Abstract
The feasibility of using Bombyx mori as model animal is attracting more attention. Whether the effect of drugs on the metabolite profiling was consistent with those in mammals was an aspect to evaluate the feasibility of B. mori as model animal. In this study, we used acetaminophen to treat Dazao fifth-instar B. mori , and its metabolites in hemolymph were detected by gas chromatography–mass spectrometry. The corresponding data were processed and analyzed by total model analysis, principal component analysis, partial least squares-discriminant analysis, orthogonal partial least squares-discriminant analysis, and finally, the difference metabolites between acetaminophen group and control group were selected and identified by our reference material database and the National Institute of Standard and Technology database. The results showed that acetaminophen administration induced elevation of metabolites related to energy source, the intermediate of cholesterol synthesis, and the metabolites related to melanization and also induced the decrease of metabolites in pathway of Krebs cycle, the cholesterol, and sitosterol, which suggested that acetaminophen administration inhibited energy metabolism and promoted the expenditure and imbalance of hormone and melanization.
Keywords: acetaminophen, Bombyx mori, horme synthesis, Krebs cycle, metabolite
Drug metabolism also known as xenobiotic metabolism is the biochemical modification of pharmaceutical substance or xenobotics by living organisms. The rate of metabolism determines the duration and intensity of a drug’s pharmacological action. During the practical research and development of drug, most of drugs screened by in vitro cell system must be tested for the desired effect on disease animal model ( Rostami-Hodjegan and Tucker 2007 ). Hence, in vivo metabolism is the final validation pathway for drug pharmacokinetics. Mammalian animal, especially animals with high homology to human, has been the first animal model for drug pharmacokinetics. However, mammalian animal is expensive and highly problematic with regard to ethical issues ( Orlans et al. 1998 , Baumans 2004 ). Scientists have been looking for low-expense invertebrate animal to replace mammalian animals as animal model for drug screening ( Berger 2009 ; Fujiyuki et al. 2010 ).
Replacement animals involved invertebrate have received more and more attentions. Caenorhabditis elegans and Drosophila have been firstly studied by many scientists. However, a limitation of C . elegans and Drosophila as replacement model organism is that their body sizes are too small for use in studies of pharmacodynamics, so these models are generally used in bacterial infection models ( Mahajan-Miklos et al. 1999 , Bernal and Kimbrell 2000 ). Bombyx mori has particular advantages compared with C . elegans, Drosophila , mammalians, and cells: low in cost, little conflict with ethical problem, rapid and convenient assay, injection into not only hemolymph but also midgut, and no danger of biohazard. Thus, some researchers have studied the possibility of silkworm as model organism used in the evaluation of bacterial infection ( Kaito et al. 2002 ), virulence factors ( Miyazaki et al. 2012 ), therapeutic effects, and pharmacokinetics of drugs ( Matsumoto et al. 2012 ). As mentioned above, most of drugs screened by in vitro cell system must be tested for the desired effect on disease animal model for drug metabolism in vivo ( Rostami-Hodjegan and Tucker 2007 ). Hence, as replacement animal, the influence of drug on its metabolism must be studied. Hamamoto et al. (2009) evaluate the feasibility of using B. mori as a model animal for screening drug candidates by detecting the effect of umbelliferone on the pathway of B. mori . Acetaminophen, chemically named N-acetyl-p-aminophenol, is a widely used over-the-counter analgesic and antipyretic, and its pharmaceutics and the effect on human are relatively clear. Hence, exploring the influence of acetaminophen on B. mori will indicate the feasibility of B . mori as replacement animal model preliminarily.
