Abstract
NK cell receptors play a critical role in the homeostasis of antigen-experienced T cells. Indeed, prolonged antigen stimulation may induce changes in the receptor repertoire of T cells to a profile that features NK cells receptors (NKRs). Chronic antigen exposure, at the same time, has been shown to trigger the loss of co-stimulatory CD28 molecules with recently reported intensified antigen thresholds of antigen-experienced CD8+ T cells.
In transplantation, NKRs have been shown to assist allograft rejection in a CD28-independent fashion. Here, we discuss a role for CD28-negative T cells that have acquired the competency of the NKR machinery, potentially promoting allorecognition either through TCR cross-reactivity or independently from TCR recognition.
Collectively, NKRs can bring about innate-like T cells by providing alternative co-stimulatory pathways that gain significance in chronic inflammation, potentially leading to resistance to CD28-targeting immunosuppressants.
Keywords: NK cell receptors, T cells, innate immunity, adaptive immunity, transplantation
Crossing the border between innate and adaptive immunity
NK cell receptors (NKRs) have traditionally been considered as key players of innate immunity. Indeed, hallmarks of innate immunity include germline-encoded receptors such as NKRs that can directly recognize peptide motifs. In NK cells, activation or inhibition is determined by the balance of inhibitory and stimulatory NKR signals referred to as missing-self and induced-self recognition [1,2]. A balance of co-stimulatory and co-inhibitory receptor signals modulating T cell activation has also been investigated more recently [3]. In contrast, hallmarks of adaptive immunity have been characterized by the formation of antigen-specific recall responses characterized by differentiation and clonal expansion, leading to immunological memory [4]. Clear lines that distinguish innate from adaptive immune features appear to fade. In addition to adaptive memory features in NK cell immunity [5,6], NKRs also appear to play a critical role in T cell immunity. T cells using innate receptors such as NKRs while expressing T cell receptors (TCRs) responded rapidly to stress and have been recognized as innate-like T cells recently [7]. Importantly, antigenic TCR signaling has been critical to initiate the development of these innate-like T cells, but attenuated subsequently. Thus, it has been suggested that the suppression of conventional TCR signaling in parallel to an acquisition of innate signaling pathways is characteristic of innate-like T cells [7].
These insights have driven concepts that propose a new distinction of lymphocytes based on antigen-experience rather than a mere partition of innate versus adaptive lymphocytes [8]. This model proposes that antigen-experienced conventional T cells and innate lymphocytes exist in a common state of differentiation with identical conserved functions that are notably distinct from conventional naïve T cells. Thus, in addition to established players of adaptive immunity including traditional T and B cells, innate-like T cell populations including γ and δ TCR chain-expressing T cells (γδ T cells), CD1d-restricted natural killer T cells (NKT cells), invariant TCR α-chain expressing NKT cells (iNKT cells) and vaguely defined non-cognate αβ TCR CD8+ T cells have been introduced [8,9].
Although the relevance for NKR co-stimulation in antigen-experienced T cell immunity has been demonstrated, changes in NKR expression patterns and functional consequences, in particular during chronic antigen exposure, have not received major attention thus far.
Here, we discuss conditions and consequences of NKR expression in T cell immunity, crosslinking antigen-experience, NKR diversity and functional implications in T cell responses.
Antigen-experience and the innate capacity in T cells
Predominantly CD8+ T cells can express a wide array of stimulatory and inhibitory NKRs (Table 1). At the same time, antigenic TCR stimulation may alter NKR expression patterns on T cells.
Table 1.
NK cell receptors, respective function and ligands
| Receptor | Species | Function | Ligand |
|---|---|---|---|
|
NKG2D (Natural Killer Group
2D) |
Human, mouse | stimulatory | MICA/B and ULBP (human), RAE-1 and H60 (mouse) |
|
KIRs (killer cell
immunoglobulin-like receptors) |
Human | Isotype-dependent: KIR2DS2 stimulatory KIR2DL1/2/3 inhibitory |
Known for various MHC I molecules: HLA-A/B/C |
| CD94/NKG2 heterodimer | Human, mouse | Isotype-dependent: NKG2A inhibitory NKG2C stimulatory |
MHC I, HLA-E (human), Qa-1 (mouse) |
|
KLRB1 (killer cell lectin-like
receptor B1) |
Human (CD161), mouse (NK1.1) |
stimulatory | CLEC2D (LLT1) |
|
KLRG1 (killer cell lectin-like
receptor G1) |
Human, mouse | inhibitory | Cadherins |
| 2B4 (CD244) | Human, mouse | stimulatory or inhibitory | CD48 |
| CD160 (BY55) | Human, mouse | stimulatory or inhibitory | MHC I, HLA-C (human), herpesvirus entry mediator |
Previously, TCR stimulation had been linked to the up-regulation of the inhibitory NKG2A isotype on human CD8+ T cells, characteristic of effector-memory CD8+ T cells with an entirely distinct TCR repertoire compared to CD94low NKG2A− CD8+ T cells [10]. In this context, NKG2A-committed clones expressed sequence-related TCRs that indicated an identical antigen specificity presumably recognizing the same or a similar peptide, thus emphasizing on the relevance of TCR specificity for NKG2A-specfic commitment. Collectively, NKG2A commitment had been considered to reflect antigen persistence as a phenomenon developmentally acquired subsequent to TCR expression and during an initial TCR-dependent antigen encounter. Eventually, this process had been suggested to prevent clonal exhaustion during which antigen persistence drives differentiated effector cells into a state of functional exhaustion. More recently, it has been shown that signaling via the TCR is critical for the development of innate-like T cells [7]. Innate-like T lymphocytes, for example, have been characterized in murine epithelial γδ T cells and responded to stimulatory NKG2D ligands in the epidermis [11]. In support, murine memory CD8+ T cells that had been challenged by bacteria, up-regulated NKG2D and were subsequently either reactivated in in the presence of IL-15 and IL-18 [12] or in an NKG2D-dependent manner [13] largely independent from antigenic TCR stimulation. TCR engagement has also been suggested to be required to enhance the differentiation of pathogen-specific memory KLRG1+ NK1.1+ CD8+ T cells that developed in the presence of IL-15 during viral or bacterial infections, providing antigen-independent responses against microbial re-infections [14].
While downstream signaling pathways of NKG2D involve the same adaptor molecule linked to CD28, inducing the PI3K/mTOR signaling cascade, activation of NKG2D/TCR in contrast to CD28/TCR has recently been suggested to increase the formation of memory precursor CD8+ T cells based on intermediate levels of mTOR complex 1 (mTORc1) activation [15]. Interestingly, augmented co-stimulation through NKG2D has also been shown to rescue memory CD8+ T cell responses in the absence of CD4+ T cell help, mainly dependent on IL-15 [16]. Furthermore, IL-15 exposure has been linked to CD28 down-regulation and memory CD8+ T cell maintenance, preparing memory CD8+ T cells for cell-cycle entry via the mTORc1 pathway and independently of antigen re-encounter [17,18]. Similarly, mTORc1 has also been involved in IL-15-mediated development and activation of NK cells [19].
