Abstract
Sirtuin1 (Sirt1) and Sirtuin3 (Sirt3) are two well-characterized members of the silent information regulator 2 (Sir2) family of proteins. Both Sirt1 and Sirt3 have been shown to play vital roles in resistance to cellular stress, but the interaction between these two sirtuins has not been fully determined. In this study, we investigated the role of Sirt1-Sirt3 axis in blood-brain barrier (BBB) permeability after ischemia in vitro. Human brain microvascular endothelial cells and astrocytes were co-cultured to model the BBB in vitro and oxygen and glucose deprivation (OGD) was performed to mimic ischemia. The results of transepithelial electrical resistance (TEER) showed that suppression of Sirt1 via siRNA or salermide significantly decreased BBB permeability, whereas Sirt3 knockdown increased BBB permeability. In addition, Sirt1 was shown to regulate Sirt3 expression after OGD through inhibiting the AMPK-PGC1 pathway. Application of the AMPK inhibitor compound C partially prevented the effects of Sirt1-Sirt3 axis on BBB permeability after OGD. The results of flow cytometry and cytochrome c release demonstrated that Sirt1 and Sirt3 exert opposite effects on OGD-induced apoptosis. Furthermore, suppression of Sirt1 was shown to attenuate mitochondrial reactive oxygen species (ROS) generation, which contribute to the Sirt1-Sirt3 axis-induced regulation of BBB permeability and cell damage. In summary, these findings demonstrate that the Sirt1-Sirt3 axis might act as an important modulator in BBB physiology, and could be a therapeutic target for ischemic stroke via regulating mitochondrial ROS generation.
Keywords: Stroke, Blood-brain barrier, Sirt1, Sirt3, Mitochondrial ROS
1. Introduction
Stroke is a leading cause of morbidity and mortality worldwide, claiming around 129,000 lives every year [1]. The blood-brain barrier (BBB) is formed by endothelial cells that are interconnected by tight junctions and adherens junctions. It is part of the neurovascular unit (NVU) and serves as an anatomical and functional barrier between the blood and the brain to maintain an optimal environment for neuronal network [2]. The BBB dysfunction has been demonstrated to play an important role in the pathophysiology of many neuronal disorders including stroke. However, the molecular mechanisms underlying the regulation of BBB permeability after ischemic stroke are not fully determined.
Sirtuins are the human and murine homologs of the silent information regulator 2 (Sir2) first discovered in Saccharomyces cerevisiae [3]. They belong to class III histone deacetylases that are dependent on nicotinamide adenine dinucleotide (NAD+) for their activity. Seven mammalian sirtuins homologs (Sirt1-7) have been identified and they are localized to the nucleus (Sirt1, Sirt6, and Sirt7), mitochondria (Sirt3, Sirt4, and Sirt5), or cytoplasm (Sirt2) [4], [5]. It now appears clear that these sirtuins deacetylate various substrates, such as transcription factors, metabolic enzymes and histones, to control many biological processes including metabolism, cell growth, apoptosis, autophagy and genetic control of ageing [6]. Sirt1 is the most widely studied sirtuin and expressed at a high level in the brain compared to other organs [7]. It was shown that inhibition of Sirt1 activity protects against neuronal injury through mitochondrial associated anti-apoptotic pathways [8], [9]. Sirt3 resides primarily in the mitochondria and has been identified as a responsive deacetylase that regulates metabolism and oxidative stress [10]. Our previous studies showed that Sirt3 attenuates hydrogen peroxide-induced oxidative stress via preservation of mitochondrial function [11], [12]. However, the interaction between Sirt1 and Sirt3 under ischemic stroke has not been fully determined.
The present study investigated the existence and function of a Sirt1-Sirt3 axis in an in vitro ischemic stroke model induced by oxygen and glucose deprivation (OGD). Human brain microvascular endothelial cell and astrocyte co-cultures were used to mimic the BBB in vitro. In addition, we also determined the role of AMPK-PGC1 pathway and mitochondrial ROS generation in the interaction between Sirt1 and Sirt3, and investigated their effects on BBB permeability by measuring transepithelial electrical resistance (TEER).
2. Materials and methods
2.1. In vitro BBB model
The in vitro BBB model was established as previously described [13]. Human brain microvascular endothelial cells (Catalog number #1000) and human astrocytes (Catalog number #1800) were purchased from ScienCell (Carlsbad, CA, USA) and co-cultured in the endothelial cell and astrocyte medium. Human astrocytes were seeded on the outside of collagen-coated 0.4 µm pore PTFE membrane transwell inserts directed upside down and allowed to adhere to the membrane overnight. Human brain microvascular endothelial cells were seeded on the inside of the insert and cells were grown to confluence to create a contact co-culture model.
2.2. Short interfering RNA (siRNA) and transfection
The specific siRNA targeted Sirt1 (Si-Sirt1, sc-40986), Sirt3 (Si-Sirt3, sc-61555), PGC1 (Si-PGC1, sc-38884) and control siRNA (Si-Control, sc-37007), which should not knock down any known proteins, were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The above siRNA molecules were transfected with Lipofectamine 2000 (Invitrogen, CA, USA) in for 48 h. After transfection, the cells were treated with OGD and subjected to various measurements.
2.3. Measurement of BBB permeability
TEER was determined as a marker of co-culture integrity and as a measure of paracellular permeability. The resistance across the membrane was measured using STX2 electrodes linked to an EVOM2 resistance meter (World Precision Instruments, Hertfordshire, UK). Three readings were taken per insert and the average value was used. A baseline TEER reading was taken (i.e. 0 h) and the percentage change from this value was calculated for subsequent readings.
2.4. OGD
The OGD protocol was used to mimic ischemia in vitro. Briefly, culture medium was removed and rinsed with PBS for three times. Cells were placed into a specialized, humidified chamber containing 5% CO2, 95% N2 at 37 °C with glucose-free RPMI medium (Invitrogen, CA, USA), which was pre-gassed with N2/CO2 (95%/5%) to remove residual oxygen. After 2 h challenge, TEER was read and the RPMI medium was replaced with the cells’ normal medium. The cells were maintained for further 22 h at 37 °C in a humidified 5% CO2 incubator to generate reperfusion, and the permeability of the BBB was assessed throughout the reperfusion period.
