Abstract
Cantharidin, a terpenoid defensive toxin mainly produced by blister beetles, is among the most widely known insect natural products in the world. However, little is known about the site of cantharidin biosynthesis in vivo. Our previous research showed that 3-hydroxy-3-methylglutary-CoA reductase (HMGR) is an essential enzyme in cantharidin biosynthesis. In this report, we further investigated cantharidin titer and HMGR mRNA expression levels in different tissues of male and female Epicauta chinensis, and performed a comparative analysis of HMGR transcript levels in male Tenebrio molitor, a Tenebrionidae beetle that cannot produce cantharidin. HMGR transcripts had a positive correlation with cantharidin production. Furthermore, the specifically high amounts of HMGR transcript and abundant cantharidin production in fat body of male E. chinensis indicated the process of cantharidin synthesis may occur in the fat body.
Keywords: HMGR, Cantharidin, Epicauta chinensis, Tenebrio molitor, fat body
Blister beetles are famous for its defensive compound, cantharidin, which is highly toxic to most animals (Dettner 1997, Ghaffarifar 2010, Khan et al. 2013). Cantharidin is a widely known insect defensive product or a sexual attractant and stimulates copulation in Epicauta nyassensis (Coleoptera: Meloidae) (Nikbakhtzadeh et al. 2007a,b, 2012). Moreover, cantharidin has only been found in blister beetles (Coleoptera: Meloidae) and oedemerid beetles (Coleopetra: Oedemeridae), where it is found in various tissues (Carrel et al. 1986a, Frenzel and Dettner 1994, Dettner 1997).
Cantharidin has 10 carbon atoms, and the structure is consistent in containing two isoprene units. However, feeding experiments using isotope-labeled acetate and mevalonate suggested that cantharidin is not formed by either a tail-to-tail or head-to-tail linkage of two isoprene units, but possibly by a more complicated process (Schlatter et al. 1968, Guenther et al. 1969). Subsequently, experiments with isotope-labeled farnesol and mevalonolactone by Peter et al. (1977a,b) showed that the C10-molecule cantharidin was biosynthesized from the C15-precursor, farnesol, which is cleaved between C(1)–C(2), C(4)–C(5) and C(7)–C(8). Woggon et al. (1983) also suggested farnesol as an intermediate in the biosynthesis of cantharidin. McCormick et al. (1986) indicated that cantharidin is most likely derived from a juvenile hormone (JH) metabolite. The inhibition of cantharidin production caused by injecting 6-fluoromevalonate (FMVA) into Lytta vesicatoria supported this opinion, as FMVA also inhibits JH biosynthesis (Carrel et al. 1986b). Ruzicka (1953) suggested that isopentenyl pyrophosphate is the biologically active isoprene unit. This hypothesis has been supported by Agranoff et al. (1960), who showed that the isomerization of isopentenyl pyrophosphate to dimethylallyl pyrophosphate by isopentenyl pyrophosphate isomerase is an essential initial step in the biosynthesis of several trans-isoprenoid compounds. Based on these results, a monoterpenoid is presumably assembled from isopentenyl diphosphenoid and dimethylallyl diphosphate derived from the mevalonate (MVA) pathway (Bellés et al. 2005). 3-Hydroxy-3-methylglutary-CoA reductase (HMGR), which catalyzes the reduction of HMG-CoA to mevalonate, is the key enzyme regulating the MVA pathway (Friesen and Rodwell 2004). Analyses of HMGR expression have been used to locate de novo biosynthesis of monoterpenoids such as aggregation pheromones in bark beetles (Hall et al. 2002a, Seybold and Tittiger 2003, Tillman et al. 2004, Bearfield et al. 2006). HMGR has been shown to be a key enzyme in the biosynthesis of cantharidin in Epicauta chinensis Laporte (Lü et al. 2016). However, little is known about where and when the cantharidin biosynthesized, or the relationship between HMGR transcripts and the amount of cantharidin produced in E. chinensis.
In most meloid species, larvae can sustainably produce cantharidin before pupation. After eclosion, males synthesize cantharidin persistently while females produce almost no cantharidin (Dettner 1987). During copulation, males transfer virtually all their cantharidin to females as a nuptial gift, and high amounts of cantharidin will accumulate in the eggs during oviposition (Sierra et al. 1976, Carrel et al. 1993, Eisner et al. 1996). This phenomenon was regarded as a self-protective mechanism to defend against predators. Several meloid species such as Epicauta mannerheimi, Meloetyplus fuscatus and Meloe proscarabaeus can mate more than once (Garófalo et al. 2011, Ghoneim 2013). It also has been argued that cantharidin can function as pheromone during courtship (Eisner et al. 1996; Nikbakhtzadeh et al. 2007a,b, 2008). Female Epicauta nyassensis may be able to discriminate the cantharidin content of males and prefer to mate with the one with the most abundant cantharidin reserve (Nikbakhtzadeh et al. 2007a). In this study, HMGR mRNA levels were considered to be an indicator to localize the site of early steps for cantharidin production in E. chinensis. We therefore measured HMGR transcriptional level and cantharidin content in different developmental stages and tissues of E. chinensis. To eliminate the differences of physiological reactions between genders in E. chinensis, another Coleoptera insect, Tenebrio molitor, which does not synthesize cantharidin, was selected as a reference Our results suggested that the fat body is the probable site for cantharidin biosynthesis.
Materials and Methods
Insect
Wild E. chinensis blister beetles were collected near Suide County (Shaanxi, China) and reared as continuous cultures in the laboratory (28 °C and 14:10 L:D) on fresh lucerne or soybean leaves. We selected adult pairs that had just finished copulation and reared them independently in separate containers under the same conditions as above. Larvae of E. chinensis were reared in the laboratory (25 °C, 14:10 L:D and 60% RH) on eggs of Locusta migratoria. About three triungulin larva were reared in a plastic cup (5 cm diameter and 8 cm high) with several egg masses and covered with soil to avoid cannibalism. Larvae were observed every 3 d and larvae collected as needed. Tenebrio molitor was reared on wheat bran and fresh cabbage leaves in an environmental chamber (25 °C, 16:8 L:D and 60% RH). Larvae were collected every 10 d until pupation. Pupae were collected and monitored for adult eclosion.
