Abstract
Whitefly biotypes B and Q are the two most damaging members of the Bemisia tabaci (Hemiptera: Aleyrodidae) species complex. Control of B. tabaci (and especially of Q) has been impaired by resistance to commonly used insecticides. To find new insecticides for B. tabaci management in China, we investigated the sensitivity of eggs, larvae, and adults of laboratory strains of B and Q (named Lab-B and Lab-Q) and field strains of Q to several insecticides. For eggs, larvae, and adults of B. tabaci and for six insecticides (cyantraniliprole, chlorantraniliprole, pyriproxyfen, buprofezin, acetamiprid, and thiamethoxam), LC 50 values were higher for Lab-Q than for Lab-B; avermectin LC 50 values, however, were low for adults of both Lab-Q and Lab-B. Based on the laboratory results, insecticides were selected to test against eggs, larvae, and adults of four field strains of B. tabaci Q. Although the field strains differed in their sensitivity to the insecticides, the eggs and larvae of all strains were highly sensitive to cyantraniliprole, and the adults of all strains were highly sensitive to avermectin. The eggs, larvae, and adults of B. tabaci Q were generally more resistant than those of B. tabaci B to the tested insecticides. B. tabaci Q eggs and larvae were sensitive to cyantraniliprole and pyriproxyfen, whereas B. tabaci Q adults were sensitive to avermectin. Field trials should be conducted with cyantraniliprole, pyriproxyfen, and avermectin for control of B. tabaci Q and B in China.
Keywords: Bemisia tabaci, insecticide resistance, developmental stage, cyantraniliprole, avermectin
The sweet potato whitefly Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae) is a destructive pest of many field and protected crops worldwide. B. tabaci is regarded as a species complex composed of at least 34 morphologically indistinguishable species, differing in host range, feeding behavior, virus transmission, insecticide resistance, or the symbionts that they harbor ( Brown et al. 1995 , Perring 2001 , De Barro et al. 2011 , Liu et al. 2012 , Pan et al. 2012 ). Among them, B. tabaci B (also known as “Middle East-Asia Minor 1 species”) and Q (also known as “Mediterranean species”) are the most invasive ( De Barro et al. 2011 ). During the last two decades, B. tabaci B, which originated in the Middle East-Asia Minor, has spread to at least 54 countries. More recently, B. tabaci Q, which originated in the Mediterranean region, has spread to at least 10 countries where it has caused severe crop damage. B. tabaci was first detected in China in the late 1940s ( Zhou 1949 ), but it was not considered a significant pest until the introduction of B. tabaci B in the 1990s ( Luo et al. 2002 ). After its introduction, B. tabaci B rapidly invaded the entire country and caused serious yield losses in many crops. The first detection of B. tabaci Q in China was in Yunnan Province in 2003 ( Zhang et al. 2005 , Chu et al. 2006 ), and Q was subsequently found in Beijing, Henan, and Shandong provinces ( Chu et al. 2006 , 2010a , b ). During the past several years, Q has gradually displaced B and become the dominant form of B. tabaci in field agricultural systems in most parts of China ( Teng et al. 2010 ; Pan et al. 2010 , 2011 ). Research has also demonstrated that Q is significantly more resistant than B to many insecticides ( Horowitz et al. 2005 , Luo et al. 2010 , Wang et al. 2010 ), which may be an important reason for the replacement of B by Q in China ( Sun et al. 2013 ).
In many cropping systems, control of B. tabaci depends on insecticides including organophosphates (OPs), carbamates, pyrethroids, insect growth regulator (IGRs), and neonicotinoids. Because of excessive insecticide application, however, B. tabaci has developed high resistance to commonly used insecticides and especially to neonicotinoids ( Nauen and Denholm 2005 , Roditakis et al. 2005 , Erdogana et al. 2008 , Ahmad et al. 2010 , Houndété et al. 2010 , Luo et al. 2010 , Wang et al. 2010 , Vassiliou et al. 2011 , Kontsedalov et al. 2012 ). Relative to sensitive strains, strains collected in Israel showed up to1,000-fold resistance to thamithoxam ( Kontsedalov et al. 2012 ), and those collected in Turkey and Crete showed 20- to 310-fold resistance to OPs, 30- to 600-fold resistance to α-cypermethrin, and 38- to 1,958-fold resistance to imidacloprid ( Erdogana et al. 2008 , Roditakis et al. 2009 ). In China, B. tabaci resistance to neonicotinoids is a serious problem. Adult B. tabaci collected from southeastern China exhibited 28- to 1,900-fold resistance to imidacloprid and 29- to 1,200-fold resistance to thiamethoxam ( Wang et al. 2010 ). Most research on resistance of B. tabaci to neonicotinoids has focused on adults rather than on eggs and larvae.
Because B. tabaci Q is the dominant whitefly species in vegetable-field ecosystems in China and is highly resistant to many insecticides, we first investigated whether the susceptibility to seven relatively new insecticides differed among eggs, larvae, and adults of one laboratory strain of Q and one of B (Lab-Q and Lab-B). On the basis of the results obtained with the laboratory strains, we selected the most effective insecticides to test against eggs, larvae, and adults of four field strains of Q.
Materials and Methods
Insecticides
The following seven commercially available insecticides were used in the bioassays: cyantraniliprole 200 g/liter SC (Cyazypyr, DuPont Crop Protection), chlorantraniliprole 200 g/liter SC (Coragen), avermectin 18 g/liter EC (Dynameca), pyriproxyfen 100 g/liter EC (Knack), buprofezin 250 g/liter WP (Applaud), acetamiprid 50 g/liter EC (Gazelle), and thiamethoxam 250 g/liter WG (Actara, Syngenta Crop Protection Company, Switzerland). Except as indicated for Cyazypyr and Actara, these insecticides were obtained from the Ministry of Agriculture Pesticide Testing Institute, China. Based on different developmental stage samples and the accuracy of LC50 calculation, respectively, for egg, larva, or adult, the highest working dilutions of each insecticide used for several dilutions were 1.28, 0.08, and 100 mg/liter (cyantraniliprole); 250, 6.4, and 8,970 mg/liter (chlorantraniliprole); 80, 50, and 0.5 mg/liter (avermectin); 2.5, 6.25, and 12,800 mg/liter (pyriproxyfen); 25,600, 25, and 8,000 mg/liter (buprofezin); 36, 40, and 400 mg/liter (acetamiprid); and 1,600, 31.2, and 400 mg/liter (thiamethoxam).
