Abstract
Background
Behçet’s disease (BD) susceptibility had been associated with single-nucleotide polymorphisms (SNPs) in IL23R–IL12RB2, IL10, STAT4, or ERAP1 locus in Japanese, Turkish, Chinese, and other populations, but not in a Korean genome-wide association study (GWAS). We aimed to fine-map BD risk association of these four loci using extensive imputation and additional genotyping for replication.
Methods
In the discovery phase, 369 patients with BD enrolled in the previous Korean GWAS and 2000 controls retrieved from a population-based cohort of healthy Koreans were imputed for their genotypes of all SNPs in the four loci using the Asian data of the 1000 Genomes Project as reference. For genotype imputation of ERAP1 SNPs, the adjacent ERAP2 SNPs were also covered. For the 10 most significantly associated SNPs (8 imputed and 2 GWAS-genotyped), an additional 84 patients with BD and 283 healthy controls were genotyped for replication. The results from the discovery and replication phases were pooled for meta-analysis using the Mantel-Haenszel test to estimate the odds ratio (OR) and 95% confidence interval (CI).
Results
An IL23R–IL12RB2 intergenic SNP rs1495965 was significantly associated with BD risk (OR (95% CI) = 1.5 (1.3, 1.7), P = 2.5 × 10−7) in the pooled meta-analysis of the discovery (1.4 (1.2, 1.7), P = 4.9 × 10−7) and replication (1.9 (1.3, 2.6), P = 6.0 × 10−4) phases. BD risk association was fine-mapped on the intergenic region rather than the two flanking genes, as rs1495966 and rs4655535, almost perfectly correlated with rs1495965 (r 2 = 0.99), were also located in the same intergenic region. Consistent with previous reports, the P values tended to be lower within IL23R than IL12RB2. On the other hand, several IL10 SNPs were suggested for association in the discovery phase but all failed in the replication phase. No SNP in ERAP1–ERAP2 and STAT4 was suggested even in the discovery phase.
Conclusions
BD susceptibility association was fine-mapped on the intergenic region between IL23R and IL12RB2 as marked by three correlated SNPs, rs1495965, rs1495966, and rs4655535.
Electronic supplementary material
The online version of this article (doi:10.1186/s13075-017-1435-5) contains supplementary material, which is available to authorized users.
Keywords: Behçet’s disease, Case-control disease-association study, ERAP1, Genotyping, Imputation, IL10, IL12RB2, IL23R, STAT4
Background
Behçet’s disease (BD) is a chronic relapsing inflammatory disease characterized by orogenital ulcers, cutaneous inflammation and uveitis. In addition to its typical muco-cutaneous and ocular manifestations, BD is a multi-system disease that also targets musculoskeletal, vascular, nervous, and gastrointestinal systems [1]. Although the etiology of BD remains unclear, it is well-established that BD is strongly associated with HLA-B*51.
Since two landmark genome-wide association studies (GWAS) performed on Japanese and Turkish populations [2, 3] identified HLA-A, IL10, and IL23R–IL12RB2 to be novel BD susceptibility loci, the association of IL10 and IL23R–IL12RB2 with BD has been replicated thereafter in various ethnic groups including the Chinese Han, Iranian, and Western Algerian populations [4–9]. The identification of IL10 and IL23R–IL12RB2 not only indicates involvement of non-HLA genes but also implies the importance of cytokine dysregulation in the pathogenesis of BD.
Additional BD susceptibility loci other than IL10 and IL23R–IL12RB2 identified by subsequent GWAS include STAT4 in the Chinese Han [10] and GIMAP in Koreans [11]. STAT4 is a transcription factor that transduces IL-12, IL-23, and type 1 interferon signals in T cells and monocytes [12]. Thus, the functional relevance of STAT4 in BD appears sensible because T helper (Th) 1 and Th17 cytokines are closely related to BD pathogenesis [13–15]. Notably, the Korean GWAS, which identified association between BD and GIMAP, failed to replicate the association with IL10, IL23R–IL12RB2, or STAT4 [11]. This lack of association could have been due to insufficient statistical power of the study (limitation in sample size or SNP density) or due to the unique ethnic background.
The imputation technique, coupled with the GWAS database, has been successfully used in studying BD genetics to achieve genome-wide fine-mapping. By enhancing SNP density and thereby helping identify the most strongly associated among virtually all SNPs in a region of interest, genotype imputation can upgrade the statistical power of GWAS. Applying this technique in the GWAS database has enabled identification of the association with STAT4 in the Turkish and Japanese populations and recessive association with two nonsynonymous ERAP1 SNPs in the Turkish population [16]. Their minor alleles were too few in the Japanese population to evaluate the recessive effect. ERAP1 homozygotes of the BD risk-associated allele conferred the risk preferentially to HLA-B*51 positive individuals, suggesting a gene-gene interaction between ERAP1 and HLA-B*51.
We hypothesized that those susceptibility genes identified in other Asian groups are associated with BD in Koreans as well and selected the genetic regions to be imputed, where their association has been confirmed in at least two Asian groups at GWAS level sample size. ERAP1 was also selected to examine a gene-gene interaction with HLA-B*51 in our population. Finally, we aimed to fine-map IL23R–IL12RB2, IL10, and STAT4 regions and ERAP1 by applying imputation technique to our Korean GWAS dataset.