Tissue Sample Preparation
Tissue samples of blister beetles were taken from adults 4 d after mating, and those of mealworm were taken from male adults 10 d after eclosion. For each experimental series, tissues of 30 male T. molitor or 20 male E. chinensis were pooled in pre-cooled tubes and immediately immersed in liquid nitrogen before storing at −80 °C until needed. All tissues were dissected in modified Ringer’s solution (Crozier 1968, Kerkut et al. 1969) and divided into three independent experimental series.
Identification of HMGR in T. molitor
Total RNA was extracted from pupae of T. molitor with the RNAiso Plus kit (TaKaRa, Dalian, China) according to the manufacturer’s instructions. The quality and concentrations of RNA were determined by NanoDrop 2000 spectrometer (Thermo, Waltham, USA). First-strand template cDNA was synthesized with PrimeScript™ II 1st Strand cDNA Synthesis Kit (TaKaRa) for RT-PCR using random 6mers in total volume 20 μl. A conserved fragment of HMGR cDNA was amplified using Ex Taq (TaKaRa) with TmHMGR-F1 and TmHMGR-R1 primers (Table 1), which were designed from conserved regions in the aligned amino acid sequences of HMGRs from other insects [Tribolium castaneum (XP_973850.1), Gastrophysa viridula (ABO37161.1), Ips paraconfusus (AAD20975.2), Chrysomela populi (ABO37162.1), Anthonomus grandis (AAF80475.2)and Dendroctonus jeffreyi (AAF80374.1)]. PCR was performed in a 50-μl reaction volumes including ∼50 ng cDNA, 10 μl 10 × Ex Taq Buffer (Mg2+ plus), 4 μl dNTP Mixture (2.5 mM), 0.5 μM of each primer and 0.25 μl Ex Taq (5 U/μl). The cycling profile was: 94 °C for 3 min, followed by 35 cycles of 94 °C for 30 s, 56 °C for 45 s, 72 °C for 75 s, and a final extension at 72 °C for 10 min. 5′- and 3′-UTRs were recovered by nested-PCR using the SMART 5′ RACE & 3′ RACE kits (TaKaRa) with gene-specific primers and adaptor primers (5′ & 3′ RACE in Table 1). The full-length ORF of TmHMGR cDNA was amplified using a pair of gene-specific primers, TmHMGR-F and TmHMGR-R, from first strand cDNA for verification. For sequence analysis, All PCR products were purified using a Gel extraction kit (Code No. BSC02S1, Biospin, Hangzhou, China), and cloned into pMD19-T vector (Code No. 6013, TaKaRa), then transformed into Trans10 chemically competent cells (TransGen, Beijing, China). Recombinant plasmids were subsequently purified for sequencing.
Table 1.
Oligonucleotide primers used for cDNA cloning and qRT-PCR
| Primers | Sequences (5′–3′) | |
|---|---|---|
| Conserved cDNA | TmHMGR-F1 | GTTGTAGTGAATTACGTAGTATTTATGACGTTYTAYCCNGC |
| TmHMGR-R1 | CGTTCCTTTGGATACCATGTTCATNCCCAT | |
| 5′ & 3′ RACE | Tm3RS1 | TTAGGCGGGTCGGTCTTGAGTGATG |
| Tm3RS2 | AGCAGAGTCGTGGGAGATGGAATGA | |
| Tm5RA1 | TGTGGGCTCTCAGTTGTTCGGCTAA | |
| Tm5RA2 | TGTGGTCTTCTTCCTTCAATGCCCT | |
| ORF | TmHMGR-F | TACTCTCACCATCAGTGAAAACTCG |
| TmHMGR-R | GTACAAAAAATACGTAAACCTAGGC | |
| qRT-PCR | TmHMGR-QS | ACAACTGAGAGCCCACATAAG |
| TmHMGR-QA | TGGGATTGTCGCTCAAGAAA | |
| TmRPS3-QS | TCTTGTTGAGGCAAGGAGTG | |
| TmRPS3-QA | GTTGTCAGGGAGAGGTTTCTT | |
| EcHMGR-QS | CAACAACGATACCCACTA | |
| EcHMGR-QA | CTCCAACTAAGCCACACT | |
| Ecactin-QS | TCTGGTCGTACAACTGGTATTG | |
| Ecactin-QA | CGTAGGATAGCATGCGGTAAA |
Sequence Analyses
Nucleotide sequencing was achieved commercially (AuGCT, Inc., Beijing, China). Sequence analysis was done with DNASTAR Inc V.7.0 software (DNASTAR Inc., Madison, WI). Protein sequence similarity searches were performed using BLASTP in GenBank (http://blast.ncbi.nlm.nih.gov/Blast.cgi). Transmembrane segments were predicted by HMMER (http://www.ebi.ac.uk/services/proteins). Predicted domains and important sites were analyzed by InterPro (http://www.ebi.ac.uk/interpro/). Sequence alignments were performed by Clustal Omega (http://www.ebi.ac.uk/Tools/msa/clustalo/) based on the selected HMGRs, and the phylogenetic tree was compiled using MEGA 6.0 software.
