Abstract
Ripart’s Anomalous Blue Polyommatus ripartii (Freyer, 1830) is one of the most seriously endangered butterfly species in central Europe, a small, relict population of which has survived in two localities in Poland. This isolated population is undoubtedly the last and northernmost remnant of a once much wider range in central Europe. P. ripartii is associated with highly xerophilous vegetation on gypsum and calcareous soils. Only active conservation measures can ensure its survival. For these to be successful, however, precise information on the butterfly’s biology, behavior and also its morphology is crucial. The first to do so, this article describes the butterfly’s egg-laying preferences, and specifies the numbers of eggs on a single shoot and their placement on it. A unique behavioral trait of the female—the secretion of oviposition-deterring pheromones—is reported. The preferred plant associations and nectar sources have been investigated, and information on overnight roosts is given. In addition, an exhaustive description of the morphologies of the egg, final instar and pupa, as well as new details of adult behavior are provided.
The main conclusion of the this study is that the existence of a stable population in the Nida Region is determined by the presence of large patches of sainfoin, which is both the larval host plant and a source of nectar for the imago. Moreover, stress is laid on the importance of Inula ensifolia L. as the secondary nectaring plant, which may facilitate dispersion among patches of suitable habitat. Finally, the study shows that searching for the easily detected eggs may be the best method for proving the existence of the species in a given locality.
Keywords: Agrodiaetus, egg, larva, pupa, biology
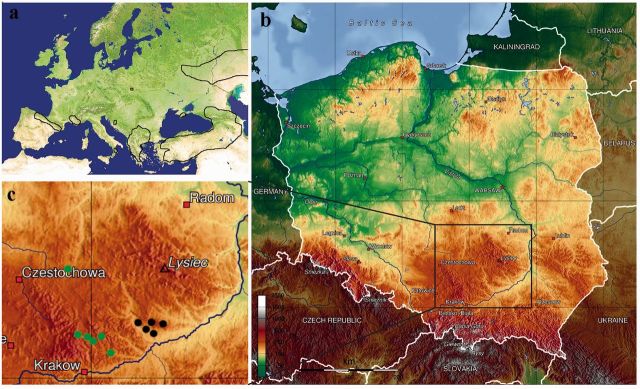
Ripart’s Anomalous Blue Polyommatus ripartii (Freyer, 1830) is one of the most seriously endangered butterfly species in central Europe, a unique, relict population of which survives in a small area in the Nida River Valley in southeastern Poland ( Fig. 1 a). The importance of protecting this population lies in its unique value for the ongoing and future study of the phylogeny and zoogeographic history of taxonomically related species.
Fig. 1.
Western Palaearctic distribution (a) and localities (b and c) of P. ripartii in Poland: green dots, records before 1970; black dots, records after 1970.
P. ripartii belongs to the large group of morphologically homogeneous but karyologically diverse species that has been separated by several authors into the independent genus Agrodiaetus ( Kandul et al. 2004 , 2007 ; Lukhtanov et al. 2005 ; Vila et al. 2010 ). In this article, the taxonomic approach of Wiemers et al. (2009) is applied. Agrodiaetus is regarded there as a member of the large, polytopic genus of Polyommatus which, except for the subgenus Lysandra , makes up a monophyletic group with a bootstrap support of 88%. In a group of superficially similar species, P. ripartii and its relatives form a separate lineage that includes a number of cryptic taxa distributed in the Mediterranean region and characterized by very small, local ranges. Vila et al. (2010) took a significant step towards understanding the internal variability of this group. The results of their thorough molecular study showed that several forms, previously regarded as separate species, are no more than geographic variants of the widely distributed P. ripartii . The problem of phylogeography of European populations has been elucidated by Dincă et al. (2013) . These authors demonstrated that three genetically differentiated lineages occur in Europe that could be considered evolutionarily significant units for conservation. The specific affinity of the Polish population has recently been elaborated by Przybyłowicz et al. (2014) , this cytological and molecular study confirmed its affiliation to the species P. ripartii .
The species’ present-day locality in Poland lies some 800 km from its nearest localities in SE Serbia, that is, far beyond the northern limit of its contiguous range. This extends from eastern Spain in the west to the mountains of central Asia in the east ( Tshikolovets 2011 ). Although the distribution in Asia is generally continuous, the pattern in Europe is entirely different. The species consists of several disjunct populations spread along the Mediterranean coast. At the European scale, the species is not yet seriously endangered: the red list published by Van Swaay et al. (2010) ranks it as being of “least concern”. However, if only EU countries are considered, its category is less favorable — “near threatened” — according to criterion ( IUCN 2001 ). Nonetheless, this evaluation does not reflect the situation and value of the Polish population, which is on the verge of extinction. Because of its isolation from the main range and its exceptional northerly location, it can serve as a valuable source of data concerning the glacial history of central European fauna.
The Polish population of P. ripartii is currently restricted to the Nida River Basin in the southeastern part of the country ( Fig. 1 b). The slow decline of this species in recent decades was recorded by Przybyłowicz (2000) . Until 1970, the species was known from several localities in the Nida Valley and the Miechów Upland, whereas all later records come only from the former region ( Fig. 1 c). It is legally protected on the strength of a regulation of the Minister of the Environment concerning the protection of animal species. The Polish Red Book of Invertebrates ( Głowaciński and Nowacki 2004 ) places the species in the category CR (“critically endangered”).