Plants
The nontransgenic cotton variety [ Gossypium herbaceum (L.), cv. Zhongmian 49] was used for bioassays and for the rearing of whitefly strains. For rearing whiteflies, Zhongmian 49 seeds were planted in a potting mix (a mixture of peat moss, vermiculite, organic fertilizer, and perlite at 10:10:10:1 by volume) and grown in whitefly-proof cages under natural light in an isolated greenhouse.
Laboratory Strains of B. tabaci
Specimens of B. tabaci B were collected on cabbage ( Brassica oleracea cv. Jingfeng 1) in Beijing, China, in 2000 as described by Feng et al. ( 2009 , 2010 ). Specimens of B. tabaci Q were collected on poinsettia ( Euphorbia pulcherrima Wild. [ex Klotz.]) in Beijing, China, in 2009. Before they were used in bioassays, the B and Q strains were maintained for at least six generations on cotton plants (cv. Zhongmian 49), which were grown under the same conditions as described earlier and without exposure to any chemical insecticides. The two new cotton strains were designated as Lab-B and Lab-Q.
Field Strains of B. tabaci
B. tabaci specimens were collected in 2012 from three host plants (eggplant, melon, and pepper) in four localities: Beijing, Shandong, Hunan, and Hubei. The specimens from each of the four localities were considered to represent one population or strain ( Table 1 ). Adult whiteflies were collected in glass tubes and then brought to the laboratory in a large plastic vials. Some of the collected specimens were directly used for adult bioassays (as described later), and others were permitted to lay eggs. Some of the eggs were used for egg bioassays (as described later), and others were permitted to develop into larvae and were used for larval assays (as described later). Adult bioassays were conducted within 2–3 h after field collection ( Roditakis et al. 2005 , Basit et al. 2013a ). After adults were collected, disabled and dead individuals at the bottom of vials were discarded. The biotype or cryptic species designation of the four whitefly strains was determined based on the specific mitochondrial cytochrome oxidase I primers ( Shatters et al. 2009 ) using 30 individuals in one sample for each strain.
Table 1.
Origins and host plants of B. tabaci strains
| Strain | Sampling location | Longitude and latitude of the sampling location | Sampling date | Host | Species |
|---|---|---|---|---|---|
| BJ | Beijing | 116° 20′ E, 39° 56′ N | May 2012 | Pepper | Q |
| SD | Shandong | 116° 23′ E, 39° 54′ N | June 2012 | Eggplant | Q |
| HN | Hunan | 113° E, 28° 11′ N | October 2012 | Melon | Q |
| HB | Hubei | 114° E, 31° N | October 2012 | Melon | Q |
Egg Bioassay
The susceptibility of eggs to insecticides was measured as described by Li et al . (2012) . When cotton seedlings grew to the first true-leaf stage (11 cm in height and 2 cm in diameter), the true leaf of each seedling was infested with 10 pairs of whitefly adults. After 24 h, the adults were removed, and the eggs were counted. Then, each leaf was inserted into a 20-ml glass scintillation vial containing water (control) or an insecticide for 20 s. After the leaves had dried in the air, the seedlings were kept in an incubator at 28°C and with a photoperiod of 16:8 (L:D) h. About 7 d later, the number of first instars on each seedling was counted and recorded. The mortality of eggs on each seedling was calculated by the formula: egg mortality = (the total number of eggs − the numbers of first instars)/the total number of eggs corresponding to each leaf. Each diluting concentration of each insecticide (and the control) had 3–4 replicate leaves, and each replicate had at least 40 eggs.
Larva Bioassay
The susceptibility of larvae to insecticides was measured as described by Li et al. (2012) ; in this bioassay, insecticide activity depended on uptake of the insecticide by the petiole of a detached leaf and its subsequent movement into the leaf blade. Briefly, leaves were infested with whiteflies as described for the egg bioassay. When the eggs developed to late second-stage instars, the second-stage instars were counted. The leaf of the cotton seedling with attached second-stage instars was detached from the seedling and inserted into a 20-ml glass scintillation vial containing water (control) or an insecticide (the leaf petiole was inserted into the water/insecticide but the leaf blade was not); the tubes containing the leaves and larvae were kept at 28°C with a photoperiod of 16:8 (L:D) h. After 6 d, the fourth-stage instars on each leaf were counted. Mortality was calculated as follows: larval mortality = (the number of late second-stage instars − the number of fourth-stage instars)/the number of late second-stage instars corresponding to each leaf. Each diluting concentration of each insecticide (and the control) had 3–4 replicate leaves, and each replicate had at least 40 larva.
Adult Bioassay
The susceptibility of adults to insecticides was measured using the method of Feng et al. ( 2009 , 2010 ) and Xie et al. (2011) . Briefly, leaf discs (22 mm in diameter) from cotton plants were dipped in the insecticide solutions (or distilled water controls) for 10 s. After drying, the leaf discs were placed with their adaxial surface downward on agar in a flat-bottomed glass tube. About 20 unsexed adults were then added to each tube. Mortality was recorded after 48 h at 28°C and with a photoperiod of 16:8 (L:D) h. Each diluting concentration of each insecticide (and the control) had 3–4 replicate tubes, and each replicate had at least 15 adults.
Statistical Analysis
Bioassay data, including LC 50 values and their 95% confidence limits, were calculated from Probit regressions using the log–concentration–response probit analysis program ( Chi 1997 ). Mortality was corrected using Abbott’s formula for each probit analysis ( Abbott 1925 ). LC 50 values of insecticides were considered significantly different ( P < 0 . 05) if their 95% confidential limits did not overlap.