Principal component analysis is a mathematical procedure that uses orthogonal transformation to covert a set of observations of possibly correlated variables into a set of values of linearly uncorrelated variables called principal components. Often, its operation can be thought of as revealing the internal structure of the data in a way that best explains the variance in the data. If a multivariate dataset is visualized as a set of coordinates in a high-dimensional data space (1 axis per variable), principal component analysis (PCA) can supply the user with a lower-dimensional picture, a “shadow” of this object when viewed from its most informative viewpoint. Partial least squares-discriminant analysis (PLS-DA) is a partial least squares regression of a set Y of binary variables describing the categories of categorical variable on a set X of predictor variables. It is a compromise between the usual discriminant analysis and a discriminant analysis on the significant principal components of the predictor variables. Karp et al. (2005) applied PLS-DA in expression proteomics, and this approach has the advantages of reduced risk of false positives and the identification of spots that are significantly altered in terms of correlated expression rather than absolute expression values. Orthogonal PLS-DA (OPLS-DA) is a supervised multiple regression analysis for identification of discrimination between different datasets referred to as X and Y. So far, proteomics approaches have proven their value to provide a broad indication of biological systems. However, as no single analytical technique is sufficient to reveal the full biochemical content of complex biological matrices, combining the strengths of established data analysis strategies is proposed. To accurately extract knowledge from multiple variables, we used three analysis methods (PCA, PLS-DA, and OPLS-DA) to extract 41 metabolites from multiple variables.
In this study, we used acetaminophen to treat Dazao fifth-instar B. mori , detected the metabolites in hemolymph of B. mori using gas chromatography–mass spectrometry (GC/MS), and analyzed data by metabonomic analysis. The results showed that acetaminophen administration induced elevation of metabolites related to energy source, the intermediate of cholesterol synthesis, and the metabolites related to melanization and also induced the decrease of metabolites in pathway of Krebs cycle, the cholesterol, and sitosterol, which suggested that acetaminophen administration promoted the energy metabolism, the expenditure, and imbalance of hormone and melanization.
Materials and Methods
B . mori Feeding and Hemolymph Obtainment
B. mori were fed mulberry leaves for 12:12 (L:D) h at 25°C each day. Fifth-instar B . mori were divided into two groups ( n = 10): acetaminophen-treated group (AP group) and control check group (CK group). Two groups of B. mori were fasted for 4 h, and acetaminophen-treated group received acetaminophen by intragastric injection administration. Then two groups were fed as common. Ten hours later, 10 hemolymph samples from each group were used in GC/MS detection, and 7 quality-control samples were treated at the same time.
Sample Pretreatment
Twenty microliters of hemolymph sample were added to 80 µl of ice-cold methanol, vortex for 30 s, kept at −20°C for 20 min, and centrifuged for 15 min at 14,000 × g , 4°C. Eighty microliters of supernatant were added into glass vial and blowed dry by nitrogen to obtain dry matter. Dry matter was added into thirty microliters of 20 mg/ml methoxyamine hydrochloride pyridine solution, oximation reaction for 90 min, then added 30 µl of O-Bis (trimethylsilyl) acetamide N (containing 1% trimethylchlorosilane) derivatizing reagent, kept closed at 70°C for 60 min. Finally, sample was taken out and kept at room temperature for 30 min for GC/MS.
GC/MS Analysis
Agilent 7890A/5975C GC/MS system and HP-5 capillary chromatogram column (Agilent J&W Scientific, Palo Alto, CA) (30 m by 0.25 mm by 0.25 µm) were used in detection. The parameters were as follows: injector temperature: 280°C; EI ionization source temperature: 230°C; quadrupole temperature: 150°C; high purity helium (purity >99.999%) as carrier gas; and sample volume: 1.0 µl. Temperature program: beginning temperature 80°C for 2 min, 10°C/min up to 320°C for 6 min. Full-scan model was used in mass detection (range 50–600 m/z). Random sequence was used in continued sample analysis to avoid the disturbance of signal fluctuation.
Data Process
Raw data were pretreated by our program including baseline filtering, peak identification, integration, retention time correction, peak alignment, and mass fragment classification and then edited in excel. The final data were two-dimension data matrix including variable (rt_mz, i.e., retention time_mass to charge ratio), sample, and integration area. Five hundred thirty-four matters were obtained. All data were normalized to total signal integration. All edited data matrix was imported into Simca-P software 11.0 for PCA, PLS-DA, and OPLS-DA.
