Abstract
Pregnant women have greater mortality and complications associated with viral infections compared to the general population, but the reason for the increased susceptibility is not well defined. Placental type I interferon (IFN) is an important immune modulator and protects the pregnancy. We hypothesized that loss of placental IFN affects the regulation of the maternal immune system, resulting in the differential response to infections observed in pregnancy. Pregnant mice lacking the IFN-α/β receptor (IFNAR) became viremic and had higher mortality compared to non-pregnant animals. Notably, an embryo with functional IFN signaling alone was sufficient to rescue the pregnant IFNAR−/− dam from virus-associated demise. Placental IFN was also an important regulator of viral replication in placental tissue and significantly affected viral transmission to the fetus. These findings highlight the role of fetal/placental IFN in the modulation of viral infection in the mother and fetus.
Introduction
The maternal immune system is significantly affected by pregnancy. The changes in immune function can result in the beneficial amelioration of some autoimmune disorders but can also impact the severity of the responses to some infections [1]. For example, pregnant women are more susceptible to infection with Toxoplasma gondii [2–4], Plasmodium falciparum [5], and Listeria monocytogenes [6]. They also exhibited increased susceptibility and/or disease severity associated with numerous types of viral infections. For example, pregnant women have higher mortality associated with varicella virus infection, which is 10 times more likely to be complicated by pneumonia during pregnancy [1, 7, 8]. They are more susceptible to rubeola (measles), and the infection is more likely to cause death [9, 10]. Furthermore, during the 2009 H1N1 influenza pandemic, pregnant women developed more severe flu-related complications, such as hospitalization and death, compared to the general population [11–19]. These same symptoms were also confirmed to have occurred during the 1918 H1N1 [20, 21] and 1957 H5N1 [22, 23] pandemics. Despite these clear differences, there is still little known about pregnancy-associated changes in the immune response to pathogens.
Several viruses have been shown to infect the placenta, including cytomegalovirus (CMV), herpes simplex viruses (HSV), human papillomaviruses, Lassa fever, and Zika virus [24–26]. The placenta itself is a robust immune organ that expresses pattern recognition receptors (PRR) and consequently responds to these pathogens at the maternal-fetal interface [27–33]. Activation of these PRRs by viral proteins can result in the activation of the transcription factor NF-ĸB and/or type I IFN. Type I IFN, through the IFN-α/β receptor (IFNAR), induces a multitude of IFN-stimulated genes that can block viral replication and activate the immune system in response to the virus [34, 35]. Interestingly, many viruses can target the IFN pathway, and successful inhibition enhances infection and viral spread. Specifically, we have shown, in our animal model of pregnancy and herpesvirus infection, that murine herpes virus-68 (MHV68) infects the placenta [28, 36–38] and inhibits the expression of type I IFN [28]. The inhibition of IFN results in an enhanced pro-inflammatory response to bacteria [37, 38], demonstrating that placental IFN is an important mediator of the immune response. Could these changes in placental immune modulation also affect maternal wellbeing?
We hypothesize that fetal-placental IFN signaling is critical for the protection of the fetus against viral infection and can also significantly affect maternal health. This could be due to changes in placental modulation of the maternal immune response, or the presence of the placenta could be an additional source of an infectious virus, thus enhancing disease severity. Specifically, we suggest that viral inhibition of placental IFN is a key mediator of these changes. To test our hypothesis, in this study, we compared disease severity between pregnant and non-pregnant mice without the type I IFNAR to determine whether IFN contributes to pregnancy-specific mortality during viral infections. Furthermore, we investigated viral replication and the severity of the maternal and fetal response to viral infections by manipulating embryonic-placental IFN. We demonstrate that loss of fetal-derived type I IFN, specifically, contributes to fetal viremia, and fetal-placental IFN signaling can affect maternal mortality.
Material and Methods
MHV68 production and quantification
MHV68 passaged and viral titer detection has been previously described [37, 39]. Results are reported as copies per 100 ng or 500 ng of DNA.