Methods
Study participants
A total of 369 Korean patients with BD (cases) enrolled in a previous GWAS [11] and 2000 age-matched and sex-matched controls retrieved from a population-based cohort of healthy Koreans (Korea Biobank Network, http://cdc.co.kr) were included in this study. For replication, a different set of 84 Korean patients with BD and 283 age-matched and sex-matched healthy controls who had not been included in the discovery phase were recruited. Patients with BD fulfilled the International Study Group diagnostic criteria for BD [17].
Genotype imputation in the discovery phase
Using the Asian dataset (CHB + JPT) of the 1000 Genomes Project as a reference panel, the missing genotypes in the four loci were inferred for 369 cases and 2000 controls after phasing the observed genotypes derived from the Korean GWAS data [11]. Before phasing, the GWAS genotypes determined by the Affymetrix genome-wide human SNP array 6.0 were screened for quality control in terms of call rate (>95%), minor allele frequency (>5%), and Hardy-Weinberg equilibrium P value in controls (>0.0001). Boundaries of imputation ranges were determined to include linkage disequilibrium (LD) blocks where the qualified GWAS SNPs of a given gene reside.
For IL10, SNPs with minor allele frequency of 1–5% were additionally included due to the paucity of GWAS SNPs in this region if they satisfy the other quality control measures and if their signal clusters showed correct call decisions in manual inspection. ERAP2 SNPs were included for high-quality imputation of ERAP1 SNP genotypes since the LD block expanded from ERAP1 to ERAP2. The MATCH v1.0.16 software (University of Michigan, Ann Arbor, MI, USA) was used to perform the imputation. While 109 SNPs of the four loci had been genotyped in the previous GWAS, 1629 additional SNPs were imputed and passed a cutoff of imputation quality, r square (Rsq) > 0.3 in this study.
Direct genotyping in the replication phase
Among the imputed SNPs, two IL10 SNPs with a lower P value for BD risk association than the GWAS SNP rs1554286 and six IL23R–IL12RB2 SNPs with a lower or similar P value than the GWAS SNP rs6677188 were selected together with the two GWAS SNPs for a replication study. Thus, 10 lead SNPs (3 in IL10 and 7 in IL23R–IL12RB2) were genotyped in an additional population of 84 BD cases and 283 controls using the Taqman® primers and probes designed by Applied Biosystems (Foster City, CA, USA).
Statistical analysis for BD association
The chi-square test or Fisher’s exact test was used to compare the allele frequencies between patient cases and healthy controls. Statistical analysis was done using the SPSS v17.0 software (SPSS Inc., Chicago, IL, USA). For meta-analysis, data were pooled and analyzed according to the Mantel-Haenszel test using the Stata v14 software (StataCrop LP, College Station, TX, USA). Between-study heterogeneity was quantified using the I 2 statistic.
Results
Clinical characteristics of study participants
The clinical manifestations of patients with BD in the discovery and replication phases are separately summarized in Table 1. Compared to the GWAS population enrolled in the discovery phase, the replication phase population had more women (57% vs. 50%), more skin lesions (96.3% vs. 89.2% for any skin lesion, P = 0.04; and 61.4% vs. 54.6% positivity in pathergy testing), but fewer eye lesions (28.8% vs 43.5%, P = 0.01). Central nervous system involvement was more frequent in the discovery phase (11.6% vs. 3.8%, P = 0.03).
Table 1.
Clinical characteristics of the enrolled patients with Behçet’s disease
| Characteristic | Discovery phase (n = 379) | Replication phase (n = 84) | P valueb |
|---|---|---|---|
| Male (%): female | 191 (50): 188 | 36 (43): 48 | |
| Age at diagnosis of BD (years)a | 41.6 ± 10.1 | 44.1 ± 11.4 | |
| Clinical manifestation (%) | |||
| Recurrent oral ulcer | 100 | 100 | |
| Recurrent genital ulcer | 74.4 | 80.0 | |
| Skin lesions | 89.2 | 96.3 | 0.04 |
| Eye lesions | 43.5 | 28.8 | 0.01 |
| Positive pathergy test | 54.6 (130/238) | 61.4 (27/44) | |
| Vascular involvement | 16.4 | 17.5 | |
| Central nervous system involvement | 11.6 | 3.8 | 0.03 |
| Joint involvement | 41.4 | 51.3 | |
BD Behçet’s disease
aAge is presented as mean ± standard deviation
bOnly P values <0.05 are presented
LD structure comparison in Asian populations
In order to see whether the 1000 Genomes Project data can be used as reference for imputing the Korean genotypes of the four loci, we first examined similarity of LD structures of HapMap-scale SNPs in the loci between the Korean genotype data of the Korean HapMap Project (equivalent to the International HapMap Project) and the Chinese and Japanese (referred to as Asian) sequence data of the 1000 Genomes Project.
The correlation coefficient (r 2) was calculated for all SNPs within each of the four loci in reference to the SNP that was most significant in the Korean GWAS (rs6677188 in IL23R–IL12RB2, rs1554286 in IL10, rs1031508 in STAT4, and rs26652 in ERAP1–ERAP2) using the Korean genotype data and Asian sequence data separately. Then, SNP distributions plotted in descending order of r 2 values were compared between the two populations (Additional file 1: Figure S1). The two plots were nearly superimposable across all SNPs in each of IL10, IL12RB–IL23R, and STAT4 loci and across the highly correlated SNPs (0.5 ≤ r 2 ≤ 1.0) in ERAP1–ERAP2.