Present knowledge of the biology and morphology of the immature stages of P. ripartii is very fragmentary. The only comprehensive article devoted exclusively to this species was published by Schurian (1976) . That author summarized all the available information scattered around the literature and provided a brief overview of this butterfly’s life cycle as observed in captivity. Later, Lafranchis et al. (2007) presented new information on the flight periods, nectar sources, habitats, life cycles, larval host plants, myrmecophily, and parasitoids of eight Polyommatus species (including P. ripartii ), based on populations from Greece and southern Spain (cited as Agrodiaetus ). These latter authors state that the flight period of the Greek populations of P. ripartii starts around mid-July and lasts a full 3 months. Preferred biotopes include poorly grazed or recently abandoned clearings and bushy grasslands with sparse trees at altitudes of 600–1,500 m. The pupal stage lasts 16–27 d in natural conditions. Three species of Onobrychis (sainfoin) were recorded as host plants: alba , arenaria , and ebenoides (but not viciifolia ). Eleven species of ants belonging to the genera Camponotus , Crematogaster , Lasius , Lepisiota , Plagiolepis , and Tapinoma were found to be associated with larvae of P. ripartii . Finally, Hyposoter notatus (Gravenhorst 1829) (Ichneumonidae: Campopleginae) was recorded as a parasitoid of this butterfly.
Some general remarks concerning the threats to the Polish population and possible measures for its conservation can be found in Buszko and Masłowski (2008) . These authors highlight the isolation and the small range of the Polish population as a factor increasing the risk of its extinction. Another serious threat is the gradual overgrowth of its xerothermic habitat by shrubs and tall herbs as a result of natural succession. The aforementioned authors state that the three most important activities towards the conservation of Polish population are maintaining the xerothermic habitat in good condition, monitoring the abundance of the food plant (sainfoin), and acquiring an understanding of the biology and life history of this local population.
Finally, some hitherto unknown facts concerning larval behavior are provided by Warecki (2010) . The caterpillar feeds from late afternoon until dusk exclusively on Onobrychis . Both younger and older larvae skeletonize the leaves. They spend the day concealed under the plants at ground level. They are facultatively myrmecophilous, being associated with Lasius alienus (Förster 1850). Pupation takes place on the ground, near the food plant, often in the tunnels of ants. The pupa is positioned with its dorsal side downwards.
As conservation efforts need to be based on a thorough knowledge of the characteristics and behavior of the taxon in question, the core objective of this article is to supply new information on the biology of this threatened population of P. ripartii . I describe hitherto unknown biological facts regarding food plant preferences and the imago’s defensive strategies. Special attention is focused on the egg laying behavior. The study analyses the distribution of eggs on a single shoot of the food plant (number of eggs, vertical position, and the morphological structure on which the egg is deposited). The results are congruent with my new findings, indirectly confirming the presence of oviposition-deterring pheromones in P. ripartii as well. In addition, I analyze the number of eggs, the final instar larva and the pupa, and present for the first time a detailed morphological characterization of these developmental stages illustrated with scanning electron micrographs (SEMs). I demonstrate the presence in this species of specialized myrmecophilous organs. The article concludes with a brief discussion of possible conservation action with respect to the Polish population of P. ripartii based on the results of this study.
Materials and Methods
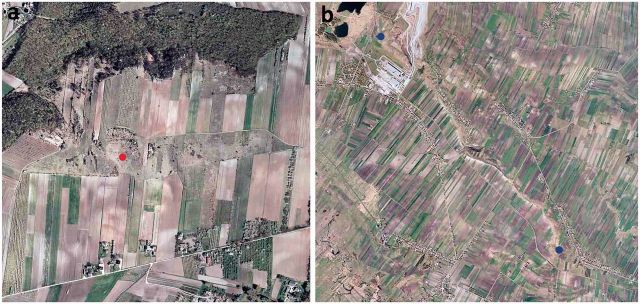
P. ripartii was recorded in Poland in 2010 and 2011 at just two localities about 12 km apart in the Nida Basin. One of them, previously unknown, was discovered 4 km east of Busko Zdrój (50° 27′57″ N 20° 46′39″S) ( Fig. 2 a). It is a small portion of an extensively farmed south-facing slope. Entirely surrounded by arable fields, the locality has an area of ∼9.3 ha. The butterflies were recorded only in its driest, westernmost part. The real value of this new locality remains unknown as only four specimens were sighted.
Fig. 2.
Present localities of P. ripartii in the Nida Basin (a and b): a—small slope near Busko Zdrój: red dot, place of observation of specimens; b—elongated, narrow ridge between Gacki and Wola Zagojska (not to the same scale): blue dots, NW and SE border of the ridge.
