Abstract
Introduction
The strong link between early-life education and subsequent reduced risk of dementia suggests that education in later life could enhance cognitive function and may reduce age-related cognitive decline and protect against dementia.
Methods
Episodic memory, working memory, executive function, and language processing performances were assessed annually over 4 years in 359 healthy older adults who attended university for a minimum of 12 months (intervention) and were compared against 100 healthy adult controls.
Results
Multiple group latent growth curve modeling revealed a significant improvement in language processing capacity over time in the intervention group. No changes were detected for episodic memory, working memory, or executive function.
Discussion
These results suggest that complex mental stimulation resulting from late-life further education results in improved crystallized knowledge but no changes to fluid cognitive functions.
Keywords: Cognitive reserve, Education, Aging, Neuropsychological, Crystallized function, Fluid function, Episodic memory, Working memory, Language processing, Executive function
Highlights
-
•
Early-life educational attainment predicts dementia.
-
•
Early-life educational attainment predicts rate of cognitive decline.
-
•
Late-life education in healthy adults improves language processing capacity.
-
•
Late-life education enhances crystallized knowledge not fluid cognitive abilities.
1. Introduction
Interventions designed to enhance and protect cognitive function are a promising non-pharmacological approach to delaying and preventing Alzheimer's disease (AD). The positive benefits of such interventions presumably occur due to an increase in cognitive reserve (CR; [1], [2]). Education, occupational attainment, and leisure activities have been shown to make both independent and overlapping contributions to CR [3]. Consequently, recent research has sought to provide a multidimensional measure of CR [4], [5], [6] to assess the relationship between CR and cognitive functioning. Bonner-Jackson et al. [6] found that higher levels of reserve are associated with a reduced rate of decline in executive function over time in prodromal Huntington's disease. Furthermore, individuals with high CR are able to sustain a higher degree of brain damage before the same level of clinical symptoms that are expressed as in individuals low in CR [5]. However, in healthy older adults or in advanced stages of AD neuropathology, it appears that CR does not influence cognitive performance [5]. Rather, CR may act as a buffer between cognitive function and brain pathology only in the early stages of AD [5].
Several studies report that CR can be enhanced or modified through environmental and lifestyle factors. Education is receiving increased research attention as a potentially modifiable lifestyle factor for reducing age-related cognitive decline (ARCD), albeit the focus has been on early-life educational attainment. Enhancement of CR through education is thought to be a result of the development of new cognitive strategies in the individual [7]. Higher levels of educational attainment at younger ages is associated with reduced risk of dementia [8], and the level of educational attainment moderates the relationship between brain pathology and neuropsychological test performance in memory, language, speed of processing, and visuospatial skills [9], [10], [11]. Higher levels of educational attainment are associated with reduced rates of decline in information processing speed [12], memory [12], [13], and general mental status [12], [14]. However, previous research has also questioned this relationship, reporting that the rate of decline across memory [15], [16], [17], processing speed [18], [19], language processing [15], [20], [21], and visuospatial skills [13], [20] is constant regardless of level of educational attainment. Despite this, reviews of the literature indicate that higher levels of education in early adulthood are associated with superior performance on measures of cognitive function [22], [23].
While there is ongoing debate and research into the relationship between educational attainment in early life and cognitive performance in later life, studies have not yet examined the potential benefit of further formal education in late adulthood in enhancing or maintaining cognitive function, potentially also contributing to resilience to decline in AD. The Tasmanian Healthy Brain Project (THBP) is designed to assess the impact of university-level education on CR and cognitive function in healthy older adults [24]. We have recently demonstrated that further education leads to a measurable increase in current CR among older adults who undertake further education [25]. The aim of the present article was to examine if the observed increase in CR among older adults undertaking further education is associated with a change in cognitive function over time.
2. Method
2.1. Participants
The THBP (Summers et al., 2013) is a prospective longitudinal study of older adults engaging in university-level education. The THBP sample was recruited progressively from 2011 to 2014 and has undertaken annual comprehensive assessments. Data analyzed in the present article were collected from 459 adults aged between 50 and 79 years who had participated in the THBP as of the 31 December, 2014. Inclusion criteria for entry into the THBP were that participants were aged 50–79 years at the time of entry and were healthy. Participants were excluded from entry into the THBP if they reported a diagnosis of a condition that is independently associated with impairments to cognitive function (dementia; multiple sclerosis; prior head injury requiring hospitalization; epilepsy; cerebrovascular complications including stroke, aneurysm, transient ischemic attacks; poorly controlled diabetes; poorly controlled hypertension or hypotension; other neurological disorders [e.g., cerebral palsy or spina bifida]; chronic obstructive pulmonary disease; heart disease; partial or total blindness; deafness; current psychiatric diagnosis) and those who presented with a medical, neurological, or psychiatric disorder that could potentially impair cognition were precluded from entry into the THBP. The project was approved by the Human Research Ethics Committee (Tasmania) Network, and further details of the study protocol have been previously published (see Summers et al. [24]).
On entry into the THBP, participants opted (non-random assignment) to participate in either a further education group (intervention) or a no further education group (control). All participants undertook baseline assessment before commencing in the THBP. Those in the intervention group (n = 359) then completed a minimum of 12 months of part-time or full-time university study, with a minimum study load of two units at undergraduate or postgraduate levels. The remaining 100 participants in the control group did not undertake any further formal education and served as a no-intervention reference group. Previous growth mixture modeling analysis of longitudinal change in CR revealed two latent classes within each of the control and the intervention groups. The latent classes identified were improved CR (55.7% of control group, 92.5% of intervention group) and stable CR (43.3% of control group, 7.5% of intervention group) [25]. Owing to insufficient sample size (n < 100) in the intervention stable CR subgroup (7.5% of intervention, n = 15), it was not possible to analyze potential differences between improved and stable CR intervention groups in cognitive function [26]. To minimize statistical bias, the 15 stable CR cases from the intervention group were excluded from the present analysis. No significant differences in cognitive performances were identified between the stable CR and improved CR subgroups of the control sample. As these control subgroups performed at equivalent levels of cognitive function, they were collapsed into a single control group for the purposes of these analyses (see Supplementary Material 1). Examination of the equivalent full-time study load (EFTSL) completed by each participants in the intervention group over the first four phases of the THBP indicates that they completed on average 110.48 EFTSL (standard deviation = 83.89, 95th confidence interval [CI] = 101.59–119.38). One unit of full-time study is 12.5% EFTSL, indicating that participants in the intervention group completed on average 8.84 full-time equivalent units of study, where 100% EFTSL equates to full-time study for 12 months.