Results
Sensitivity of Eggs, Larvae, and Adults of Two Laboratory Strains to Seven Insecticides
Bioassay results showed that insecticide toxicity depended on the insecticide, the B. tabaci developmental stage, and B. tabaci strain (Lab-B or Lab-Q). Except in the case of adults exposed to avermectin, the LC 50 values were significantly higher (as indicated by nonoverlapping 95% fiducial limits) for the Lab-Q strain than for the Lab-B strain ( Table 2 ). This was especially evident for eggs exposed to cyantraniliprole and pyriproxyfen; the cyantraniliprole LC 50 values were 210-fold higher for Lab-Q than for Lab-B, and the pyriproxyfen LC 50 values were 77-fold higher for Lab-Q than for Lab-B. In the case of adults and avermectin, however, LC 50 values were low for both Lab-Q and Lab-B. The DMS index was used to indicate the relative sensitivity of each whitefly stage to each insecticide: DMS = the LC 50 of a developmental stage/the LC 50 of the most sensitive stage; a smaller value for the DMS index indicates a greater relative sensitivity. According to the DMS index ( Table 2 ) and insecticide dose–mortality probit curves ( Fig. 1 ), regardless of strain, the egg was the most sensitive to pyriproxyfen and acetamiprid; the larva was the most sensitive to chlorantraniliprole, buprofezin, and thiamethoxam; and the adult was the most sensitive to avermectin. Overall, cyantraniliprole was the most toxic of the seven insecticides to Lab-B eggs and Lab-Q larvae, and avermectin was toxic to both Lab-B and Lab-Q adults ( Table 2 ; Fig. 1 ).
Table 2.
Sensitivity of eggs, larvae, and adults of two laboratory strains of B. tabaci (Lab-Q and Lab-B) to seven insecticides
| In secticide | Stage | Strain | n a | LC 50 (95% FL) (mg/liter) b | Lab-Q LC 50 /Lab-B LC 50 | DMS c | Slope (SE) |
|---|---|---|---|---|---|---|---|
| Cyantraniliprole (anthranilic diamide) | Egg | Lab-B | 2,811 | 0.000632 (0.000471−0.000847) a | 1.00 | 0.937 (0.0728) | |
| Lab-Q | 1,541 | 0.133 (0.0914−0.183) b | 210 | 10.6 | 0.891 (0.0891) | ||
| Larva | Lab-B | 1,902 | 0.00156 (0.00109−0.00221) a | 2.46 | 1.27 (0.0974) | ||
| Lab-Q | 1,719 | 0.0126 (0.0110−0.0143) b | 8.08 | 1.00 | 2.05 (0.115) | ||
| Adult | Lab-B | 574 | 5.91 (5.56−6.29) a | 9,351 | 1.978 (0.231) | ||
| Lab-Q | 502 | 96.3 (67.3−139) b | 16.3 | 7,643 | 1.20 (0.213) | ||
| Chlorantraniliprole (anthranilic diamide) | Egg | Lab-B | 1,226 | 21.1 (8.61−66.5) a | 161 | 0.892 (0.175) | |
| Lab-Q | 1,135 | 227 (161−321) b | 10.8 | 306 | 0.853 (0.159) | ||
| Larva | Lab-B | 1,175 | 0.131 (0.0762−0.205) a | 1.00 | 2.06 (0.178) | ||
| Lab-Q | 1,368 | 0.743 (0.653−0.836) b | 5.67 | 1.00 | 1.09 (0.0869) | ||
| Adult | Lab-B | 381 | 582 (240−1,450) a | 4,443 | 1.01 (0.196) | ||
| Lab-Q | 451 | 4,567 (3,656−5,708) b | 7.85 | 6,147 | 2.09 (0.314) | ||
| Avermectin (macrocyclic lactone) | Egg | Lab-B | 1,853 | 32.5(29.2−36.3) a | 367 | 1.10 (0.105) | |
| Lab-Q | 1,068 | 337(284−401) b | 10.4 | 3,370 | 0.756 (0.175) | ||
| Larva | Lab-B | 2349 | 23.5 (18.9−29.3) a | 266 | 0.807 (0.0680) | ||
| Lab-Q | 1,013 | 62.6 (34.9−116) b | 2.66 | 626 | 0.905 (0.150) | ||
| Adult | Lab-B | 453 | 0.0885(0.0618−0.127) a | 1.00 | 1.97 (0.285) | ||
| Lab-Q | 432 | 0.100 (0.0658−0.150) a | 1.13 | 1.00 | 2.20 (0.249) | ||
| Pyriproxyfen (IGR) | Egg | Lab-B | 2,094 | 0.00293 (0.00224−0.00384) a | 1.00 | 2.14 (0.133) | |
| Lab-Q | 2,508 | 0.225 (0.139−0.356) b | 76.8 | 1.00 | 0.435 (0.0429) | ||
| Larva | Lab-B | 1,338 | 0.341 (0.251−0.438) a | 116 | 0.666 (0.105) | ||
| Lab-Q | 1,266 | 1.65 (1.47−1.85) b | 4.84 | 7.33 | 0.962 (0.0928) | ||
| Adult | Lab-B | 434 | 1,461 (1,020−2,090) a | 49,8635 | 2.44 (0.262) | ||
| Lab-Q | 946 | 8,832 (7,958−9,802) b | 6.05 | 39,253 | 1.08 (0.149) | ||
| Buprofezin (IGR) | Egg | Lab-B | 2,278 | 3,634 (2,644−5,006) a | 5,040 | 0.915 (0.0930) | |
| Lab-Q | 1,326 | 30,300 (23,488−39,107) b | 8.34 | 5,782 | 1.27 (0.164) | ||
| Larva | Lab-B | 1,391 | 0.721 (0.512−0.969) a | 1.00 | 1.04 (0.111) | ||
| Lab-Q | 2,470 | 5.24 (4.00−6.84) b | 7.27 | 1.00 | 0.833 (0.0569) | ||
| Adult | Lab-B | 581 | 1,473 (1,120−1,937) a | 2,043 | 1.97 (0.177) | ||
| Lab-Q | 588 | 5,156 (4,395−6,050) b | 3.50 | 984 | 1.57 (0.223) | ||
| Acetamiprid (neonicotinoid) | Egg | Lab-B | 1,071 | 1.83 (1.38−2.37) a | 1.00 | 1.46 (0.120) | |
| Lab-Q | 942 | 8.96 (7.76−10.3) b | 4.90 | 1.00 | 1.84 (0.146) | ||
| Larva | Lab-B | 3,616 | 3.60 (2.95−4.36) a | 1.97 | 0.684 (0.0436) | ||
| Lab-Q | 2,927 | 23.9 (19.9−28.6) b | 6.64 | 2.67 | 1.10 (0.0786) | ||
| Adult | Lab-B | 351 | 3.28 (2.21−4.82) a | 1.79 | 3.88 (0.552) | ||
| Lab-Q | 387 | 22.0 (16.3−29.7) b | 6.71 | 2.46 | 1.47 (0.198) | ||
| Thiamethoxam (neonicotinoid) | Egg | Lab-B | 1,457 | 131 (90.0−192) a | 125 | 0.535 (0.0853) | |
| Lab-Q | 941 | 861 (727−1020) b | 6.57 | 184 | 1.58 (0.171) | ||
| Larva | Lab-B | 1,111 | 1.05 (0.820−1.35) a | 1.00 | 1.21 (0.125) | ||
| Lab-Q | 611 | 4.68 (1.84−11.0) b | 4.46 | 1.00 | 1.75 (0.229) | ||
| Adult | Lab-B | 382 | 17.9 (9.56−33.8) a | 17.0 | 1.12 (0.176) | ||
| Lab-Q | 715 | 91.5 (50.7−164) b | 5.11 | 19.6 | 0.767 (0.0689) |
a n , number of eggs used in the bioassay, including controls.