The largest and the most important locality, in fact the only refugium of the comparatively large, stable population of P. ripartii , is the elongated but rather narrow xerothermic gypsum outcrop between the villages of Gacki and Wola Zagojska ( Fig. 2 b). The study was carried out at this site. Some 5 km in length, the outcrop extends diagonally from the north-west (50° 27′24″ N 20° 35′50″ S) towards the south-east (50° 25′14″ N 20° 38′03″ S). Although rather long, the locality is very narrow, the minimum width being <20 m in some places. It has an overall area of around 42 ha. The site supports mostly xerothermic associations from the class F estuco -B rometea Br. Bl. et R.Tx. and is surrounded by arable fields and fallow land. Both adults and immatures of P. ripartii were observed and studied at this site in 2010 and 2011. The adult larvae were observed in May and the butterflies were on the wing in July, when the oviposition preferences, behavior, nectar plants, and habitat preferences were also recorded. The eggs were counted twice: in the field at the beginning and end of August. During the second count shoots bearing the empty egg shells were collected, the shells were later counted in the laboratory. This was not done in the field for two reasons: 1) by that time almost all the eggs had already hatched and only fragments of shells remained on the shoots, so there was no risk of destroying developing embryos; 2) remnants of eggs are much more difficult to locate on the shoots because they have turned brown; moreover, they are often small, and are covered with small particles, spider threads, or fungal spores. Each time 600 shoots were examined. Counting in the field was done with the aid of a 5× hand magnifying glass. For the laboratory counts and measurements of eggs, a stereoscopic binocular Leica MZ75 equipped with an ocular micrometer was used.
For a more accurate description of the taxon, detailed macro photographs and SEMs were taken (see Figs. 3 and 4 ). For eggs and pupae, structures from dried specimens were mounted on a tiny block of colloidal graphite and then coated in gold. The larvae, preserved in 70% alcohol, were fixed by treatment with osmium tetroxide and then coated in gold. The samples were analyzed and the photographs taken using a JOEL JSM 5500 LV scanning microscope. The macro photographs in the field were taken using a Canon EOS 50 d camera with additional equipment (ring flash, tele macro lenses).
Fig. 3.
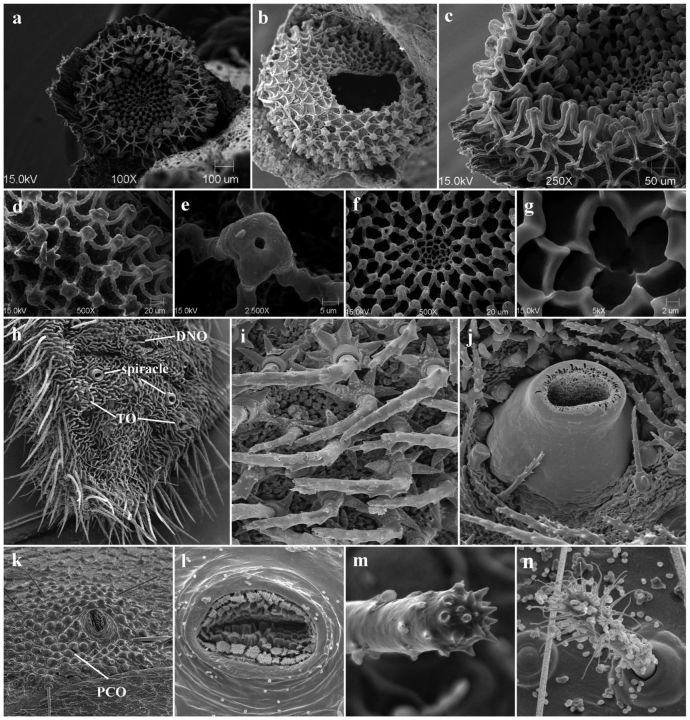
SEMs of the P. ripartii egg (a–g), larva (h–j), and pupa (k–n). (a) general shape and structure of the freshly laid egg; (b) egg shell after hatching; (c) concentric zones with three different types of reticulation; d, lateral zone with stalks and low ribs; (e) aeropyle; (f) transitional zone; (g) micropylar pit; (h) terminal segments of the last instar (DNO, TO); (i) seta with stellate base; (j) raised spiracle with semielliptical opening; (k) spiracle surrounded by PCO; (l) lamellate, branched papillae of spiracle; (m) cremaster seta with apical, blunt spine-like projections; (n) dendritic seta on the fifth abdominal segment.
Fig. 4.
P. ripartii . (a) location of eggs (black dots) on dry shoots of O. viciifolia ; (b) last- instar larva with prominent TO; (c) female displaying “gliding” behavior; (d) ditto; (e) typical roosting site; (f) resting position.
The morphological terms used to describe the eggs follow Downey and Allyn (1981) , and those used for the larval morphology are taken from Duarte et al. (2001) .
Results
Morphology .
Egg
The freshly laid egg ( Fig. 3 a) is whitish at first but then gradually turns pale grey. The spherical and discoidally flattened shape is typical of the Polyommatinae. The central portion bearing the micropylar area is significantly depressed. The diameter is ∼0.75 mm ( n = 16, σ = 0.03) mm and the marginal area is 0.29 mm in height ( n = 16, σ = 0.02). The chorion is covered by a characteristic reticulation forming three different, concentric zones ( Figs 3 b and c). The outermost, lateral region occupying the marginal area is made up of numerous, prominent stalks, and low ribs extending star-like from their base ( Fig. 3 d). The number of ribs associated with a single stalk varies from 4 to 8 (average 5). The terminal portion of the stalk is slightly widened and distinctly flattened. The aeropyle (respiratory opening of the egg) is located in its central part ( Fig. 3 e). The aeropylar opening is polygonal rather than circular, and the number of bends depends on the number of associated ribs. The transitional zone covers most of the central depression ( Fig. 3 f). The overall structure is much denser with much lower, narrower ribs and smaller, less prominent stalks. These last are mostly spherical with scarce, thinly scattered aeropyles. The ribs are connected to four, exceptionally five stalks. According to Downey and Allyn (1981) this zone is typical of the Polyommatini and is much less frequent in the other groups of Lycaenidae. The inner zone constitutes the micropylar region (rosette), conspicuously depressed into the chorion. Its irregularly discoid surface is divided into numerous cells outlined by elevated ribs. They are wedge shaped and arranged in a number of indistinct circles (annuli). The cells in the inner annuli are narrower than those in the outer ones. The micropyle makes up the central pit of the rosette. It bears three openings forming a triangle ( Fig. 3 g). The number of openings is equal to the number of surrounding cells.