Quantitative RT-PCR Analysis
Expression of HMGR in different tissues or stages of T. molitor and E. chinensis was analyzed by relative quantitative real-time PCR (qRT-PCR). Aliquots of cDNA which were synthesized from total RNA isolated from different samples with PrimeScript RT reagent Kit with gDNA Eraser (TaKaRa). Actin of blister beetles and RPS3 of mealworms were used as an internal control gene for normalization. Primers specific to the reference gene and HMGR of T. molitor and E. chinensis were designed from the identified PCR fragments using Primer 3 Software (http://www.simgene.com/Primer3) (Table 1). PCR amplification and fluorescence detection were performed using a Cylcer iQ Real-time PCR System (Bio-Rad iQ5 Hercules, CA) with a SYBR Premix Ex Taq Kit (TaKaRa). The protocol was: 10 s at 95 °C; 40 cycles of 30 s at 95 °C, 30 s at 60 °C followed by melting curve analysis. The whole process of qRT-PCR was according to Bustin et al. (2009). The data was analyzed according to the 2−ΔΔCT method (Livak and Schmittgen 2001).
Isolation and Detection of Cantharidin
Cantharidin from tissues or adults of E. chinensis were extracted and analyzed as described previously (Lü et al. 2016), with the following modifications. Cantharidin was quantified by gas chromatography (GC) using a GC-2010 Plus instrument (SHIMADZU, Kyoto, Japan) with an HP-5 capillary column (15 m × 0.53 mm × 1.5 μm) and flame ionization detector. Conditions of GC were as follows: the temperature program started at 140 °C and maintained for 2 min then increased by 12 °C/min to 240 °C and maintained at 240 °C for 5 min; injector and detector temperature were both 250 °C. Nitrogen was applied as a carrier gas at a constant flux of 30 ml/min, hydrogen and air at 2.0 kg/cm2 and 2.55 kg/cm2, respectively. About 1 μl of each sample was injected for each of three independent replicates. Cantharidin was quantified by comparing peaks with those of a standard curve of pure cantharidin (Sigma, Aldrich).
Statistical Analysis
All results were displayed as mean ± SEM and compared using one-way analysis of variance (one-way ANOVA, LSD method; SPSS 19 software).
Results
Isolation and Characterization of HMGR cDNA in T. molitor
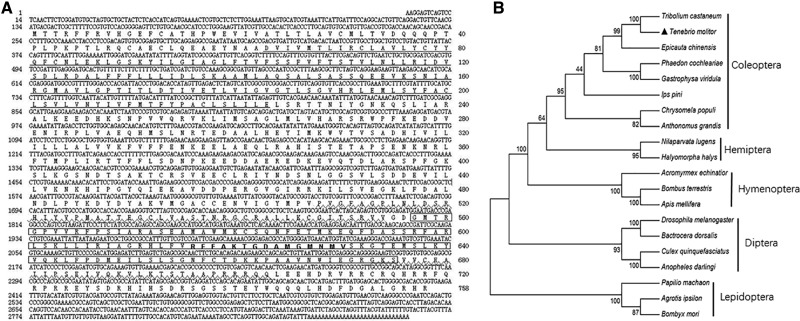
A combination of using degenerate RACE-PCR yielded a 2,881-bp cDNA from T. molitor consisting of a 2,277-bp ORF flanked by 133 and 471 bp for 5′- and 3′-UTRs, respectively. The ORF encoded 758 amino acid residues with a predicted molecular weight of 85.4 kDa and theoretical pI of 8.26. Comparisons with database entries of GenBank by using BLASTX obtained the highest Bit scores to HMGR sequences from Tribolium castaneum (TcHMGR) with 89% identity and 92% positive, and from Epicauta chinensis HMGR (EcHMGR) with 77% identity and 87% positive. We therefore named this clone TmHMGR cDNA (GenBank accession no. KU885424). The deduced amino acid sequence of TmHMGR has a sterol-sensing domain at 63-223 (Fig. 1A). The catalytic domain is at 508-554 and 673-703, respectively. The NAD or NADP-binding domain and conserved site of TmHMGR was identical to HMGR from leaf beetle (Burse et al. 2008). A phylogenetic tree of HMGRs from 20 insect species is shown in Fig. 1B.
Fig. 1.
Sequence analysis of Tenebrio molitor 3-hydroxy-3-methyl glutaryl coenzyme A reductase. (A) cDNA and deduced amino acid sequence of TmHMGR. Sterol-sensing domain is underlined. The catalytic domain is underlined with wave line. The NAD/NADP-binding domain is boxed. The conserved site of HMGR is in bold. (B) Phylogenetic relationship of TmHMGR with sequence from insects. The tree was constructed using the maximums likelihood method with a bootstrap test with 1000 replicates. Acromyrmex echinatior EGI58498.1, Agrotis ipsilon CAA08775.1, Anopheles darlingi ETN66344.1, Anthonomus grandis AAF80475.2, Apis mellifera XP_016773303.1, Bactrocera dorsalis JAC38805.1, Bombyx mori NP_001093298.1, Bombus terrestris NP_001295235.1, hrysomela populi ABO37162.1, Culex quinquefasciatus EDS41396.1, Drosophila melanogaster NP_732900.1, Epicauta chinensis AGF87101.1, Gastrophysa viridula ABO37161.1, Halyomorpha halys XP_014280269.1, Ips pini AAL09351.1, Nilaparvata lugens AGC79113.1, Papilio machaon KPJ10638.1, Phaedon cochleariae ABO37160.1, Tribolium castaneum KYB27676.1.