b For each stage treated with each insecticide, different lowercase letters indicate significant difference between Lab-B and Lab-Q strains.
c DMS = (the LC 50 of a developmental stage)/(the LC 50 of the most susceptible stage) for Lab-B (left column) or Lab-Q (right column).
Fig. 1.
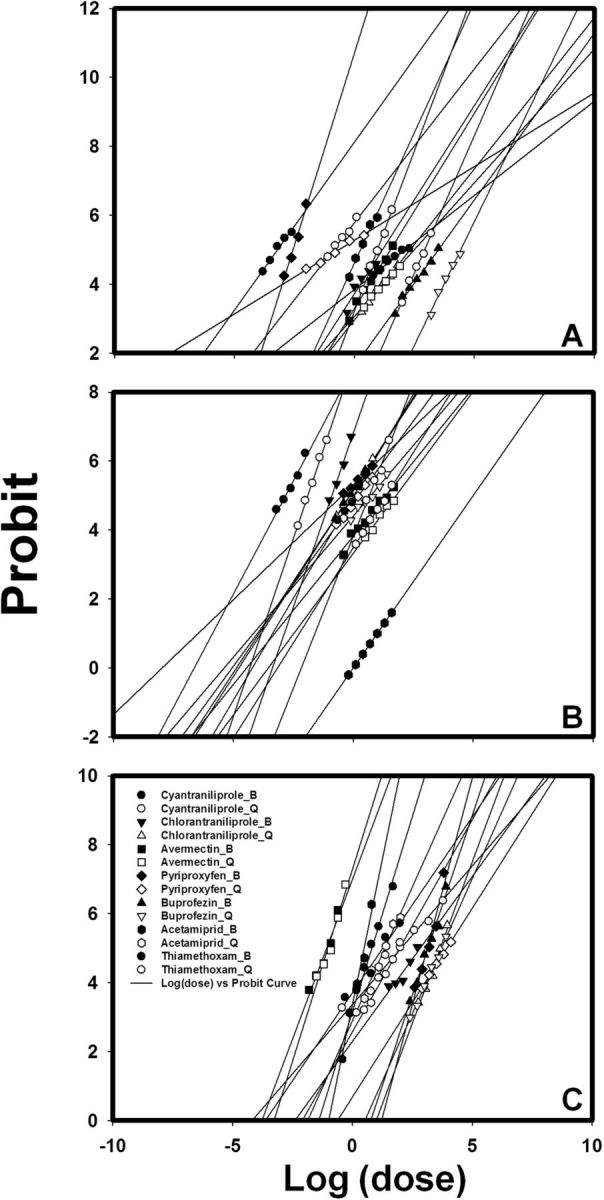
Insecticide dose–mortality probit curves of two laboratory B. tabaci strains (Lab-Q and Lab-B) to seven insecticides. (A) B/Q difference in egg stage. (B) B/Q difference in larval stage. (C) B/Q difference in adult stage.
Sensitivity of Eggs, Larvae, and Adults of Four Field Strains to Selected Insecticides
The four field strains were determined to be B. tabaci Q ( Table 1 ). On the basis of the DMS indices for the seven insecticides and three developmental stages, we selected the insecticides that were most effective against each stage to use in bioassays with the four field strains. Three insecticides (cyantraniliprole, pyriproxyfen, and acetamiprid) were used for eggs, five insecticides (cyantraniliprole, chlorantraniliprole, buprofezin, pyriproxyfen, and thiamethoxam) were used for larvae, and two insecticides (avermectin and acetamiprid) were used for adults.
Eggs
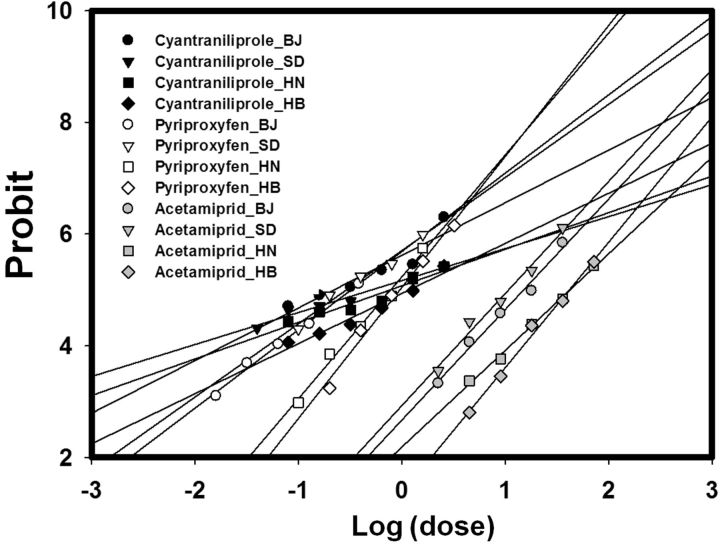
Cyantraniliprole and pyriproxyfen were more toxic than acetamiprid to eggs ( Table 3 ; Fig. 2 ). For all three insecticides, the LC 50 values were significantly lower for the BJ strains than for the HN and HB strains; LC 50 values were also lower for the SD strain in the case of pyriproxyfen and acetamiprid. For cyantraniliprole, the LC 50 values of the HN and HB strains were 5.74- and 8.65-fold greater, respectively, than the LC 50 of the Lab-Q strain ( Table 3 ).