2.5. Flow cytometry
Cells were harvested 24 h after OGD as described. Cells were washed twice with ice-cold PBS and re-suspended in binding buffer. The cell suspension was transferred into a tube and double-stained with Annexin V-FITC and propidium iodide (PI) at room temperature in the dark for 15 min, according to the Annexin V apoptosis detection kit instructions (Roche Applied Bioscience, Indianapolis, IN, USA). The percentage of apoptotic cells was quantified by flow cytometry at 530 nm and 600 nm to measure green Annexin V-FITC and red PI fluorescence respectively (FACSCalibur, BD Biosciences, San Jose, CA, USA). Further differentiation between early and late apoptosis was studied by Annexin V and PI staining in different quadrants: AV+/PI+, the late phase apoptotic cells and AV+/PI−, the early phase apoptotic cells.
2.6. Subcellular fractionation for cytoplasm and mitochondria
Cells were washed with ice-cold PBS for three times and lysed with a lysis buffer containing protease inhibitors. The cell lysate was centrifuged for 10 min at 750g at 4 °C, and the pellets containing the nuclei and unbroken cells were discarded. The supernatant was then centrifuged at 15 000g for 15 min. The resulting supernatant was removed and used as the cytosolic fraction. The pellet fraction containing mitochondria was further incubated with PBS containing 0.5% Trition X-100 for 10 min at 4 °C. After centrifugation at 16 000g for 10 min, the supernatant was collected as mitochondrial fraction.
2.7. Measurement of mitochondrial ROS
Mitochondrial superoxide production was measured using the MitoSOX Red Kit (Invitrogen, Carlsbad, CA, USA). Briefly, mitochondria were isolated and 20 μg of mitochondria in 50 μl of respiration buffer was loaded into 96-well plate. One hundred microliters of ice cold respiration buffer was supplemented with 2 μM MitoSOX, 5 μM antimycin A and 4 μM ADP. Fifty micromoles salermide or 1 μM FeTCCP was added into indicated wells before loading into the FLUOstar Omega Microplate Reader. The basal level of fluorescence intensity was measured at 355 nm and 590 nm and fluorescence intensity was measured at 5-min intervals.
2.8. Western blot analysis
Equivalent amounts of protein (40 μg per lane) were loaded and separated by 10% SDS-PAGE gels, and transferred to polyvinylidene difluoride (PVDF) membranes. Membranes were blocked with 5% skimmed milk solution in tris-buffered saline with 0.1% Triton X-100 (TBST) for 1 h, and then incubated overnight at 4 °C with the primary Sirt1 (1:1000), Sirt3 (1:1000), p-AMPK (1:400), AMPK (1:600), PGC1 (1:600), cytochrome c (1:800), 3-NT (1:800), COX V (1:600), Tubulin (1:1000) or β-actin (1:1000) antibody dilutions in TBST. After that the membranes were washed and incubated with secondary antibody for 1 h at room temperature. Immunoreactivity was detected with Super Signal West Pico Chemiluminescent Substrate (Thermo Scientific, Rockford, IL, USA). Image J (Scion Corporation) was used to quantify the optical density of each band.
2.9. Statistical analysis
Statistical analysis was performed using SPSS 16.0, a statistical software package. Statistical evaluation of the data was performed by one-way analysis of variance (ANOVA) followed by Bonferroni's multiple comparisons. A value of p < 0.05 was considered statistically significant.
3. Results
3.1. Sirt1 and Sirt3 exert opposite effects on BBB permeability
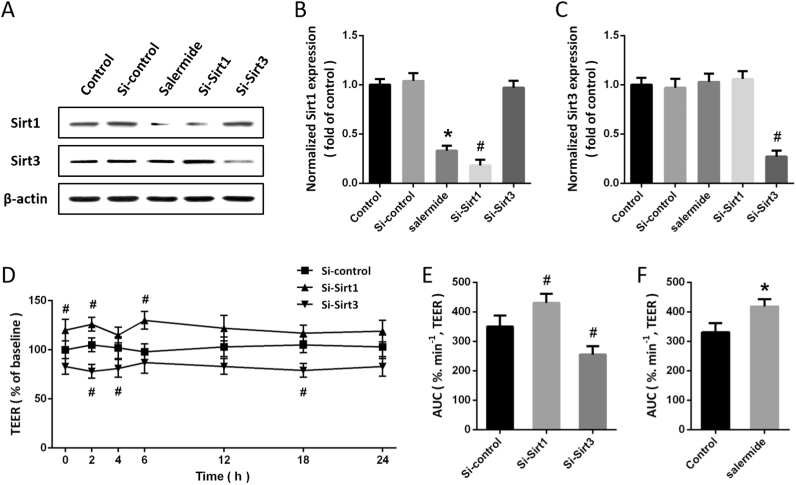
To investigate the role of Sirt1 and Sirt3 in our model, the expression of Sirt1 was inhibited by Si-Sirt1 transfection or salermide treatment, while the expression of Sirt3 was suppressed by Si-Sirt3 transfection. The results of western blot showed that both Si-Sirt1 and salermide significantly decreased Sirt1 expression, and the Sirt3 level in Si-Sirt3 group was lower than that in Si-control group (Figs. 1A-C). As shown in Fig. 1D, the BBB permeability in vitro was detected by measuring TEER. The results showed that Sirt1 knockdown significantly increased TEER, whereas Si-Sirt3 decreased TEER compared with Si-control (Fig. 1E). In addition, treatment with salermide also decreased BBB permeability as evidenced by increased TEER (Fig. 1F).
Fig. 1.
Sirt1 and Sirt3 exert opposite effects on BBB permeability. Cells were transfected with Si-Sirt1, Si-Sirt3 or Si-control for 48 h, or treated with salermide (50 μM) for 24 h, and the expression of Sirt1 and Sirt3 was detected by western blot (A) and calculated (B and C). The effects of Si-Sirt1 and Sirt3 on BBB permeability were shown in D and E, and the effect of salermide on BBB permeability was shown in F. Data are shown as mean ± SEM. *p < 0.05 vs. Control. #p < 0.05 vs. Si-control.