2.2. Materials
Participants in the THBP completed a comprehensive testing battery. For detailed project protocol, refer to Summers et al. [24]. The Dementia Rating Scale, 2nd edition (DRS-2; [27]); the Hospital Anxiety and Depression Scale (HADS; [28]), Lubben Social Network Scale-18 (LSNS; [29]); and the Medical Health Status Questionnaire [24] were administered to ensure that participants were free from dementia and of sound psychological and physical health. A composite proxy measure of prior CR (derived from estimated full-scale intelligence quotient [IQ], prior education, occupational, and lifestyle experiences) was calculated for each participant to examine the influence of early-life experiences on current cognitive function (see Ward et al. [4]; Supplementary Material 1).
2.2.1. Neuropsychological performance
The neuropsychological test battery comprised 14 tests encompassing four broad cognitive domains: episodic memory (Logical Memory [LMI, LMII; [30]] test, Rey Auditory Verbal Learning Test [RAVLT; [31]], and Paired Associates Learning [PAL; [32]]), working memory (Digit Span [33], Letter-Number Sequencing [33], Spatial Span [SSP; [32]], and Spatial Working Memory [SWM; [32]] tests), executive function (Trail Making Test Trail B [TMT B; [34]], 24-item Victoria version Stroop Color-Word Test [Stroop C; 34], and Rapid Visual Processing [RVP A′; [32]]), and language processing (vocabulary [33], comprehension [33], and Boston Naming Test [35]). Composite scores were created for each cognitive domain by principal components analysis (PCA) consistent with an approach used in previous work by this group ([36]; see also Supplementary Material 1). To create the domain composite scores, the z-scores from relevant tests were multiplied by the factor coefficients produced from the PCAs. To this effect, cognitive domain composite scores represent decline or improvement over time relative to the sample mean at baseline.
2.3. Procedure
After obtaining consent, the elements of the full THBP test battery used in the present analysis were administered to each participant in the following order: WTAR, DRS-2, Medical Health Questionnaire, PAL, RAVLT, LMI, SSP, Digit Span, SWM, Letter-Number Sequencing, LMII, vocabulary, comprehension, Boston Naming Test, RVP A’, STROOP C, TMT B, and HADS. An approximate 20-minute delay occurred between the administration of LMI and LMII. Lifetime Experience Questionnaire (LEQ; [37]), WTAR, and DNA data were only collected once, at baseline. The full THBP took approximately 4 hours to complete, and subjects were encouraged to take short breaks as needed to avoid fatigue [24]. Participants were reassessed at 1-year intervals (±1 month) for a total of 4 years (baseline-T0, T1, T2, and T3).
2.4. Analysis
Prior CR was calculated for each participant using factor analysis defined regression coefficients [4]. Four separate PCAs were then conducted to compute composite scores for each cognitive domain at baseline (see Supplementary Material 1 for full description) consistent with the approach used in previous studies of the THBP [36].
2.4.1. Multiple group latent growth curve modeling
Multiple group latent growth curve modeling (LGCM) was conducted using Mplus 7.4 [38] maximum likelihood estimation (see Supplementary Material 1 for full description). Prior CR and participant age (years) were included as covariates in all models. In all models, time was parameterized with time scores that represented years because study entry and the intercept loadings of the four time points were fixed at one. In each model, the intercept term represented the mean of each respective cognitive domain score, the linear growth term represented the annual rate of change in score, and the quadratic growth term indicated the change in the rate of change (accelerating or decelerating change).
2.4.2. Model fit
Model fit was assessed using multiple statistics. Likelihood-ratio chi-square is a popular statistic to assess overall fit; however, it is sensitive to sample size and prone to type II error in the case of large sample sizes [39]. Other measures we examined for model fit included root mean squared error of approximation (RMSEA) with <0.07 indicating good fit and <0.03 indicating excellent fit [40]; and, comparative fit index (CFI) with values of ≥ 0.95 indicative of good fit [41].
3. Results
3.1. Descriptive data
The sample consisted of 444 older adults, aged between 50 and 79 years at baseline (Table 1). Analysis of demographic variables revealed that the intervention group was significantly younger than the control group at baseline (t(442) = 3.84, P < .001). No group differences were detected in global cognition, level of anxiety, or level of depression. Examination of the relationship between age and neuropsychological performance at each of the four time points revealed no meaningful correlations (correlations of a moderate, r ≥ 0.5, or greater magnitude [42] considered meaningful given the large sample size). Despite this, age was retained as a covariate in the growth models to control for possible age dependent interactions with change in cognitive performance over time. Baseline test performances for each group are presented in Table 1. Owing to the well-documented relationship between education [22] and other aspects of life experience and cognitive function [1], prior CR was also included in all models as a covariate.
Table 1.