Animals: viral infection
All animals were maintained in the Yale University School of Medicine Animal Facility under specific pathogen-free conditions. All procedures were approved by the Yale University Institutional Animal Care and Use Committee, and between 6 and 10 mice were used in each group to obtain statistical power. The IFNAR−/− and C57BL/6 mice were obtained from the Jackson Laboratory (Bar Harbor, ME). The IFNAR−/− (B6.129S2-Ifnar1tm1Agt/Mmjax) mice were backcrossed onto C57BL6/J for 3 generations preceding experiments. Adult mice (8–12 wks of age) with vaginal plugs were infected i.p. at embryonic day (E)8.5 post-conception with 1 × 105 PFU MHV68 in 100 μl of DMEM or DMEM alone (vehicle). Viral infection was quantified by analyzing the copy number for MHV68 ORF53 using qPCR [39].
Animals: breeding crosses and genotyping
To produce litters with IFNAR+/− and IFNAR−/− pups, IFNAR−/− males were bred to IFNAR+/− females. Animals were infected with MHV68 at E8.5, as described above, and sacrificed at E15.5 to determine viral titers in placentas and fetal samples. Each fetus was paired with its placenta and genotyped using the genotyping protocol described for stock number 32045-JAX through the Jackson Laboratory. The following primers were used: common (9850): 5′-CGAGGCGAAGTGGTTAAAAG-3′; Wild-type (wt) reverse (9851): 5′-ACGGATCAACCTCATTCCAC-3′; and mutant (oIMR8963): 5′-AATTCGCCAATGACAAGACG-3′.
Animals: embryo transfers
C57BL6/J served as donor animals, while IFNAR−/− were pseudo-pregnant recipients. Donor mice, 3–4-week-old weanlings, received 5 IU pregnant mare serum gonadotropin (National Hormone and Peptide Program) on protocol day (D)1, followed by 5 IU human chorionic gonadotropin (CG10; Sigma) 46 h later, on D3. Subsequently, donor females were placed with intact C57BL6/J males overnight (1:1 ratio) and were removed by 9 AM on D4. Pregnancy was confirmed by observation of vaginal mucus plugs (E0.5). The recipient females (10–12 wks old) were bred with vasectomized males on D5 and were removed the following morning (D6). Initiation of pseudo-pregnancy in recipients was confirmed by the evidence of a vaginal mucus plug on the morning of D6, pseudo-pregnant day 0.5. E3.5 blastocysts with intact zonas were flushed from the uteri of E3.5 donors with M2 medium (Ambion). Blastocysts were washed by moving into 3 separate microdrops of M2 and placed in a microdrop of KSOM (Ambion) under embryo-tested oil (Irvine Scientific). Between 16 and 20 embryos were transferred into P2.5 recipients utilizing a trans-cervical, non-surgical embryo transfer device (ParaTechs).
Statistics
Differences between means (3 groups or more) were determined using ANOVA, and differences between two groups were analyzed using independent t-test functions of GraphPad inSTAT statistical software (La Jolla, CA). Differences under curves were analyzed with GraphPad to determine statistical differences in survival. A p-value of ≤0.05 was considered significant, and data are presented as mean +/− standard error of the mean (SEM).
Results and Discussion
Mortality is higher in pregnant ifnar−/− compared to non-pregnant ifnar−/− mice infected with MHV68
MHV68 is a natural murine pathogen that is not lethal to wt mice [38]. However, non-pregnant ifnar−/− mice are more susceptible to MHV68 infection and show ~50% mortality within six weeks of infection. The susceptibility of pregnant wt or ifnar−/− mice to MHV-68 is unknown. To address this issue, we infected pregnant (E8.5) and non-pregnant C57BL6/J (wt) and ifnar−/− mice with 105 PFU MHV68 and monitored their responses. Infected pregnant wt mice did not show any sign of illness, and all survived (Figure 1A). Strikingly, 100% of the pregnant ifnar−/− mice succumbed within 5 days post-infection with MHV68 (Figure 1B). Similar to previous reports, the non-pregnant ifnar−/− mice did not show any sign of mortality during the same period (Figure 1B). We observed a decrease in survival only after 10 d post-infection (Figure 1B) [40, 41], demonstrating that the loss of type I IFN signaling during pregnancy was more detrimental to pregnant animals.
Figure 1. Loss of IFNAR results in higher mortality in pregnant mice compared to non-pregnant mice infected with virus.