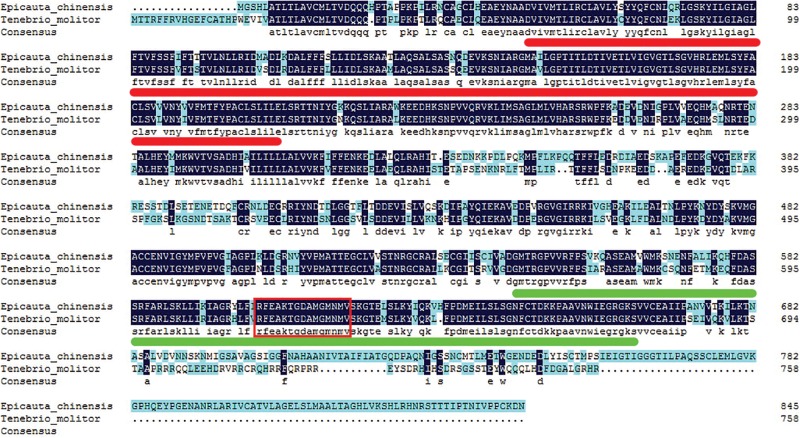
The deduced amino acid sequences of TmHMGR and EcHMGR were aligned. The analysis result revealed that TmHMGR shared 77% sequence identity with E. chinensis HMGR and it showed a high degree of sequence conservation in the sterol-sensing domain and the NAD or NADP-binding domain (Fig. 2). A HMGR conserved site (RFEAKTGDAMGMNMV) was found to be 100% conserved between these two proteins.
Fig. 2.
Alignment of deduced amino acid sequence of HMGR from E. chinensis and T. molitor. Sterol-sensing domain is indicated with red line under the sequences. The NAD or NADP-binding domain is indicated with green line. The conserved site of HMGR is marked with red rectangle.
Developmental Expression Profiles of HMGR in T. molitor and E. chinensis
The expression levels of HMGR in different stages of T. molitor and E. chinensis were determined by qRT-PCR. To improve the accuracy of the data, we measured cantharidin content and HMGR transcript levels in male blister beetles from zero to seventh day after mating again according to our previous research (Lü et al. 2016). In male blister beetles the expression level of HMGR gradually increased from day zero to the seventh day after copulation, and was relatively lower over the first three days (Fig. 3A). The expression level was 4.7-fold higher at the seventh day compared to that of day 0. However, in females, HMGR mRNA levels were highest on day 0, ∼9- to 120-fold higher than those on the first to seventh days after copulation. The highest expression of HMGR was restricted to the female just after mating, with much lower expression levels on the other tested days (Fig. 3B).
Fig. 3.
HMGR relative expression levels in different stages of Epicauta chinensis and Tenebrio molitor. (A) Male E. chinensis 0–7 d after mating. (B) Female E. chinensis 0–7 d after mating. (C) Egg and different instars of E. chinensis larvae. (D) Different developmental stages of Tenebrio molitor larvae. RNA was isolated from five adults and at least 10 eggs or larvae (±SEM, N = 3). Different letters on the error bars show significant differences (P ≤ 0.05).
HMGR mRNA levels in E. chinensis larvae were high only in the third and fourth instars, but remained at low levels from egg to the second instar, and the fifth instar (Fig. 3C). In T. molitor, HMGR mRNA levels were the lowest at the egg stage and highest at 10 d after hatch, with lowered levels from 20th day after hatch to pupa stage (Fig. 3D).
Tissue-Specific Expression Profiles of HMGR in T. molitor and E. chinensis
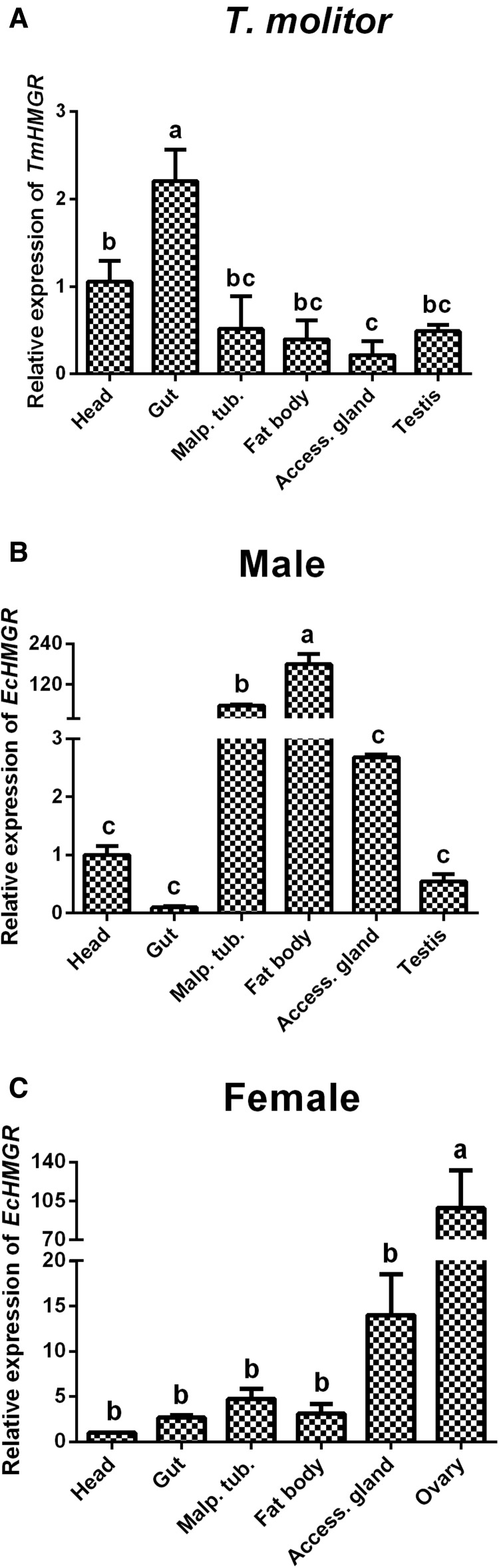
HMGR mRNA can be detected in all tissues from T. molitor, with similar expression levels except for the gut (Fig. 4A). The HMGR expression level of the gut was ∼10-fold higher than the lowest transcript level in the accessory gland. We also measured HMGR mRNA levels in male and female blister beetles 4 d after mating. In males, HMGR mRNA levels were highest in the fat body, ∼180-fold higher than that in the head (Fig. 4B). However, in female E. chinensis, the highest expression of HMGR was found in the ovary, other tissues remaining at much lower levels (Fig. 4C).