Table 3.
Sensitivity of the eggs of four field strains of B. tabaci strains to selected insecticides
| Insecticide | Strain | n a | LC 50 (95% FL) (mg/liter) | RR b | Slope (SE) | |
|---|---|---|---|---|---|---|
| Cyantraniliprole (anthranilic diamide) | Egg | BJ | 1,449 | 0.231 (0.0857−0.431) a | 1.74 | 0.845 (0.0881) |
| SD | 1,902 | 0.525 (0.282−1.13) ab | 3.95 | 0.558 (0.0707) | ||
| HN | 2,125 | 0.763 (0.492−1.30) b | 5.74 | 0.669 (0.0653) | ||
| HB | 1,759 | 1.15 (0.851−1.67) b | 8.65 | 0.964 (0.0924) | ||
| Pyriproxyfen (IGR) | Egg | BJ | 1,423 | 0.330 (0.285−0.385) a | 1.47 | 1.39 (0.160) |
| SD | 1,005 | 0.282 (0.196−0.383) a | 1.25 | 1.26 (0.133) | ||
| HN | 1,625 | 0.792 (0.655−0.953) b | 3.52 | 2.22 (0.173) | ||
| HB | 1,967 | 0.919 (0.805−1.04) b | 4.08 | 2.18 (0.139) | ||
| Acetamiprid (neonicotinoid) | Egg | BJ | 1,319 | 15.1 (12.0−19.0) a | 1.69 | 2.00 (0.164) |
| SD | 1,393 | 10.9 (8.73−13.5) a | 1.22 | 1.97 (0.144) | ||
| HN | 1,525 | 41.9 (38.1−46.1) b | 4.68 | 1.78 (0.157) | ||
| HB | 1,685 | 41.8 (35.2−49.6) b | 4.67 | 2.09 (0.183) |
a n , number of eggs used in bioassay, including controls.
b RR, resistance ratio = (the LC 50 of field population)/(the LC 50 of Lab-Q) corresponding to the same developmental stage.
Fig. 2.
Insecticide dose–mortality probit curves of the four field B. tabaci eggs to three selected insecticides (cyantraniliprole, pyriproxyfen, and acetamiprid).
Larvae
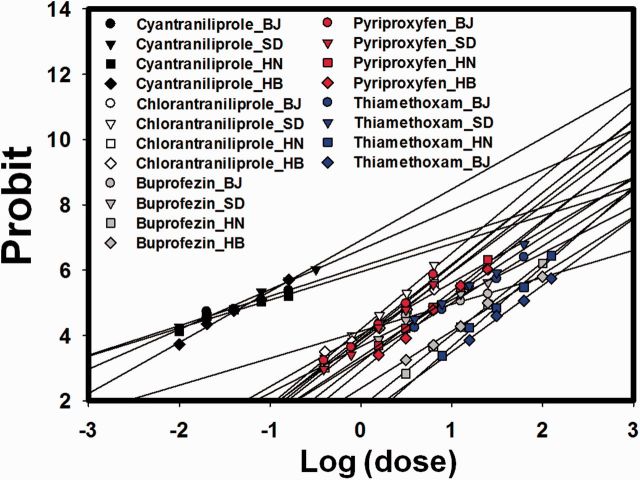
Cyantraniliprole was highly toxic to the larvae of the four field strains, i.e., all LC 50 values were <0.1 mg/liter ( Table 4 ; Fig. 3 ). Sensitivity to the insecticides differed among the strains. For example, the LC 50 values of the SD strain were significantly lower than those of the HN strain for all five insecticides and were also lower than those of the HB strain except in the case of cyantraniliprole. For thiamethoxam, the LC 50 values of the HN and HB strains were 11.6- and 7.61-fold greater, respectively, than that of the Lab-Q strain. Sensitivities of HN and HB strains did not significantly differ to cyantraniliprole, chlorantraniliprole, buprofezin, or pyriproxyfen. However, the HN strain was significantly less sensitive than the HB strain to thiamethoxam ( Table 4 ).
Table 4.
Sensitivity of the larvae of four field strains of B. tabaci strains to selected insecticides
| Insecticide | Strain | n a | LC 50 (95% FL) (mg/liter) | RR b | Slope (SE) | |
|---|---|---|---|---|---|---|
| Cyantraniliprole (anthranilic diamide) | Larvae | BJ | 1,194 | 0.0594 (0.0402−0.0880) ab | 4.71 | 0.855 (0.117) |
| SD | 2,490 | 0.048 (0.0412−0.0559) a | 3.81 | 1.26 (0.0696) | ||
| HN | 1,554 | 0.0807 (0.0573−0.115) b | 6.40 | 0.811 (0.0978) | ||
| HB | 1,556 | 0.0590 (0.0491−0.0710) ab | 4.68 | 1.53 (0.121) | ||
| Chlorantraniliprole (anthranilic diamide) | Larvae | BJ | 2,010 | 3.10 (2.85−3.37) b | 4.17 | 2.32 (0.156) |
| SD | 2,248 | 2.28 (2.01−2.58) a | 3.07 | 2.45 (0.137) | ||
| HN | 1,709 | 3.57 (3.01−4.25) b | 4.80 | 2.08 (0.168) | ||
| HB | 1,487 | 3.96 (3.33−4.75) b | 5.33 | 1.76 (0.164) | ||
| Buprofezin (IGR) | Larvae | BJ | 1,038 | 11.9 (9.82−14.4) a | 2.27 | 0.76 (0.0847) |
| SD | 1,126 | 8.56(7.39−9.93) a | 1.63 | 1.40 (0.129) | ||
| HN | 1,502 | 27.0 (25.9−28.2) b | 5.15 | 2.14 (0.142) | ||
| HB | 1,498 | 30.8 (23.8−40.0) b | 5.88 | 1.70 (0.122) | ||
| Pyriproxyfen (IGR) | Larvae | BJ | 1,629 | 2.96 (2.55−3.42) a | 1.79 | 2.46 (0.197) |
| SD | 1,704 | 3.67 (3.28−4.10) a | 2.22 | 2.28 (0.203) | ||
| HN | 1,785 | 7.07 (6.19−8.06) b | 4.28 | 2.23 (0.137) | ||
| HB | 1,655 | 8.16 (6.90−9.63) b | 4.95 | 2.28 (0.148) | ||
| Thiamethoxam (neonicotinoid) | Larvae | BJ | 1,376 | 9.80 (7.09−13.4) a | 2.09 | 1.71 (0.121) |
| SD | 1,558 | 8.02 (5.87−10.9) a | 1.71 | 1.81 (0.122) | ||
| HN | 1,806 | 35.6 (30.1−42.0) b | 7.61 | 2.43 (0.142) | ||
| HB | 1,384 | 54.1 (44.1−66.4) c | 11.6 | 1.98 (0.169) |
a n , number of eggs used in bioassay, including controls.
b RR, resistance ratio = (the LC 50 of field population)/(the LC 50 of Lab-Q) corresponding to the same developmental stage.