In parallel to processes that alter NKR expression through antigenic TCR stimulation, prolonged antigen stimulation through complete TCR/CD28 engagement has been shown to trigger the loss of CD28 co-stimulatory molecules on T cells [20]. Moreover, TCR down-regulation on murine antigen-experienced memory CD8+ T cells and augmented antigen thresholds during successive recall engagements have been reported [21], all in support of conditional innate-like features that may compensate for the loss of conventional stimulatory signals on antigen-experienced T cells.
NKR-expressing T cells in aging and chronic inflammation
Although chronic antigen exposure may exhaust classical CD28/TCR activity leading to the expression of NKRs on specific T cell clones, clear mechanisms driving those events remain unclear. Most notably, an augmented expression of NKRs on some T cells may provide, at least in part, the capacity for antigen-independent, innate-like responses. Therefore, phenotypic shifts that determine the functional capacity of these T cells to respond in an innate, NK cell-like fashion may involve two pathways: i) an absolute increase of NKRs expressed on T cells may boost NK cell-like function through an augmented density, and, ii) the down-regulation of conventional adaptive pathways involving co-stimulatory molecules such as CD28, or, a compromised TCR engagement may result in an enhanced innate function, affecting the signaling balance by favoring NKR pathways as alternate co-stimulatory signals through the lack of CD28/TCR on a clonal basis. Those mechanisms may substitute classical co-stimulatory signals and promote allorecognition either by TCR cross-reactivity or completely independent from TCR recognition (Fig. 1).
Figure 1. Key Figure: T cells acquire innate characteristics by expressing NK cell receptors subsequent to chronic antigen challenge.
Antigen-presenting cells (APC) stimulate TCR/CD28-mediated signals and activation (blue). Antigen-experienced memory T cells may lose CD28 and require an augmented antigen threshold over time, thus supporting a resistance to classical adaptive stimulatory pathways (grey). Chronic antigen challenge, in turn, may induce the expression of NK cell receptors (NKRs) on some T cell clones (pink), ultimately facilitating the response of antigen-experienced T cells based on acquired NKR signaling . Those mechanisms may compensate for the lost capacity of conventional adaptive pathways (purple).
APC , antigen-present ing cell; TCR , T cell receptor; Ag, antigen; sNKR, stimulatory NK cell receptor; iNKR, inhibiting NK cell receptor ; SL, stimulatory ligand; IL, inhibiting ligand.
It has been recognized that particularly CD8+ T cells increase their NKR expression patterns subsequent to viral or bacterial stimuli [22,23]. Furthermore, aging and chronic inflammation lead to an expansion of NKR-expressing T cells [24,25]. Strikingly, virus-specific CD8+ T cells have been reported to up-regulate 29 stimulatory and inhibitory NKRs during the acute phase of cytomegalovirus (CMV) reactivation in renal transplant recipients; 19/29 NKRs remained elevated one year after cessation of viral replication [26]. In unrelated allogeneic stem cell transplantation, the expansion of Granzyme Bhigh CD28low CD57high CD8+ effector-memory T cells during the course of CMV reactivation had been accompanied by a contraction in TCRβ diversity and increased clonality in the effector-memory compartment [27]. This process is suggestive of previous antigen-specific activation that lead to the oligoclonal T cell expansion of (CMV-specific) effector-memory T cells – albeit with a qualitatively compromised TCR repertoire when compared to naïve T cells. Moreover, age and CMV positivity had not only been linked to the expansion of specific CD8+ CD56+ NKT-like subsets, but also to an increased functional responsiveness to the superantigen staphylococcal enterotoxin B [28]. In aging, particularly CD57-expressing NKT-like cell population displayed an augmented functional responsiveness. Similar shifts in NKR patterns have been shown to occur within the adaptive T cell compartment of the maturing immune system characterized by the expression of CD57 [29]. While mature CD8+ T cells acquired both stimulatory and inhibitory NKRs, NK cells had acquired inhibitory receptors. Therefore, it had been suggested that enhanced NKR signaling in NKR-expressing T cells may be linked to a compromised antigen-specificity and -dependency. Clinically, an expression of CD57 has been observed on pre-transplant PD1− CD28− CD4+ T cells implicated in CD28 co-stimulatory blockade-resistant rejections after renal transplantation [30]. Similarly, an expansion of terminally differentiated effector-memory CD27− CD28− CD8+ T cells and restricted TCR Vβ diversity correlated with the expression of CD57, clinically linked to long-term kidney graft dysfunction [31]. However, NKR patterns had not been assessed in both studies. Lately, CD57+ CD8+ T cells have also been shown to predict cutaneous squamous cell carcinomas in immunosuppressed patients [32].
The role of NKRs and NK cells in alloimmunity
In transplantation, an increase of antigen-experienced memory T cells is based on a pre-existing pool of memory T cells or, alternatively, representative of a de-novo antigen-experienced T cell population in response to alloantigens [33]. Earlier, allospecific memory T cells have been shown to become activated independently of homing mechanisms to secondary lymphoid organ, potentially bypassing the need of co-stimulatory signals by classical antigen-presenting cells [34]. Dendritic cells constitute a critical population of antigen-presenting cells. Following transplantation, host- as well as donor organ-derived dendritic cells can capture alloantigens, traffic to secondary lymphoid organs and present these to recipient T cells. In this context, dendritic cells stimulate naïve T cells by processing and presenting antigens on MHC molecules and providing additional co-stimulatory surface molecules such as CD40, CD80 and CD86.
Initially, NK cell transcripts had been linked to antibody-mediated rejection (AMR) in renal transplantation. More detailed analyses helped to conceptualize a complement-independent pathway of AMR involving NK cells [35–37]. In experimental studies, a detrimental involvement of NK cells in chronic graft injury has been suggested in both kidney and heart transplantation [38,39]. Conversely, peripheral transcripts linked to NK cells have also been detected in recipients developing operational tolerance after liver transplantation [40]. In several murine transplant models, NK cells have been shown to prolong graft survival when administering different combinations of co-stimulatory blockade involving anti-CD154 (CD40L), thereby inhibiting co-stimulatory CD40-CD40L interactions on T cells [41–44]. Therefore, it had been suggested that the inhibition of CD40/CD40L signaling on alloreactive dendritic cells and/or T cells by a CD154-specific monoclonal antibody may turn these cells susceptible to NK cell killing rather than increasing NK cell activity [41]. In addition, murine CD27low NK cells had been linked to a compromised expansion of IFN-γ–producing memory CD8+ T cells [44]. However, it remains to be determined whether comparable scenarios are also involved in clinical organ transplantation.