3.2. Sirt1 regulates Sirt3 expression through AMPK-PGC1 pathway after OGD
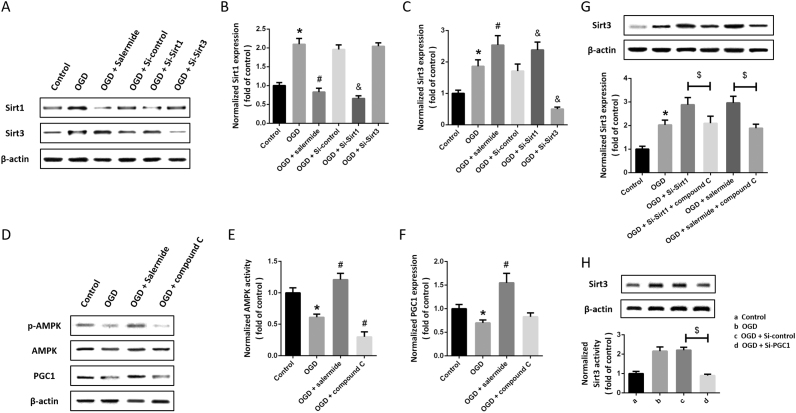
Next, we performed OGD to mimic ischemia in vitro, and detected the expression of Sirt1 and Sirt3 by western blot (Fig. 2A). We found that the expression of Sirt1 and Sirt3 were both significantly increased by OGD (Fig. 2B). The expression of Sirt3 after OGD was further increased by Si-Sirt1 and salermide (Fig. 2C), whereas Si-Sirt3 had no effect on Sirt1 expression after OGD (Fig. 2B). As shown in Fig. 2D, we also detected the expression of AMPK and PGC1. The results showed that the expression of PGC1 and phosphorylation of AMPK were decreased by OGD, but increased by salermide (Fig. 2E). Blocking AMPK activity using compound C significantly decreased AMPK phosphorylation and PGC1 expression (Fig. 2F). To further investigate the mechanisms underlying Sirt1-induced inhibition on Sirt3 expression, we used compound C to inhibit AMPK activity after OGD and Sirt1 suppression. The results showed that Sirt1 blockage-induced increase in Sirt3 expression after OGD was partially reversed by compound C (Fig. 2G). In addition, knockdown of PGC1 expression using Si-PGC1 significantly decreased Sirt3 expression in OGD-treated cells (Fig. 2H).
Fig. 2.
Sirt1 regulates Sirt3 expression through AMPK-PGC1 pathway after OGD. Cells were transfected with siRNA for 48 h or pretreated with salermide (50 μM) for 30 min, and exposed to OGD. The expression of Sirt1 and Sirt3 was detected by western blot (A) and calculated (B and C). Cells were pretreated with salermide (50 μM) or compound C (5 μM) for 30 min and exposed to OGD. The expression of Sirt1 and Sirt3 was detected by western blot (D) and calculated (E and F). Cells were transfected with siRNA for 48 h with or without compound C, or pretreated with salermide (50 μM) for 30 min with or without compound C, and exposed to OGD. The expression of Sirt3 was detected by western blot (G). Cells were transfected with Si-control or Si-PGC1 for 48 h and exposed to OGD. The expression of Sirt3 was detected by western blot (H). Data are shown as mean ± SEM. *p < 0.05 vs. Control. #p < 0.05 vs. OGD. &p < 0.05 vs. Si-control. $p < 0.05.
3.3. Sirt1-Sirt3 axis contributes to OGD-induced hyperpermeability
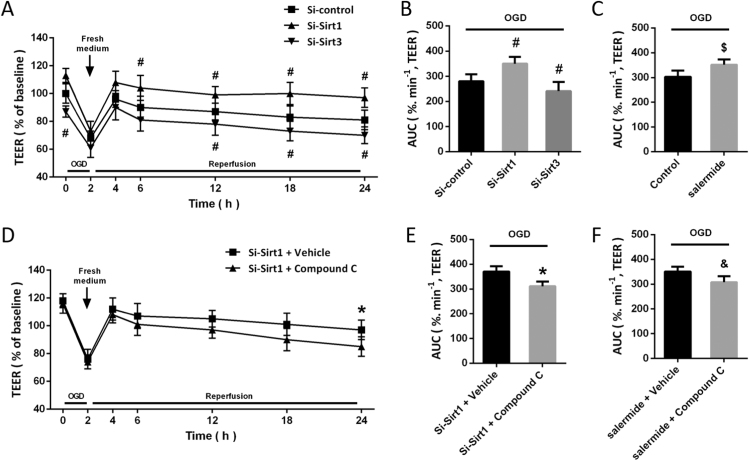
We measured TEER in OGD-injured cells up to 24 h, and the results showed that exposing the cells to 2 h OGD increased permeability as shown by a reduction in TEER of approximately 42% (Fig. 3A). Sirt1 knockdown significantly increased TEER after OGD, whereas Si-Sirt3 decreased TEER compared with that in Si-control transfected and OGD-injured cells (Fig. 3B). As shown in Fig. 3C, a similar result was also observed after salermide treatment. To investigate the role of AMPK-PGC1 pathway, cells were treated with Si-Sirt1 or salermide in the presence or absence of compound C (Fig. 3D). The results showed that both Si-Sirt1- and salermide-induced increase in TEER were significantly attenuated by compound C (Figs. 3E and 3F).
Fig. 3.
Sirt1-Sirt3 axis contributes to OGD-induced hyperpermeability. The effects of Si-Sirt1 and Si-Sirt3 on BBB permeability after OGD were shown in A and B, and the effect of salermide on BBB permeability after OGD was shown in C. The effect of compound C on BBB permeability after OGD were shown in D and E, and the effects of salermide and compound C on BBB permeability after OGD were shown in F. Data are shown as mean ± SEM. #p < 0.05 vs. Si-control. #p < 0.05 vs. Si-Sirt1 + Vehicle. $p < 0.05 vs. Control. &p < 0.05 vs. salermide + Vehicle.