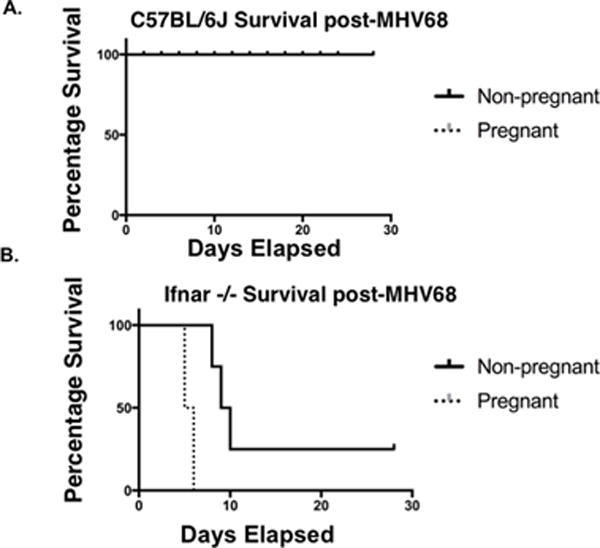
All pregnant animals were infected with 105 PFU MHV68 i.p. on E8.5. (A) C57BL6/J pregnant versus non-pregnant survival (n=10). (B) ifnar −/− pregnant versus non-pregnant survival (n=8).
Placental and fetal IFN signaling can protect the mother from fetal viremia in the absence of maternal IFNAR
Because one obvious difference between pregnant and non-pregnant mice is the presence of the placental/fetal unit, we next determined whether the loss of IFN signaling in the embryo/placenta, specifically, contributed to the increased mortality observed in the pregnant ifnar−/− mice. For this, wt embryos were transferred into pseudo-pregnant ifnar−/− mice that were then infected with MHV68 at day E8.5, and animals were monitored for signs of illness or mortality (Figure 2A). Strikingly, the presence of wt embryos completely prevented MHV68-induced maternal mortality of pregnant ifnar−/− mice (Figure 2B). Further, the ifnar−/− mothers with wt embryos had significantly lower viral titers in the spleen (10,000 times lower), compared to ifnar−/− mothers carrying ifnar−/− pups. The viral titers observed in the ifnar−/− mothers with wt embryos were similar to splenic titers observed in wt mothers and embryos (Figure 3).
Figure 2. Placental IFN is sufficient to protect IFNAR−/− mothers from virus-associated mortality.

(A) Experimental design: wt embryos were transferred into pseudo-pregnant ifnar−/− recipients and received MHV68 on E8.5. (B) wt embryos protected IFNAR−/− dams from virus-induced mortality (n=6).
Figure 3. Effect of placental IFN on maternal viral titers.

Maternal viral infection was evaluated in the spleen of wt and ifnar−/− dams carrying wt or ifnar−/− embryos. The dam’s splenic MHV68 titers are 8.88 × 105 when the embryo and dam are wt; the dam’s splenic MHV68 titers are 2.87 × 1010 when the embryo and dam are ifnar−/−, and the dam’s splenic titers are reduced to 8.12 × 106 when the embryo is wt and the dam is ifnar−/− (n=5) *p = 0.0314.
Loss of fetal-placental IFNAR results in viral transmission to the fetus
To test the role of fetal-placental IFNAR signaling in controlling local viral replication, we infected wt and ifnar−/− mice at E8.5 and compared viral titers of the placentas and fetuses at E15.5. As expected, wt animals had significantly lower placental titers compared to ifnar−/− mice (Figure 4A); furthermore, there was no viral transmission to wt fetuses (Figure 4B). In contrast, we found significant MHV68 viral titers in the placenta and fetus of ifnar−/− mice (the viral titers exceeded 1010 in ifnar−/− placentas and fetuses) (Figure 4).
Figure 4. Type I IFN inhibits placental viral infection and viral transmission to the fetus.

Wt and ifnar−/− pregnant mice were exposed to MHV68 on E8.5, and viral titers were determined on E15.5. Placental (A) and fetal (B) MHV68 titers are significantly higher in ifnar−/− mice compared to wt mice (n=6). *p <0.001.