Analysis Setting
Data were processed by Unit Variance Scaling and mean centered to obtain straightforward results in Simca-P software. Data were analyzed by automodel fitting to obtain reliable number of preliminary component. Also, cross-validation ( n = 7) was used to establish model for preventing model from overfitting.
Screening and Identification of Difference Metabolites
Difference metabolites were obtained by combining variable importance in the projection of the first preliminary component in OPLS-DA model with P value (threshold 0.05) in Student’s t -test. The identification of difference metabolites was done by searching own reference chemical data and the National Institute of Standard and Technology commercial data (comparing retention time or retention index of mass spectrum and chromatograph).
Results and Discussions
Review of Chromatogram
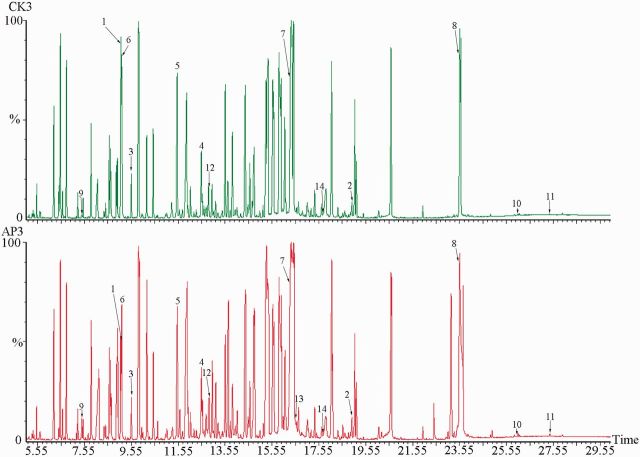
All total ion chromatograms were reviewed, and the results showed strong signal, large peak capacity, and good reproducibility for retention time. The representative total ion chromatogram is shown in Fig. 1 .
Fig. 1.
Representative total ion chromatogram from CK3 and AP3. (1) Glycine; (2) tryptophan; (3) fumaric acid; (4) α-ketoglutaric acid; (5) malic acid; (6) succinic acid; (7) glucose; (8) trehalose; (9) β-alanine; (10) cholesterol; (11) sitosterol; (12) β-Hydroxy-β-methylglutaric acid; (13) tyrosine; and (14) dopa.
General Model Analysis
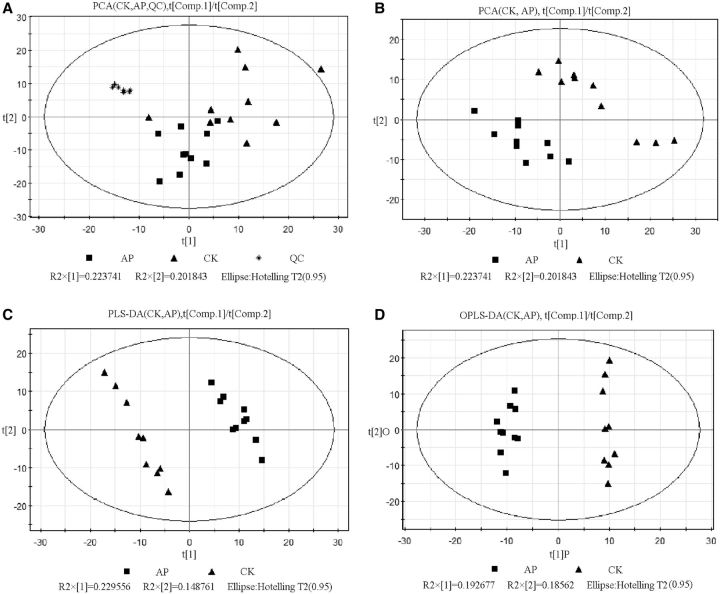
The principal component analysis for samples shows the reliability of test method in general, the metabolic difference among groups, and the viability of samples within group. This study firstly did PCA for CK group sample, AP group sample, and quality control (QC) sample and obtained five principal components; the cumulative rate of interpretation is 0.682. Generally, the cumulative rate of interpretation is >0.4 means the reliability of model, cumulative rate of interpretation 0.682 shows this model is reliable. Hence, the model established by us could be used to test the metabolic difference among groups in general. PCA scores plot is shown in Fig. 2 A. In Fig. 2 A, t[1] (x-axis) and t[2] (y-axis) represent the scores plot of the first principal component and the scores plot of the second principal component, respectively. The scores plot showed QC sample gathered in a narrow principal component plot (*), while CK and AP samples scattered in a wide principal component plot (▴, ▪), CK samples scattered in a wider plot than AP samples, which indicated that variation in CK was higher than AP group, thus suggested that the influence on B. mori mainly resulted from drug (acetaminophen) treatment. In further analysis, QC samples were excluded.