3.4. Sirt1-Sirt3 mediates OGD-induced cellular damage in the BBB model
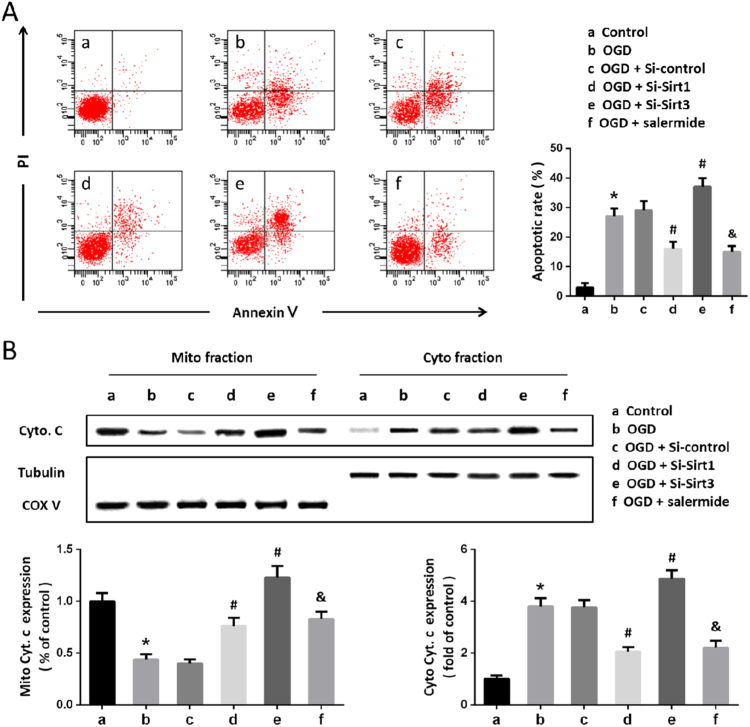
To determine whether the Sirt1-Sirt3 axis-induced regulation on BBB permeability was associated with cell injury, we detected apoptosis by flow cytometry (Fig. 4A). The results showed that OGD significantly increased both early and late apoptotic cell death, which were attenuated by Si-Sirt1 and salermide, but further increased by Si-Sirt3 transfection. In addition, we also detected the levels of cytochrome c protein in mitochondrial and cytosolic fraction by western blot (Fig. 4B). We found that Sirt1 suppression using Si-Sirt1 or salermide markedly decreased cytosolic cytochrome c and increased mitochondrial cytochrome c after OGD. In contrast, knockdown of Sirt3 significantly increased OGD-induced cytochrome c release, indicating a protective role of Sirt3 in our in vitro BBB model.
Fig. 4.
Sirt1-Sirt3 mediates OGD-induced cellular damage in the BBB model. Cells were transfected with siRNA for 48 h or pretreated with salermide (50 μM) for 30 min, and exposed to OGD. The apoptotic cell death was detected by flow cytometry (A), and the levels of cytochrome c protein were detected by western blot (B). Data are shown as mean ± SEM. *p < 0.05 vs. a. #p < 0.05 vs. b. &p < 0.05 vs. b.
3.5. Mitochondrial ROS generation is involved in Sirt1-Sirt3 axis-induced regulation of BBB permeability and cellular damage
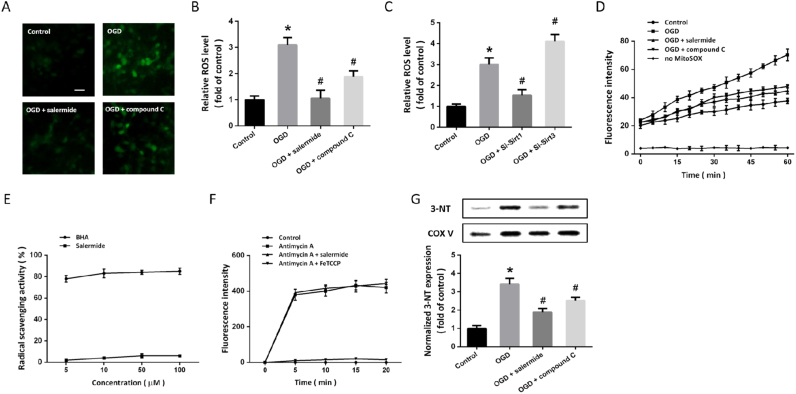
To investigate the potential role of ROS generation in our in vitro model, DHE probe was used to detected intracellular ROS levels (Fig. 5A). Increased levels of ROS were observed in OGD-treated cells, whereas both salermide and compound C significantly alleviated ROS accumulation (Fig. 5B). Similarly, knockdown of Sirt1 expression significantly reduced ROS generation after OGD, whereas Si-Sirt3 increased ROS levels in OGD-treated cells (Fig. 5C). When cytoplasm harvested after OGD were incubated with MitoSOX-loaded normal mitochondria, an increase in O2•- was found and was partially prevented by salermide and compound C (Fig. 5D). Next, scavenging of DPPH was performed, and we found that salermide was not a radical scavenger, as compared with BHA (Fig. 5E). To further determine whether salermide directly inhibited intramitochondrial ROS generation, we assayed its effect on MitoSOX-loaded mitochondria treated with antimycin A. As shown in Fig. 5F, the increased ROS readout was not altered by salermide. The results of western blot showed that mitochondrial 3-NT levels were markedly suppressed by salermide and compound C after OGD (Fig. 5G).
Fig. 5.
Effects of Sirt1-Sirt3 axis on ROS generation. Cells were treated with salermide (50 μM) or compound C (5 μM) and exposed to OGD. Intracellular ROS generation was detected by DHE probe (A) and calculated (B). Cells were transfected with siRNA for 48 h and exposed to OGD. ROS levels were measured (C). Mitochondrial superoxide was measured by MitoSOX up to 60 min after OGD (D). Cells were treated with DPPH (50 μM) with different concentrations of salermide or BHA (5, 10, 50 or 100 μM), and radical scavenging activity was determined (E). Cells were treated with Antimycin with or without salermide or FeTCCP, and mitochondrial superoxide was determined (F). Cells were treated with salermide or compound C and exposed to OGD. The expression of 3-NT in isolated mitochondria was detected by western blot (G). Scale bar = 20 µm. Data are shown as mean ± SEM. *p < 0.05 vs. Control. #p < 0.05 vs. OGD.