Next, we bred ifnar−/− males with ifnar+/− females. This resulted in litters with half of the embryos/placentas lacking a functional IFNAR (homozygous ifnar−/−) in a mother with functional IFNAR (ifnar+/−) and half of the embryos/placenta with a functional IFNAR (heterozygous ifnar+/−) in a heterozygous (ifnar+/−) mother. Pregnant females were infected with MHV68 on E8.5; at E15.5, the fetal-placental units were collected and genotyped, and viral titers were quantified in the individual placentas and fetuses. Although the mothers had a functional IFNAR, viral titers were significantly higher in placenta and fetus lacking IFNAR signaling (homozygous ifnar−/−) compared to those with a functional IFNAR (heterozygous ifnar+/−) (Figure 5). Therefore, despite sharing the same maternal phenotype (functional IFNAR), the susceptibility to infection was increased in fetal/placental units lacking a functional IFNAR (Figure 5).
Figure 5. Placental type I IFN regulates viral transmission to the placenta and fetus.

ifnar+/− females were bred with ifnar−/− males. Pregnant mice were infected with MHV68 at E8.5, and embryos were collected and genotyped at E15. Representative samples of embryonic genotypes from IFNAR−/− embryos (single band) or IFNAR+/− embryos (double band) and associated viral titers in their respective placentas (A) and fetuses (B). Viral titers from homozygous ifnar−/− and heterozygous ifnar+/− embryos. IFNAR−/− embryos have higher viral titers even when the mother is a heterozygote (n=5–7).
In summary, this study demonstrates placental/fetal IFN signaling regulates fetal viral transmission and has the potential to modulate maternal viral infection. It is well established that many viruses inhibit IFN signaling as a mechanism to evade the host immune response. Therefore, a virus that successfully inhibits placental IFN could be much more effective at infecting the fetus. We also provided evidence that the fetus/placenta can affect the mother by demonstrating that fetal/placental IFN signaling was sufficient to protect the mother from viremia and death. We postulate that if placental IFN is inhibited, this could also affect placental function and placental regulation of the maternal immune response; this is the basis of ongoing studies.
Acknowledgments
We are grateful to A. F. Parlow, Scientific Director at Los Angeles Biomedical Research Institute National Hormone & Peptide Program for the Pregnant Mare Serum Gonadotropin used for superovulation protocols for embryo transfer experiments. Also, we extend our gratitude to JoAnn Bilyard for the careful editing of this manuscript.
Funding Statement
This study is in part funded by grants from the National Institute of Health, 3N01 HD23342 and the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services.
Footnotes
Disclosure
The authors report no conflicts of interest.
References
- 1.Jamieson DJ, Theiler RN, Rasmussen SA. Emerging infections and pregnancy. Emerg Infect Dis. 2006;12:1638–1643. doi: 10.3201/eid1211.060152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Avelino MM, Campos D, Jr, do Carmo Barbosa de Parada J, de Castro AM, et al. Pregnancy as a risk factor for acute toxoplasmosis seroconversion. Eur J Obstet Gynecol Reprod Biol. 2003;108:19–24. doi: 10.1016/s0301-2115(02)00353-6. [DOI] [PubMed] [Google Scholar]
- 3.Kravetz JD, Federman DG. Toxoplasmosis in pregnancy. Am J Med. 2005;118:212–216. doi: 10.1016/j.amjmed.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 4.Avelino MM, Campos D, Jr, Parada JB, Castro AM. Risk factors for Toxoplasma gondii infection in women of childbearing age. Braz J Infect Dis. 2003;8:164–174. doi: 10.1590/s1413-86702004000200007. [DOI] [PubMed] [Google Scholar]