Fig. 2.
Scores plot of PCA for CK, AP, and QC groups (A) and scores plots of PCA (B), PLS-DA (C), and OPLS-DA(D) for CK and AP groups. t[1] represents the first principal component, t[2] represents the second principal component, t[1]P represents principal component of OPLS-DA, and t[2]O represents orthogonal component of OPLS-DA.
PCA Between AP and CK Group
Four principal components were obtained from fitting analysis by Simca-P software, and the cumulative rate of interpretation is 0.602, which suggested the reliability of model. The scores plot of PCA is shown in Fig. 2 B, and all samples were within 95% confidence interval (Hotelling T 2 ellipse) and showed no outlier samples. Figure 2 B showed PCA scores plot of AP and CK group located lower left side and upper right side, respectively, which suggested that two groups had significant metabolic difference.
PLS-DA Between AP Group and CK Group
Two principal component by PLS-DA method, R2Y = 0.987, Q2 = 0.943, the scores plot is shown in Fig. 2 C. t[1] (x-axis) and t[2] (y-axis) represented the scores plot of the first principal component and the scores plot of the second principal component, respectively. R2Y is the interpretation rate of model and is 0.987, which suggested that PLS-DA model could interpret the difference between two groups appropriately. Also, the predictive rate of model ( Q2 ) was 0.943, which showed good predictive capacity. Figure 2 C showed that samples from two groups were located in different sites, which suggested that two samples from two groups had significant metabolic difference.
OPLS-DA Between AP Group and CK Group
One principal component (P, R2Y = 0.888) and one orthogonal component (O, R2Y = 0.099) were calculated from OPLS-DA analysis, and its quality index was R2Y = 0.987, Q2 = 0.91, which showed that OPLS-DA model was very reliable. The scores plot is shown in Fig. 2 D. Two samples from AP group and CK group were located in the plus side and minus side, respectively, which indicated that two groups had significant metabolic difference in OPLS-DA scores plot.
Difference Metabolites and Their Structure Identification Between AP Group and CK Group
Difference metabolites were very reliable because of filtering irrelevant orthosignal. Difference metabolites were obtained by combining variable importance in the projection of the first principal component in OPLS-DA model (threshold > 1) with P value (threshold 0.05) in Student’s t -test. In this study, 41 difference metabolites were obtained by screening, the level of 18 metabolites decreased and 23 metabolites increased.
We screened the metabolites with the absolute value of fold change > 0.5 and obtained 10 metabolites. We found these 10 metabolites were mainly related to three metabolic processes: glycolysis and Krebs cycle, sterol, and melanization. Then we selected metabolites related to these three processes and obtained 14 metabolites that showed the metabolic change induced by acetaminophen in B. mori was mainly related to carbohydrate catabolism, hormone metabolism, and pigmentation ( Table 1 ). The levels of substances involved in Krebs cycle including fumaric acid, α-ketoglutaric acid, malic acid, and succinic acid were decreased significantly, and the corresponding fold change [log 2 (AP/CK)] was −0.49, −0.25, −0.47, and −0.34, respectively. The level of trihalose, glucose, and β-alanine increased, and fold change [log 2 (AP/CK)] was 2.17, 2.03, and 1.27, respectively. The level of the intermediate for cholesterol, beta-hydroxy-β-methylglutaric acid, increased (fold change [log 2 (AP/CK)] 0.67), while fold change [log 2 (AP/CK)] of cholesterol and sisterol was −0.86 and −0.51, respectively. The levels of the metabolites related to melanization, tyrosine and dopa, increased, and their fold changes were ∞ and 2.59, respectively.