Association analysis in the discovery phase
In the IL23R–IL12RB2 locus ranging from − 22.7 kb of IL23R to + 54.3 kb of IL12RB2, 37 SNPs had been genotyped in the previous GWAS [11] and 509 additional SNPs were imputed in this study for association tests (Fig. 1). The most significantly associated SNP was rs4655535 (imputed, OR = 1.4 (1.2, 1.7), P = 0.000033), which had a sixfold lower P value than the GWAS-genotyped SNP rs6677188 (P = 0.00020), which had the same OR and 95% CI (Table 2).
Fig. 1.
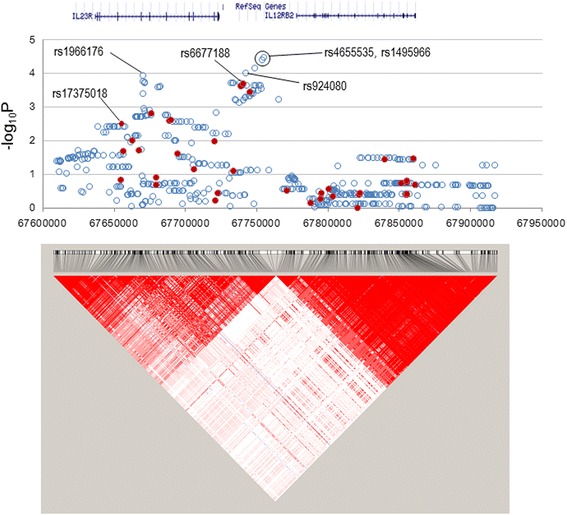
Manhattan plot for the IL23R–IL12RB2 locus. All imputed (solid red circles) and genome-wide association study (GWAS)-genotyped (open blue circles) SNPs are plotted together with a linkage disequilibrium structure below
Table 2.
Allelic association of SNPs after imputation in the discovery phase
| Locus | SNP | Risk allele (%) | HWE P | Rsqc | Allelic association | ||
|---|---|---|---|---|---|---|---|
| BD cases (n = 738) | Controls (n = 4000) | OR (95% CI) | P | ||||
| IL23R–IL12RB2 | rs4655535 (G > T)a | 467 (63) | 2201 (55) | 0.00023 | 0.40 | 1.4 (1.2, 1.7) | 0.000033 |
| rs1495966 (T > C)a | 467 (63) | 2204 (55) | 0.00042 | 0.40 | 1.4 (1.2, 1.7) | 0.000038 | |
| rs1495965 (C > T)a | 466 (63) | 2203 (55) | 0.00050 | 0.40 | 1.4 (1.2, 1.7) | 0.000049 | |
| rs6665569 (T > C)a | 465 (53) | 2210 (55) | 0.047 | 0.48 | 1.4 (1.2, 1.6) | 0.000094 | |
| rs1966176 (G > A)a | 506 (69) | 2442 (61) | 0.61 | 0.87 | 1.4 (1.2, 1.7) | 0.00011 | |
| rs6677188 (T > A)a,b | 588 (80) | 2919 (73) | 0.034 | NA | 1.4 (1.2, 1.8) | 0.00020 | |
| rs924080 (T > C)a | 590 (80) | 2940 (73) | 0.11 | 0.77 | 1.4 (1.2, 1.7) | 0.00022 | |
| IL10 | rs1518110 (A > C) | 563 (76) | 2728 (68) | 0.47 | 0.91 | 1.5 (1.2, 1.8) | 0.000012 |
| rs1518111 (T > C)a | 563 (76) | 2728 (68) | 0.47 | 0.91 | 1.5 (1.2, 1.8) | 0.000012 | |
| rs1800871 (A > G) | 563 (76) | 2728 (68) | 0.47 | 0.87 | 1.5 (1.2, 1.8) | 0.000012 | |
| rs1800872 (T > G)a | 563 (76) | 2728 (68) | 0.47 | 0.87 | 1.5 (1.2, 1.8) | 0.000012 | |
| rs3024490 (A > C) | 563 (76) | 2728 (68) | 0.47 | 0.85 | 1.5 (1.2, 1.8) | 0.000012 | |
| rs1554286 (A > G)a,b | 551 (76) | 2720 (68) | 0.47 | NA | 1.5 (1.2, 1.8) | 0.000030 | |
SNP single-nucleotide polymorphism, BD Behçet’s disease, HWE Hardy-Weinberg equilibrium, Rsq r square, OR odds ratio, CI confidence interval, NA not applicable
aThe ten SNPs were genotyped in the subsequent replication phase
bThe two SNPs had been genotyped in the previous genome-wide association study [11] and the genotypes of the others were imputed in this study
cRsq is an imputation quality metric estimated for each imputed SNP
Furthermore, four other SNPs (rs1495966, rs1495965, rs6665569, and rs1966176 in ascending order of P value) had lower P values (0.000038 ≤ P ≤ 0.00011) and rs924080 had a similar P value (P = 0.00022) compared to rs6677188. These SNPs were all located within the intergenic region except for rs1966176 located within the IL23R gene as shown in Fig. 1. Subsequently, seven SNPs (rs4655535, rs1495966, rs1495965, rs6665569, rs1966176, rs924080, and rs6677188) were chosen to be genotyped for replication of BD risk association. As seen in Turkish and Iranian studies [3, 8], many SNPs located within the IL23R gene had lower P values than any SNP located within the IL12RB2 gene, although it needs to be verified whether the BD risk-associated intergenic SNPs affect expression of IL23R, IL12RB2, or both in future studies.