Fig. 3.
Insecticide dose–mortality probit curves of the four field B. tabaci larvae to five selected insecticides (cyantraniliprole, chlorantraniliprole, buprofezin, pyriproxyfen, and thiamethoxam).
Adults
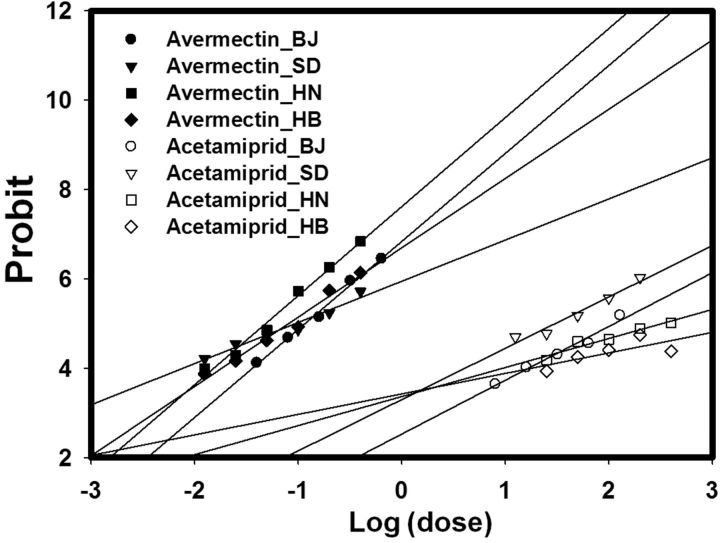
Adults from the four field strains did not exhibit resistance to avermectin ( Table 5 ; Fig. 4 ). All LC 50 values were <0.2 mg/liter, and the ratio of LC 50 values of the field strains to the Lab-Q strain was about 1. In the case of acetamiprid, however, the LC 50 values of the HN and HB strains were 14.8- and 19.0-fold greater, respectively, than that of the Lab-Q strain ( Table 5 ).
Table 5.
Sensitivity of the adults of four field strains of B. tabaci strains to selected insecticides
| Insecticide | Strain | n a | LC 50 (95% FL) (mg/liter) | RR b | Slope (SE) | |
|---|---|---|---|---|---|---|
| Avermectin (macrocyclic lactone) | Adults | BJ | 530 | 0.118 (0.0985−0.142) b | 1.18 | 1.98 (0.205) |
| SD | 380 | 0.0932 (0.0571−0.152) ab | 0.932 | 0.940 (0.157) | ||
| HN | 703 | 0.0509 (0.0415−0.0623) a | 0.509 | 2.05 (0.171) | ||
| HB | 788 | 0.0842 (0.0651−0.109) b | 0.842 | 1.60 (0.143) | ||
| Acetamiprid (neonicotinoid) | Adults | BJ | 566 | 112 (70.0−183) b | 5.09 | 1.26 (0.212) |
| SD | 349 | 30.7 (20.4−45.9) a | 1.40 | 1.15 (0.217) | ||
| HN | 655 | 326 (162−667) bc | 14.8 | 0.644 (0.158) | ||
| HB | 985 | 417 (218−807) c | 19.0 | 0.862 (0.237) |
a n , number of eggs used in bioassay, including controls.
b RR, resistance ratio = (the LC 50 of field population)/(the LC 50 of Lab-Q) corresponding to the same developmental stage.
Fig. 4.
Insecticide dose–mortality probit curves of the four field B. tabaci adults to two selected insecticides (avermectin and acetaminprid).
Discussion
Differences in Sensitivity to Insecticides Between B. tabaci B and Q
As noted earlier, previous research has documented that B. tabaci Q has been displacing non-Q whiteflies in many regions of China including Shanxi, Henan, Hubei, Jiangsu, Zhejiang, Hunan, and Hainan provinces ( Teng et al. 2010 ). Chu et al. ( 2010a , b ) reported that the ratio of numbers of B. tabaci Q to numbers of B. tabaci B has been increasing and that Q is displacing B on cotton, eggplant, and other plants in Shandong Province of China. Recently, Hu et al. (2011) found that B. tabaci Q was the dominant species in the Yangtze River Valley and eastern coastal areas, and Pan et al. (2011) revealed that Q represented 100.0% of 44 populations from 14 agricultural areas in China. Displacement among insects is generally a complex process that may be mediated by many intrinsic and environmental factors such as host plant, climate, and application of insecticides ( Reitz and Trumble 2002 ). Some assessments of insecticide resistance of B. tabaci B and Q showed that Q is significantly more resistant than B to nearly all commonly used insecticides. For example, the resistance of the Q population collected from Yunnan Province was 450-fold greater to imidacloprid and 300-fold greater to thiamethoxam compared with the resistance of B ( Wang et al. 2010 ). Specimens collected from Xinjiang, Beijing, Zhejiang, Jiangsu, and Hubei provinces indicated that B. tabaci B strains remained largely sensitive, whereas Q strains were resistant to acetamiprid, imidacloprid, and thiamethoxam ( Luo et al. 2010 ). Rao et al. (2012) reported that the four B. tabaci Q strains from China that were studied were moderately or strongly resistant to three of four neonicotinoid insecticides tested, whereas the one B strain that was studied was sensitive to all four insecticides ( Rao et al. 2012 ). But previous research of the greater resistance of Q versus B seems to mainly focus on adults rather than on eggs and larvae. In our assays with laboratory strains of B and Q, Q adults were less sensitive than B adults to six of seven insecticides (both strains were highly sensitive to the seventh insecticide, avermectin). Meanwhile, our assays with laboratory strains also revealed that Q eggs and larvae were less sensitive than B eggs and larvae to all seven insecticides. Given the heavy use of pesticides for whitefly control, it would appear that this difference in sensitivity to insecticides may have contributed to the displacement of B by Q in many locations in China.