- 5.Okoko BJ, Enwere G, Ota MOC. The epidemiology and consequences of maternal malaria: a review of immunological basis. Acta Trop. 2003;87:193–205. doi: 10.1016/s0001-706x(03)00097-4. [DOI] [PubMed] [Google Scholar]
- 6.Gellin BG, Broome CV, Bibb WF, Weaver RE, Gaventa S, Mascola L. The epidemiology of listeriosis in the United States-1986. Listeriosis Study Group. Am J Epidemiol. 1991;133:392–401. doi: 10.1093/oxfordjournals.aje.a115893. [DOI] [PubMed] [Google Scholar]
- 7.Haake DA, Zakowski PC, Haake DL, Bryson YJ. Early treatment with acyclovir for varicella pneumonia in otherwise healthy adults: retrospective controlled study and review. Rev Infect Dis. 1990;12:788–798. doi: 10.1093/clinids/12.5.788. [DOI] [PubMed] [Google Scholar]
- 8.Paryani SG, Arvin AM. Intrauterine infection with varicella-zoster virus after maternal varicella. N Engl J Med. 1986;314:1542–1546. doi: 10.1056/NEJM198606123142403. [DOI] [PubMed] [Google Scholar]
- 9.Atmar RL, Englund JA, Hammill H. Complications of measles during pregnancy. Clin Infect Dis. 1992;14:217–226. doi: 10.1093/clinids/14.1.217. [DOI] [PubMed] [Google Scholar]
- 10.Christensen PE, Schmidt H, Bang HO, Andersen V, Jordal B, Jensen O. An epidemic of measles in southern Greenland, 1951; measles in virgin soil. III. Measles and tuberculosis. Acta Med Scand. 1953;144:450–454. doi: 10.1111/j.0954-6820.1953.tb15718.x. [DOI] [PubMed] [Google Scholar]
- 11.Anderson BL, Rouse DJ, Fitzsimmons C. Clinical characteristics of pregnant women with influenza-like illness during the 2009 H1N1 pandemic and use of a standardized management algorithm. Am J Obstet Gynecol. 2011;204(6 Suppl 1):S31–37. doi: 10.1016/j.ajog.2011.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Creanga AA, Johnson TF, Graitcer SB, Hartman LK, Al-Samarrai T, Schwarz AG, Chu SY, Sackoff JE, Jamieson DJ, Fine AD, Shapiro-Mendoza CK, Jones LE, Uyeki TM, Balter S, Bish CL, Finelli L, Honein MA. Severity of 2009 pandemic influenza A (H1N1) virus infection in pregnant women. Obstet Gynecol. 2010;115:717–726. doi: 10.1097/AOG.0b013e3181d57947. [DOI] [PubMed] [Google Scholar]
- 13.Creanga AA, Kamimoto L, Newsome K, D’Mello T, Jamieson DJ, Zotti ME, Arnold KE, Baumbach J, Bennett NM, Farley MM, Gershman K, Kirschke D, Lynfield R, Meek J, Morin C, Reingold A, Ryan P, Schaffner W, Thomas A, Zansky S, Finelli L, Honein MA. Seasonal and 2009 pandemic influenza A (H1N1) virus infection during pregnancy: a population-based study of hospitalized cases. Am J Obstet Gynecol. 2011;204(6 Suppl 1):S38–S45. doi: 10.1016/j.ajog.2011.02.037. [DOI] [PubMed] [Google Scholar]
- 14.Ellington SR, Hartman LK, Acosta M, Martinez-Romo M, Rubinson L, Jamieson DJ, Louie J. Pandemic 2009 influenza A (H1N1) in 71 critically ill pregnant women in California. Am J Obstet Gynecol. 2011;204(6 Suppl 1):S21–S30. doi: 10.1016/j.ajog.2011.02.038. [DOI] [PubMed] [Google Scholar]
- 15.Jamieson DJ, Honein MA, Rasmussen SA, Williams JL, Swerdlow DL, Biggerstaff MS, Lindstrom S, Louie JK, Christ CM, Bohm SR, Fonseca VP, Ritger KA, Kuhles DJ, Eggers P, Bruce H, Davidson HA, Lutterloh E, Harris ML, Burke C, Cocoros N, Finelli L, MacFarlane KF, Shu B, Olsen SJ, Novel Influenza A (H1N1) Pregnancy Working Group H1N1 2009 influenza virus infection during pregnancy in the USA. Lancet. 2009;374(9688):451–458. doi: 10.1016/S0140-6736(09)61304-0. [DOI] [PubMed] [Google Scholar]