In the IL10 locus ranging from − 8.4 kb to + 23.1 kb, four SNPs had been genotyped [11] and 34 SNPs were imputed (Fig. 2). The most significantly associated SNPs were rs1518110, rs1518111, rs1800871, rs1800872, and rs3024490 (all imputed, OR (95% CI) = 1.5 (1.2, 1.8), P = 0.000012), which were perfectly correlated (r 2 = 1.0) with each other (Table 2). These five SNPs had only a slightly lower P value (0.000030) and the same OR (95% CI) (1.5 (1.2, 1.8)]), compared to the GWAS-genotyped rs1554286 (Table 2). Among these six highly correlated SNPs, genotyping of any one would be sufficient for the replication study, but three SNPs, rs1518111, rs1800872, and rs1554286, were genotyped in the replication phase just for redundant assurance.
Fig. 2.
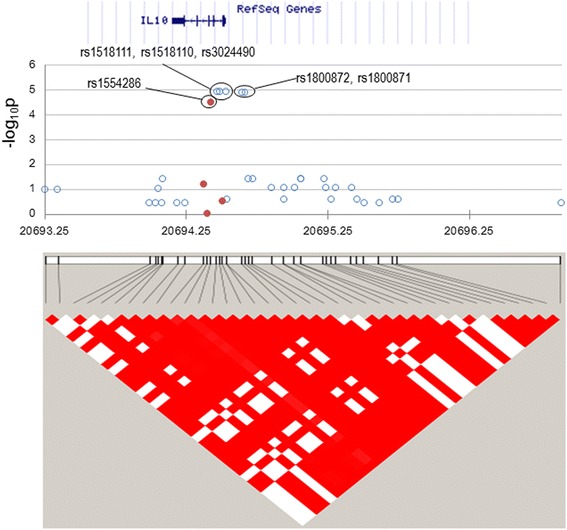
Manhattan plot for the IL10 locus. All imputed (solid red circles) and genome-wide association study (GWAS)-genotyped (open blue circles) SNPs are plotted together with a linkage disequilibrium structure below
In the STAT4 locus ranging from − 4.7 kb to + 9.4 kb, 25 SNPs had been genotyped [11] and 239 SNPs were imputed, but no SNPs were associated with BD risk in this Korean population (Fig. 3). In the ERAP1–ERAP2 locus ranging from − 14.0 kb of ERAP1 to + 112.0 kb of ERAP2, 43 SNPs had been genotyped [11] and 847 SNPs were imputed but no SNPs were associated (Fig. 4). Minor allele homozygotes of two ERAP1 SNPs, rs17482078, and rs10050860, had been associated with Turkish BD [16], but their minor allele frequencies were similar between BD cases and controls (4.9% vs. 4.4% for rs17482078; 4.9% vs. 4.5% for rs10050860, respectively) without such homozygote carriers in this study.
Fig. 3.
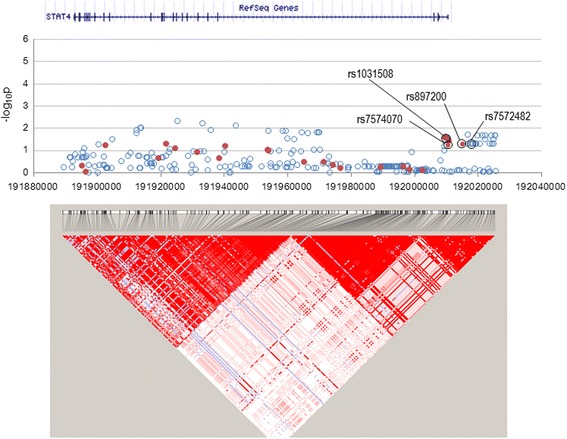
Manhattan plot for STAT4 locus. All imputed (solid red circles) and genome-wide association study (GWAS)-genotyped (open blue circles) SNPs are plotted together with a linkage disequilibrium structure below
Fig. 4.