Sensitivity of Eggs, Larvae, and Adults of Four Field Strains of B. tabaci Q to Selected Insecticides
As noted earlier, the management of B. tabaci in China has mainly relied on chemical insecticides, and owing to excessive application, B. tabaci has developed high resistance to commonly used insecticides; this is especially true for adults and neonicotinoid insecticides ( Luo et al. 2010 , Wang et al. 2010 ). At the same time, B. tabaci Q has displaced B as the predominant B. tabaci in China ( Hu et al. 2011 , Pan et al. 2011 ). One of the aims of this study was to evaluate new insecticides and to include eggs and larvae, which are not generally included in tests for insecticide sensitivity in China. Our data indicate that cyantraniliprole and pyriproxyfen should be effective for control of eggs and larvae of B. tabaci Q. Cyantraniliprole, which is an anthranilic diamide that binds to ryanodine receptors ( Jeanguenat 2013 ), was introduced into China in 2013 but has not yet been registered for the control of whiteflies in China. Based on B. tabaci collected from southern Florida and Arizona in 2008 and 2009, anthranilic diamides are highly effective against eggs and larvae and exhibit no cross-resistance with neonicotinoids or pyriproxyfen ( Li et al. 2012 , Caballero et al. 2013 ). In other studies of novel insecticides, cyantraniliprole was effective against whitefly eggs and larvae and also against aphids and thrips ( Jacobson and Kennedy 2011 , Foster et al. 2012 , Li et al. 2012 , Caballero et al. 2013 ). Pyriproxyfen can suppress hatching of B. tabaci eggs and cause larval mortality ( Ishaaya and Horowitz 1995 , Lee et al. 2002 ), but its effectiveness has been compromised in some areas of Israel and Arizona by the buildup of resistance after 8 yr of application ( Horowitz et al. 1999 , 2002 , 2003 ). Pyriproxyfen was registered for use in 2008 (pesticide registration database in China) in China, where no evidence of pyriproxyfen resistance in whiteflies has yet to be reported. When tested against the four field strains of Q in this study, pyriproxyfen was as toxic as cyantraniliprole to eggs but was slightly less toxic to larvae. Based on the above results, cyantraniliprole followed by pyriproxyfen appear to be the best choices for controlling eggs/larvae but care must be taken to avoid selecting for resistance.
In this study, the B. tabaci Q adults collected from four field locations exhibited relatively high resistance to the neonicotinoid insecticide acetamiprid. This is consistent with reports of field trials with other neonicotinoid insecticides. For example, Ma et al. (2007) reported that B. tabaci Q adults collected from the Xinjiang Uygur autonomous region from 2003 to 2005 showed low to moderate resistance to imidacloprid. Wang et al. (2010) reported that B. tabaci Q adults collected from southeastern of China from 2008 to 2009 had moderate to high resistance to imidacloprid and thiamethoxam. The resistance of B. tabaci Q to neonicotinoids has reduced the ability of farmers to control this pest. Although mixtures of neonicotinoids and IGRs were recently found to be effective against B. tabaci , these mixtures may not be suitable for long-term management ( Basit et al. 2013a , b ). It follows that an insecticide specific to whitefly adults is needed. In this study, avermectin was highly toxic to adults of laboratory and field strains, a finding that is consistent with some previous field data ( Luo et al. 2010 , Wang et al. 2010 ). It, therefore, seems possible that avermectin could replace neonicotinoid insecticides for the management of whitefly adults.
Except in the case of avermectin against adults and cyantraniliprole against larvae, the field strains HN and HB were less sensitive than the other two field strains (BJ and SD) to the tested insecticides. In the locations where HN and HB were collected, an alternating application of avermectin and cyantraniliprole should be tried.
In summary, cyantraniliprole, pyriproxyfen, and avermectin could be useful insecticides for whitefly management in China. The novel insecticide cyantraniliprole seems especially promising. All three insecticides warrant further investigation in the field.
Acknowledgments
This research was supported by the National Science Fund for Distinguished Young Scholars (31025020); the Special Fund for Agro-scientific Research in the Public Interest (201303028); the National Science & Technology pillar program (2012BAD19B01), and the Beijing Key Laboratory for Pest Control and Sustainable Cultivation of Vegetables.