Sample demographic information as a function of group
| At baseline | Control N at T0 = 100 |
Intervention N at T0 = 344 | Independent samples t-test |
Effect size |
|---|---|---|---|---|
| Mean (SD) | P | d | ||
| Female N (%) | 63 (63%) | 238 (69.2%) | (χ2) = .24 | n/a |
| Baseline age | 62.49 (6.24) | 59.59 (6.77) | <.001∗∗ | 1.14 |
| Prior CR | −0.36 (2.28) | 0.14 (2.26) | .054 | 0.33 |
| WTAR Est FSIQ | 112.49 (5.05) | 112.56 (5.49) | .908 | 0.03 |
| Prior education (years) | 13.53 (2.65) | 14.28 (2.69) | .015∗ | 0.46 |
| LEQ young adult specific | 15.22 (7.08) | 16.16 (7.82) | .282 | 0.34 |
| LEQ young adult nonspecific | 24.62 (5.28) | 24.98 (5.47) | .560 | 0.16 |
| LEQ midlife specific | 19.22 (4.76) | 18.83 (5.01) | .486 | 0.18 |
| LEQ midlife nonspecific | 24.45 (4.37) | 24.53 (4.37) | .898 | 0.04 |
| LEQ midlife continuing education | 7.49 (7.47) | 10.75 (8.34) | <.001∗∗ | 1.16 |
| DRS-2 AEMSS | 11.91 (2.27) | 11.93 (2.10) | .943 | 0.01 |
| HADS–anxiety | 5.51 (2.91) | 5.24 (3.14) | .444 | 0.16 |
| HADS–depression | 2.82 (2.32) | 2.42 (2.27) | .125 | 0.26 |
| LMI immediate recall total | 47.34 (7.63) | 48.45 (8.42) | .237 | 0.39 |
| LMII delayed recall total | 29.79 (6.40) | 30.15 (6.50) | .621 | 0.14 |
| RAVLT 1–5 recall total | 51.97 (8.55) | 53.60 (8.92) | .106 | 0.55 |
| PAL first trial memory score | 17.73 (3.78) | 18.60 (3.15) | .022∗ | 0.47 |
| Letter-Number Sequencing | 11.45 (2.56) | 11.68 (2.33) | .415 | 0.15 |
| Digit Span | 11.96 (2.90) | 11.83 (2.82) | .677 | 0.08 |
| SSP Length | 5.51 (1.12) | 5.83 (1.21) | .018∗ | 0.30 |
| SWM between errors | 26.86 (19.27) | 25.53 (18.49) | .530 | 0.31 |
| RVP A′ | 0.9052 (0.057) | 0.9145 (0.046) | .093 | 0.04 |
| Stroop C time | 26.89 (8.17) | 25.91 (7.58) | .260 | 0.04 |
| Vocabulary | 56.06 (6.53) | 57.25 (5.40) | .066 | 0.05 |
| Comprehension | 25.84 (3.82) | 26.43 (3.06) | .112 | 0.32 |
| Boston Naming Test | 57.62 (2.21) | 57.69 (3.15) | .845 | 0.04 |
Abbreviations: CR, cognitive reserve; DRS-2 AEMSS, Mattis Dementia Rating Scale age- and education-corrected Mayo scaled score; HADS, Hospital Anxiety and Depression Scale; LEQ, Lifetime Experience Questionnaire; LM, Logical Memory; PAL, Paired Associates Learning; RAVLT, Rey Auditory Verbal Learning Test; RVP, Rapid Visual Processing; SD, standard deviation; SSP, Spatial Span; SWM, Spatial Working Memory; WTAR (Est FSIQ), Wechsler Test of Adult Reading Scale estimated full-scale IQ.
NOTE. ∗P <.05; ∗∗P <.01.
3.2. Episodic memory
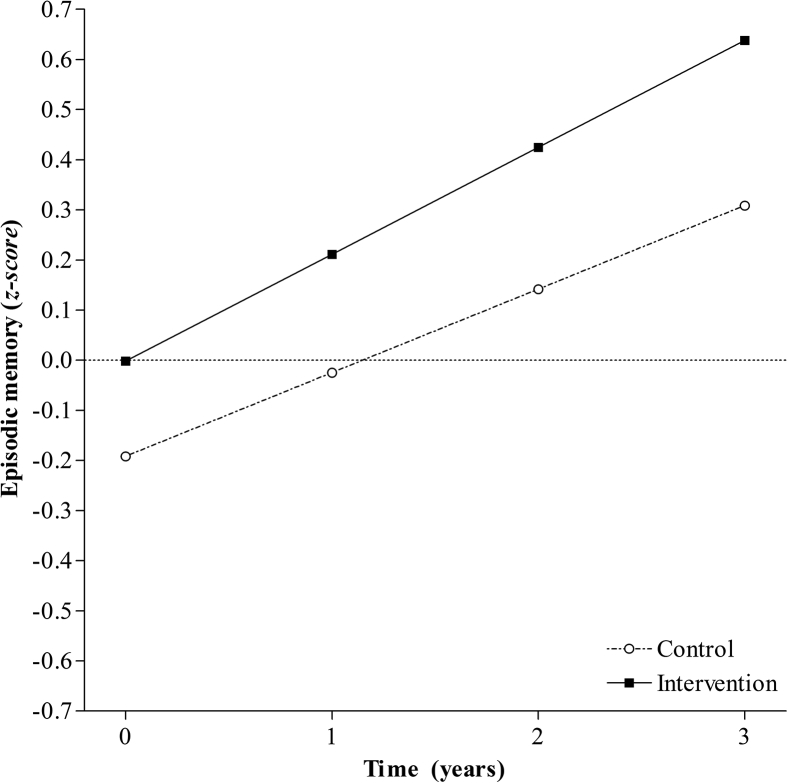
Linear and quadratic models were a good fit of the data for both groups (Table 2). In both groups, the linear models were initially inadmissible because of negative variances on the linear growth factor. As the negative variance was small and nonsignificant, variance was fixed at zero. The linear model was then simultaneously fitted to both groups, with the linear growth factor variance fixed at zero. The model was a good fit of the data (χ2(22, N= 444) = 28.64, P = .16, RMSEA = 0.037, CFI = 0.992). A significant negative mean intercept was detected in the control group but not in the intervention group. In addition, the linear term was positive and significant in both groups. This suggests that after accounting for prior CR and age, episodic memory scores improved over time and were significantly lower at baseline in the control group compared with the intervention group (Fig. 1 and Supplementary Table 3).
Table 2.