- 16.Louie JK, Acosta M, Jamieson DJ, Honein MA, California Pandemic (H1N1) Working Group Severe 2009 H1N1 influenza in pregnant and postpartum women in California. N Engl J Med. 2010;362:27–35. doi: 10.1056/NEJMoa0910444. [DOI] [PubMed] [Google Scholar]
- 17.Marcelin G, Aldridge JR, Duan S, Ghoneim HE, Rehg J, Marjuki H, Boon AC, McCullers JA, Webby RJ. Fatal outcome of pandemic H1N1 2009 influenza virus infection is associated with immunopathology and impaired lung repair, not enhanced viral burden, in pregnant mice. J Virol. 2011;85:11208–11219. doi: 10.1128/JVI.00654-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rasmussen SA, Kissin DM, Yeung LF, MacFarlane K, Chu SY, Turcios-Ruiz RM, Mitchell EW, Williams J, Fry AM, Hageman J, Uyeki TM, Jamieson DJ, Pandemic Influenza and Pregnancy Working Group Preparing for influenza after 2009 H1N1: special considerations for pregnant women and newborns. Am J Obstet Gynecol. 2011;204(6 Suppl 1):S13–S20. doi: 10.1016/j.ajog.2011.01.048. [DOI] [PubMed] [Google Scholar]
- 19.Siston AM, Rasmussen SA, Honein MA, Fry AM, Seib K, Callaghan WM, Louie J, Doyle TJ, Crockett M, Lynfield R, Moore Z, Wiedeman C, Anand M, Tabony L, Nielsen CF, Waller K, Page S, Thompson JM, Avery C, Springs CB, Jones T, Williams JL, Newsome K, Finelli L, Jamieson DJ, Pandemic H1N1 Influenza in Pregnancy Working Group Pandemic 2009 influenza A(H1N1) virus illness among pregnant women in the United States. JAMA. 2010;303:1517–1525. doi: 10.1001/jama.2010.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Watanabe T, Kawaoka Y. Pathogenesis of the 1918 pandemic influenza virus. PLoS Pathog. 2011;7:e1001218. doi: 10.1371/journal.ppat.1001218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harris JW. Influenza in pregnant women. JAMA. 1919;72:978–980. [Google Scholar]
- 22.Hardy JM, Azarowicz EN, Mannini A, Medearis DN, Jr, Cooke RE. The effect of Asian influenza on the outcome of pregnancy, Baltimore, 1957–1958. Am J Public Health Nations Health. 1961;51:1182–1188. doi: 10.2105/ajph.51.8.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Freeman D, Barno A. Deaths from Asian influenza associated with pregnancy. Am J Obstet Gynecol. 1959;78:1172–1175. doi: 10.1016/0002-9378(59)90570-8. [DOI] [PubMed] [Google Scholar]
- 24.Gomez LM, Ma Y, Ho C, McGrath CM, Nelson DB, Parry S. Placental infection with human papillomavirus is associated with spontaneous preterm delivery. Hum Reprod. 2008;23:709–715. doi: 10.1093/humrep/dem404. [DOI] [PubMed] [Google Scholar]
- 25.Kwon JY, Romero R, Mor G. New insights into the relationship between viral infection and pregnancy complications. Am J Reprod Immunol. 2014;71:387–390. doi: 10.1111/aji.12243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chatterjee A, Chartrand SA, Harrison CJ, Felty-Duckworth A, Bewtra C. Severe intrauterine herpes simplex disease with placentitis in a newborn of a mother with recurrent genital infection at delivery. J Perinatol. 2001;21:559–564. doi: 10.1038/sj.jp.7210573. [DOI] [PubMed] [Google Scholar]
- 27.Abrahams VM, Schaefer TM, Fahey JV, Visintin I, Wright JA, Aldo PB, Romero R, Wira CR, Mor G. Expression and secretion of antiviral factors by trophoblast cells following stimulation by the TLR-3 agonist, Poly(I : C) Hum Reprod. 2006;21:2432–2439. doi: 10.1093/humrep/del178. [DOI] [PubMed] [Google Scholar]