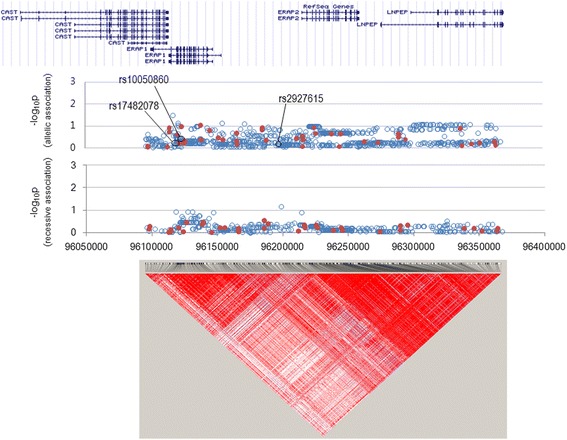
Manhattan plot for the ERAP1–ERAP2 locus. All imputed (solid red circles) and genome-wide association study (GWAS)-genotyped (open blue circles) SNPs are plotted together with a linkage disequilibrium structure below. The top panel represents the allelic association and the bottom one represents the recessive association
Association analysis in the replication phase
The above-mentioned seven IL23R–IL12RB2 SNPs and three IL10 SNPs were genotyped in a replication population consisting of 89 BD cases and 283 healthy controls of Korean ethnicity (Table 3). All SNPs in IL23R–IL12RB2 maintained BD risk association with P values (0.00062 ≤ P ≤0.0049) lower than the significance level for multiple testing, α = 0.05/10 = 0.005 (for 10 SNPs), except for rs6665569 (P = 0.0063). The OR values of these seven SNPs were higher with experimentally determined genotype data in the replication phase (1.6 ≤ OR ≤2.1) than with imputed genotype data in the discovery phase (OR = 1.4), although their 95% CI overlapped.
Table 3.
Allelic association of SNPs in the replication phase
| Locus | SNP | BD cases, risk allele/total (%) | Controls, risk allele/total (%) | Allelic association | |
|---|---|---|---|---|---|
| OR (95% CI) | P | ||||
| IL23R–IL12RB2 | rs1495965 (C > T) | 106/168 (63) | 272/566 (48) | 1.9 (1.3, 2.6) | 0.00062 |
| rs1495966 (T > C) | 106/168 (63) | 264/548 (48) | 1.8 (1.3, 2.6) | 0.00071 | |
| rs4655535 (G > T) | 107/168 (64) | 277/566 (49) | 1.8 (1.3, 2.6) | 0.00078 | |
| rs924080 (T > C) | 143/168 (85) | 413/566 (73) | 2.1 (1.3, 3.4) | 0.0013 | |
| rs1966176 (G > A) | 118/168 (70) | 322/562 (57) | 1.8 (1.2, 2.6) | 0.0026 | |
| rs6677188 (T > A) | 142/168 (85) | 419/566 (74) | 1.9 (1.2, 3.0) | 0.0049 | |
| rs6665569 (T > C) | 103/168 (61) | 271/550 (49) | 1.6 (1.2, 2.3) | 0.0063 | |
| IL10 | rs1518111 (A > G) | 120/168 (71) | 408/566 (72) | 1.0 (0.7, 1.4) | 0.87 |
| rs1800872 (A > C) | 120/168 (71) | 408/566 (72) | 1.0 (0.7, 1.4) | 0.87 | |
| rs1554286 (T > C) | 120/168 (71) | 396/554 (71) | 1.0 (0.7, 1.5) | 0.99 | |
SNP single-nucleotide polymorphism, BD Behçet’s disease, OR odds ratio, CI confidence interval
However, the association between the three IL10 SNPs and risk of BD was no longer significant in the replication phase, as the P values were much higher than a marginal significance level of α = 0.05 (Table 3). Association test results were the same for the three highly correlated SNPs when they were re-genotyped for 20 randomly chosen cases of BD and 42 controls, with alternatively designed primers and probes in a blinded manner, rejecting any possibility of technical or sampling errors in genotyping.
Meta-analysis
The discovery and replication phase data of the seven lead SNPs in the IL23R–IL12RB2 locus were combined for meta-analysis (Table 4). All seven SNPs were significantly associated with BD susceptibility, as their P values passed a significance level for multiple testing of all 1629 imputed and 109 genotyped SNPs, α = 0.05/1738 = 2.9 × 10−5: the P value was an order of magnitude lower for three SNPs, rs1495965 (P = 2.5 × 10−7), rs4655535 (P = 2.6 × 10−7), and rs1495966 (P = 2.9 × 10−7), than the other four SNPs (P ≥ 2.1 × 10−6). The three lowest P value SNPs were almost perfectly correlated with each other (r 2 = 0.99) and all located in the intergenic region between IL23R and IL12RB2.
Table 4.