Table 1.
The influence of acetaminophen administration on the metabolics of B. Mori
| Compounds | VIP value (OPLS-DA) | P value ( t -test) | Fold change a [log 2 (AP/CK)] |
|---|---|---|---|
| Glycolysis and Krebs cycle | |||
| Glycine | 1.75 | 1.08E-04 | −0.99 |
| Tryptophan | 1.52 | 1.59E-03 | −0.73 |
| Fumaric acid | 1.71 | 1.75E-04 | −0.49 |
| α-Ketoglutaric acid | 1.48 | 2.25E-03 | −0.25 |
| Malic acid | 2.00 | 6.67E-07 | −0.47 |
| Succinic acid | 1.68 | 2.59E-04 | −0.34 |
| Glucose | 2.01 | 7.89E-08 | 2.03 |
| Trehalose | 1.49 | 4.74E-05 | 2.17 |
| β-Alanine | 1.74 | 1.15E-04 | 1.27 |
| Sterol | |||
| Cholesterol | 1.26 | 1.25E-02 | 0.86 |
| Sitosterol | 1.04 | 4.51E-02 | −0.51 |
| β-Hydroxy-β-methylglutaric acid | 1.64 | 4.16E-04 | 0.67 |
| Melanization | |||
| Tyrosine | 1.35 | ∞ | |
| Dopa | 1.56 | 1.03E-03 | 2.59 |
VIP, variable importance in the projection.
a The binary logarithm for the ratio of AP to CK.
Discussions
To explore the effect of the acetaminophen on the metabolite profile in B. mori , we detected the metabolites in hemolymph of B. mori treated with acetaminophen (AP group) or with normal saline (CK group) using GC/MS, then found the significant difference of metabolites between AP group and CK group by PCA, PLS-DA, and OPLS-DA. Forty-one difference metabolites between AP group and CK group were identified using databases. These difference metabolites were mainly related to Krebs cycle, hormone, and melanization.
Glycine is derived from serine, which is derived from 3-phospho- d -glycerate, an intermediate of glycolysis. Tryptophan can be oxidized into the precursor of nicotinamide adenine dinucleotide (NAD) and NAD phosphate (NADP). When tryptophan changes into precursor of NAD and NADP, the catabolism of glycogen decreases. In our study, the level of glycine and tryptophan decreased, which suggested the depletion of substances in glycolysis and the inhibition in glycolysis.
The levels of substances involved in Krebs cycle including fumaric acid, α-ketoglutaric acid, malic acid, and succinic acid were decreased significantly, which suggested that acetaminophen administration might inhibit catabolism. Besides, the level of glucose, trehalose, and β-alanine increased, which indicated that acetaminophen administration induced higher glucose, trehalose, and β-alanine. Trehalose is a main blood sugar for insect, which is synthesized by fat body of B. mori and hydrolyzed into two molecule of glucose to be absorbed and used by organism. Beta-alanine is the intermediate product in Krebs cycle and can transfer into pyruvic acid and enter into Krebs cycle. Therefore, the changes of above substances generally suggested that acetaminophen administration inhibited energy consuming and producing. In vitro experiment showed that acetaminophen inhibited NADH-linked respiration reversely, whereas the metabolites inhibited all mitochondrial respiration, apparently in the complex III region of the respiratory in mouse liver mitochondria ( Ramsay et al. 1998 ). In vivo study for CD-1 mice showed that inhibition of mitochondrial respiration, especially glutamate- and succinate-supported respiration, was an early event in acetaminophen-induced hepatoxicity ( Donnelly et al. 1994 ). Also, the metabonomic analysis of urine and plasma in chimeric mice and normal mice revealed alterations of endogenous metabolites, which were the intermediates involved in the Krebs cycle ( Yamamoto et al. 2007 ). Our study also showed the similar results as the above study, which suggested that the influence of the acetaminophen on Krebs cycle might be similar or conserved in B . mori and mammals.