Fig. 4.
Relative expression of HMGR in different tissues of Epicauta chinensis and Tenebrio molitor. (A) Male T. molitor 10 d after emergence. (B) Male E. chinensis 4 d after mating. (C) Female E. chinensis 4 d after mating. Different letters on the error bars show significant differences (P ≤ 0.05).
Detection and Distribution of Cantharidin in E. chinensis
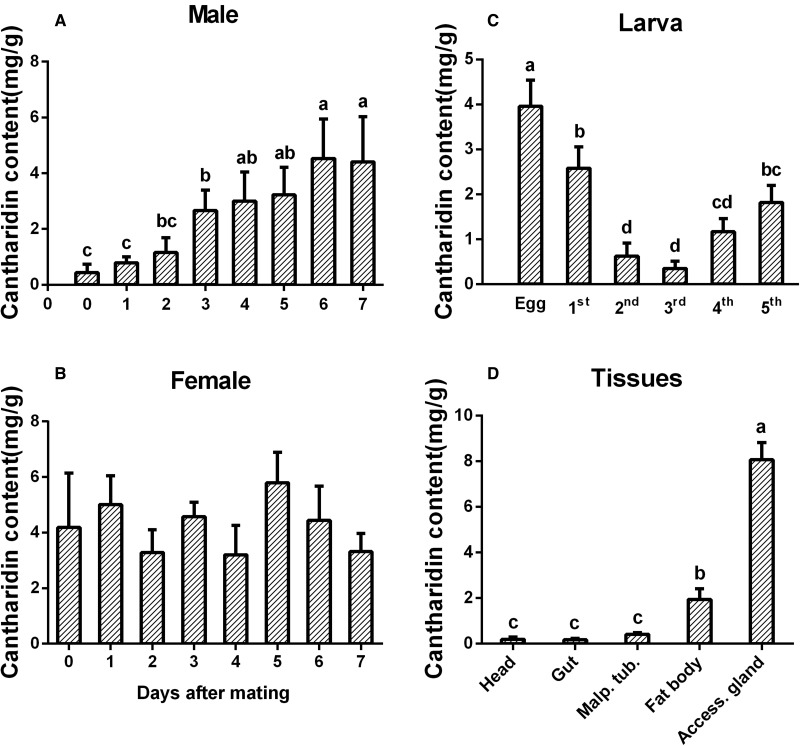
Cantharidin in all tested samples was determined by gas chromatography. During the 7 d after mating, the cantharidin content in male E. chinensis accumulated in a pattern similar to that of EcHMGR mRNA. On the seventh day after mating, the content of cantharidin reached 4.41 mg/g (dry weight) which was over 10 times higher compared to that on day 0 (Fig. 5A). In addition, Cantharidin content slowly accumulated over the first three days after mating, and then appeared to accumulate more slowly thereafter. In females, cantharidin content was maintained at ∼4 mg/g from day 0 to day 7 after mating, with no significant difference between samples (Fig. 5B).
Fig. 5.
Identification of cantharidin content in various stages and tissues of Epicauta chinensis. (A) Males 0–7 d after mating. (B) Females 0–7 d after mating. (C) Different developmental stages. (D) Tissues of male E. chinensis 4 d after mating. Cantharidin was extracted from five adults and at least 10 eggs or larvae (± SEM, N = 3). Different letters on the error bars show significant differences (P ≤ 0.05).
Furthermore, cantharidin was detectable in all the first five larval instars, with the highest relative content of 3.96 mg/g at the egg stage (Fig. 5C). Cantharidin content was lowest in the second and third instars, which was only a tenth of that in eggs, and gradually increased in the fourth and fifth instar larvae.
We also measured cantharidin amounts in various tissues from male blister beetles. The accessory gland had the highest cantharidin content, which was over 40-fold higher than that contained in head (0.19 mg/g) (Fig. 5D). Unexpectedly, the fat body showed the second highest cantharidin content (∼1.95 mg/g), making it significantly different from other tissues.
Discussion
3-Hydroxy-3-methylglutary-CoA reductase is the key enzyme to regulate the MVA pathway for biosynthesis of terpenes. The reaction catalyzed by HMGR is the rate-limiting step in synthesizing the carbon skeleton of sterols and other terpenoids (Friesen and Rodwell 2004). In beetles, HMGR is involved in production of various terpenoids, including ipsdienol in Ips species (Ivarsson et al. 1993; Bearfield et al. 2006, 2009), frontalin in Dendroctonus jeffreyi (Hall et al. 2002b), iridoids in Chrysomelina species larvae (Burse et al. 2007, 2008) and diterpene defense compounds in the termite Nasutitermes takasagoensis (Hojo et al. 2012). HMGR mRNA levels correlate with terpenoid production and helped determine the tissues where various terpenoids are made. HMGR is highly expressed in the anterior midgut, which is the site of frontalin production in male Dendroctonus jeffreyi (Hall et al. 2002b). Furthermore, Burse et al. (2007) proved that, for Phaedon cochleariae and Gastrophysa viridula which can produce iridoids, the expression of HMGR in fat body was higher than in other tissues. They also pointed out that 8-hydroxygeraniol-8-O-β-d-glucoside (the precursor of iridoid) and geraniol content in fat body paralleled HMGR expression in P. cochleariae and G. viridula. However, no significant difference was detected in HMGR expression and glucoside content in all tissues of Chrysomela populi, a sequestering species producing salicylaldehyde substitute iridoids (Burse et al. 2007).