Meta-analysis on allelic associations of IL23R–IL12RB2 SNPs
| SNP | Phase | BD cases, risk allele/total | Controls, risk allele/total | Weight % | I 2 % | OR (95% CI) | P |
|---|---|---|---|---|---|---|---|
| rs1495965 | Dis. | 467/738 (63) | 2203/4000 (55) | 83.7 | 35 | 1.41 (1.20, 1.65) | 0.000049 |
| Rep. | 106/168 (63) | 272/566 (48) | 16.3 | 1.85 (1.30, 2.63) | 0.00060 | ||
| Total | 573/906 (63) | 2475/4566 (54) | 1.47 (1.27, 1.70) | 0.00000025 | |||
| rs4655535 | Dis. | 467/738 (63) | 2201/4000 (55) | 83.7 | 27 | 1.41 (1.20, 1.66) | 0.000033 |
| Rep. | 107/168 (64) | 277/566 (49) | 16.3 | 1.83 (1.28, 2.61) | 0.00080 | ||
| Total | 574/906 (63) | 2478/4566 (54) | 1.47 (1.27, 1.71) | 0.00000026 | |||
| rs1495966 | Dis. | 467/738 (63) | 2204/4000 (55) | 83.4 | 0 | 1.40 (1.19, 1.64) | 0.000038 |
| Rep. | 106/168 (63) | 264/548 (48) | 16.6 | 1.84 (1.29, 2.62) | 0.00070 | ||
| Total | 573/906 (63) | 2464/4548 (54) | 1.47 (1.27, 1.70) | 0.00000029 | |||
| rs1966176 | Dis. | 506/738 (69) | 2443/4000(61) | 83.6 | 5 | 1.39 (1.18, 1.64) | 0.00011 |
| Rep. | 118/168 (70) | 322/562 (57) | 16.4 | 1.76 (1.21, 2.55) | 0.0026 | ||
| Total | 624/906 (69) | 2765/4562 (61) | 1.45 (1.24, 1.69) | 0.0000021 | |||
| rs6665569 | Dis. | 465/738 (53) | 2210/4000 (55) | 83.0 | 0 | 1.38 (1.17, 1.62) | 0.000094 |
| Rep. | 103/168 (61) | 271/550 (49) | 17.0 | 1.63 (1.15, 2.32) | 0.0063 | ||
| Total | 568/906 (63) | 2481/4550 (55) | 1.42 (1.23, 1.65) | 0.0000030 | |||
| rs924080 | Dis. | 590/738 (80) | 2940/4000 (74) | 86.4 | 62 | 1.44 (1.18, 1.74) | 0.00022 |
| Rep. | 143/168 (85) | 413/566 (73) | 13.6 | 2.12 (1.33, 3.37) | 0.0013 | ||
| Total | 733/906 (81) | 3353/4566 (73) | 1.53 (1.28, 1.83) | 0.0000032 | |||
| rs6677188 | Dis. | 590/738 (80) | 2937/4000 (73) | 85.8 | 34 | 1.44 (1.19, 1.75) | 0.00019 |
| Rep. | 142/168 (85) | 419/566 (74) | 14.2 | 1.92 (1.21, 3.03) | 0.0049 | ||
| Total | 732/906 (81) | 3356/4566 (73) | 1.51 (1.26, 1.80) | 0.0000060 |
SNP single-nucleotide polymorphism, BD Behçet’s disease, CI confidence interval, Dis. discovery phase, OR odds ratio, Rep. replication phase
Discussion
This study investigated the previously BD-associated IL23R–IL12RB2, IL10, STAT4, and ERAP1 loci for fine-mapping by using comprehensive imputation for discovering candidate SNPs and genotyping them in additional cases and controls for independent replication of the association in Koreans. Among the four loci, only IL23R–IL12RB2 was confirmed for association with BD susceptibility in the pooled meta-analysis of the discovery and replication phases, consistent with several previous studies [2–4, 7].
More importantly, association between BD risk and the IL23R–IL12RB2 locus was fine-mapped on the intergenic region rather than the IL23R or IL12RB2 gene, as the most significant association (P = 10−7) was observed with three almost perfectly correlated SNPs (r 2 = 0.99), rs1495965, rs1495966, and rs4655535 located in the intergenic region, which may contain regulatory sequences for expression of IL23R, IL12RB2, or both. These three SNPs were in complete LD (D’ = 1.0) with another intergenic SNP, rs924080, which has been associated with IL23R expression. More specifically, the risk-associated allele A of rs924080 was associated with enhanced expression of IL23R, IL6, and TNFα in the previous Turkish study [18], although not in the previous Chinese study [6], where IL23R and IL17 mRNA levels were affected by rs12141431 instead. It is plausible to hypothesize that genetic polymorphisms in the IL23R–IL12RB2 locus are associated with upregulated Th17 axis in BD. The importance of Th17 cells and cytokines in BD has been shown in many studies. Elevated levels of circulating Th17 cells or Th17 cytokines in BD have been reported [13, 14, 19, 20] and successful anti-TNFα treatment was found to decrease Th17 differentiation [15]. However, the mechanism by which the BD-associated SNPs alter disease susceptibility remains to be clarified.
Several IL10 SNPs were positive for association with BD in the discovery phase but all failed in the replication phase. Association between BD and IL10 was previously evident with rs1518111 in the Turkish GWAS [3] but was evident with rs1800871 and rs1800872 in the Japanese GWAS [2], whereas nominal association was observed with rs1518111 and rs1554286 in an Iranian population [7], which was not replicated in another Iranian population [8], indicating contrasting results in different studies. In this study in Koreans, the association test results were drastically different between the discovery and replication phases. This discrepancy could have been caused by heterogeneity of the enrolled subjects, among other causes. For example, the discovery phase population had significantly more eye and central nervous system lesions and fewer skin lesions than the replication phase population. It might be that the IL10 polymorphism is preferentially involved in eye or central nervous system lesions, since the prevalence of the IL10 polymorphism in patients with BD was higher in the discovery phase (76%) than in the replication phase (71%) whereas its prevalence was similar in controls in both phases (68% in the discovery phase vs. 71–72% in the replication phase). Another possible cause could be that the sample size was too small in the replication phase, i.e. the statistical power was insufficient. A more homogenous, larger sample will help further determine the significance of IL10 polymorphisms in Koreans with BD.