Fit indices of separate group analysis latent growth curve modeling with prior cognitive reserve and age entered as covariates
| Cognitive domain | Group | N | Chi-square test |
RMSEA | SRMR | CFI | Δχ2 difference |
||
|---|---|---|---|---|---|---|---|---|---|
| χ2 | df | P | P | ||||||
| Episodic memory | |||||||||
| Control | Linear | 100 | 9.279 | 11 | .60 | <0.001 | 0.035 | 1.00 | NS |
| Quadratic | 100 | 7.719 | 8 | .46 | <0.001 | 0.032 | 1.00 | ||
| Intervention | Linear | 344 | 16.602 | 9 | .06 | 0.050 | 0.024 | 0.988 | <.05 |
| Quadratic | 344 | 5.381 | 6 | .50 | <0.001 | 0.014 | 1.00 | ||
| Working memory | |||||||||
| Control | Linear | 100 | 10.063 | 11 | .53 | <0.001 | 0.048 | 1.00 | NS |
| Quadratic | 100 | 8.507 | 8 | .39 | 0.025 | 0.047 | 0.998 | ||
| Intervention | Linear | 343 | 11.163 | 11 | .43 | 0.007 | 0.023 | 1.00 | NS |
| Quadratic | 343 | 10.743 | 8 | .22 | 0.032 | 0.021 | 1.00 | ||
| Executive function | |||||||||
| Control | Linear | 100 | 6.320 | 9 | .71 | <0.001 | 0.046 | 1.00 | NS |
| Quadratic | 100 | 5.088 | 6 | .53 | <0.001 | 0.043 | 1.00 | ||
| Intervention | Linear | 343 | 5.898 | 11 | .88 | <0.001 | 0.033 | 1.00 | NS |
| Quadratic | 343 | 3.966 | 8 | .86 | <0.001 | 0.030 | 1.00 | ||
| Language Processing | |||||||||
| Control | Linear | 100 | 18.596 | 11 | .07 | 0.083 | 0.091 | 0.963 | NS |
| Quadratic | 100 | 17.299 | 8 | .03 | 0.108 | 0.090 | 0.955 | ||
| Intervention | Linear | 344 | 12.094 | 9 | .21 | 0.032 | 0.049 | 0.993 | NS |
| Quadratic | 344 | 7.591 | 6 | .27 | 0.028 | 0.035 | 0.996 | ||
Abbreviations: CFI, comparative fit index; RMSEA, root mean squared error of approximation; SRMR, standardized root mean square residual; df, degrees of freedom; NS, non-significant difference.
Fig. 1.
Model-predicted episodic memory trajectories over 4 years for individuals in the control group and the intervention group.
3.3. Working memory
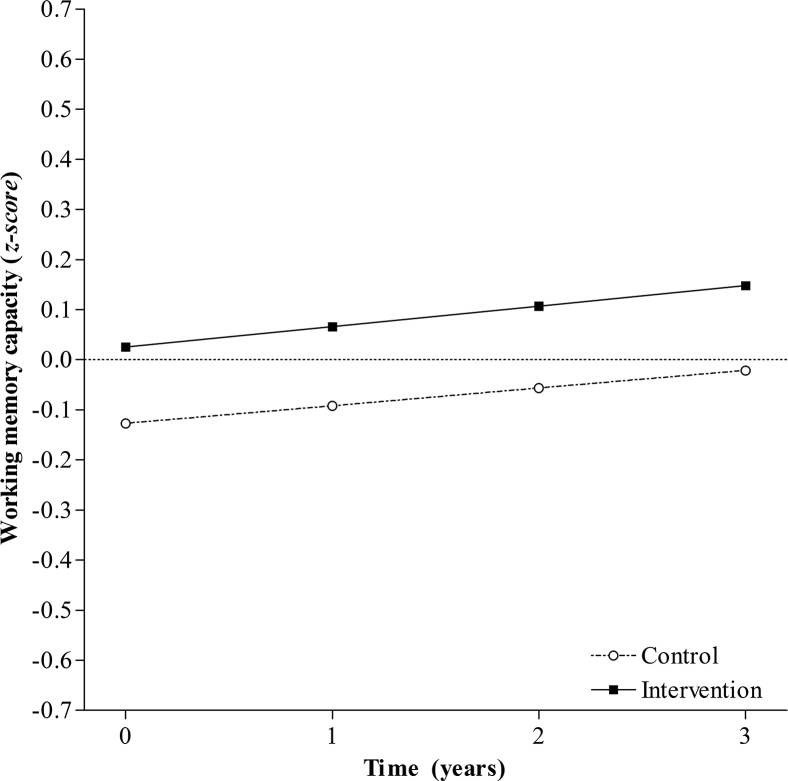
For both the control and intervention groups, the linear and quadratic models provided adequate fit of the working memory data; however, the quadratic model did not significantly improve data fit (Table 2). Negative variances in the linear growth term required variance to be fixed at zero. The estimated simultaneous model fit the data well (χ2(22, N= 443) = 21.23, P = .51, RMSEA = <.001, CFI = 1.00), with no significant difference of the intercept from zero in either group (Fig. 2 and Supplementary Table 3). For both groups, the linear term was positive but attained significance in the intervention group. This suggests that after accounting for age and prior CR, working memory scores improved over time in the intervention group but remained stable in the control group (Fig. 2 and Supplementary Table 3).
Fig. 2.
Model-predicted working memory trajectories over 4 years for individuals in the control group and the intervention group.