In E. chinensis, previous research had demonstrated that HMGR is a key enzyme in regulating the biosynthesis of cantharidin (Lü et al. 2016). In the present study, HMGR mRNA levels were considered to be an indicator to localize the site of early steps for cantharidin production in E. chinensis. We therefore measured HMGR transcriptional level and cantharidin content in different developmental stages of E. chinensis and T. molitor. Furthermore, the different HMGR expression levels in tissues of male and female E. chinensis, and tissues from male E. chinensis and T. molitor were detected to explore the site of cantharidin production. We also profiled HMGR mRNA levels in T. molitor, a beetle that does not produce cantharidin, to provide an additional data set for comparison.
In the previous study, HMGR expression appeared to correlate with cantharidin production (Lü et al. 2016). In the present research, a more detailed study was conducted to affirm the correlation between HMGR expression and cantharidin content. The increased HMGR expression level corresponded to the increasing cantharidin amounts in male E. chinensis (Figs. 3A and 5A). These results indicated that HMGR transcript expression correlated to cantharidin biosynthesis in males. In contrast cantharidin content in females remained at a relatively constant level during the seven days after mating. This result is consistent with the fact that females cannot produce cantharidin, and likely represents the storage of transferred cantharidin following mating. However, the HMGR transcript was high in females just after mating, then decreased to a low level till the seventh day after mating (Fig. 3C).
A curious phenomenon was found in E. chinensis larvae. The cantharidin content was lowest in the second and third instar (Fig. 5A) in contrast to the peak value of the HMGR transcript in the third and fourth instar larvae (Fig. 3B). Mature female blister beetles usually endow their embryos with most of their cantharidin to protect eggs from potential predators (Carrel and Eisner 1974, Carrel et al. 1993). Therefore, we speculate that the high amounts of cantharidin content in eggs and triungulin larvae were from the female adult. Furthermore, the increased amounts of cantharidin content in the fourth and fifth instar larvae may be due to synthesis, which would require increased HMGR activity. This is supported by the observed HMGR transcript levels in third and fourth instars. From these results, we can further confirm that HMGR transcript has the positive correlation to cantharidin production.
In previous studies, HMGR mRNA levels have been used to locate tissues related to pheromone production (Ivarsson et al. 1998; Hall et al. 2002a,b; Burse et al. 2007). In this study, we compared the mRNA expression levels of HMGR in different tissues of males and females during the cantharidin synthesizing phase. HMGR transcript levels were significantly high in the fat body and Malpighian tubules in males, ∼60-fold higher than levels in other tissues (Fig. 4B). In females, the highest expression levels were detected in the ovary and the accessory gland, with expression levels in fat body and Malpighian tubules much lower, only approximately one-twentieth of the transcript level in the ovary. HMGR influences the development of the ovary and accessory gland in Blattella germanica (Burse et al. 2007). We speculate that the high expression observed in blister beetle ovaries may similarly be connected to ovary development. Consequently, we speculate that either the fat body or the Malpighian tubules may be the tissue for cantharidin synthesis in E. chinensis.
To eliminate the differences of physiological reactions between genders in E. chinensis, we selected another Coleoptera insect, Tenebrio molitor, which cannot produce cantharidin, as a reference. Surprisingly, the highest expression of HMGR in T. molitor was found in the gut, nearly twofold higher than the mRNA level in the head (Fig. 4A). Furthermore, HMGR mRNA levels in Malpighian tubules, accessory gland and fat body were low. It is much different from the expression profile in male E. chinensis with highest expression in fat body and Malpighian tubules. This comparison further supports that the increased transcript level of HMGR in Malpighian tubules and the fat body in male blister beetles may be involved with cantharidin production.
We speculated that the tissue with the highest levels of cantharidin may also be the site of cantharidin biosynthesis. We found that the accessory gland had the highest cantharidin content, and the fat body had the second highest level (Fig. 5D). A previous study (McCormick and Carrel 1987) reported that the accessory gland is the storage gland for cantharidin, not the site of the cantharidin biosynthesis. Cantharidin is transported to the accessory gland via the hemolymph. In our study the fat body tissues were taken from male blister beetles during a time of high cantharidin biosynthesis and we found a high cantharidin content and abundant HMGR transcript levels in the fat body indicating that the synthesis of cantharidin occurs in the fat body. We speculate that cantharidin is then transported to the accessory gland. More studies are needed to further explore the function of fat body on the biosynthesis of cantharidin in E. chinensis and the function of high EcHMGR transcript levels in Malpighian tubules.
In summary, we identified and characterized the HMGR gene from T. molitor. Furthermore, the temporal and spatial expression patterns of EcHMGR and TmHMGR were detected. We also determined the cantharidin content in different stages and tissues of male E. chinensis. The EcHMGR transcript showed high positive correlation with cantharidin production in male blister beetles. The cantharidin detection revealed that abundant cantharidin amounts were found in fat body compared to other tissues except for the accessory gland in blister beetles. Furthermore, HMGR transcript comparisons between tissues from male T. molitor, male and female E. chinensis showed that EcHMGR expression level is significantly high in fat body from male E. chinensis. Combined with significantly high cantharidin amounts in fat body, we speculated that the fat body in E. chinensis maybe involve in the process of cantharidin biosynthesis.
Acknowledgments
We thank Chen Yang for collecting and raising beetles. We sincerely appreciate J.R. Schrock (Emporia State University, Emporia, KS) for revising the manuscript. This research was supported by the Special Fund for the Public Interest (Agriculture) (200903052), the “13115” Sci-Tech Innovation Project of Shaanxi Province (2009ZDKG-06), the National Natural Science Foundation of China (31101442) and the Fundamental Research Funds for the Central Universities (2452015113 and 2452013QN044).