4. Discussion
Disruption of the BBB and followed cerebral edema are the key pathogenic events leading to neurological dysfunction and death after ischemic stroke. In this study, we reported the existence of a Sirt1-Sirt3 axis in the brain microvascular endothelial cell and astrocyte co-cultures, and demonstrated that Sirt1 regulated Sirt3 expression via the AMPK-PGC1 pathway after OGD. The activation of Sirt1-Sirt3 axis was involved in the increased BBB permeability, as measured by transepithelial electrical resistance. In addition, inhibition of Sirt1-Sirt3 cascades could exert protective effects against OGD-induced apoptotic cell death in our in vitro model.
As the most widely studied sirtuin, Sirt1 modulates multiple downstream genes and adapts cell metabolism to new requirements in response to cellular stress [14]. Activation of Sirt1 by gene overexpression or pharmacological activators has been demonstrated to exert protective effects against neurodegenerative diseases, but the role of Sirt1 in ischemic stroke is still a matter of controversy. Sirt1 could bind to the mitochondrial uncoupling protein 2 (UCP-2) and reduce UCP-2 transcription, leading to increased mitochondrial oxidative phosphorylation [15], [16]. A previous study showed that Sirt1 attenuated the decrease in Bcl-2 expression and inhibited caspase-3 cleavage in OGD-injured neurons [17]. Moreover, Sirt1 was also shown to mediate the neuroprotective effect of phosphoribosyltransferase (Nampt), which is a rate-limiting enzyme in the salvage pathway to synthesize NAD+ in neuronal cells [18]. However, the detrimental effects of Sirt1 in the brain have also been reported recently. For example, inhibition of Sirt1 with nicotinamide or sirtinol, or knockdown of Sirt1 expression protected cultured neurons against oxidative stress [8]. These effects were associated with regulation of IGF-1 and ERK pathways. In addition, Sirt1 was also shown to impair transcription of many protective genes, including grp78 and gadd34, via deacetylating the ER stress associated transcription factor XBP-1 [19]. Our present data showed that activation of Sirt1 was associated with increased BBB permeability in both normal and ischemic conditions in vitro. Importantly, inhibition of Sirt1 with salermide or Si-Sirt1 attenuated apoptosis, which was measured by flow cytometry and western blot analysis. All these results indicated that Sirt1 exerted detrimental effects in the in vitro BBB model.
Sirt3 expression is highest in metabolically active tissues, such as the brain, heart, liver and skeletal muscle [20], [21], [22]. As a mitochondria-specific sirtuin, Sirt3 directly binds and deacetylates various metabolic and respiratory enzymes to regulate mitochondrial function. The beneficial effects of Sirt3 under cellular stress conditions have been extensively studied. Sirt3 regulates subunits of mitochondrial electron transport chain (ETC) complexes and tricarboxylic acid (TCA) cycle enzymes to increase production of energy equivalents during caloric restriction (CR), fasting and exercise [23]. It also deacetylates manganese superoxide dismutase (MnSOD) and isocitrate dehydrogenase 2 (IDH2) and enhances their abilities to scavenge ROS [24], [25]. Thus, Sirt3 was able to inhibit apoptosis by lowering ROS generation and preventing components of the mitochondrial permeability transition pore (mPTP) [26], [27]. Our previous studies showed that Sirt3 attenuates hydrogen peroxide-induced oxidative stress through preservation of mitochondrial function in HT22 cells [11]. We also found that overexpression of Sirt3 by lentivirus transfection protects cortical neurons against oxidative stress through regulating mitochondrial calcium and mitochondrial biogenesis [12]. In line with our results, Sirt3 was recently found to directly deacetylase and stabilize 8-xoguanine-DNA glycosylase 1 (OGG1) to promote mitochondrial DAN (mtDNA) repair [28]. More recently, Cheng et al. showed that neurons lacking the mitochondrial Sirt3 are more vulnerable to oxidative stress and apoptosis [29]. In this study, we found that knockdown of Sirt3 aggravated the OGD-induced BBB disruption and apoptosis, which further confirmed the protective role of Sirt3 in our in vitro ischemia model.
Under stress conditions, the expression and activity of Sirt3 can be regulated by several upstream molecules, among which PGC1 is an important one. PGC1 activates nuclear transcriptors to upregulate the expression of nuclear encoded mitochondrial proteins, making it a key regulator of mitochondrial function [30], [31]. Previous studies showed that PGC1 exert stimulatory effect on Sirt3 promoter and act as an upstream activator of Sirt3 expression in hepatocytes and muscle cells [32]. In contrast, Sirt3 could also protect neuronal cells through PGC1-mediated antioxidative activities [33], [34]. Increased levels of PGC1 and Sirt3 were shown to modulate mitochondrial metabolic activity and oxidative stress regulator pathway activation, acting as ROS suppressors by increasing the expression and activity of glutathione peroxidase (GPX) and superoxide dismutase 2 (SOD2) [35], [36]. Our previous studies also showed that overexpression of Sirt3 protect cortical neurons against oxidative stress through regulating PGC1-mediated mitochondrial biogenesis [12]. Thus, we speculated that the activated PGC1, together with the upregulated Sirt3, inhibited OGD-induced oxidative stress to preserve the BBB permeability in our in vitro model. To determine this possibility, we measured cellular ROS generation using DHE probe and detected mitochondrial ROS by MitoSOX. As expected, both DHE and MitoSOX fluorescence intensities were significantly inhibited by salermide and compound C. This effect could be due to salermide be a radical or ROS scavenger causing removal of ROS or it could be due to salermide with generation of ROS by interfering with ROS precurosors with mitochondria [37]. Further studies demonstrated that salermide is not a radical scavenger as compared to BHA, a well-known antioxidant [38]. Thus, the Sirt1-Sirt3 axis could preserve the BBB permeability via inhibiting mitochondrial ROS generation, which might be associated with blocking the interaction of cytosolic oxidative stress cascades with mitochondria.