Larva
Only the larva of the last instar was examined. Its entire upper surface is covered with numerous setae. The cuticle (hardly visible and only partially examined) is densely armoured with minute, slightly elongate warts. There are two kinds of setae: one kind is long, forming lateral bands along both sides of the larva, whereas the other is much shorter with a stellate base ( Fig. 3 i). The setae are regularly distributed and cover the thorax and abdomen. Three types of specialized myrmecophilous organs are found on the terminal segments of this instar ( Fig. 3 h). Pore cupola organs (PCO) — wart-like glandular structures — are present in both the larvae and pupae of many lycaenids ( Fiedler 1991 ). A pair of tentacular organs (TOs) is located exteroposteriorly from the spiracles on the eighth abdominal segment ( Fig. 4 b). They are membranous and readily eversible on living larvae. However, no specimens with the everted organ were available for detailed SEM examination, and only partly retracted organs are presented. The dorsal nectary organ (DNO) is located on the posterodorsal portion of the seventh abdominal segment. The raised spiracles taper gradually towards the semielliptical opening ( Fig. 3 j).
Pupa
The mature larva pupates in the upper layer of the soil or between the dead parts of plants (leaves, stems). It lies on its dorsal side ( Warecki 2010 ). The ∼11–12-mm-long pupa is barrel shaped. The pupal cuticle has scattered short setae with circular bases and are denser on the ventral areas of the abdomen. On each segment of the lateral zone of the abdomen there are spiracles ( Fig. 3 k) that are densely filled with lamellate, branched papillae. The papillae are covered with numerous, short, thick spines ( Fig. 3 l). The spiracle is surrounded by an area of densely located PCO ( Fig. 3 k) similar to those found in the last instar larva. According to Duarte et al. (2001) , this supports the hypothesis of myrmecophily during the pupal stage. Among these organs characteristic dendritic setae were detected on the fifth abdominal segment ( Fig. 3 n). They are thought to secrete ant-attractive chemicals ( Balmer and Pratt 1988 ). The last abdominal segment bears the cremaster. This consists of several rather short, straight, blunt-ended setae with apical, blunt spine-like projections ( Fig. 3 m).
Biology.
The entire Polish population of P. ripartii survives in just two localities in the Nida Valley, separated by a distance of ∼12 km. The butterflies are restricted to a specific type of vegetation, determined by edaphic factors. At both sites the dominant habitat type comprises a number of xerothermic associations from the class F estuco -B rometea Br. Bl. et R.Tx. In Poland, the growing conditions for O. viciifolia are favorable in this association, and although it is not treated as a characteristic species of any association, it is abundant in different plant communities. Apart from Onobrychis , I.ensifolia (L.) (Swordleaf Inula) is very important to P. ripartii . On large patches of its localities, this plant forms, within F estuco -B rometea , a distinct association — Inuletum ensifoliae Kozł. — of which it is one of the distinguishing species. This botanical association is characteristic of the shallow soils on chalk, limestone, or unconsolidated material containing highly calcareous topsoil. Besides the Nida Valley, this association has been identified only in the Lublin Upland and the western Volhynian Upland in SE, and E Poland. Despite the fact that the butterfly has not been found in those regions, this association can be treated as a sign of potentially suitable biotopes. This clue should be used in the future search for new localities of P. ripartii in the Nida region and elsewhere.
The caterpillar of P. ripartii is a narrow oligophage. The host plants recorded to date ( Lafranchis et al. 2007 , Buszko and Masłowski 2008 ) are restricted to the genus Onobrychis : O. alba Waldst. & Kit. (absent in Poland), O. arenaria (Kit.) DC., and O. ebenoides Boiss & Sprun. O. arenaria (Siberian Sainfoin), which is rare and very local, has been mentioned from just a few localities in the Nida Valley, the region where the butterfly lives. O. viciifolia Scop. (Common Sainfoin) is the most common species, growing abundantly in many dry and sunny spots ( Zając and Zając 2001 ). It is certainly the main and probably the only food plant of P. ripartii in Poland.
P. ripartii is a monovoltine species, the flight period of the Nida population being restricted to July. The earliest date recorded from museum specimens is 21 June 1996, whereas the latest one is 15 August 1961. A very exceptional finding was made during the fieldwork in 2011, when a freshly emerged specimen was sighted as early as 11 June. However, in spite of a thorough search at this locality, no more specimens could be found, and the next imagoes were not seen until the first days of July — their usual period of occurrence. This suggests that the June observation was of a specimen with an exceptionally atypically modified ontogenetic development.