Alloreactive CD8+ T cells that expressed multiple stimulatory and inhibitory NKRs restricted to HLA-E, a non-classical HLA class Ib molecule, had been linked to CMV reactivation in kidney-transplanted patients [45]. HLA-E is a critical ligand for CD94/NKG2 heterodimers and HLA-E restricted CD8+ T cells had been shown to attack allogeneic endothelial cells in vitro, suggesting that a HLA-E restricted subset of CD8+ T cells may play a role in both, control of CMV infection and graft damage. Moreover, HLA-E-restricted CD8+ T cells displayed broad TCR-dependent cytolytic activities towards phytohemagglutinin-induced blasts derived from HLA-unmatched donors in vitro [46]. CMV-associated CD28− CD4+ T cells expressing CD57, NKG2D and perforin in kidney-transplanted patients attacked glomerular endothelial cells in an NKG2D-dependent manner in vitro, although no direct evidence for HLA-alloreactivity had been reported [47]. Viral infections in transplanted immunocompromised patients may therefore recruit NK cell-like T cells, suggesting a role of NK cell-like CD8+ T cells and/or CD4+ T cells during both antiviral defenses and rejection.
Importantly, NKRs have been shown to assist allograft rejection in a CD28-independent fashion [48,49]. More recently, NK cell signatures have also been implicated in T cell-mediated rejections subsequent to renal transplantation [50]. However, it remains unclear if these NK cell transcripts reflect an increasing presence of NK cells or rather a phenomenon of T cells expressing NKRs as suggested by the authors.
T cell responses independently of CD28/TCR engagement
NKRs play an important role in alloimmunity during CD28 blockade or subsequent to the loss of CD28 expression on T cells by providing alternative stimulatory signals subsequent to encountering their ligands on allogeneic cells. Recent findings suggest that CD8+ memory T cells have the potential to down-regulate their surface TCR expression while acquiring augmented levels of protein tyrosine phosphatases that suppress TCR signal transduction [21]. Furthermore, impaired TCR signal transduction has also been linked to aging in clinical and experimental systems [51]. Indeed, consequences of CD28 loss previously recognized in transplantation may also be linked to changes in NKR profiles on T cells [52].
Innate-like, cytokine-dependent activation
Chronic antigen exposure during viral infections has been shown to generate innate-like CD8+ T cells that respond to cytokines in a TCR-independent fashion [53]. Furthermore, it has been demonstrated that CD8+ T cells responded in an antigen-independent, innate-like fashion when exposed to secondary viral stimulations [54]. The activation of iNKT cells, characterized by the invariant Vα14Jα18 TCR sequence had been triggered by cytokines in the absence of antigenic stimulation during viral or bacterial infections [55]. Clinically, iNKT cells acquire transient innate responsiveness through IL-12 and IL-18 exposure subsequent to weak TCR stimulation [56].
Collectively, it appears that tonic TCR signaling is not required for iNKT cell homeostasis, lineage identity, and rapid cytokine secretion [57]. Similarly, antigen-experienced memory T cell homeostasis does not require the contact of TCR with MHC molecules to maintain numbers, identity, and functional capacities in the absence of antigen [57,58].
Experimental suppression of the CD28 pathway
The two-signal model of T cell activation characterized initially the necessity of a secondary co-stimulatory signal such as CD28, in addition to TCR ligation [59]. Therefore, TCR signaling in the absence of additional co-stimulatory signals has been shown to be insufficient for the activation of T cells while triggering activation-induced cell death [60]. Thus, when suppressing the conventional CD28 co-stimulatory pathway, distinct co-stimulatory molecules may substitute secondary signals and gain functional relevance.
In a landmark study on NK cell involvement in alloimmunity, CD28-deficient mice experienced prolonged cardiac allograft survival when NK1.1-expressing cells were depleted [48]. Notably, increased frequencies of graft infiltrating NK1.1-expressing cells in CD28−/− hosts were found more pronounced on NK1.1+ T cells rather than NK1.1+ NK cells. Moreover, CD4+ T cell infiltration was significantly affected in CD28−/− hosts, reducing the overall CD4/CD8 T cell ratio. Similarly, depletion of 2B4-expressing cells resulted into prolonged cardiac allograft survival of CD28−/− recipients, although a role of classical CD1+ NKT cells in supporting rejection had been excluded in this model [61]. In the same cardiac transplant model, CD8+ T cell graft infiltrates had significantly increased in CD28−/− hosts compared to wild type animals [62]. Thus, rejection in CD28−/− hosts had been linked to the presence of CD8+ T cells while depletion of CD4+ T cells did not entirely prevent rejection. Furthermore, inhibition of NKG2D prolonged CD28-independent graft survival in this model, supporting an involvement of NKG2D co-stimulation [49]. Intragraft up-regulation of the stress-inducible NKG2D ligand RAE-1 has been reported in several experimental studies [49,63]. Intriguingly, experimental induction of RAE-1 in pancreatic islets led to antigen-independent, NKG2D-mediated recruitment of adoptively transferred CD8+ T cells, in older mice also associated with islet inflammation [64]. In another experimental study, blockade of CD160 ligands through CD160-Ig prolonged cardiac allograft survival and reduced frequencies of systemic effector/memory CD8+ T cells in CD28−/− but not in CD8−/− hosts; although effect specificity for the CD160 receptor had not been demonstrated in the study [65]. While others have characterized inhibitory capacities of CD160 in human CD4+ T cells, stimulatory effects have been reported in activated human CD28− CD4+ CD8+ T cell clones and murine NK cells [66–68].
In clinical organ transplantation, interventions targeting CD28 co-stimulation include CTLA-4-Ig, a fusion protein that inhibits T cell co-stimulation through the blockade of CD28 ligands CD80 and CD86. Although graft survival was comparable to that in calcineurin inhibitor treated patients, CTLA-Ig-treatment had been linked with an increased incidence and severity of acute rejections [69]. As CTLA-4-Ig simultaneously blocks ligands for the co-inhibitory CTLA-4 receptor on T cells, immunosuppressive strategies have recently also focused on a selective blockade of CD28. Interestingly, selective blockade of CD28 preserved CTLA-4 signaling and triggered the up-regulation of the murine co-inhibitory 2B4 receptor on alloreactive CD8+ T cells, thereby controlling antigen-specific CD8+ T cell responses [70]. However, genetic deletion of 2B4 on donor-reactive CD8+ T cells in untreated animals did not affect alloreactive CD8+ T cell responses. In the T cell compartment, 2B4 receptor expression has been reported on most γδ T cells and predominantly in the effector/memory subset of CD8+ T cells while its gene expression appeared enhanced in old human CD8+ T cells [51,71,72]. The stimulatory and/or inhibitory function of 2B4 remains to be discussed controversially as human 2B4 has been suggested to transduce activating signals rather than an inhibitory cascade in murine effector T and NK cells [73].