Recently, Sirt1 was demonstrated to play an important role in human endothelial pathology. Sirt1 significantly decreased in human endothelial cells after hydrogen peroxide (H2O2) exposure [39]. Sirt1 also reduced the ionomycin-induced ICAM-1 expression and attenuated the downregulation of thrombomodulin after particulate matter treatment [40], [41]. As expected, our results showed that inhibition of Sirt1 was associated with the decreased BBB permeability in our co-cultures with or without OGD exposure. However, Sirt3 exerted the opposite effect. Importantly, Sirt1 acted as an upstream inhibitory regulator on Sirt3 expression in our in vitro model. In line with our results, a recent study showed that Sirt1-silenced cells show an increased Sirt3 expression, which was dependent on the presence of SP1 and ZF5 recognition sequences on Sirt3 promoter [42]. As the link between Sirt1 and Sirt3 are not fully determined, there must exist signaling cascades mediating the possible Sirt1-Sirt3 crosstalk. As a serine/threonine kinase that senses energy status in various cells and tissues, AMPK is an important downstream molecular of Sirt1 to regulate cellular metabolism [43]. Previous studies and our present data also showed that Sirt3 function as a downstream target of PGC1, which is directly regulated by AMPK and exerts protective effects under oxidative stress [44], [45], [46]. Thus, we used commercially available inhibitors of Sirt1 and AMPK, salermide and compound C, to further confirm the involvement of Sirt1-AMPK-PGC1 signaling in the Sirt1-Sirt3 axis. In vitro assays have demonstrated that 50 μM salermide and 5 μM compound C were effective in blocking Sirt1 and AMPK activity, respectively [47]. Our results showed that the effects of Sirt1 on PGC1 and Sirt3 expression were partially prevented by both salermide and compound C. In addition, these two inhibitors were also effective in nullifying Sirt1-induced effects on BBB permeability and cell apoptosis. All these data strongly suggest that the link between Sirt1 and Sirt3 in our model is mediated by the AMPK-PGC1 pathway.
5. Conclusion
In summary, this study demonstrated that Sirt1 and Sirt3 exert opposite effects on BBB permeability in vitro in human cells. After OGD, Sirt1 inhibited Sirt3 expression through the AMPK-PGC1 pathway, causing mitochondrial ROS generation, which in turn leads to decreased BBB permeability and cell apoptosis. In view of these results, the Sirt1-Sirt3 axis might be an ideal target to modulate BBB permeability after ischemic stroke.
Conflict of interest
The authors declare that they have no conflict of interest.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 81371447, No. 81671303, No. 81430043, No. 81701932, No. 81301037) and Shaanxi Province Natural Science Foundation Research Program (No. 2014JM4131).
Contributor Information
Zhou Fei, Email: fmmuzhoufei@163.com.
Xiao-Fan Jiang, Email: fmmujiangxiaofan@163.com.
References
- 1.Mozaffarian D., Benjamin E.J., Go A.S., Arnett D.K., Blaha M.J., Cushman M., Das S.R., de Ferranti S., Despres J.P., Fullerton H.J., Howard V.J., Huffman M.D., Isasi C.R., Jimenez M.C., Judd S.E., Kissela B.M., Lichtman J.H., Lisabeth L.D., Liu S., Mackey R.H., Magid D.J., McGuire D.K., Mohler E.R., 3rd, Moy C.S., Muntner P., Mussolino M.E., Nasir K., Neumar R.W., Nichol G., Palaniappan L., Pandey D.K., Reeves M.J., Rodriguez C.J., Rosamond W., Sorlie P.D., Stein J., Towfighi A., Turan T.N., Virani S.S., Woo D., Yeh R.W., Turner M.B. Heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation. 2016;133(4):e38–e360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 2.Schoknecht K., David Y., Heinemann U. The blood-brain barrier-gatekeeper to neuronal homeostasis: clinical implications in the setting of stroke. Semin. Cell Dev. Biol. 2015;38:35–42. doi: 10.1016/j.semcdb.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 3.Alhazzazi T.Y., Kamarajan P., Verdin E., Kapila Y.L. SIRT3 and cancer: tumor promoter or suppressor? Biochim. Biophys. Acta. 2011;1816(1):80–88. doi: 10.1016/j.bbcan.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Satoh A., Imai S. Systemic regulation of mammalian ageing and longevity by brain sirtuins. Nat. Commun. 2014;5:4211. doi: 10.1038/ncomms5211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Michan S. Acetylome regulation by sirtuins in the brain: from normal physiology to aging and pathology. Curr. Pharm. Des. 2013;19(38):6823–6838. doi: 10.2174/1381612811319380014. [DOI] [PubMed] [Google Scholar]
- 6.Park S.H., Ozden O., Jiang H., Cha Y.I., Pennington J.D., Aykin-Burns N., Spitz D.R., Gius D., Kim H.S. Sirt3, mitochondrial ROS, ageing, and carcinogenesis. Int. J. Mol. Sci. 2011;12(9):6226–6239. doi: 10.3390/ijms12096226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Michishita E., Park J.Y., Burneskis J.M., Barrett J.C., Horikawa I. Evolutionarily conserved and nonconserved cellular localizations and functions of human SIRT proteins. Mol. Biol. Cell. 2005;16(10):4623–4635. doi: 10.1091/mbc.E05-01-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Y., Xu W., McBurney M.W., Longo V.D. SirT1 inhibition reduces IGF-I/IRS-2/Ras/ERK1/2 signaling and protects neurons. Cell Metab. 2008;8(1):38–48. doi: 10.1016/j.cmet.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao Y., Luo P., Guo Q., Li S., Zhang L., Zhao M., Xu H., Yang Y., Poon W., Fei Z. Interactions between SIRT1 and MAPK/ERK regulate neuronal apoptosis induced by traumatic brain injury in vitro and in vivo. Exp. Neurol. 