P. ripartii is a typical heliophile, whose activity is strictly dependent on the local weather. The butterflies are active throughout the day in appropriate, i.e., warm and sunny, weather. In these conditions, they become active around 09:30 h and start looking for nectar. In good weather this activity continues until about 19:00 h. For roosting the butterflies choose dense patches of grasses or other tall herbs ( Fig. 4 e), resting on the terminal parts of spikes or shoots. In windy conditions, they select shorter shoots protected by surrounding, taller stems, although they still prefer the terminal parts of these plants ( Fig. 4 f).
Field observations in the larger locality, on the 5 km long gypsum ridge near Wola Zagojska, revealed two species of plants serving as preferred nectar sources for the imagoes ( Table 1 ). The principal one, usually the most often visited, is O. viciifolia . In sites with many flowering plants, the inflorescences of this species were always selected first, other species almost always being ignored. The second nectar source was Inula ensifolia (L.). But in places where both plants were equally numerous, Inula was very rarely chosen, certainly much less often than Onobrychis . Nonetheless, the butterflies were observed many times on extensive, dense patches of Inula , where this plant was then the accepted nectar source. At sites where Inula significantly outnumbered Onobrychis , the butterflies did not look for scattered individuals of the latter but fed on Inula . Although feeding, the butterflies “jumped” from flower to flower in the nearest neighborhood, only rarely undertaking longer flights of up to several metres.
Table 1.
Preferred nectar plants of P. ripartii and the time budget during feeding
| Observation (min) | No. of flowers visited (“jumps”) | Mean time spent on one shoot (min) | No. of Onobrychis flowers visited | No. of Inula flowers visited | Proportion (%) Onobrychis—Inula | Dominant nectar plant |
|---|---|---|---|---|---|---|
| 56 | 38 | 1.5 | 0 | 38 | 0–100 | Inula |
| 54 | 20 | 2.7 | 18 | 2 | 90–10 | Onobrychis |
| 16 | 16 | 1 | 14 | 2 | 87.5–12.5 | Onobrychis |
| 30 | 31 | >1 | 5 | 26 | 16–84 | Inula |
Only one accidental nectar plant — yellow lucerne Medicago falcata (L.) — has been recorded, on which a feeding butterfly was observed just once. Warecki (2010) adds two other species: lucerne Medicago sativa (L.) and yellow bedstraw Galium verum (L.).
Comparative observations were made to estimate the proportions of flowers visited ( Table 1 ) and the average time spent on a single plant. The results show that the butterflies spent an average of ∼90 s on a single inflorescence (flower head). Feeding at one particular site usually lasted for ½–1 h. During this time, the butterflies sometimes stopped feeding and rested on the plants for a while. They were then much less active and spent time cleaning their antennae and legs.
Observations revealed two defensive strategies displayed by the butterflies while nectaring. With several fast and shallow wingbeats, they repelled much smaller insects (flies, bees) that wanted to land on the same flower; this behavior was observed several times. A different strategy was adopted against much larger insects like bumble bees. On landing on the same plant (flower), these did not respect the presence of the butterfly. The unexpected contact between the two insects resulted in the butterfly immediately dropping into the dense vegetation on the ground without opening its wings; after a moment, however, it flew up and away. This behavior was observed on two occasions.
Another intriguing behavior following oviposition was noticed. Before leaving the shoot, the female walked slowly forwards and downwards beneath the inflorescence, wiping the end of her abdomen against both sides of the shoot ( Fig. 4 c and d). These abdominal movements resembled gliding. The probable explanation for this behavior is that the female had secreted oviposition-deterring pheromones to inform other females that eggs had already been laid on this shoot. Indirect confirmation of this explanation is the fact that in most cases no more than 1 egg is laid per shoot. This is a common strategy among, e.g., beetles and hymenopterans ( Nufio and Papaj 2001 , Anderson 2002 ), but there is very little evidence of such behavior in Lepidoptera, especially in butterflies ( Schoonhoven 1990 , Garcia-Barros and Fartmann 2009 ). This finding is probably the best-documented evidence of a putative oviposition-deterring pheromones in the Lycaenidae. This new observation may confirm the supposition of Sielezniew and Stankiewicz-Fiedurek (2013) that this kind of signaling is more common in Lycaenidae than hitherto recognized. The oviposition-deterring pheromones may reduce larval competition by enabling females to choose shoots on which their larvae are most likely to survive after hatching. The durability of the chemical signals left by P. ripartii females remains unknown. The general evidence for butterflies suggests that these marks may last no more than 1–2 d in Cupido minimus (Fuessly) ( Thomas and Lewington 2010 ), or that they may be very stable, as in the genus Pieris ( Schoonhoven 1990 ). With the reservation that the presence of oviposition-deterring pheromones in Lepidoptera is very poorly understood and somewhat speculative, the existence of such a mechanism is very probable. The present study shows that female behavior as well as the pattern in which only a single egg is present on almost 70% of shoots (and 90% of shoots have no more than two eggs) strongly suggest that there should be some mechanism preventing excessively dense egg laying. The absence of communication between females would certainly result in a much more uniform distribution of eggs on shoots. My observations are also indirectly confirmed by the other studies cited above.
Examination of florets in late August corroborates the information that the species hibernates as a first-instar larva amongst dry plant remains ( Tolman and Lewington 1999 ). These observations concur with those of Warecki (2010) , who also investigated the Nida Valley population, but contradict the general information given by Buszko and Masłowski (2008) , according to whom the species overwinters as an egg.