Collectively, these experimental studies demonstrate the relevance of NKR-mediated responses in CD28 co-stimulatory-independent pathways leading to allograft rejection.
Loss of CD28 in aging and chronic inflammation
Aging, chronic infections, cancer and vascular diseases have all been linked to a loss of CD28 on T cells associated with a ‘natural’ down-regulation of CD28 following repeated CD28/TCR-mediated activation. The expression of NKRs including killer cell immunoglobulin-like receptors (KIRs) on CD4+ T cells are predominantly acquired in (memory) CD28− CD4+ T cell subsets [74,75]. Indeed, NKR-mediated activation of pro-inflammatory CD28− CD4+ T clones has been linked to a number of disease processes, including acute coronary syndrome (ACS). CD28− CD4+ T clones thereby exhibited TCR-independent, KIR2DS2-mediated lysis of human umbilical vein endothelial cells when KIR2DS2 was ligated in vitro, suggesting a role against endothelial cells that express the KIR ligand HLA-C in vivo [76]. Furthermore, TCR-mediated cytotoxicity of ACS-associated CD28− CD4+ T cell clones has been shown to be conditionally dependent on KIR2DS2 stimulation, also demonstrating specificity for HLA-C-encoded molecules [77]. In rheumatoid arthritis patients, CD28− CD4+ T cells exerted an augmented effector function under suboptimal TCR-mediated stimulation in vitro when 2B4, CD226 and/or NKG2D had been ligated [75]. However, neither NKR signals alone, nor the collective NKR stimulation could activate these T cells in absence of TCR stimulation, suggesting that both an adequate balance of NKR signals and the presence of secondary innate signals through cytokines are required for their activation. An increased NKG2D expression has also been documented for IL-15-stimulated CD28− CD4+ T cells with amplified pathogenic properties, and for CMV-specific CD28− CD4+ T cells that became activated under NKG2D ligation and suboptimal TCR stimulation [78,79].
A loss of CD28 on CD8+ T cells correlated with the acquisition of a wide array of NKRs in melanoma patients [80]. Interestingly, a CD56-positive CD8+ T cell subset has been shown capable of killing tumor target cells in a distinct 2B4-dependent way, notably, without the involvement of the TCR [81]. Intestine memory/effector CD28− CD8+ T cells expressed NKG2D selectively subsequent to IL-15 exposure [82]. Thus, NKG2D engagement enhanced T cell activation while initiating NK cell-like cytolysis under suboptimal TCR engagement and independently of IL-15 [82]. The high IL-15 exposure in patients with celiac disease facilitated intraepithelial CD8+ T cells of TCR-independent NKG2D-mediated cytolysis, a mechanism that has also been observed in IL-15 stimulated peripheral effector CD8+ T cells in healthy individuals [83]. Subsequent work emphasized on the relevance of NKG2D-mediated activity in NK-like T cells, a process that was strictly dependent on initial TCR signals [84]. Furthermore, engagement of NKG2D triggered TCR-independent NK-like cytolysis on CD4− iNKT cells [85]. In addition, NKG2D engagement provided co-stimulatory signals for iNKT-cell activation that sparked off conventional pathways of suboptimal CD1d- or TCR-mediated activation.
During the latent stages of chronic infections, inhibitory KLRG1 expression has been identified on the majority of virus epitope-specific CD8+ T cells. In particular CD57+ CD28− CD8+ T cells had been shown to co-express KLRG1 and perforin [86]. A functional relevance of KLRG1 receptor expression through inhibiting TCR-mediated proliferation had been observed in KLRG1-expressing CD27− CD28− CD8+ T cells that accumulated during aging [87].
Taken together, these studies underscore the functional relevance for phenotypic expression of NKRs on CD28− T cells. In parallel to increasing defects within the TCR and conventional co-stimulatory pathways of senescent T cells, NKRs may provide stimulatory and/or inhibitory signals in pathogen-specific T cell clones.
Consequences for immunosuppression
With the introduction of immunosuppressive regimes targeting CD28-dependent co-stimulatory pathways, the NKR repertoire may gain central relevance during CD28-independent rejection. As memory T cells may resist tolerance induction by CD28 ⁄ CD154 co-stimulatory blockade [88], it appears critical to elucidate alternative mechanistic pathways of antigen-experienced T cell activation.
The immunosuppressive capacity of CTLA-4-Ig had been attenuated during progressive T cell maturation, emphasizing that T cells may acquire CD28-independent effector functions with maturation [89]. Moreover, IL-15 has been shown to induce proliferation of alloreactive CD28− memory CD8+ T cells that were subsequently resistant to CTLA-4-Ig blockade [90]. Recently, it has also been suggested that the loss of CD28 subsequent to viral infections facilitated the acquisition of co-stimulatory-independent alloimmune responses [91]. In support, viral infections prior to transplantation have been shown to abrogate skin graft survival following CTLA-4-Ig/anti-CD154 treatment [92].
While the secretion of cytokines by human NK cells in vitro had been suppressed by calcineurin inhibitors but not by mTOR inhibitors, NK cells in immunosuppressed patients displayed an activated phenotype and retained their functional capacity [93]. Of additional relevance, CD28− CD4+ T cells had been resistant to both calcineurin inhibitor and mTOR inhibitor treatment [94]. Moreover, cell-cycle entry but not antigen-independent enhancement of granzyme B had been reduced by mTOR inhibitors in IL-15-primed memory CD8+ T cells that were exposed to inflammation [19].
Eventually, excessive and chronic immunosuppression may support subclinical infections, potentially contributing to the accumulation of oligoclonally expanded CD57-positive and/or innate-like T cells [32], which may escape immunosuppression due to an altered CD28/TCR responsiveness and alternative co-stimulatory pathways.
Concluding Remarks
Activation and suppression of specific antigen-experienced T cell clones appears affected by the delicate balance of stimulatory and inhibitory NK cells receptors. The threshold for T cell activation on CD28-negative clones may be compensated by NKR-mediated co-stimulation, which can ultimately modify the susceptibility of CD28-targeting immunosuppressive interventions and potentially support co-stimulatory blockade-resistant transplant rejection. T cell maturation and senescence are critical components of physiological and pathological processes that are tightly regulated by activating signals. Pathogen-specific T cell clones have the capacity, under the influence of chronic antigen exposure to develop a TCR-independent status in which NKRs substitute classical co-stimulatory signals that promote allorecognition either through TCR cross-reactivity or completely independent from TCR recognition (Fig. 2.).