2012;237(2):489–498. doi: 10.1016/j.expneurol.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 10.Jin L., Galonek H., Israelian K., Choy W., Morrison M., Xia Y., Wang X., Xu Y., Yang Y., Smith J.J., Hoffmann E., Carney D.P., Perni R.B., Jirousek M.R., Bemis J.E., Milne J.C., Sinclair D.A., Westphal C.H. Biochemical characterization, localization, and tissue distribution of the longer form of mouse SIRT3. Protein Sci.: Publ. Protein Soc. 2009;18(3):514–525. doi: 10.1002/pro.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dai S.H., Chen T., Wang Y.H., Zhu J., Luo P., Rao W., Yang Y.F., Fei Z., Jiang X.F. Sirt3 attenuates hydrogen peroxide-induced oxidative stress through the preservation of mitochondrial function in HT22 cells. Int. J. Mol. Med. 2014;34(4):1159–1168. doi: 10.3892/ijmm.2014.1876. [DOI] [PubMed] [Google Scholar]
- 12.Dai S.H., Chen T., Wang Y.H., Zhu J., Luo P., Rao W., Yang Y.F., Fei Z., Jiang X.F. Sirt3 protects cortical neurons against oxidative stress via regulating mitochondrial Ca2+ and mitochondrial biogenesis. Int. J. Mol. Sci. 2014;15(8):14591–14609. doi: 10.3390/ijms150814591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hind W.H., Tufarelli C., Neophytou M., Anderson S.I., England T.J., O'Sullivan S.E. Endocannabinoids modulate human blood-brain barrier permeability in vitro. Br. J. Pharmacol. 2015;172(12):3015–3027. doi: 10.1111/bph.13106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petegnief V., Planas A.M. SIRT1 regulation modulates stroke outcome. Transl. Stroke Res. 2013;4(6):663–671. doi: 10.1007/s12975-013-0277-y. [DOI] [PubMed] [Google Scholar]
- 15.Della-Morte D., Dave K.R., DeFazio R.A., Bao Y.C., Raval A.P., Perez-Pinzon M.A. Resveratrol pretreatment protects rat brain from cerebral ischemic damage via a sirtuin 1-uncoupling protein 2 pathway. Neuroscience. 2009;159(3):993–1002. doi: 10.1016/j.neuroscience.2009.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bordone L., Motta M.C., Picard F., Robinson A., Jhala U.S., Apfeld J., McDonagh T., Lemieux M., McBurney M., Szilvasi A., Easlon E.J., Lin S.J., Guarente L. Sirt1 regulates insulin secretion by repressing UCP2 in pancreatic beta cells. PLoS Biol. 2006;4(2):e31. doi: 10.1371/journal.pbio.0040031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yan W., Fang Z., Yang Q., Dong H., Lu Y., Lei C., Xiong L. SirT1 mediates hyperbaric oxygen preconditioning-induced ischemic tolerance in rat brain. J. Cereb. Blood Flow Metab.: Off. J. Int. Soc. Cereb. Blood Flow Metab. 2013;33(3):396–406. doi: 10.1038/jcbfm.2012.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abdellatif M. Sirtuins and pyridine nucleotides. Circ. Res. 2012;111(5):642–656. doi: 10.1161/CIRCRESAHA.111.246546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang F.M., Chen Y.J., Ouyang H.J. Regulation of unfolded protein response modulator XBP1s by acetylation and deacetylation. Biochem. J. 2011;433(1):245–252. doi: 10.1042/BJ20101293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lombard D.B., Alt F.W., Cheng H.L., Bunkenborg J., Streeper R.S., Mostoslavsky R., Kim J., Yancopoulos G., Valenzuela D., Murphy A., Yang Y., Chen Y., Hirschey M.D., Bronson R.T., Haigis M., Guarente L.P., Farese R.V., Jr., Weissman S., Verdin E., Schwer B. Mammalian Sir2 homolog SIRT3 regulates global mitochondrial lysine acetylation. Mol. Cell. Biol. 2007;27(24):8807–8814. doi: 10.1128/MCB.01636-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahn B.H., Kim H.S., Song S., Lee I.H., Liu J., Vassilopoulos A., Deng C.X., Finkel T. A role for the mitochondrial deacetylase Sirt3 in regulating energy homeostasis. Proc. Natl. Acad. Sci. USA. 2008;105(38):14447–14452. doi: 10.1073/pnas.0803790105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gurd B.J., Holloway G.P., Yoshida Y., Bonen A. In mammalian muscle, SIRT3 is present in mitochondria and not in the nucleus; and SIRT3 is upregulated by chronic muscle contraction in an adenosine monophosphate-activated protein kinase-independent manner. Metab.: Clin. Exp. 2012;61(5):733–741. doi: 10.1016/j.metabol.2011.09.016. [DOI] [PubMed] [Google Scholar]
- 23.Kincaid B., Bossy-Wetzel E. Forever young: SIRT3 a shield against mitochondrial meltdown, aging, and neurodegeneration. Front. Aging Neurosci. 2013;5:48. doi: 10.3389/fnagi.2013.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qiu X., Brown K., Hirschey M.D., Verdin E., Chen D. Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation. Cell Metab. 2010;12(6):662–667. doi: 10.1016/j.cmet.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 25.Yu W., Dittenhafer-Reed K.E., Denu J.M. SIRT3 protein deacetylates isocitrate dehydrogenase 2 (IDH2) and regulates mitochondrial redox status. J. Biol. Chem. 2012;287(17):14078–14086. doi: 10.1074/jbc.M112.355206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bause A.S., Haigis M.C. SIRT3 regulation of mitochondrial oxidative stress. Exp. Gerontol. 2013;48(7):634–639. doi: 10.1016/j.exger.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 27.Giralt A., Villarroya F. SIRT3, a pivotal actor in mitochondrial functions: metabolism, cell death and aging. Biochem. J. 2012;444(1):1–10. doi: 10.1042/BJ20120030. [DOI] [PubMed] [Google Scholar]