During the fieldwork, special attention was paid to recording egg laying behavior as well as egg location and abundance. These observations confirmed historical information ( Powell 1903 ) that a fertile female starts to look for a suitable site to attach the egg by walking downwards from the top to the base of the inflorescence. During all the phases of this activity she is standing or moving head downwards. Having found a good site, the female always lays a single egg and then moves on. In most cases only one egg is laid on one shoot (75.5% of 216 eggs on 1,200 shoots examined). The egg is usually attached to the base of the lancet bract in the basal half of the inflorescence ( Fig. 4 a) ( Table 2 ). This location was observed on 71 (94.75) of 75 fresh shots and on 42 (87.5%) of 48 dry shoots with a single egg attached. Only 4 (5.3%) and 6 (12.5%) eggs were located in the upper portion of fresh and dry shoots, respectively. The location of the eggs in the lower part of the inflorescence can be explained as protection against grazing by herbivores and detachment of the terminal part of the shoot by different factors. The egg is attached to the inner surface to protect the egg and to provide maximum camouflage. In Onobrychis , after the fruits (short legumes) have been produced, the entire legume drops to the ground together with its supporting pedicel. The fresh shoots, just after the fruits and their pedicels have dropped, are the ones favored by the females ( Table 3 ). Of 216 eggs recorded on 1,200 shoots, as many as 194 (89.8%) were laid on the dry, basal part of the bract. Only in one case was a single egg laid below the inflorescence; in addition, 13 eggs were observed on the legumes, and a single egg was seen to be attached to the dry remnant of the calyx just once.
Table 2.
Vertical position of P. ripartii egg on O. viciifolia inflorescences with single eggs
| No. of single eggs | Lower part | Upper part | |
|---|---|---|---|
| fresh shoots | 75 | 71 (94.7%) | 4 (5.3%) |
| dry shoots | 48 | 42 (87.5%) | 6 (12.5%) |
Table 3.
Morphological structure on which the eggs of P. ripartii are deposited
| Total number of eggs | Basal part of bract | Pedicel | Dry calyx | Legume | Shoot below inflorescence | |
|---|---|---|---|---|---|---|
| Fresh shoots | 153 (100%) | 147 (96.1%) | 0 (0.0%) | 1 (0.6%) | 5 (3.3%) | 0 (0.0%) |
| Dry shoots | 63 (100%) | 47 (74.6%) | 7 (11.1%) | 0 (0.0%) | 8 (12.7%) | 1 (1.6%) |
A precise count was carried out to determine the statistical parameters concerning egg location. On one day in early August, 6 counts of 100 shoots were carried out in adjacent sites: the total number of eggs per sample and the differences in their location were calculated (see Tables 2–4 ). On 600 fresh shoots examined, a total of 153 eggs attached to 96 shoots were found. Sixty five of the shoots had only a single egg attached to them (67.7%). Shoots with two eggs made up an additional 21.9% (21 shoots). Larger numbers of eggs were found on single shoots: 3 eggs on 2 shoots (2.1%), 4 eggs on 4 shoots (4.2%), 5 eggs on 1 shoot (1.0%), 6 eggs on 2 shoots (2.1%), and the highest number of 7 eggs on 1 shoot (1.0%). There were an average of 16 eggs per sample, and the mean number of eggs per shoot was 0.16, which implies that, statistically, eggs were laid on every sixth shoot.
Table 4.
The number of eggs of P. ripartii on fresh and dry shoots of O. viciifolia in the Nida region
| No. of shoots inspected | No. of shoots with eggs | Total number of eggs | Shoots with one egg | Shoots with two eggs | Shoots with three eggs | Shoots with four eggs | Shoots with five eggs | Shoots with six eggs | Shoots with seven eggs | |
|---|---|---|---|---|---|---|---|---|---|---|
| Fresh shoots | 600 | 96 (100%) | 153 | 65 (67.7%) | 21 (21.9%) | 2 (2.1%) | 4 (4.2%) | 1 (1.0%) | 2 (2.1%) | 1 (1.0%) |
| Dry shoots | 600 | 55 (100%) | 63 | 48 (87.3%) | 6 (10.9%) | 1 (1.8%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
A similar experiment was carried out in late August, when 600 dry shoots were collected. Fifty five eggs were detected in this sample, far fewer than at the beginning of August. This was to be expected; however, as 3 wk after being laid, the eggs would have been exposed to a variety of destructive factors and conditions. The fruits and pedicels had also become detached from most shoots, and in any case some very small eggshell remnants could have gone undetected. In addition, the terminal portions of several shoots were broken. Despite these differences, the same egg location pattern was obtained ( Table 4 ), 48 of the 55 shoots had single eggs on them, all of which had been laid in the basal part. Three eggs were detected on only one of the 600 shoots, and single eggs were found exclusively in the terminal parts of just 7 shoots.
Myrmecophily.
The P. ripartii larvae observed in the field were frequently attended by ants of the species L. alienus (Först.) and L. niger (L.). Both ant species are common in Poland, and L. alienus is an oligotrophic species preferring dry grasslands with limestone soils — typical P. ripartii biotopes ( Radczenko et al. 2004 ). L. alienus has already been recorded by Warecki (2010) , but this is the first time L. niger was seen in association with P. ripartii caterpillars. In Greek populations of P. ripartii , only Lasius paralienus Seifert has been reported to exhibit such behavior ( Lafranchis et al. 2007 ). Field observations support earlier data suggesting the facultative myrmecophily of this butterfly ( Lafranchis et al. 2007 ).
