x. [DOI] [PubMed] [Google Scholar]
- 5.Zhang D., Zhang L., Zheng Y. Circulating zonulin levels in newly diagnosed Chinese type 2 diabetes patients. Diabetes Res. Clin. Pract. 2014;106(2):312–318. doi: 10.1016/j.diabres.2014.08.017. [DOI] [PubMed] [Google Scholar]
- 6.Küme T., Acar S., Tuhan H. A. The relationship between serum zonulin level and clinical and laboratory parameters of childhood obesity. J. Clin. Res. Pediatr. Endocrinol. 2017;9(1):31–38. doi: 10.4274/jcrpe.3682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Damms-Machado A., Louis S., Schnitzer A. Gut permeability is related to body weight, fatty liver disease, and insulin resistance in obese individuals undergoing weight reduction. Am. J. Clin. Nutr. 2017;105(1):127–135. doi: 10.3945/ajcn.116.131110. [DOI] [PubMed] [Google Scholar]
- 8.Mokkala K., Pellonperä O., Röytiö H. Increased intestinal permeability, measured by serum zonulin, is associated with metabolic risk markers in overweight pregnant women. Metabolism. 2017;69:43–50. doi: 10.1016/j.metabol.2016.12.015. [DOI] [PubMed] [Google Scholar]
- 9.Ciccia F., Guggino G., Rizzo A. Dysbiosis and zonulin upregulation alter gut epithelial and vascular barriers in patients with ankylosing spondylitis. Ann. Rheum. Dis. 2017 doi: 10.1136/annrheumdis-2016-210000. (pii: annrheumdis-2016-210000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li C., Gao M., Zhang W. Zonulin regulates intestinal permeability and facilitates enteric bacteria permeation in coronary artery disease. Sci. Rep. 2016;6:29142. doi: 10.1038/srep29142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vanuytsel T., Vermeire S., Cleynen I. The role of Haptoglobin and its related protein, Zonulin, in inflammatory bowel disease. Tissue Barriers. 2013;1(5):e27321. doi: 10.4161/tisb.27321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Silverberg M.S., Satsangi J., Ahmad T. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can. J. Gastroenterol. 2005;19(Suppl A):5A–36A. doi: 10.1155/2005/269076. [DOI] [PubMed] [Google Scholar]
- 13.Fasano A. Intestinal zonulin: open sesame! Gut. 2001;49(2):159–162. doi: 10.1136/gut.49.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clemente M.G., De Virgiliis S., Kang J.S. Early effects of gliadin on enterocyte intracellular signalling involved in intestinal barrier function. Gut. 2003;52(2):218–223. doi: 10.1136/gut.52.2.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fasano A. Regulation of intercellular tight junctions by zonula occludens toxin and its eukaryotic analogue zonulin. Ann. N. Y. Acad. Sci. 2000;915:214–222. doi: 10.1111/j.1749-6632.2000.tb05244.x. [DOI] [PubMed] [Google Scholar]
- 16.Guttman J.A., Finlay B.B. Tight junctions as targets of infectious agents. Biochim. Biophys. Acta. 2009;1788(4):832–841. doi: 10.1016/j.bbamem.2008.10.028. [DOI] [PubMed] [Google Scholar]
- 17.Buhner S., Buning C., Genschel J. Genetic basis for increased intestinal permeability in families with Crohn's disease: role of CARD15 3020insC mutation? Gut. 2006;55(3):342–347. doi: 10.1136/gut.2005.065557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Birrenbach T., Böcker U. Inflammatory bowel disease and smoking: a review of epidemiology, pathophysiology, and therapeutic implications. Inflamm. Bowel Dis. 2004;10(6):848–859. doi: 10.1097/00054725-200411000-00019. [DOI] [PubMed] [Google Scholar]
- 19.(a) Benjamin Smokers with active Crohn´s disease have a clinically relevant dysbiosis of the gastrointestinal microbiota. Inflamm. Bowel Dis. 2012;18:1092–1100. doi: 10.1002/ibd.21864. [DOI] [PubMed] [Google Scholar]; (b) Severs Smoking is associated with extra-intestinal manifestations in inflammatory bowel disease. J. Crohn's Colitis. 2016:455–461. doi: 10.1093/ecco-jcc/jjv238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Begon J., Juillerat P., Cornuz J. Smoking and digestive tract: a complex relationship. Part 2: intestinal microblota and cigarette smoking. Rev. Med. Suisse. 2015;11(478):1304–1306. [PubMed] [Google Scholar]
- 21.Biedermann L., Zeitz J., Mwinyi J. Smoking cessation induces profound changes in the composition of the intestinal microbiota in humans. PLoS One. 2013;8(3):e59260. doi: 10.1371/journal.pone.0059260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Biedermann L., Brülisauer K., Zeitz J. Smoking cessation alters intestinal microbiota: insights from quantitative investigations on human fecal samples using FISH. Inflamm. Bowel Dis. 2014;20(9):149. doi: 10.1097/MIB.0000000000000129. [DOI] [PubMed] [Google Scholar]