- 28.Cheng Y., Ren X., Gowda A.S., Shan Y., Zhang L., Yuan Y.S., Patel R., Wu H., Huber-Keener K., Yang J.W., Liu D., Spratt T.E., Yang J.M. Interaction of Sirt3 with OGG1 contributes to repair of mitochondrial DNA and protects from apoptotic cell death under oxidative stress. Cell Death Dis. 2013;4:e731. doi: 10.1038/cddis.2013.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheng A., Yang Y., Zhou Y., Maharana C., Lu D., Peng W., Liu Y., Wan R., Marosi K., Misiak M., Bohr V.A., Mattson M.P. Mitochondrial SIRT3 Mediates Adaptive Responses of Neurons to Exercise and Metabolic and Excitatory Challenges. Cell Metab. 2016;23(1):128–142. doi: 10.1016/j.cmet.2015.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wenz T. Regulation of mitochondrial biogenesis and PGC-1alpha under cellular stress. Mitochondrion. 2013;13(2):134–142. doi: 10.1016/j.mito.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 31.Wenz T. PGC-1alpha activation as a therapeutic approach in mitochondrial disease. IUBMB Life. 2009;61(11):1051–1062. doi: 10.1002/iub.261. [DOI] [PubMed] [Google Scholar]
- 32.Kong X., Wang R., Xue Y., Liu X., Zhang H., Chen Y., Fang F., Chang Y. Sirtuin 3, a new target of PGC-1alpha, plays an important role in the suppression of ROS and mitochondrial biogenesis. PLoS One. 2010;5(7):e11707. doi: 10.1371/journal.pone.0011707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gusdon A.M., Callio J., Distefano G., O'Doherty R.M., Goodpaster B.H., Coen P.M., Chu C.T. Exercise increases mitochondrial complex I activity and DRP1 expression in the brains of aged mice. Exp. Gerontol. 2017;90:1–13. doi: 10.1016/j.exger.2017.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Q., Li L., Li C.Y., Pei Z., Zhou M., Li N. SIRT3 protects cells from hypoxia via PGC-1alpha- and MnSOD-dependent pathways. Neuroscience. 2015;286:109–121. doi: 10.1016/j.neuroscience.2014.11.045. [DOI] [PubMed] [Google Scholar]
- 35.Someya S., Yu W., Hallows W.C., Xu J., Vann J.M., Leeuwenburgh C., Tanokura M., Denu J.M., Prolla T.A. Sirt3 mediates reduction of oxidative damage and prevention of age-related hearing loss under caloric restriction. Cell. 2010;143(5):802–812. doi: 10.1016/j.cell.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bell E.L., Guarente L. The SirT3 divining rod points to oxidative stress. Mol. Cell. 2011;42(5):561–568. doi: 10.1016/j.molcel.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huo Y., Win S., Than T.A., Yin S., Ye M., Hu H., Kaplowitz N., Antcin H protects against acute liver injury through disruption of the interaction of c-Jun-N-terminal kinase with mitochondria. Antioxid. Redox Signal. 2017;26(5):207–220. doi: 10.1089/ars.2016.6833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu X., Wang X., Pang N., Zhu W., Zhao X., Wang F., Wu F., Wang J. APA-style human milk fat analogue from silkworm pupae oil: enzymatic production and improving storage stability using alkyl caffeates. Sci. Rep. 2015;5:17909. doi: 10.1038/srep17909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kao C.L., Chen L.K., Chang Y.L., Yung M.C., Hsu C.C., Chen Y.C., Lo W.L., Chen S.J., Ku H.H., Hwang S.J. Resveratrol protects human endothelium from H(2)O(2)-induced oxidative stress and senescence via SirT1 activation. J. Atheroscler. Thromb. 2010;17(9):970–979. doi: 10.5551/jat.4333. [DOI] [PubMed] [Google Scholar]
- 40.Jia Y., Gao P., Chen H., Wan Y., Zhang R., Zhang Z., Yang R., Wang X., Xu J., Liu D. SIRT1 suppresses PMA and ionomycin-induced ICAM-1 expression in endothelial cells. Sci. China Life Sci. 2013;56(1):19–25. doi: 10.1007/s11427-012-4407-7. [DOI] [PubMed] [Google Scholar]
- 41.Wu Z., Liu M.C., Liang M., Fu J. Sirt1 protects against thrombomodulin down-regulation and lung coagulation after particulate matter exposure. Blood. 2012;119(10):2422–2429. doi: 10.1182/blood-2011-04-350413. [DOI] [PubMed] [Google Scholar]
- 42.Carnevale I., Pellegrini L., D'Aquila P., Saladini S., Lococo E., Polletta L., Vernucci E., Foglio E., Coppola S., Sansone L., Passarino G., Bellizzi D., Russo M.A., Fini M., Tafani M. SIRT1-SIRT3 Axis regulates cellular response to oxidative stress and etoposide. J. Cell. Physiol. 2016 doi: 10.1002/jcp.25711. [DOI] [PubMed] [Google Scholar]
- 43.Ou H.C., Hsieh Y.L., Yang N.C., Tsai K.L., Chen K.L., Tsai C.S., Chen I.J., Wu B.T., Lee S.D. Ginkgo biloba extract attenuates oxLDL-induced endothelial dysfunction via an AMPK-dependent mechanism. J. Appl. Physiol. 1985;114(2):274–285. doi: 10.1152/japplphysiol.00367.2012. (2013) [DOI] [PubMed] [Google Scholar]
- 44.Yechoor V.K., Patti M.E., Ueki K., Laustsen P.G., Saccone R., Rauniyar R., Kahn C.R. Distinct pathways of insulin-regulated versus diabetes-regulated gene expression: an in vivo analysis in MIRKO mice. Proc. Natl. Acad. Sci. USA. 2004;101(47):16525–16530. doi: 10.1073/pnas.0407574101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guan Y., Cui Z.J., Sun B., Han L.P., Li C.J., Chen L.M. Celastrol attenuates oxidative stress in the skeletal muscle of diabetic rats by regulating the AMPK-PGC1alpha-SIRT3 signaling pathway. Int. J. Mol. Med. 2016;37(5):1229–1238. doi: 10.3892/ijmm.2016.2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen Y., Qing W., Sun M., Lv L., Guo D., Jiang Y. Melatonin protects hepatocytes against bile acid-induced mitochondrial oxidative stress via the AMPK-SIRT3-SOD2 pathway. Free Radic. Res. 2015;49(10):1275–1284. doi: 10.3109/10715762.2015.1067806. [DOI] [PubMed] [Google Scholar]
- 47.Zhou G., Myers R., Li Y., Chen Y., Shen X., Fenyk-Melody J., Wu M., Ventre J., Doebber T., Fujii N., Musi N., Hirshman M.F., Goodyear L.J., Moller D.E. Role of AMP-activated protein kinase in mechanism of metformin action. J. Clin. Investig. 2001;108(8):1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]