Conservation.
For decades or possibly even centuries traditional farming has sustained the Nida Basin population of P. ripartii . The changes in these practices, threaten not only this but the majority of endangered species in Europe. However, in the context of modern, intensive agriculture, the management of this seriously endangered population is impossible without detailed knowledge of its biology, behavior and the key factors determining its stable existence. The current study has provided much new and significant information and presents hitherto unknown details that could be applied in future action plans for this population. The Nida Basin is rich in numerous remnants of steppe-like habitats, which may be considered in the future as potential relocation localities for P. ripartii . Habitat preferences are among the key factors determining this butterfly’s ability to colonize (or recolonize) other locations in the Nida region. These factors are intimately connected with the occurrence of suitable plants. The availability of nectaring plants should be thoroughly examined before any practical action is undertaken. This work has shown that the existence of a stable population is limited by the presence of Onobrychis , which acts both as larval host plant and a nectar source for the imago. However, the butterfly can also use Inula ensifolia for nectaring, which may facilitate dispersion among patches of suitable habitat. These findings can be used for improving habitat quality in potential locations where the artificial planting of Onobrychis and Inula seeds is feasible. These plants might be sown on the margins of selected localities on abandoned, extremely dry remnants of old arable fields.
Data on the location, density and morphology of the eggs of this species are presented for the first time. They can be treated as a starting point for further research into the condition of different populations. This information may also be useful for monitoring the results of possible future active conservation measures. Although the presence of the butterflies does not necessarily confirm their colonization of a locality, and the search for widely scattered caterpillars can be difficult, the search for eggs — fairly easily detected within a reasonable time — can supply important comparative data. The data presented here on the distribution pattern of eggs and their generally low density per shoot is also important information for future conservation efforts. It implies that to establish any long-lasting locality for P. ripartii a rather large plot covered with sainfoin should be prepared. The existing restricted patches of the plant are certainly too small to allow the Nida population to expand.
The species is on the verge of extinction in Poland, and the data presented here should be regarded as a significant step towards the active conservation of this remarkable population. Further research should concentrate on estimating the population size and the migratory abilities of this species. The significance of artificial sainfoin plantations set up as feeding places that might be utilized by the butterflies migrating between suitable patches of chalk grassland should also be tested.
Acknowledgments
I would like to thank Jacek Kazimierczak for his assistance during the field work. Krzysztof Fiołek kindly edited the images and prepared the plates. I am grateful to Olga Woźnicka (Institute of Zoology, Jagiellonian University, Kraków) for preparing the material and taking the SEMs. The linguistic correction was kindly done by Peter Senn. The work was supported by a Ministry of Science and Higher Education (grant NN304319536).
References Cited
- Anderson P. 2002. . Oviposition pheromones in herbivorous and carnivorous insects . InHilker M., Meiners T. (eds.), Chemoecology of insect eggs and egg deposition . Blackwell, Oxford; . [Google Scholar]
- Balmer G. R., Pratt G. F. . 1988. . A survey of the last instar larvae of the Lycaenidae of California . J. Res. Lepid. 27 : 1 – 81 . [Google Scholar]
- Buszko J., Masłowski J. . 2008. . Motyle dzienne Polski , pp. 274 . Koliber, Nowy Sącz . [Google Scholar]
- Dincă V., Runquist M., Nilsson M., Vila R. . 2013. . Dispersal, fragmentation and isolation shape the phylogeography of the European lineages of Polyommatus ( Agrodiaetus ) ripartii (Lepidoptera: Lycaenidae) . Biol. J. Linn. Soc. 109 : 817 – 829 . [Google Scholar]
- Downey J. C., Allyn A. C. . 1981. . Chorionic Sculpturing in Eggs of Lycaenidae . Part I. Bull. Allyn Mus. 61 : 1 – 29 . [Google Scholar]
- Duarte M., Almeida G. L., Casagrande M. M., Mielke O. . 2001. . Notes on the last instar larva and pupa of Hemiargus hanno (Stoll) (Lepidoptera, Lycaenidae, Polyommatinae) . Rev. Brasil. Zool. 18 : 1097 – 1105 . [Google Scholar]
- Fiedler K. 1991. . Systematic, evolutionary, and ecological implications of myrmecophily within Lycaenidae (Insecta: Lepidoptera: Papilionoidea) . Bonner Zoologische Monographien 31 : 1 – 210 . [Google Scholar]