We found no association with STAT4 and ERAP1–ERAP2 loci. Alteration of STAT4 signaling or antigen presentation of pathogenic peptides by SNPs [16] may not be a major disease susceptibility mechanism in Koreans. However, there is a possibility that the rare risk alleles may have been undetected by this fine mapping due to limited statistical power.
Conclusion
Association was confirmed between susceptibility to BD and the IL23R–IL12RB2 locus, and was fine-mapped on the intergenic region rather than the two flanking genes, suggesting association with altered expression of IL23R, IL12RB2, or both. The other three loci, IL10, STAT4, and ERAP1–ERAP2, were not confirmed in this study with Koreans.
Acknowledgments
Funding
This study was supported by a grant of the Korea Health Technology R&D Project funded by the Ministry of Health & Welfare through the Korea Health Industry Development Institute (HI14C1277). It was also funded by the National Research Council of Science and Technology (DRC-14-2-KRISS).
Availability of data and materials
The datasets supporting the conclusions of this article are available as follows. The GWAS dataset of 2000 controls can be requested at Korea Biobank Network (http://cdc.go.kr/). The GWAS dataset of Korean patients with BD and the datasets generated during the current study are available from the corresponding author on a reasonable request.
Abbreviations
- BD
Behçet’s disease
- CI
Confidence interval
- GWAS
Genome-wide association study
- HWE
Hardy-Weinberg equilibrium
- LD
Linkage equilibrium
- OR
Odds ratio
- SNP
Single-nucleotide polymorphism
- Th
T helper
- TNF
Tumor necrosis factor
Additional file
Figure S1. LD structure similarities of the four loci (IL10, IL23R–IL12RB2, STAT4, or ERAP1) between Korean and Asian (Chinese and Japanese) populations. Using the Korean and Asian genotype databases, the correlation coefficient value (r 2) was calculated for all the SNPs within each of the four loci in reference to the SNP found to be most significant in the Korean GWAS (rs1554286 in IL10, rs6677188 in IL23R–IL12RB2, rs1031508 in STAT4, and rs26652 in ERAP1-ERAP2). The Y axis shows r 2 between the reference SNP and the other SNPs within each gene. The X axis shows SNPs ranked in the descending order of r 2 values. In each plot, open circles represent SNPs from the Korean genotype database and solid squares those from the Asian database. (TIF 65 kb)
Authors’ contributions
EHK was involved in the study design and data interpretation and drafted the manuscript. SK performed imputation-based genotyping for the discovery phase and was involved in the data interpretation. MYP helped in sorting the GWAS database for the discovery phase and imputation-based genotyping. CJY performed replication genotyping. MJK, IAC, YJH, EYL, and EBL helped in collecting the patient clinical data and critical editing of the manuscript. CK and YWS were involved in the study design, data interpretation, and critical editing of the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
All study participants provided written informed consent for participation in the study, and the study was approved by the Institutional Review Boards of Seoul National University Hospital (H-1401-101-549) and Seoul National University Bundang Hospital (B-1504-296-303).
Consent for publication
All study participants provided written informed consent for publication.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1186/s13075-017-1435-5) contains supplementary material, which is available to authorized users.
Contributor Information
Eun Ha Kang, Email: kangeh@snubh.org.
Sewon Kim, Email: raphael30@kaist.ac.kr.
Min Young Park, Email: minmin@dnalink.com.
Ji Yong Choi, Email: jy9793@gmail.com.
In Ah Choi, Email: sylph014@hanmail.net.
Min Jung Kim, Email: fairytaie@naver.com.
You-Jung Ha, Email: hayouya@snubh.org.
Eun Young Lee, Email: elee@snu.ac.kr.
Yun Jong Lee, Email: yn35@snu.ac.kr.
Eun Bong Lee, Email: leb7616@snu.ac.kr.
Changwon Kang, Email: ckang@kaist.ac.kr.
Yeong Wook Song, Email: ysong@snu.ac.kr.