3.4. Executive function
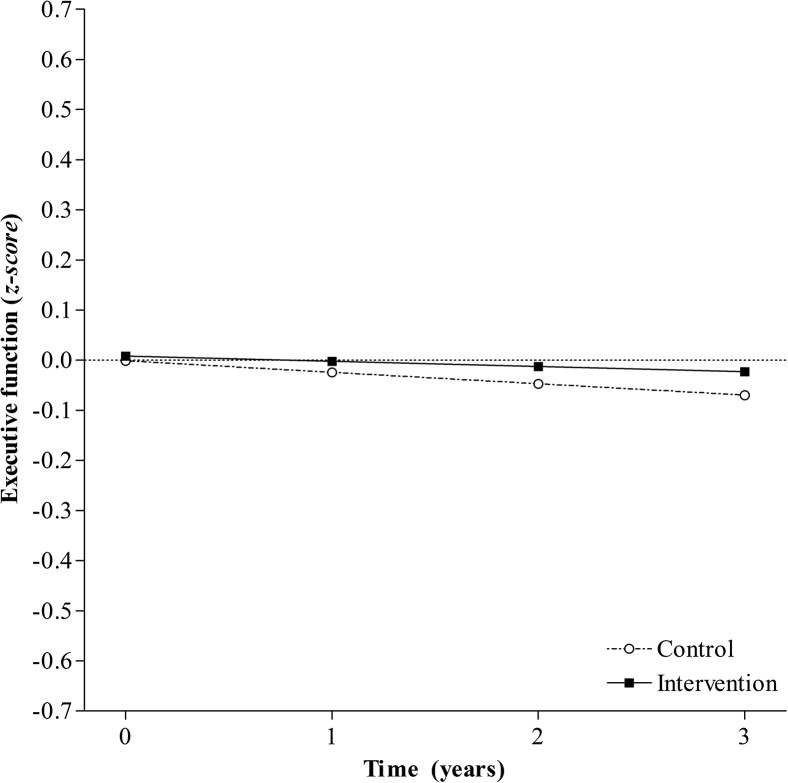
Both linear and the quadratic models were a good fit of the data for the control group; however, the quadratic model did not significantly improve data fit (Table 2). For the purpose of the multiple group analysis, the linear model was used for both groups to avoid potential over-fitting a quadratic model to the control group. The linear model was a good fit applied simultaneously to both groups (χ2(22, N= 444) = 13.011, P = .93, RMSEA = < 0.001, CFI = 1.00), with the intercept of both groups not significantly different from zero. The linear growth term was negative in both groups indicating a nonsignificant downward trend (Fig. 3 and Supplementary Table 3). After adjusting for the effect of age and prior CR, the mean baseline score for both groups was not significantly different to zero (Fig. 3 and Supplementary Table 3). In addition, both groups continued to display a nonsignificant negative linear term, indicating stability of executive function score over the 4 years (Fig. 3 and Supplementary Table 3).
Fig. 3.
Model-predicted executive function trajectories over 4 years for individuals in the control group and the intervention group.
3.5. Language processing
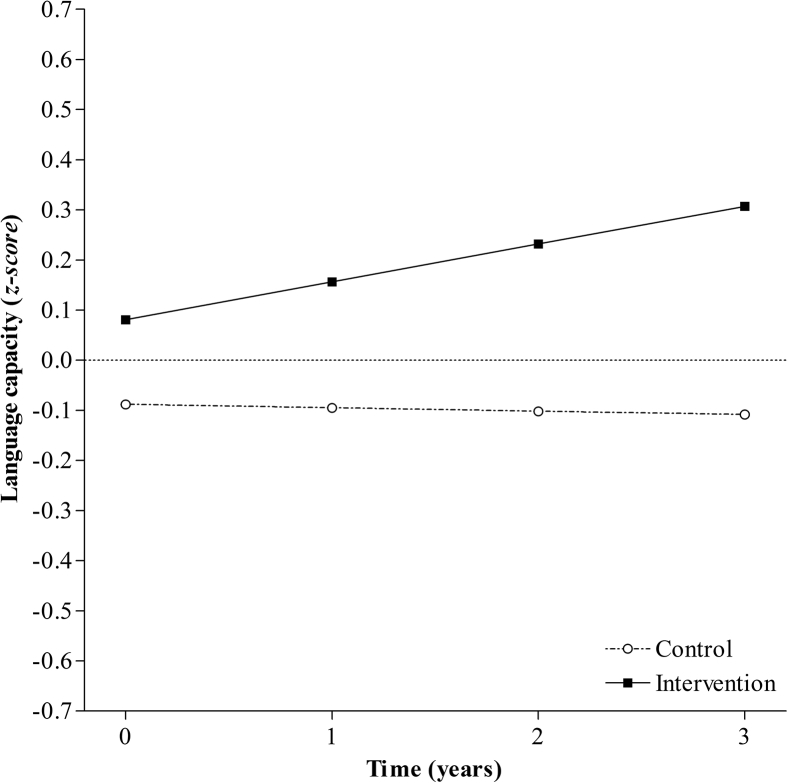
A linear model provided adequate fit of the data for both groups and was not improved by a quadratic model (Table 2); however, variance of the linear growth factor was fixed at zero to avoid an inadmissible model. The linear model with linear growth factor variance fixed at zero was fitted simultaneously to both groups resulting in a good fit of the data (χ2(22, N= 444) = 37.215, P = .02, RMSEA = 0.056, CFI = 0.977). While the control group had a negative intercept and the intervention group had a positive intercept, language processing score at baseline was not significantly different from zero in either group (see Fig. 4 and Supplementary Table 3). The negative slope in the control group was not significant, indicating no stable decline in language processing score after accounting for age and prior CR. However, the intervention group displayed significant growth in language processing score over time after accounting for age and prior CR (Fig. 4 and Supplementary Table 3). These results indicate a significant between group differences in the rate of change over time, with the intervention group displaying a significant increase in language processing score compared to the control group that displayed no change over time.
Fig. 4.
Model-predicted language processing trajectories over 4 years for individuals in the control group and the intervention group.
4. Discussion
The results of this study indicate that the intervention (further education) group displayed higher baseline language capacity than the control group and also displayed a significant linear increase in language processing capacity over the first 4 years compared with no change over time detected in the control group. Episodic memory performance significantly increased in both the control and intervention (further education) groups, whereas only the intervention group displayed a significant improvement in working memory capacity. Importantly, there were no significant differences between the control and the intervention group in the rate of change over time in episodic memory, working memory, or executive function in the first 4 years following engaging in further education.
That there was no increase in language processing capacity detected in the control group discounts the possibility that the increased language processing capacity observed in the intervention group is an artifact of familiarity or practice effects. The language processing composite measure, which comprised vocabulary and other acquired knowledge-based tasks, would appear to tap into crystallized knowledge. No group differences or change over time was detected across measures of executive function, episodic memory, or working memory, which are likely to tap into fluid cognitive abilities. It seems possible that in the context of formal education such as university-based education, an environment predicated on the acquisition of new information triggers enhancement of crystallized, knowledge-based, cognitive functions such as language processing capacity but not fluid cognitive functions such as executive function, working memory, or episodic memory. A potential counter-explanation of the observed increase in language function following university-level education in older adults is that this increase may be a product of increased social interaction rather than academic skills development. To test this, we explored whether a difference in the social networks of the control and intervention groups was observed over the course of the study. The results (see Supplementary Tables 4 and 5; Supplementary Fig. 1) of a two-group linear LGCM of the Lubben Social Network Scale score for each group revealed no significant change in social networks over time in either group. These results support the interpretation that the increase in CR following university education is the most likely contributor to increased language capacity and not an increase in social interaction.