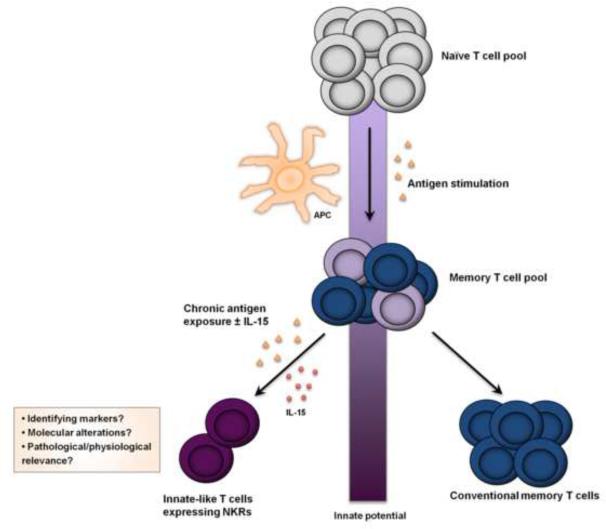
Figure 2. Clonal development of innate-like T cells is impacted by chronic antigen exposure and cytokine environment.
Subsequent to an initial antigen encounte r (yellow) facilitated by antigen-presenting cells (APCs), some na·ive T cells evolve into memory T cells (blue) . With chronic antigenic TCR stimulation and/or the presence of cytokines including IL-15 (red), specific T cell clones (light purple) within the pool of memory T cells may either develop characteristics of innate-like T cells (dark purple) or remain conventional memory T cells. Changes in the innate T cell potential are depicted by the purple colored shading of the vertical bar.
Taken together, changes in NKR density and activation, in addition to alterations of classical T cell signaling may impact adaptive immune responses and raise questions on broader immunological implications (see Outstanding Questions). Consequences of those processes may account for the observed resistance to CD28-targeting immunosuppressants or co-stimulation blockade-resistant rejection.
Outstanding Questions Box.
Does antigenic TCR stimulation, completed CD28 ligation and/or cytokine stimuli need to exceed a particular threshold to initiate the development of innate-like T cells?
Is the expansion of innate-like T cells pre-programmed or solely dependent on antigen-experience?
What molecular alterations encompass the development of innate-like T cells?
What defines the phenotypic and functional repertoire/markers of innate-like T cells?
What criteria will distinguish physiological and pathological roles exerted by innate-like T cells?
What means and mechanisms can be developed to suppress innate-like T cells during transplantation or autoimmunity?
Conceptually, does the generation of innate-like T cells represent an evolutionary adaption process that is reciprocal to the loss of adaptive features during age and chronic antigen exposure?
While prolonged antigen stimulation can induce the loss of co-stimulatory CD28 molecules, diminished TCR expression/increased antigen thresholds in antigen-experienced CD8+ T cells have been reported.
Prolonged antigenic TCR-dependent activation may lead to the development of innate-like T cells that express functional NK cell receptors.
Suppression of classical TCR signaling in parallel to an acquisition of NK cell receptor signaling pathways may be characteristic of innate-like T cells.
CD57 can reflect the maturity of T cells and correlates with both an augmented expression in addition to a modified functional responsiveness of NK cell receptors.
Clinically, chronic immunosuppression may promote the development of innate-like T cells by supporting infections that involve antigen exposure. Due to their unconventional functional repertoire, innate-like T cells may escape CD28-targeting immunosuppressants.
Acknowledgements
This work has been supported by grants from the National Institutes of Health (R01AG039449 to S.G.T.), the German Research Foundation (SFB738 project B3/B8 to C.S.F.), German Ministry of Education and Research (BMBF 01EO1302 to C.S.F.) and German Center for Infection Research (TTU-IICH 07.803 to C.S.F.). M.S. was supported by the German National Academic Foundation and International Academy of Life Sciences. M.Q. received support from the German Research Foundation (DFG).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ljunggren HG, Kärre K. In search of the “missing self”: MHC molecules and NK cell recognition. Immunol Today. 1990;11:237–44. doi: 10.1016/0167-5699(90)90097-s. [DOI] [PubMed] [Google Scholar]
- 2.Bauer S, et al. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science. 1999;285:727–9. doi: 10.1126/science.285.5428.727. [DOI] [PubMed] [Google Scholar]
- 3.Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol. 2013;13:227–42. doi: 10.1038/nri3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Janeway CA. Approaching the Asymptote? Evolution and Revolution in Immunology. Cold Spring Harb Symp Quant Biol. 1989;54:1–13. doi: 10.1101/sqb.1989.054.01.003. [DOI] [PubMed] [Google Scholar]
- 5.O’Leary JG, et al. T cell- and B cell-independent adaptive immunity mediated by natural killer cells. Nat Immunol. 2006;7:507–16. doi: 10.1038/ni1332. [DOI] [PubMed] [Google Scholar]
- 6.Vivier E, et al. Innate or adaptive immunity? The example of natural killer cells. Science. 2011;331:44–9. doi: 10.1126/science.1198687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wencker M, et al. Innate-like T cells straddle innate and adaptive immunity by altering antigen-receptor responsiveness. Nat Immunol. 2014;15:80–7. doi: 10.1038/ni.2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bedoui S, et al. Parallels and differences between innate and adaptive lymphocytes. Nat Immunol. 2016;17:490–4. doi: 10.1038/ni.3432. [DOI] [PubMed] [Google Scholar]
- 9.Kastenmüller W, et al. A spatially-organized multicellular innate immune response in lymph nodes limits systemic pathogen spread. Cell. 2012;150:1235–48. doi: 10.1016/j.cell.2012.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jabri B, et al. TCR specificity dictates CD94/NKG2A expression by human CTL. Immunity. 2002;17:487–99. doi: 10.1016/s1074-7613(02)00427-2. [DOI] [PubMed] [Google Scholar]
- 11.Strid J, et al. Acute upregulation of an NKG2D ligand promotes rapid reorganization of a local immune compartment with pleiotropic effects on carcinogenesis. Nat Immunol. 2008;9:146–154. doi: 10.1038/ni1556. [DOI] [PubMed] [Google Scholar]