References
- 1.Sakane T, Takeno M, Suzuki N, Inaba G. Behçet’s disease. N Engl J Med. 1999;341:1284–91. doi: 10.1056/NEJM199910213411707. [DOI] [PubMed] [Google Scholar]
- 2.Mizuki N, Meguro A, Ota M, Ohno S, Shiota T, Kawagoe T, et al. Genome-wide association studies identify IL23R-IL12RB2 and IL10 as Behçet’s disease susceptibility loci. Nat Genet. 2010;42:703–6. doi: 10.1038/ng.624. [DOI] [PubMed] [Google Scholar]
- 3.Remmers EF, Cosan F, Kirino Y, Ombrello MJ, Abaci N, Satorius C, et al. Genome-wide association study identifies variants in the MHC class I, IL10, and IL23R-IL12RB2 regions associated with Behçet’s disease. Nat Genet. 2010;42:698–702. doi: 10.1038/ng.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jiang Z, Yang P, Hou S, Du L, Xie L, Zhou H, et al. IL-23R gene confers susceptibility to Behcet’s disease in a Chinese Han population. Ann Rheum Dis. 2010;69:1325–8. doi: 10.1136/ard.2009.119420. [DOI] [PubMed] [Google Scholar]
- 5.Wu Z, Zheng W, Xu J, Sun F, Chen H, Li P, et al. IL10 polymorphisms associated with Behçet’s disease in Chinese Han. Hum Immunol. 2014;75:271–6. doi: 10.1016/j.humimm.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 6.Yu H, Zheng M, Zhang L, Li H, Zhu Y, Cheng L, et al. Identification of susceptibility SNPs in IL10 and IL23R-IL12RB2 for Behçet’s disease in Han Chinese. J Allergy Clin Immunol. 2016;139:621–7. doi: 10.1016/j.jaci.2016.05.024. [DOI] [PubMed] [Google Scholar]
- 7.Xavier JM, Shahram F, Davatchi F, Rosa A, Crespo J, Abdollahi BS, et al. Association study of IL10 and IL23R-IL12RB2 in Iranian patients with Behçet’s disease. Arthritis Rheum. 2012;64:2761–72. doi: 10.1002/art.34437. [DOI] [PubMed] [Google Scholar]
- 8.Carapito R, Shahram F, Michel S, Le Gentil M, Radosavljevic M, Meguro A, et al. On the genetics of the Silk Route: association analysis of HLA, IL10, and IL23R-IL12RB2 regions with Behçet’s disease in an Iranian population. Immunogenetics. 2015;67:289–93. doi: 10.1007/s00251-015-0841-6. [DOI] [PubMed] [Google Scholar]
- 9.Khaib Dit Naib O, Aribi M, Idder A, Chiali A, Sairi H, Touitou I, et al. Association analysis of IL10, TNF-α, and IL23R-IL12RB2 SNPs with Behçet’s disease risk in Western Algeria. Front Immunol. 2013;4:342. doi: 10.3389/fimmu.2013.00342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hou S, Yang Z, Du L, Jiang Z, Shu Q, Chen Y, et al. Identification of a susceptibility locus in STAT4 for Behçet’s disease in Han Chinese in a genome-wide association study. Arthritis Rheum. 2012;64:4104–13. doi: 10.1002/art.37708. [DOI] [PubMed] [Google Scholar]
- 11.Lee YJ, Horie Y, Wallace GR, Choi YS, Park JA, Choi JY, et al. Genome-wide association study identifies GIMAP as a novel susceptibility locus for Behcet’s disease. Ann Rheum Dis. 2013;72:1510–6. doi: 10.1136/annrheumdis-2011-200288. [DOI] [PubMed] [Google Scholar]
- 12.Korman BD, Kastner DL, Gregersen PK, Remmers EF. STAT4: genetics, mechanisms, and implications for autoimmunity. Curr Allergy Asthma Rep. 2008;8:398–403. doi: 10.1007/s11882-008-0077-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shimizu J, Takai K, Fujiwara N, Arimitsu N, Ueda Y, Wakisaka S, et al. Excessive CD4+ T cells co-expressing interleukin-17 and interferon-γ in patients with Behçet’s disease. Clin Exp Immunol. 2012;168:68–74. doi: 10.1111/j.1365-2249.2011.04543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geri G, Terrier B, Rosenzwajg M, Wechsler B, Touzot M, Seilhean D, Tran TA, Bodaghi B, Musset L, Soumelis V, Klatzmann D, Cacoub P, Saadoun D. Critical role of IL-21 in modulating TH17 and regulatory T cells in Behçet disease. J Allergy Clin Immunol. 2011;128:655–64. [DOI] [PubMed]
- 15.Sugita S, Kawazoe Y, Imai A, Yamada Y, Horie S, Mochizuki M. Inhibition of Th17 differentiation by anti-TNF-alpha therapy in uveitis patients with Behçet’s disease. Arthritis Res Ther. 2012;14:R99. doi: 10.1186/ar3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kirino Y, Bertsias G, Ishigatsubo Y, Mizuki N, Tugal-Tutkun I, Seyahi E, et al. Genome-wide association analysis identifies new susceptibility loci for Behçet’s disease and epistasis between HLA-B*51 and ERAP1. Nat Genet. 2013;45:202–7. doi: 10.1038/ng.2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Criteria for diagnosis of Behçet’s disease International Study Group for Behçet’s Disease. Lancet. 1990;335:1078–80. [PubMed] [Google Scholar]
- 18.Cavuş F, Ulusoy C, Örçen A, Gül A, Tüzün E, Vural B. Increased IL-23 receptor, TNF-α and IL-6 expression in individuals with the IL23R-IL12RB2 locus polymorphism. Immunol Lett. 2014;160:96–8. doi: 10.1016/j.imlet.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 19.Na SY, Park MJ, Park S, Lee ES. Up-regulation of Th17 and related cytokines in Behçet’s disease corresponding to disease activity. Clin Exp Rheumatol. 2013;31(Suppl 77):32–40. [PubMed] [Google Scholar]
- 20.Kim J, Park JA, Lee EY, Lee YJ, Song YW, Lee EB. Imbalance of Th17 to Th1 cells in Behçet’s disease. Clin Exp Rheumatol. 2010;28(Suppl 60):S16–9. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets supporting the conclusions of this article are available as follows. The GWAS dataset of 2000 controls can be requested at Korea Biobank Network (http://cdc.go.kr/). The GWAS dataset of Korean patients with BD and the datasets generated during the current study are available from the corresponding author on a reasonable request.