- Garcia-Barros E., Fartmann T. . 2009. . Butterfly oviposition: sites, behaviour and modes, pp. 29–42 . InSettele J., Shreeve T. G., Konvicka M., Van Dyck H. (eds.), Ecology of butterflies in Europe . Cambridge University Press, Cambridge; . [Google Scholar]
- Głowaciński Z., Nowacki J. . 2004. . Polish Red Data Book . Invertebrates, pp. 447. IOP PAN & AR Poznań . [Google Scholar]
- IUCN . 2001. . IUCN Red List Categories and Criteria: Version 3.1 , pp. ii + 33 . IUCN Species Survival Commission. IUCN, Gland, Switzerland and Cambridge, UK . [Google Scholar]
- Kandul N. P., Lukhtanov V. A., Dantchenko A. V., Coleman J.W.S., Sekercioglu C. H., Haig D., Pierce N. E. . 2004. . Phylogeny of Agrodiaetus Hübner, 1822 (Lepidoptera: Lycaenidae) inferred from mtDNA sequences of COI and COII and nuclear sequences of EF1-a: karyotype diversification and species radiation . Syst. Biol. 53 : 278 – 298 . [DOI] [PubMed] [Google Scholar]
- Kandul N. P., Lukhtanov V. A., Pierce N. E. . 2007. . Karyotypic diversity and speciation in Agrodiaetus butterflies . Evolution 61 : 546 – 559 . [DOI] [PubMed] [Google Scholar]
- Lafranchis T., Gil T. F., Lafranchis A. . 2007. . New data on the ecology of 8 taxa of Agrodiaetus H übner , 1822 from Greece and Spain: hostplants, associated ants and parasitoids (Lepidoptera: Lycaenidae . Hymenoptera. Diptera). Atalanta 38 : 189 – 197 . [Google Scholar]
- Lukhtanov V. A., Kandul N. P., Plotkin J. B., Dantchenko A. V., Haig D., Pierce N. E. . 2005. . Reinforcement of pre-zygotic isolation and karyotype evolution in Agrodiaetus butterflies . Nature 436 : 385 – 389 . [DOI] [PubMed] [Google Scholar]
- Nufio C. R., Papaj D. R. . 2001. . Host marking behavior in phytophagous insects and parasitoids . Entomol. Exp. Appl. 99 : 273 – 293 . [Google Scholar]
- Powell H. 1903. . The egg-laying habits of Polyommatus admetus var. ripartii , with description of its ovum . Entomol. Rec. J. Var. 16 : 92 – 94 . [Google Scholar]
- Przybyłowicz Ł. 2000. . Polish butterflies of the subgenus Polyommatus ( Agrodiaetus ) (Lepidoptera: Lycaenidae) . Polskie Pismo Entomologiczne 69 : 329 – 334 . [Google Scholar]
- Przybyłowicz Ł., Lukhtanov V., Lachowska-Cierlik D. . 2014. . Towards the understanding of the origin of the Polish remote population of Polyommatus ( Agrodiaetus ) ripartii (Lepidoptera: Lycaenidae) based on karyology and molecular phylogeny . J. Zool. Syst. Evol. Res. 52 : 44 – 51 . [Google Scholar]
- Radczenko A., Czechowska W., Czechowski W. . 2004. . Mrówki—Formicidae. Klucze do Oznaczania Owadów Polski, 24 (63), pp. 138. Polskie Towarzystwo Entomologiczne, Toruń . [Google Scholar]
- Schoonhoven L. M. 1990. . Host-marking pheromones in Lepidoptera, with special reference to two Pieris spp . J. Chem. Ecol. 16 : 3043 – 3052 . [DOI] [PubMed] [Google Scholar]
- Schurian K. 1976. . Beiträge zur Biologie der Gattung Agrodiaetus 1. Agrodiaetus ripartii Freyer (Lep., Lycaenidae) . Entomologische Zeitschrift 86 : 196 – 200 . [Google Scholar]
- Sielezniew M., Stankiewicz-Fiedurek A. M. . 2013. . Behavioural evidence for a putative oviposition-deterring pheromone in the butterfly, Phengaris (Maculinea) teleius (Lepidoptera: Lycaenidae) . Eur. J. Entomol. 110 : 71 – 80 . [Google Scholar]
- Thomas J. A., Lewington R. . 2010. . The Butterflies of Great Britain & Ireland , pp. 288 . British Wildlife Publishing, Milton on Stour; . [Google Scholar]
- Tolman T., Lewington R. . 1999. . Butterflies of Britain & Europe . Collins Field Guide, pp. 320 . HarperCollins Pub Ltd, London . [Google Scholar]
- Tshikolovets V. V. 2011. . Butterflies of Europe & the Mediterranean area , pp. 1 – 544 . Publ. by V. Tshikolovets, Pardubice; . [Google Scholar]
- Van Swaay C., Cuttelod A., Collins S., Maes D., Lopez Munguira M., Šašić M., Settele J., Verovnik R., Verstrael T., Warren M., et al. . 2010. . European red list of butterflies , pp. 48 . Publications Office of the European Union; , Luxembourg: . [Google Scholar]
- Vila R., Lukhtanov V. A., Talavera G., Gil T. F., Pierce N. E. . 2010. . How common are dot-like distribution ranges? Taxonomical oversplitting in Western European Agrodiaetus (Lepidoptera, Lycaenidae) revealed by chromosomal and molecular markers . Biol. J. Linn. Soc. 101 : 130 – 154 . [Google Scholar]
- Warecki A. 2010. . Motyle dzienne Polski . Atlas bionomii, pp. 320. Koliber . [Google Scholar]
- Wiemers M., Keller A., Wolf M. . 2009. . ITS2 secondary structure improves phylogeny estimation in a radiation of blue butterflies of the subgenus Agrodiaetus (Lepidoptera: Lycaenidae: Polyommatus) . BMC Evol. Biol. 9 : 300 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zając A., Zając M. (eds.). 2001. . Distribution atlas of vascular plants in Poland , pp. 715 . Laboratory of Computer Chorology, Institute of Botany, Jagiellonian University, Kraków [Google Scholar]