Xiao et al. (2009) reported exogenous trehalose was positive in increasing the resistibility of silkworm and suggested that trehalose might be an important carbohydrate involved in stress metabolism. In our study, acetaminophen induced higher level of trehalose, which also suggested that trehalose might play a role in stress metabolism.
Cholesterol widely exists in animal and is necessary for multiple physiological activities. Beta-hydroxy-β-methylglutaric acid is the intermediate for cholesterol. Gu et al. (2013) reported that the cholesterol level of fifth-instar B . mori was lower than other phases. In our study, acetaminophen induced lower cholesterol level and higher beta-hydroxy-β-methylglutaric acid, which suggested that acetaminophen administration induced cholesterol expenditure for more stress physiological activities. Sitosterol belongs to lipids, and it is the precursor of ecdyson, which is not synthesized by B. mori , must be uptaken from food. Beta-sitosterol is the sterol preference of B. mori in a few sterols including beta-sitosterol, ergosterol, cholesterol, and stigmasterol ( Nagata et al. 2006 ). Acetaminophen induced lower level of sitosterol, and suggested acetaminophen administration led to imbalance of sterol in B. mori .
Tyrosine hydroxylase can catalyze l -tyrosine into l -dopa, which is the precursor for dopamine and melanin. Liu et al. (2010) reported that repression of tyrosine hydroxylase is responsible for the sex-linked chocolate mutation of the silkworm, B. mori . In our study, acetaminophen administration induced higher level of tyrosine and dopa, which suggested that acetaminophen promoted pigmentation of B. mori .
In summary, we used acetaminophen to treat B. mori , and analyzed the metabolic profile of hemolymph, the results showed that there were difference metabolites between group treated with acetaminophen and control group, and the difference metabolites included substances in glucose glycolysis and Krebs cycle, metabolite-related sterol and melanization. This study is a preliminary study for B . mori as replacement animal, and the detailed metabolites induced by acetaminophen between B. mori and mice need to be studied.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (grant 31172264), National High-Tech R&D Program of China (863 Program) (grant 2011AA100306), Natural Science Foundation of Jiangsu Province, China (project no. BK2011298), Provincial Key Technology R&D Program of Jiangsu, China (project no. BE2011327-1), and Priority Academic Program Development of Jiangsu Higher Education Institutions, China.
References Cited
- Baumans V. 2004. . Use of animals in experimental research: an ethical dilemma? Gene Ther. 11 ( Suppl 1 ): S64 – S66 . [DOI] [PubMed] [Google Scholar]
- Berger J. 2009. . Preclinical testing on insects predicts human haematotoxic potentials . Lab. Anim. 43 : 328 – 332 . [DOI] [PubMed] [Google Scholar]
- Bernal A., Kimbrell. D. A. 2000. . Drosophila Thor participates in host immune defense and connects a translational regulator with innate immunity . Proc. Natl. Acad. Sci. USA. 97 : 6019 – 6024 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly P. J., Walker R. M., Racz. W. J. 1994. . Inhibition of mitochondrial respiration in vivo is an event in acetaminophen-induced hepatoxicity . Arch. Toxicol. 68 : 110 – 118 . [DOI] [PubMed] [Google Scholar]
- Fujiyuki T., Imamura K., Hamamoto H., Sekimizu. K. 2010. . Evaluation of therapeutic effects and pharmacokinetics of antibacterial chromogenic agents in a silkworm model of Staphylococcus aureus infection . Drug Discov. Ther. 4 : 349 – 354 . [PubMed] [Google Scholar]