- 12.Soudja SM, et al. Inflammatory monocytes activate memory CD8(+) T and innate NK lymphocytes independent of cognate antigen during microbial pathogen invasion. Immunity. 2012;37:549–62. doi: 10.1016/j.immuni.2012.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chu T, et al. Bystander-activated memory CD8 T cells control early pathogen load in an innate-like, NKG2D-dependent manner. Cell Rep. 2013;3:701–8. doi: 10.1016/j.celrep.2013.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ruiz AL, et al. NK1.1+ CD8+ T cells escape TGF-β control and contribute to early microbial pathogen response. Nat Commun. 2014;5:5150. doi: 10.1038/ncomms6150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McQueen B, et al. NKG2D and CD28 receptors differentially activate mTOR to alter murine effector CD8(+) T cell differentiation. Immunology. 2015 doi: 10.1111/imm.12563. DOI: 10.1111/imm.12563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zloza A, et al. NKG2D signaling on CD8+ T cells represses T-bet and rescues CD4-unhelped CD8+ T cell memory recall but not effector responses. Nat Med. 2012;18:422–8. doi: 10.1038/nm.2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alves NL, et al. IL-15 induces antigen-independent expansion and differentiation of human naive CD8+ T cells in vitro. Blood. 2003;102:2541–6. doi: 10.1182/blood-2003-01-0183. [DOI] [PubMed] [Google Scholar]
- 18.Richer MJ, et al. Inflammatory IL-15 is required for optimal memory T cell responses. J Clin Invest. 2015;125:3477–90. doi: 10.1172/JCI81261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marçais A, et al. The metabolic checkpoint kinase mTOR is essential for IL-15 signaling during the development and activation of NK cells. Nat Immunol. 2014;15:749–57. doi: 10.1038/ni.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vallejo AN, et al. Modulation of CD28 expression: distinct regulatory pathways during activation and replicative senescence. J Immunol. 1999;162:6572–9. [PubMed] [Google Scholar]
- 21.Mehlhop-Williams ER, Bevan MJ. Memory CD8+ T cells exhibit increased antigen threshold requirements for recall proliferation. J Exp Med. 2014;211:345–56. doi: 10.1084/jem.20131271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Assarsson E, et al. CD8+ T Cells Rapidly Acquire NK1.1 and NK Cell-Associated Molecules Upon Stimulation In Vitro and In Vivo. J Immunol. 2000;165:3673–3679. doi: 10.4049/jimmunol.165.7.3673. [DOI] [PubMed] [Google Scholar]
- 23.McMahon CW, et al. Viral and bacterial infections induce expression of multiple NK cell receptors in responding CD8(+) T cells. J Immunol. 2002;169:1444–52. doi: 10.4049/jimmunol.169.3.1444. [DOI] [PubMed] [Google Scholar]
- 24.Tarazona R, et al. Increased expression of NK cell markers on T lymphocytes in aging and chronic activation of the immune system reflects the accumulation of effector/senescent T cells. Mech Ageing Dev. 2000;121:77–88. doi: 10.1016/s0047-6374(00)00199-8. [DOI] [PubMed] [Google Scholar]
- 25.Peralbo E, et al. Invariant NKT and NKT-like lymphocytes: two different T cell subsets that are differentially affected by ageing. Exp Gerontol. 2007;42:703–8. doi: 10.1016/j.exger.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 26.van Stijn A, et al. Human Cytomegalovirus Infection Induces a Rapid and Sustained Change in the Expression of NK Cell Receptors on CD8+ T Cells. J Immunol. 2008;180:4550–4560. doi: 10.4049/jimmunol.180.7.4550. [DOI] [PubMed] [Google Scholar]
- 27.Suessmuth Y, et al. CMV reactivation drives posttransplant T-cell reconstitution and results in defects in the underlying TCRβ repertoire. Blood. 2015;125:3835–50. doi: 10.1182/blood-2015-03-631853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hassouneh F, et al. Effect of age and latent CMV infection on CD8+ CD56+ T cells (NKT-like) frequency and functionality. Mech Ageing Dev. 2016 doi: 10.1016/j.mad.2015.12.003. DOI: 10.1016/j.mad.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 29.Strauss-Albee DM, et al. Coordinated regulation of NK receptor expression in the maturing human immune system. J Immunol. 2014;193:4871–9. doi: 10.4049/jimmunol.1401821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Espinosa J, et al. CD57(+) CD4 T cells underlie belatacept-resistant allograft rejection. Am J Transplant. 2015 doi: 10.1111/ajt.13613. DOI: 10.1111/ajt.13613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yap M, et al. Expansion of highly differentiated cytotoxic terminally differentiated effector memory CD8+ T cells in a subset of clinically stable kidney transplant recipients: a potential marker for late graft dysfunction. J Am Soc Nephrol. 2014;25:1856–68. doi: 10.1681/ASN.2013080848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bottomley MJ, et al. CD8+ Immunosenescence Predicts Post-Transplant Cutaneous Squamous Cell Carcinoma in High-Risk Patients. J Am Soc Nephrol. 2015 doi: 10.1681/ASN.2015030250. DOI: 10.1681/ASN.2015030250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li XC, et al. Memory T cells in transplantation - progress and challenges. Curr Opin Organ Transplant. 2013;18:387–92. doi: 10.1097/MOT.0b013e3283626130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chalasani G, et al. Recall and propagation of allospecific memory T cells independent of secondary lymphoid organs. Proc Natl Acad Sci U S A. 2002;99:6175–80. doi: 10.1073/pnas.092596999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hidalgo LG, et al. NK cell transcripts and NK cells in kidney biopsies from patients with donor-specific antibodies: evidence for NK cell involvement in antibody-mediated rejection. Am J Transplant. 2010;10:1812–22. doi: 10.1111/j.1600-6143.2010.03201.x. [DOI] [PubMed] [Google Scholar]
- 36.Hirohashi T, et al. A novel pathway of chronic allograft rejection mediated by NK cells and alloantibody. Am J Transplant. 2012;12:313–21. doi: 10.1111/j.1600-6143.2011.03836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Venner JM, et al. The molecular landscape of antibody-mediated kidney transplant rejection: evidence for NK involvement through CD16a Fc receptors. Am J Transplant. 2015;15:1336–48. doi: 10.1111/ajt.13115. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Z-X, et al. Natural Killer Cells Mediate Long-term Kidney Allograft Injury. Transplantation. 2015;99:916–24. doi: 10.1097/TP.0000000000000665. [DOI] [PubMed] [Google Scholar]
- 39.Zhang Z-X, et al. Natural Killer Cells Play a Critical Role in Cardiac Allograft Vasculopathy in an Interleukin-6–Dependent Manner. Transplantation. 2014;98:1029–1039. doi: 10.1097/TP.0000000000000405. [DOI] [PubMed] [Google Scholar]
- 40.Li L, et al. A common peripheral blood gene set for diagnosis of operational tolerance in pediatric and adult liver transplantation. Am J Transplant. 2012;12:1218–28. doi: 10.1111/j.1600-6143.2011.03928.x. [DOI] [PubMed] [Google Scholar]