- 28.Racicot K, Kwon JY, Aldo P, Abrahams V, El-Guindy A, Romero R, Mor G. Type I interferon regulates the placental inflammatory response to bacteria and is targeted by virus: mechanism of polymicrobial infection-induced preterm birth. Am J Reprod Immunol. 2016 Apr;75:451–60. doi: 10.1111/aji.12501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abrahams VM, Aldo PB, Murphy SP, Visintin I, Koga K, Wilson G, Romero R, Sharma S, Mor G. TLR6 modulates first trimester trophoblast responses to peptidoglycan. J Immunol. 2008;180:6035–6043. doi: 10.4049/jimmunol.180.9.6035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abrahams VM, Visintin I, Aldo PB, Guller S, Romero R, Mor G. A role for TLRs in the regulation of immune cell migration by first trimester trophoblast cells. J Immunol. 2005;175:8096–8104. doi: 10.4049/jimmunol.175.12.8096. [DOI] [PubMed] [Google Scholar]
- 31.Mor G, Romero R, Aldo PB, Abrahams VM. Is the trophoblast an immune regulator? The role of Toll-like receptors during pregnancy. Crit Rev Immunol. 2005;25:375–388. doi: 10.1615/critrevimmunol.v25.i5.30. [DOI] [PubMed] [Google Scholar]
- 32.Hauguel-de Mouzon S, Guerre-Millo M. The placenta cytokine network and inflammatory signals. Placenta. 2006;27:794–798. doi: 10.1016/j.placenta.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 33.Keelan JA, Blumenstein M, Helliwell RJ, Sato TA, Marvin KW, Mitchell MD. Cytokines, prostaglandins and parturition--a review. Placenta. 2003;24(Suppl A):S33–S46. doi: 10.1053/plac.2002.0948. [DOI] [PubMed] [Google Scholar]
- 34.Teijaro JR, Ng C, Lee AM, Sullivan BM, Sheehan KC, Welch M, Schreiber RD, de la Torre JC, Oldstone MB. Persistent LCMV infection is controlled by blockade of type I interferon signaling. Science. 2013 Apr 12;340:207–211. doi: 10.1126/science.1235214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilson EB, Yamada DH, Elsaesser H, Herskovitz J, Deng J, Cheng G, Aronow BJ, Karp CL, Brooks DG. Blockade of chronic type I interferon signaling to control persistent LCMV infection. Science. 2013;340:202–207. doi: 10.1126/science.1235208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leeming GH, Kipar A, Hughes DJ, Bingle L, Bennett E, Moyo NA, Tripp RA, Bigley AL, Bingle CD, Sample JT, Stewart JP. Gammaherpesvirus infection modulates the temporal and spatial expression of SCGB1A1 (CCSP) and BPIFA1 (SPLUNC1) in the respiratory tract. Lab Invest. 2015;95:610–624. doi: 10.1038/labinvest.2014.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cardenas I, Means RE, Aldo P, Koga K, Lang SM, Booth CJ, Manzur A, Oyarzun E, Romero R, Mor G. Viral infection of the placenta leads to fetal inflammation and sensitization to bacterial products predisposing to preterm labor. J Immunol. 2010;185:1248–1257. doi: 10.4049/jimmunol.1000289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cardenas I, Mor G, Aldo P, Lang SM, Stabach P, Sharp A, Romero R, Mazaki-Tovi S, Gervasi M, Means RE. Placental viral infection sensitizes to endotoxin-induced pre-term labor: a double hit hypothesis. Am J Reprod Immunol. 2011;65:110–117. doi: 10.1111/j.1600-0897.2010.00908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cardenas I, Aldo P, Koga K, Means R, Lang S, Mor G. Subclinical viral infection in pregnancy leads to inflammatory process at the placenta with non-lethal fetal damage. Am J Reprod Immunol. 2009;61:397. [Google Scholar]
- 40.Barton ES, Lutzke ML, Rochford R, Virgin HW., 4th Alpha/beta interferons regulate murine gammaherpesvirus latent gene expression and reactivation from latency. J Virol. 2005;79:14149–14160. doi: 10.1128/JVI.79.22.14149-14160.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dutia BM, Allen DJ, Dyson H, Nash AA. Type I interferons and IRF-1 play a critical role in the control of a gammaherpesvirus infection. Virology. 1999;261:173–179. doi: 10.1006/viro.1999.9834. [DOI] [PubMed] [Google Scholar]