Lower levels of linguistic capacity in later life have been associated with higher rates of decline in general cognitive function, as well as higher rates of decline across a range of specific cognitive functions including semantic memory, episodic memory, and spatial function [43]. Lower levels of linguistic ability in early life have also been shown to be associated with late-life cognitive impairments [44] and the presence of the hallmark pathological features of Alzheimer's dementia [45]. Crystallized knowledge, such as vocabulary, is one of the few cognitive functions, which does not show evidence of substantial ARCD outside of neurodegenerative disease [46], possibly due to ongoing lifetime exposure to new words [47]. In contrast, fluid abilities including episodic memory, reasoning, spatial skills, and numeric ability show minimal change until the age of 60 years after which decline begins and then accelerates in the late sixth and early seventh decades of life [46]. Considering that the majority of the participants in the THBP are currently in their early-mid 60's, they are younger than the age at which an acceleration in ARCD is reported to occur. In addition, many cognitive functions show minimal decline over a 5- to 10-year period [46]. As such, the 4-year duration of the present study may be of insufficient duration to detect a subtle rate of decline. It is not until an acceleration in ARCD is observed in the THBP sample that definitive conclusions can be drawn regarding whether the late-life education intervention exerts a protective influence against ARCD and risk for neurodegenerative diseases.
Longitudinal research studies investigating the role of early-life educational attainment in ARCD using modeling approaches similar to that used in the present study have failed to identify an association between level of educational attainment in early life and the rate of decline in late life across a range of measures of executive function, working memory, or episodic memory [15], [17], [19]. Yet the same studies consistently reveal an association between level of early-life educational attainment and cognitive performance, reporting that individuals with higher levels of educational attainment in early life continue to perform at a superior level of function in later life across measures of general cognitive function and specific domains [15], [17], [19]. It remains possible that the late-life education initiated increase in CR identified in the THBP study [25] may be sufficient to reduce the rate of ARCD over the medium to longer term and may exert a level of protection of cognitive function in the presence of neurodegeneration.
The THBP is not a randomized control trial, rather on entry into the THBP participants elected to undertake the education intervention or not undertake the education intervention (control group). Owing to ethical constraints, it was not possible to undertake a randomized control trial using late-life education as an intervention, where participants would be randomly assigned to undertaking university study or not for periods of more than 12 months duration. Furthermore, entrance requirements for university courses precluded the allocation of participants to dose or level of dose (i.e., duration of course and course level/subject area). The inability to apply randomized control trial methodology to the THBP has the potential to introduce bias in one group over the other due to prior educational requirements for entry into university and differences with motivational factors for engaging in education as an intervention. That is, the method of recruitment of participants into the THBP may have unavoidably led to a more highly educated sample than exists in the wider community of similarly aged individuals. Entry into Australian universities requires completion of a High School Certificate of Education (or equivalent), which equates to a total of 12 years of school education. However, to enable the broadest range of participants to be involved in the THBP, participants were able to complete a university bridging program to meet university entry prerequisites. Despite this, the mean number of years of education attainment was over 13.5 years, suggesting most participants had undertaken post-secondary school education before commencing the THBP. In contrast, the average number of years of education completed by Australian adults born in the 1950s and 1960s is approximately 11.7–11.9 years [48]. The solution we applied was to collect extensive demographic information and comprehensive assessment of cognitive function, psychological health, social factors, and medical history on entry into the THBP. This information enables detailed comparisons between intervention and control group to be made with group differences in pre-existing attributes being controlled for in statistical analyses. Finally, the choice of university-level education for the intervention in the THBP was made as it has the property of dose, whereby the education a person undertakes varies in both dosage quantity (amount of study completed) and strength (university level). Identifying a relationship between undertaking late-life university education and cognitive function demonstrates that mental effort exerted in later life (independent of the form of this mental activity) is of potential benefit.
In conclusion, the results of present study indicate that in older adults engaging in formal further education resulted in improved language processing capacity, without an effect of late-life education on episodic memory, working memory, or executive function relative to a no-education control group. Combined with our previous findings of improved CR in older adults who undertake further late-life education [25], the present study demonstrating an improvement in language processing suggests that late-life education may be an intervention suitable for developing relative resistance to aging-related cognitive decline and to the effects of neurodegenerative pathology on brain function.
Research in Context.
-
1.
Systematic review: Level of early-life educational attainment predicts rate of age-related cognitive decline (ARCD) and dementia. However, to date, no research has explored the effect of late-life education on ARCD and dementia risk. The Tasmanian Healthy Brain Project is a prospective longitudinal study exploring late-life education in healthy older adults.
-
2.
Interpretation: Healthy older adults completing at least 12-month university-level education compared with a control reference group displayed a significant 4-year linear increase in language processing but not episodic memory, working memory, or executive functions. These results suggest an enhancement of crystallized knowledge but not fluid cognitive abilities.
-
3.
Future directions: This study builds upon our previous finding that late-life education increases cognitive reserve, which then results in increased crystallized knowledge. Future research with the Tasmanian Healthy Brain Project cohort will examine whether these late-life education benefits modify the trajectory of ARCD and risk for dementia.
Acknowledgments
M.E.T. received a University of Tasmania Postgraduate Research scholarship and supplemental postgraduate scholarships from the Wicking Dementia Research and Education Centre and the Alzheimer's Australia Dementia Research Foundation. This project is funded by National Health and Medical Research Council (NHRMC) Project grants (1003645 and 1108794), as well as the JO & JR Wicking Trust (Equity Trustees). M.J.S. reports personal fees from Eli Lily (Australia) Pty Ltd and grants from Novotech Pty Ltd, outside the submitted work. All other authors report nothing to disclose.
Footnotes
Megan E. Thow has previously published under the name Megan E. Lenehan.
Supplementary data related to this article can be found online at http://dx.doi.org/10.1016/j.dadm.2017.08.004.