References Cited
- Agranoff B., Eggerer H., Henning U., Lynen F.. 1960. Biosynthesis of terpenes VII. Isopentenyl pyrophosphate isomerase. J. Biol. Chem. 235: 326–332. [PubMed] [Google Scholar]
- Bearfield J. C., Henry A. G., Tittiger C., Blomquist G. J., Ginzel M. D.. 2009. Two regulatory mechanisms of monoterpenoid pheromone production in Ips spp. of bark beetles. J. Chem. Ecol. 35: 689–697. [DOI] [PubMed] [Google Scholar]
- Bearfield J., Keeling C., Young S., Blomquist G., Tittiger C.. 2006. Isolation, endocrine regulation and mRNA distribution of the 3‐hydroxy‐3‐methylglutaryl coenzyme A synthase (HMG‐S) gene from the pine engraver, Ips pini (Coleoptera: Scolytidae). Insect Mol Biol. 15: 187–195. [DOI] [PubMed] [Google Scholar]
- Bellés X., Martín D., Piulachs M.-D.. 2005. The mevalonate pathway and the synthesis of juvenile hormone in insects. Annu. Rev. Entomol. 50: 181–199. [DOI] [PubMed] [Google Scholar]
- Burse A., Schmidt A., Frick S., Kuhn J., Gershenzon J., Boland W.. 2007. Iridoid biosynthesis in Chrysomelina larvae: fat body produces early terpenoid precursors. Insect Biochem. Mol. Biol. 37: 255–265. [DOI] [PubMed] [Google Scholar]
- Burse A., Frick S., Schmidt A., Buechler R., Kunert M., Gershenzon J., Brandt W., Boland W.. 2008. Implication of HMGR in homeostasis of sequestered and de novo produced precursors of the iridoid biosynthesis in leaf beetle larvae. Insect Biochem. Mol. Biol. 38: 76–88. [DOI] [PubMed] [Google Scholar]
- Bustin S. A., Benes V., Garson J. A., Hellemans J., Huggett J., Kubista M., Mueller R., Nolan T., Pfaffl M. W., Shipley G. L.. 2009. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 55: 611–622. [DOI] [PubMed] [Google Scholar]
- Carrel J. E., Eisner T.. 1974. Cantharidin: potent feeding deterrent to insects. Science 183: 755–757. [DOI] [PubMed] [Google Scholar]
- Carrel J. E., Doom J. P., McCormick J. P.. 1986a. Identification of cantharidin in false blister beetles (Coleoptera, Oedemeridae) from Florida. J. Chem. Ecol. 12: 741–747. [DOI] [PubMed] [Google Scholar]
- Carrel J., Doom J., McCormick J.. 1986b. Cantharidin biosynthesis in a blister beetle: inhibition by 6-fluoromevalonate causes chemical disarmament. Experientia 42: 853–854. [DOI] [PubMed] [Google Scholar]
- Carrel J., McCairel M., Slagle A., Doom J., Brill J., McCormick J.. 1993. Cantharidin production in a blister beetle. Experientia 49: 171–174. [DOI] [PubMed] [Google Scholar]
- Crozier R. 1968. An acetic acid dissociation, air-drying technique for insect chromosomes, with aceto-lactic orcein staining. Stain Technol. 43: 171–173. [DOI] [PubMed] [Google Scholar]
- Dettner K. 1987. Chemosystematics and evolution of beetle chemical defenses. Annu. Rev. Entomol. 32: 17–48. [Google Scholar]
- Dettner K. 1997. Inter-and intraspecific transfer of toxic insect compound cantharidin, pp. 115–145. InVertical food web interactions. Springer, Berlin Heidelberg. [Google Scholar]
- Eisner T., Smedley S. R., Young D. K., Eisner M., Roach B., Meinwald J.. 1996. Chemical basis of courtship in a beetle (Neopyrochroa flabellata): Cantharidin as “nuptial gift”. Proc. Natl. Acad. Sci. 93: 6499–6503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenzel M., Dettner K.. 1994. Quantification of cantharidin in canthariphilous Ceratopogonidae (Diptera), Anthomyiidae (Diptera) and cantharidin-producing Oedemeridae (Coleoptera). J. Chem. Ecol. 20: 1795–1812. [DOI] [PubMed] [Google Scholar]
- Friesen J. A., Rodwell V. W.. 2004. The 3-hydroxy-3-methylglutaryl coenzyme-A (HMG-CoA) reductases. Genome Biol. 5: 248.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garófalo C. A., Camillo E., Serrano J. C.. 2011. Reproductive aspects of Meloetyphlus fuscatus a meloid beetle cleptoparasite of the bee Eulaema nigrita (Hymenoptera, Apidae, Euglossini). Apidologie 42: 337–348. [Google Scholar]
- Ghaffarifar F. 2010. Leishmania major: in vitro and in vivo anti-leishmanial effect of cantharidin. Exp. Parasitol. 126: 126–129. [DOI] [PubMed] [Google Scholar]
- Ghoneim K. 2013. Behavioral characterization of blister beetles (Coleoptera: Meloidae) in the world: a bibliographic review. Int. J. Soc. Behav. Sci. 1: 33–48. [Google Scholar]
- Guenther H., Ramstad E., Floss H.. 1969. On the biosynthesis of cantharidin. J. Pharm. Sci. 58: 1274–1274.. [DOI] [PubMed] [Google Scholar]
- Hall G. M., Tittiger C., Andrews G. L., Mastick G. S., Kuenzli M., Luo X., Seybold S. J., Blomquist G. J.. 2002a. Midgut tissue of male pine engraver, Ips pini, synthesizes monoterpenoid pheromone component ipsdienol de novo. Die Naturwiss. 89: 79–83. [DOI] [PubMed] [Google Scholar]