- 41.Beilke JN, et al. NK cells promote islet allograft tolerance via a perforin-dependent mechanism. Nat Med. 2005;11:1059–65. doi: 10.1038/nm1296. [DOI] [PubMed] [Google Scholar]
- 42.Yu G, et al. NK cells promote transplant tolerance by killing donor antigen-presenting cells. J Exp Med. 2006;203:1851–8. doi: 10.1084/jem.20060603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van der Touw W, et al. NK cells are required for costimulatory blockade induced tolerance to vascularized allografts. Transplantation. 2012;94:575–84. doi: 10.1097/TP.0b013e318264d3c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lantow M, et al. CD27low natural killer cells prolong allograft survival in mice by controlling alloreactive CD8+ T cells in a T-bet-dependent manner. Transplantation. 2015;99:391–9. doi: 10.1097/TP.0000000000000585. [DOI] [PubMed] [Google Scholar]
- 45.Allard M, et al. HLA-E-Restricted Cross-Recognition of Allogeneic Endothelial Cells by CMV-Associated CD8 T Cells: A Potential Risk Factor following Transplantation. PLoS One. 2012;7:e50951. doi: 10.1371/journal.pone.0050951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Romagnani C, et al. Identification of HLA-E-specific alloreactive T lymphocytes: a cell subset that undergoes preferential expansion in mixed lymphocyte culture and displays a broad cytolytic activity against allogeneic cells. Proc Natl Acad Sci U S A. 2002;99:11328–33. doi: 10.1073/pnas.172369799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shabir S, et al. CMV-associated CD4(+) CD28(null) cells in NKG2D-dependent glomerular endothelial injury and kidney allograft dysfunction. Am J Transplant. 2015 doi: 10.1111/ajt.13614. DOI: 10.1111/ajt.13614. [DOI] [PubMed] [Google Scholar]
- 48.Maier S, et al. Inhibition of natural killer cells results in acceptance of cardiac allografts in CD28−/− mice. Nat Med. 2001;7:557–62. doi: 10.1038/87880. [DOI] [PubMed] [Google Scholar]
- 49.Kim J, et al. The activating immunoreceptor NKG2D and its ligands are involved in allograft transplant rejection. J Immunol. 2007;179:6416–20. doi: 10.4049/jimmunol.179.10.6416. [DOI] [PubMed] [Google Scholar]
- 50.Venner JM, et al. Molecular landscape of T cell-mediated rejection in human kidney transplants: prominence of CTLA4 and PD ligands. Am J Transplant. 2014;14:2565–76. doi: 10.1111/ajt.12946. [DOI] [PubMed] [Google Scholar]
- 51.Chen G, et al. T cell aging: a review of the transcriptional changes determined from genome-wide analysis. Front Immunol. 2013;4:121. doi: 10.3389/fimmu.2013.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mou D, et al. CD28 negative T cells: is their loss our gain? Am J Transplant. 2014;14:2460–6. doi: 10.1111/ajt.12937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Freeman BE, et al. Regulation of innate CD8+ T-cell activation mediated by cytokines. Proc Natl Acad Sci U S A. 2012;109:9971–6. doi: 10.1073/pnas.1203543109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Suarez-Ramirez JE, et al. CD8 T cells in innate immune responses: using STAT4-dependent but antigen-independent pathways to gamma interferon during viral infection. MBio. 2014;5:e01978–14. doi: 10.1128/mBio.01978-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Holzapfel KL, et al. Antigen-dependent versus -independent activation of invariant NKT cells during infection. J Immunol. 2014;192:5490–8. doi: 10.4049/jimmunol.1400722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang X, et al. Human invariant natural killer T cells acquire transient innate responsiveness via histone H4 acetylation induced by weak TCR stimulation. J Exp Med. 2012;209:987–1000. doi: 10.1084/jem.20111024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vahl JC, et al. NKT cell-TCR expression activates conventional T cells in vivo, but is largely dispensable for mature NKT cell biology. PLoS Biol. 2013;11:e1001589. doi: 10.1371/journal.pbio.1001589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Boyman O, et al. Homeostatic maintenance of T cells and natural killer cells. Cell Mol Life Sci. 2012;69:1597–608. doi: 10.1007/s00018-012-0968-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lafferty KJ, Cunningham AJ. A new analysis of allogeneic interactions. Aust J Exp Biol Med Sci. 1975;53:27–42. doi: 10.1038/icb.1975.3. [DOI] [PubMed] [Google Scholar]
- 60.Kirchhoff S, et al. TCR-mediated up-regulation of c-FLIPshort correlates with resistance toward CD95-mediated apoptosis by blocking death-inducing signaling complex activity. J Immunol. 2000;165:6293–300. doi: 10.4049/jimmunol.165.11.6293. [DOI] [PubMed] [Google Scholar]
- 61.McNerney ME, et al. Role of natural killer cell subsets in cardiac allograft rejection. Am J Transplant. 2006;6:505–13. doi: 10.1111/j.1600-6143.2005.01226.x. [DOI] [PubMed] [Google Scholar]
- 62.Szot GL, et al. Different mechanisms of cardiac allograft rejection in wildtype and CD28-deficient mice. Am J Transplant. 2001;1:38–46. doi: 10.1034/j.1600-6143.2001.010108.x. [DOI] [PubMed] [Google Scholar]
- 63.Zhang Z-X, et al. NK cells induce apoptosis in tubular epithelial cells and contribute to renal ischemia-reperfusion injury. J Immunol. 2008;181:7489–98. doi: 10.4049/jimmunol.181.11.7489. [DOI] [PubMed] [Google Scholar]
- 64.Markiewicz MA, et al. RAE1ε ligand expressed on pancreatic islets recruits NKG2D receptor-expressing cytotoxic T cells independent of T cell receptor recognition. Immunity. 2012;36:132–41. doi: 10.1016/j.immuni.2011.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.D’Addio F, et al. CD160Ig fusion protein targets a novel costimulatory pathway and prolongs allograft survival. PLoS One. 2013;8:e60391. doi: 10.1371/journal.pone.0060391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Agrawal S, et al. Cutting edge: MHC class I triggering by a novel cell surface ligand costimulates proliferation of activated human T cells. J Immunol. 1999;162:1223–6. [PubMed] [Google Scholar]
- 67.Cai G, et al. CD160 inhibits activation of human CD4+ T cells through interaction with herpesvirus entry mediator. Nat Immunol. 2008;9:176–85. doi: 10.1038/ni1554. [DOI] [PubMed] [Google Scholar]