Supplementary data
References
- 1.Stern Y. Cognitive reserve. Neuropsychologia. 2009;47:2015–2028. doi: 10.1016/j.neuropsychologia.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychological Soc : JINS. 2002;8:448–460. [PubMed] [Google Scholar]
- 3.Foubert-Samier A., Catheline G., Amieva H., Dilharreguy B., Helmer C., Allard M. Education, occupation, leisure activities, and brain reserve: A population-based study. Neurobiol Aging. 2012;33:423.e15–423.e25. doi: 10.1016/j.neurobiolaging.2010.09.023. [DOI] [PubMed] [Google Scholar]
- 4.Ward D.D., Summers M.J., Saunders N.L., Vickers J.C. Modeling cognitive reserve in healthy middle-aged and older adults: the Tasmanian Healthy Brain Project. Int Psychogeriatrics. 2015;27:579–589. doi: 10.1017/S1041610214002075. [DOI] [PubMed] [Google Scholar]
- 5.Serra L., Musicco M., Cercignani M., Torso M., Spanò B., Mastropasqua C. Cognitive reserve and the risk for Alzheimer's disease: A longitudinal study. Neurobiol Aging. 2015;36:592–600. doi: 10.1016/j.neurobiolaging.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 6.Bonner-Jackson A., Long J.D., Paulsen J.S., Westervelt H., Tremont G., Aylward E. Cognitive reserve and brain reserve in prodromal Huntington's disease. J Int Neuropsychological Soc : JINS. 2013;19:739–750. doi: 10.1017/S1355617713000507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manly J.J., Byrd D., Touradji P., Sanchez D. Literacy and cognitive change among ethnically diverse elders. Int J Psychol. 2004;39:47–60. [Google Scholar]
- 8.Brayne C., Ince P.G., Keage H.A.D., McKeith I.G., Matthews F.E., Polvikoski T., EClipSE Collaborative Members Education, the brain and dementia: Neuroprotection or compensation? Brain. 2010;133:2210–2216. doi: 10.1093/brain/awq185. [DOI] [PubMed] [Google Scholar]
- 9.Rentz D.M., Locascio J.J., Becker J.A., Moran E.K. Cognition, reserve, and amyloid deposition in normal aging. Ann Neurol. 2010;67:353–364. doi: 10.1002/ana.21904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dufouil C., Alpérovitch A., Tzourio C. Influence of education on the relationship between white matter lesions and cognition. Neurology. 2003;60:831–836. doi: 10.1212/01.wnl.0000049456.33231.96. [DOI] [PubMed] [Google Scholar]
- 11.Bennett D.A., Wilson R.S., Schneider J.A., Evans D.A., de Leon C.F., Arnold S.E. Education modifies the relation of AD pathology to level of cognitive function in older persons. Neurology. 2003;60:1909–1915. doi: 10.1212/01.wnl.0000069923.64550.9f. [DOI] [PubMed] [Google Scholar]
- 12.Bosma H., van Boxtel M.P., Ponds R.W., Houx P.J. Mental work demands protect against cognitive impairment: MAAS prospective cohort study. Exp Aging Res. 2003;29:33–45. doi: 10.1080/03610730303710. [DOI] [PubMed] [Google Scholar]
- 13.Cullum S., Huppert F.A., McGee M., Dening T. Decline across different domains of cognitive function in normal ageing: Results of a longitudinal population-based study using CAMCOG. Int J Geriatr Psychiatry. 2000;15:853–862. doi: 10.1002/1099-1166(200009)15:9<853::aid-gps211>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 14.Alley D., Suthers K., Crimmins E. Education and cognitive decline in older Americans: Results from the AHEAD sample. Res Aging. 2007;29:73–94. doi: 10.1177/0164027506294245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Dijk K.R., Van Gerven P.W., Van Boxtel M.P., Van der Elst W., Jolles J. No protective effects of education during normal cognitive aging: Results from the 6-year follow-up of the Maastricht aging study. Psychol Aging. 2008;23:119–130. doi: 10.1037/0882-7974.23.1.119. [DOI] [PubMed] [Google Scholar]
- 16.Proust-Lima C., Amieva H., Letenneur L., Orgogozo J.-M., Jacqmin-Gadda H., Dartigues J.-F. Gender and education impact on brain aging: A general cognitive factor approach. Psychol Aging. 2008;23:608–620. doi: 10.1037/a0012838. [DOI] [PubMed] [Google Scholar]
- 17.Der G., Allerhand M., Starr J.M., Hofer S.M., Deary I.J. Age-related changes in memory and fluid reasoning in a sample of healthy old people. Aging Neuropsychol Cogn. 2010;17:55–70. doi: 10.1080/13825580903009071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Christensen H., Hofer S.M., Mackinnon A.J., Korten A.E., Jorm A.F., Henderson A.S. Age is no kinder to the better educated: Absence of an association investigated using latent growth techniques in a community sample. Psychol Med. 2001;31:15–28. doi: 10.1017/s0033291799002834. [DOI] [PubMed] [Google Scholar]
- 19.Zahodne L.B., Glymour M.M., Sparks C., Bontempo D., Dixon R.A., MacDonald S.W.S. Education does not slow cognitive decline with aging: 12-year evidence from the Victoria Longitudinal Study. J Int Neuropsychological Soc : JINS. 2011;17:1039–1046. doi: 10.1017/S1355617711001044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seeman T.E., Huang M.-H., Bretsky P., Crimmins E., Launer L., Guralnik J.M. Education and APOE-e4 in longitudinal cognitive decline: MacArthur Studies of Successful Aging. The Journals Gerontol Ser B, Psychol Sci Social Sci. 2005;60:P74–P83. doi: 10.1093/geronb/60.2.p74. [DOI] [PubMed] [Google Scholar]
- 21.Tucker-Drob E.M., Johnson K.E., Jones R.N. The cognitive reserve hypothesis: A longitudinal examination of age-associated declines in reasoning and processing speed. Developmental Psychol. 2009;45:431–446. doi: 10.1037/a0014012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anstey K., Christensen H. Education, activity, health, blood pressure and apolipoprotein E as predictors of cognitive change in old age: A review. Gerontology. 2000;46:163–177. doi: 10.1159/000022153. [DOI] [PubMed] [Google Scholar]