- Hall G., Tittiger C., Blomquist G., Andrews G., Mastick G., Barkawi L., Bengoa C., Seybold S.. 2002b. Male Jeffrey pine beetle, Dendroctonus jeffreyi, synthesizes the pheromone component frontalin in anterior midgut tissue. Insect Biochem. Mol. Biol. 32: 1525–1532. [DOI] [PubMed] [Google Scholar]
- Hojo M., Maekawa K., Saitoh S., Shigenobu S., Miura T., Hayashi Y., Tokuda G., Maekawa H.. 2012. Exploration and characterization of genes involved in the synthesis of diterpene defence secretion in nasute termite soldiers. Insect Mol. Biol. 21: 545–557. [DOI] [PubMed] [Google Scholar]
- Ivarsson P., Schlyter F., Birgersson G.. 1993. Demonstration of de novo pheromone biosynthesis in Ips duplicatus (Coleoptera: Scolytidae): inhibition of ipsdienol and E-myrcenol production by compactin. Insect Biochem. Mol. Biol. 23: 655–662. [Google Scholar]
- Ivarsson P., Tittiger C., Blomquist C., Borgeson C. E., Seybold S. J., Blomquist G. J., Högberg H.-E.. 1998. Pheromone precursor synthesis is localized in the metathorax of Ips paraconfusus Lanier (Coleoptera: Scolytidae). Die Naturwiss. 85: 507–511. [Google Scholar]
- Kerkut G., Pitman R., Walker R.. 1969. Iontophoretic application of acetylcholine and GABA onto insect central neurones. Comp. Biochem. Physiol. 31: 611–633. [DOI] [PubMed] [Google Scholar]
- Khan R. A., Liu J. Y., Rashid M., Wang D., Zhang Y. L.. 2013. Cantharidin impedes activity of glutathione S-transferase in the midgut of Helicoverpa armigera Hübner. Int. J. Mol. Sci. 14: 5482–5500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K. J., Schmittgen T. D.. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25: 402–408. [DOI] [PubMed] [Google Scholar]
- Lü S., Jiang M., Huo T., Li X., Zhang Y.. 2016. 3-hydroxy-3-methyl glutaryl coenzyme A reductase: an essential actor in the biosynthesis of cantharidin in the blister beetle Epicauta chinensis Laporte. Insect Mol. Biol. 25: 58–71. [DOI] [PubMed] [Google Scholar]
- McCormick J., Carrel J.. 1987. Cantharidin biosynthesis and function in meloid beetles Pheromone Biochemistry, Academic Press, Orlando, FL, pp. 307–350. [Google Scholar]
- McCormick J. P., Carrel J. E., Doom J. P.. 1986. Origin of oxygen atoms in cantharidin biosynthesized by beetles. J. Am. Chem. Soc. 108: 8071–8074. [Google Scholar]
- Nikbakhtzadeh M. R., Hemp C., Ebrahimi B.. 2007a. Further evidence for the role of Cantharidin in the mating behaviour of blister beetles (Coleoptera: Meloidae). Integr. Biosci. 11: 141–146. [Google Scholar]
- Nikbakhtzadeh M. R., Dettner K., Boland W., Gade G., Dotterl S.. 2007b. Intraspecific transfer of cantharidin within selected members of the family Meloidae (Insecta: Coleoptera). J. Insect Physiol. 53: 890–899. [DOI] [PubMed] [Google Scholar]
- Nikbakhtzadeh M. R., Dettner K., Boland W., Hemp C.. 2008. The possible role of antennal cuticular pores in the sexual behaviour of Cyaneolytta sp.(Coleoptera: Meloidae). Mitt. Dtsch. Ges. Allg. Angew. Ent. 16: 179–183. [Google Scholar]
- Nikbakhtzadeh M., Vahedi M., Vatandoost H., Mehdinia A.. 2012. Origin, transfer and distribution of cantharidin-related compounds in the blister beetle Hycleus scabiosae. J. Venom. Anim. Toxins Including Trop. Dis. 18: 88–96. [Google Scholar]
- Peter M., Waespe H., Woggon W., Schmid H.. 1977a. Incorporation experiments with (3H and 14C) doubly labelled farnesols into cantharidin. Helvetica Chim. Acta. 60: 1262.. [DOI] [PubMed] [Google Scholar]
- Peter M., Woggon W., Schmid H.. 1977b. Identification of farnesol as an intermediate in the biosynthesis of cantharidin from mevalonolactone. Helvetica Chim. Acta. 60: 2756–2762. [DOI] [PubMed] [Google Scholar]
- Ruzicka L. 1953. The isoprene rule and the biogenesis of terpenic compounds. Experientia 9: 357–367. [DOI] [PubMed] [Google Scholar]
- Schlatter C., Waldner E., Schmid H.. 1968. Zur Biosynthese des Cantharidins. I. Experientia 24: 994–995. [DOI] [PubMed] [Google Scholar]
- Seybold S. J., Tittiger C.. 2003. Biochemistry and molecular biology of de novo isoprenoid pheromone production in the Scolytidae. Annu. Rev. Entomol. 48: 425–453. [DOI] [PubMed] [Google Scholar]
- Sierra J., Woggon W.-D., Schmid H.. 1976. Transfer of cantharidin (1) during copulation from the adult male to the female Lytta vesicatoria (‘Spanish flies’). Experientia 32: 142–144. [Google Scholar]
- Tillman J. A., Lu F., Goddard L. M., Donaldson Z. R., Dwinell S. C., Tittiger C., Hall G. M., Storer A. J., Blomquist G. J., Seybold S. J.. 2004. Juvenile hormone regulates de novo isoprenoid aggregation pheromone biosynthesis in pine bark beetles, Ips spp., through transcriptional control of HMG-CoA reductase. J. Chem. Ecol. 30: 2459–2494. [DOI] [PubMed] [Google Scholar]
- Woggon W.-D., Hauffe S. A., Schmid H.. 1983. Biosynthesis of cantharidin: evidence for the specific incorporation of C-4 and C-11′ of farnesol. J. Chem. Soc. Chem. Commun. 6: 272–274. [Google Scholar]