- Gu L. J., Gao G. T., Duan A. L., Zhao J. M., Zhang. Y. F. 2013. . Analysis for cholesterol level in 871 Bombyx mori . Sci. Technol. Ind. 3 : 350 – 353 . [Google Scholar]
- Hamamoto H., Tonoike A., Narushima K., Horie R., Sekimizu. K. 2009. . Silkworm as a model animal to evaluate drug candidate toxicity and metabolism . Comp. Biochem. Physiol. C Toxicol. Pharmacol. 149 : 334 – 339 . [DOI] [PubMed] [Google Scholar]
- Kaito C., Akimitsu N., Watanabe H., Sekimizu. K. 2002. . Silkworm larvae as an animal model of bacterial infection pathogenic to humans . Microb. Pathog. 32 : 183 – 190 . [DOI] [PubMed] [Google Scholar]
- Karp N. A., Griffin J. L., Lilley. K. S. 2005. . Application of partial least squares discriminant analysis to two-dimensional difference gel studies in expression proteomics . Proteomics 5 : 81 – 90 . [DOI] [PubMed] [Google Scholar]
- Liu C., Yamamoto K., Cheng T. C., Kadono-Okuda K., Narukawa J., Liu S. P., Han Y., Futahashi R., Kidokoro K., Noda H., et al. . 2010. . Repression of tyrosine hydroxylase is responsible for the sex-linked chocolate mutation of the silkworm, Bombyx mori . Proc. Natl. Acad. Sci. USA. 107 : 12980 – 12985 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahajan-Miklos S., Tan M. W., Rahme L. G., Ausubel F. M. . 1999. . Molecular mechanisms of bacterial virulence elucidated using a Pseudomonas aeruginosa - Caenorhabditis elegans pathogenesis model . Cell 96 : 47 – 56 . [DOI] [PubMed] [Google Scholar]
- Matsumoto Y., Miyazaki S., Fukunaga D. H., Shimizu K., Kawamoto S., Sekimizu. K. 2012. . Quantitative evaluation of cryptococcal pathogenesis and antifungal drugs using a silkworm infection model with Cryptococcus neoformans . J. Appl. Microbiol. 112 : 138 – 146 . [DOI] [PubMed] [Google Scholar]
- Miyazaki S., Matsumoto Y., Sekimizu K., Kaito. C. 2012. . Evaluation of Staphylococcus aureus virulence factors using a silkworm model . FEMS Microbiol. Lett. 326 : 116 – 124 . [DOI] [PubMed] [Google Scholar]
- Nagata S., Omori Y., Nagasawa H. . Dietary sterol preference in the silkworm, Bombyx mori . 2006. . Biosci . Biotechnol. Biochem. 70 : 3094 – 3098 . [DOI] [PubMed] [Google Scholar]
- Orlans F. B., Beauchamp T. L., Dresser R., Morton D. B., Gluck J. P. . 1998. . The human use of animals: case studies in ethical choice . Oxford University Press; , New York, NY: . [Google Scholar]
- Ramsay R. R., Rashed M. S., Nelson S. D. . 1998. . In vitro effects of acetaminophen metabolites and analogs on the respiration of mouse liver mitochondria . Arch. Biochem. Biophys. 273 : 449 – 457 . [DOI] [PubMed] [Google Scholar]
- Rostami-Hodjegan A., Tucker. G. T. 2007. . Simulation and prediction of in vivo drug metabolism in human populations from in vitro data . Nat. Rev. Drug Discov. 6 : 140 – 148 . [DOI] [PubMed] [Google Scholar]
- Xiao L. R., Zhang Y. H., Zhou A. L., Xiao W. F., Xiao J. S., He. J. 2009. . Primary test on the role of exogenous trehalose to silkworm’s resistance and main economical traits . Chin. Soc. Sericultural Sci. Symp. 2009 : 356 – 362 . [Google Scholar]
- Yamamoto T., Tmonizawa K., Fujikawa M., Sato Y., Yamada H., Horii. I. 2007. . Evaluation of human hepatocyte chimeric mice as a model for toxicological investigation using panomic approaches—effect of acetaminophen on the expression profiles of protein and endogenous metabolites in liver, plasma and urine . J. Toxicol. Sci. 32 : 205 – 215 . [DOI] [PubMed] [Google Scholar]