- 68.Tu TC, et al. CD160 is essential for NK-mediated IFN-γ production. J Exp Med. 2015;212:415–429. doi: 10.1084/jem.20131601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vincenti F, et al. A phase III study of belatacept-based immunosuppression regimens versus cyclosporine in renal transplant recipients (BENEFIT study) Am J Transplant. 2010;10:535–46. doi: 10.1111/j.1600-6143.2009.03005.x. [DOI] [PubMed] [Google Scholar]
- 70.Liu D, et al. 2B4 (CD244) induced by selective CD28 blockade functionally regulates allograft-specific CD8+ T cell responses. J Exp Med. 2014;211:297–311. doi: 10.1084/jem.20130902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nakajima H, et al. Activating interactions in human NK cell recognition: the role of 2B4-CD48. Eur J Immunol. 1999;29:1676–83. doi: 10.1002/(SICI)1521-4141(199905)29:05<1676::AID-IMMU1676>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 72.Speiser DE, et al. The Activatory Receptor 2B4 Is Expressed In Vivo by Human CD8+ Effector T Cells. J Immunol. 2001;167:6165–6170. doi: 10.4049/jimmunol.167.11.6165. [DOI] [PubMed] [Google Scholar]
- 73.Waggoner SN, Kumar V. Evolving role of 2B4/CD244 in T and NK cell responses during virus infection. Front Immunol. 2012;3:377. doi: 10.3389/fimmu.2012.00377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Snyder MR, et al. Formation of the Killer Ig-Like Receptor Repertoire on CD4+CD28null T Cells. J Immunol. 2002;168:3839–3846. doi: 10.4049/jimmunol.168.8.3839. [DOI] [PubMed] [Google Scholar]
- 75.Fasth AER, et al. Activating NK-cell receptors co-stimulate CD4(+)CD28(−) T cells in patients with rheumatoid arthritis. Eur J Immunol. 2010;40:378–87. doi: 10.1002/eji.200939399. [DOI] [PubMed] [Google Scholar]
- 76.Nakajima T, et al. T-cell-mediated lysis of endothelial cells in acute coronary syndromes. Circulation. 2002;105:570–5. doi: 10.1161/hc0502.103348. [DOI] [PubMed] [Google Scholar]
- 77.Zal B, et al. Differential pathways govern CD4+ CD28− T cell proinflammatory and effector responses in patients with coronary artery disease. J Immunol. 2008;181:5233–41. doi: 10.4049/jimmunol.181.8.5233. [DOI] [PubMed] [Google Scholar]
- 78.Sáez-Borderías A, et al. Expression and function of NKG2D in CD4+ T cells specific for human cytomegalovirus. Eur J Immunol. 2006;36:3198–206. doi: 10.1002/eji.200636682. [DOI] [PubMed] [Google Scholar]
- 79.Broux B, et al. IL-15 amplifies the pathogenic properties of CD4+CD28− T cells in multiple sclerosis. J Immunol. 2015;194:2099–109. doi: 10.4049/jimmunol.1401547. [DOI] [PubMed] [Google Scholar]
- 80.Casado JG, et al. CD8 T cells expressing NK associated receptors are increased in melanoma patients and display an effector phenotype. Cancer Immunol Immunother. 2005;54:1162–71. doi: 10.1007/s00262-005-0682-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Costello RT, et al. A novel mechanism of antitumor response involving the expansion of CD3+/CD56+ large granular lymphocytes triggered by a tumor-expressed activating ligand. Leukemia. 2002;16:855–60. doi: 10.1038/sj.leu.2402488. [DOI] [PubMed] [Google Scholar]
- 82.Roberts AI, et al. NKG2D receptors induced by IL-15 costimulate CD28− negative effector CTL in the tissue microenvironment. J Immunol. 2001;167:5527–30. doi: 10.4049/jimmunol.167.10.5527. [DOI] [PubMed] [Google Scholar]
- 83.Meresse B, et al. Coordinated induction by IL15 of a TCR-independent NKG2D signaling pathway converts CTL into lymphokine-activated killer cells in celiac disease. Immunity. 2004;21:357–66. doi: 10.1016/j.immuni.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 84.von Geldern M, et al. TCR-independent cytokine stimulation induces non-MHC-restricted T cell activity and is negatively regulated by HLA class I. Eur J Immunol. 2006;36:2347–58. doi: 10.1002/eji.200535387. [DOI] [PubMed] [Google Scholar]
- 85.Kuylenstierna C, et al. NKG2D performs two functions in invariant NKT cells: direct TCR-independent activation of NK-like cytolysis and co-stimulation of activation by CD1d. Eur J Immunol. 2011;41:1913–23. doi: 10.1002/eji.200940278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ibegbu CC, et al. Expression of killer cell lectin-like receptor G1 on antigen-specific human CD8+ T lymphocytes during active, latent, and resolved infection and its relation with CD57. J Immunol. 2005;174:6088–94. doi: 10.4049/jimmunol.174.10.6088. [DOI] [PubMed] [Google Scholar]
- 87.Henson SM, et al. KLRG1 signaling induces defective Akt (ser473) phosphorylation and proliferative dysfunction of highly differentiated CD8+ T cells. Blood. 2009;113:6619–28. doi: 10.1182/blood-2009-01-199588. [DOI] [PubMed] [Google Scholar]
- 88.Li XC, et al. Costimulatory pathways in transplantation: challenges and new developments. Immunol Rev. 2009;229:271–293. doi: 10.1111/j.1600-065X.2009.00781.x. [DOI] [PubMed] [Google Scholar]
- 89.Xu H, et al. The allo- and viral-specific immunosuppressive effect of belatacept, but not tacrolimus, attenuates with progressive T cell maturation. Am J Transplant. 2014;14:319–32. doi: 10.1111/ajt.12574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Traitanon O, et al. IL-15 induces alloreactive CD28(−) memory CD8 T cell proliferation and CTLA4-Ig resistant memory CD8 T cell activation. Am J Transplant. 2014;14:1277–89. doi: 10.1111/ajt.12719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mou D, et al. Viral-induced CD28 loss evokes costimulation independent alloimmunity. J Surg Res. 2015;196:241–6. doi: 10.1016/j.jss.2015.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Williams MA, et al. Characterization of Virus-Mediated Inhibition of Mixed Chimerism and Allospecific Tolerance. J Immunol. 2001;167:4987–4995. doi: 10.4049/jimmunol.167.9.4987. [DOI] [PubMed] [Google Scholar]
- 93.Hoffmann U, et al. NK Cells of Kidney Transplant Recipients Display an Activated Phenotype that Is Influenced by Immunosuppression and Pathological Staging. PLoS One. 2015;10:e0132484. doi: 10.1371/journal.pone.0132484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Demmers MWHJ, et al. Substantial proliferation of human renal tubular epithelial cell-reactive CD4+CD28null memory T cells, which is resistant to tacrolimus and everolimus. Transplantation. 2014;97:47–55. doi: 10.1097/01.TP.0000435697.31148.b2. [DOI] [PubMed] [Google Scholar]