- 23.Lenehan M.E., Summers M.J., Saunders N.L., Summers J.J., Vickers J.C. Relationship between education and age-related cognitive decline: A review of recent research. Psychogeriatrics. 2015;15:154–162. doi: 10.1111/psyg.12083. [DOI] [PubMed] [Google Scholar]
- 24.Summers M.J., Saunders N.L., Valenzuela M.J., Summers J.J., Ritchie K., Robinson A. The Tasmanian Healthy Brain Project (THBP): A prospective longitudinal examination of the effect of university level education in older adults in preventing age-related cognitive decline and reducing the risk of dementia. Int Psychogeriatrics. 2013;25:1145–1155. doi: 10.1017/S1041610213000380. [DOI] [PubMed] [Google Scholar]
- 25.Lenehan M.E., Summers M.J., Saunders N.L., Summers J.J., Ward D.D., Ritchie K. Sending your grandparents to university increases cognitive reserve: The Tasmanian Healthy Brain Project. Neuropsychology. 2016;30:525–531. doi: 10.1037/neu0000249. [DOI] [PubMed] [Google Scholar]
- 26.Curran P.J., Obeidat K., Losardo D. Twelve frequently asked questions about growth curve modeling. J Cogn Development. 2010;11:121–136. doi: 10.1080/15248371003699969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jurica P.J., Leitten C.L., Mattis S. Psychological Assessment Resources Inc; Florida: 2001. Dementia Rating Scale-2 (DRS-2): Professional Manual. [Google Scholar]
- 28.Snaith R.P. The Hospital Anxiety and Depression Scale. Health Qual Life Outcomes. 2003;1:29. doi: 10.1186/1477-7525-1-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lubben J., Gironda M. Centrality of social ties to the health and well being of older adults. In: Berkman B., Harooytan L., editors. Social work and health care in an aging world. Springer; New York: 2003. pp. 319–350. [Google Scholar]
- 30.Wechsler D. The Psychological Corporation; 1997. Wechsler Memory Scale - third edition (WMS-III): Administration and scoring manual. [Google Scholar]
- 31.Lezak M.D., Howieson D.B., Bigler E.D., Tranel D. 5th ed. Oxford University Press; Oxford: 2012. Neuropsychological Assessment. [Google Scholar]
- 32.Cambridge Cognition Limited . Cambridge Cognition Limited; Cambridge: 2012. CANTABeclipse Test Administration Guide; p. 334. [Google Scholar]
- 33.Wechsler D. The Psychological Corporation; 1997. Wechsler adult intelligence scale - third edition (WAIS-III): Administration and scoring manual. [Google Scholar]
- 34.Strauss E., Sherman E.M.S., Spreen O. 3rd ed. Oxford University Press; New York: 2006. A compendium of neuropsychological tests: Administration, norms, and commentary. [Google Scholar]
- 35.Kaplan E., Goodglass H., Weintraub S. Lea & Febiger; Philadelphia, PA: 1983. Boston Naming Test. [Google Scholar]
- 36.Ward D.D., Summers M.J., Saunders N.L., Janssen P., Stuart K.E., Vickers J.C. APOE and BDNF Val66MET polymorphisms combine to influence episodic memory function in older adults. Behav Brain Res. 2014;271:309–315. doi: 10.1016/j.bbr.2014.06.022. [DOI] [PubMed] [Google Scholar]
- 37.Valenzuela M.J., Sachdev P. Assessment of complex mental activity across the lifespan: development of the Lifetime of Experiences Questionnaire (LEQ) Psychol Med. 2007;37:1015–1025. doi: 10.1017/S003329170600938X. [DOI] [PubMed] [Google Scholar]
- 38.Muthén B.O., Muthén L.K. 7th ed. Muthén & Muthén; Los Angeles, CA: 1998-2012. Mplus User's Guide. [Google Scholar]
- 39.Hooper D., Coughlan J., Mullen M.R. Structural equation modelling: Guidelines for determining model fit. Electron J Business Res Methods. 2008;6:53–60. [Google Scholar]
- 40.Steiger J.H. Understanding the limitations of global fit assessment in structural equation modeling. Personal Individual Differences. 2007;42:893–898. [Google Scholar]
- 41.Hu L.T., Bentler P.M. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria verses new alternatives. Struct Equation Model. 1999;6:1–55. [Google Scholar]
- 42.Cohen J. 2nd ed. Lawrence Erlbaum Associates; Hillsdale, New Jersey: 1988. Statistical power analysis for the behavioural sciences. [Google Scholar]
- 43.Farias S.T., Chand V., Bonnici L., Baynes K., Harvey D., Mungas D. Idea density measured in late life predicts subsequent cognitive trajectories: Implications for the measurement of cognitive reserve. Journals Gerontol Ser B-Psychological Sci Social Sci. 2012;67:677–686. doi: 10.1093/geronb/gbr162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Riley K.P., Snowdon D.A., Desrosiers M.F., Markesbery W.R. Early life linguistic ability, late life cognitive function, and neuropathology: Findings from the Nun Study. Neurobiol Aging. 2005;26:341–347. doi: 10.1016/j.neurobiolaging.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 45.Snowdon D.A., Kemper S.J., Mortimer J.A., Greiner L.H., Wekstein D.R., Markesbery W.R. Linguistic ability in early life and cognitive function and Alzheimer's disease in late life: Findings from the Nun Study. JAMA - J Am Med Assoc. 1996;275:528–532. [PubMed] [Google Scholar]
- 46.Hedden T., Gabrieli J.D. Insights into the ageing mind: A view from cognitive neuroscience. Nat Rev Neurosci. 2004;5:87–96. doi: 10.1038/nrn1323. [DOI] [PubMed] [Google Scholar]
- 47.Hartshorne J.K., Germine L.T. When does cognitive functioning peak? The asynchronous rise and fall of different cognitive abilities across the life span. Psychol Sci. 2015;26:433–443. doi: 10.1177/0956797614567339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kelley J, Evans MDR. Trends in educational attainment in Australia; 1996. Available from: www.international-survey.org/wwa_pub/articles/hst-ed5.htm.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.