References Cited
- Abbott W. S. 1925. . A method of computing the effectiveness of an insecticide . J. Econ. Entomol. 18 : 265 – 267 . [Google Scholar]
- Ahmad M., Arif M. I., Naveed M. . 2010. . Dynamics of resistance to organophosphate and carbamate insecticides in the cotton whitefly Bemisia tabaci (Hemiptera: Aleyrodidae) from Pakistan . J. Pest Sci. 83 : 409 – 420 . [Google Scholar]
- Basit M., Saeed S., Saleem M. A., Denholm I., Shah M. . 2013a. . Detection of resistance, cross-resistance, and stability of resistance to new chemistry insecticides in Bemisia tabaci (Homoptera: Aleyrodidae) . J. Econ. Entomol. 106 : 1414 – 22 . [DOI] [PubMed] [Google Scholar]
- Basit M., Saeed S., Saleem S. A., Sayyed A. H. . 2013b. . Can resistance in Bemisia tabaci (Homoptera: Aleyrodidae) be overcome with mixtures of neonicotinoids and insect growth regulators? Crop Prot. 44 : 135 – 141 . [Google Scholar]
- Brown J. K., Frohlich D. R., Rosell R. C. . 1995. . The sweet-potato or silverleaf whiteflies: biotypes of Bemisia tabaci or a species complex . Annu. Rev. Entomol. 40 : 511 – 534 . [Google Scholar]
- Caballero R., Cyman S., Schuster D. J., Portillo H. E., Slater R. . 2013. . Baseline susceptibility of Bemisia tabaci (Genn.) biotype B in southern Florida to cyantraniliprole . Crop Prot. 44 : 104 – 108 . [Google Scholar]
- Chi H. 1997. . Computer program for the probit analysis . National Chung Hsing University; , Taichung, Taiwan: . [Google Scholar]
- Chu D., Wan F. H., Zhang Y. J., Brown J. K. . 2010a. . Change in the biotype composition of Bemisia tabaci in Shandong Province of China from 2005 to 2008 . Environ. Entomol. 39 : 1028 – 1036 . [DOI] [PubMed] [Google Scholar]
- Chu D., Zhang Y. J., Wan F. H. . 2010b. . Cryptic invasion of the exotic Bemisia tabaci biotype Q occurred widespread in Shandong Province of China . Fla. Entomol. 93 : 203 – 207 . [Google Scholar]
- Chu D., Zhang Y. J., Brown J. K., Cong B., Xu B. Y., Wu Q. J., Zhu R. G. . 2006. . The introduction of exotic Q biotype of tabaci from the Mediterranean region into China on ornamental crops . Fla. Entomol. 89 : 168 – 174 . [Google Scholar]
- De Barro P. J., Liu S. S., Boykin L. M. . 2011. . A statement of species status of Bemisia tabaci .Annu. Rev. Entomol. 56 : 1 – 19 . [DOI] [PubMed] [Google Scholar]
- Erdogana C., Moores G. D., Gurkanc M. O., Gorman K. J., Denholm I. . 2008. . Insecticide resistance and biotype status of populations of the tobacco whitefly Bemisia tabaci (Hemiptera: Aleyrodidae) from Turkey . Crop Prot. 27 : 600 – 605 . [Google Scholar]
- Feng Y. T., Wu Q. J., Xu B. Y., Wang S. L., Chang X. L., Xie W., Zhang Y. J. . 2009. . Fitness costs and morphological change of laboratory-selected thiamethoxam resistance in the B-type Bemisia tabaci (Hemiptera: Aleyrodidae) . J. Appl. Entomol. 133 : 466 – 472 . [Google Scholar]
- Feng Y. T., Wu Q. J., Wang S. L., Chang X. L., Xie W., Xu B. Y., Zhang Y. J. . 2010. . Cross-resistance study and biochemical mechanisms of thiamethoxam resistance in B-biotype Bemisia tabaci (Hemiptera: Aleyrodidae) . Pest Manag. Sci. 66 : 313 – 318 . [DOI] [PubMed] [Google Scholar]
- Foster S. P., Denholm I., Rison J. L., Portillo H. E., Margaritopoulis J., Slater R. . 2012. . Susceptibility of standard clones and European field populations of the green peach aphid, Myzus persicae , and the cotton aphid, Aphis gossypii (Hemiptera: Aphididae), to the novel anthranilic diamide insecticide cyantraniliprole . Pest Manag. Sci. 68 : 629 – 633 . [DOI] [PubMed] [Google Scholar]
- Horowitz A. R., Mendelson Z., Cahill M., Denholm I., Ishaaya I. . 1999. . Managing resistance to the insect growth regulator, pyriproxyfen, in Bemisia tabaci . Pestic. Sci. 55 : 272 – 276 . [Google Scholar]
- Horowitz A. R., Kontsedalov S., Denholm I., Ishaaya I. . 2002. . Dynamics of insecticide resistance in Bemisia tabaci : a case study with an insect growth regulator . Pest Manag. Sci. 58 : 1096 – 1100 . [DOI] [PubMed] [Google Scholar]
- Horowitz A. R., Gorman K., Ross G., Denholm I. . 2003. . Inheritance of pyriproxyfen resistance in the whitefly, Bemisia tabaci (Q Biotype) . Arch. Insect Biochem. Physiol. 54 : 177 – 186 . [DOI] [PubMed] [Google Scholar]
- Horowitz A. R., Kontsedalov S., Khasdan V., Ishaaya I. . 2005. . Biotypes B and Q of Bemisia tabaci and their relevance to neonicotinoid and pyriproxyfen resistance . Arch. Insect Biochem. Physiol. 58 : 216 – 225 . [DOI] [PubMed] [Google Scholar]
- Houndété T. A., Kétoh G. K., Hema O. S., Brévault T., Glitho I. A., Martin T. . 2010. . Insecticide resistance in field populations of Bemisia tabaci (Hemiptera: Aleyrodidae) in West Africa . Pest Manag. Sci. 66 : 1181 – 1185 . [DOI] [PubMed] [Google Scholar]
- Hu J., De Barro P., Zhao H., Wang J., Nardi F., Liu S. S. . 2011. . An extensive field survey combined with a phylogenetic analysis reveals rapid and widespread invasion of two alien whiteflies in China . PLoS One 6 : e16061 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishaaya I., Horowitz A. R. . 1995. . Pyriproxyfen, a novel insect growth regulator for controlling whiteflies: mechanism and resistance management . Pest Manag. Sci. 43 : 227 – 232 . [Google Scholar]
- Jacobson A. L., Kennedy G. G. . 2011. . The effect of three rates of cyantraniliprole on the transmission of tomato spotted wilt virus by Frankliniella occidentalis and Frankliniella fusca (Thysanoptera: Thripidae) to Capsicum annuum . Crop Prot. 30 : 512 – 515 . [Google Scholar]
- Jeanguenat A. 2013. . The story of a new insecticidal chemistry class: the diamides . Pest Manag. Sci. 69 : 7 – 14 . [DOI] [PubMed] [Google Scholar]
- Kontsedalov S., Abu-Moch F., Lebedev G., Czosnek H., Horowitz A. R., Ghanim M. . 2012. . Bemisia tabaci biotype dynamics and resistance to insecticides in Israel during the years 2008-2010 . J. Integr. Agric. 11 : 312 – 320 . [Google Scholar]
- Lee Y. S., Park E. C., Kim J. H., Kim G. H. . 2002. . Comparative toxicities of pyriproxyfen and thiamethoxam against the sweetpotato whitefly, Bemisia tabaci (Homoptera: Aleyrodidae) . J. Asia Pac. Entomol. 5 : 117 – 122 . [Google Scholar]
- Li X. C., Degain B. A., Harpold V. S., Marçon P. G., Nichols R. L., Fournier A. J., Naranjo S. E., Palumbo J. C., Ellsworth P. C. 2012. . Baseline susceptibilities of B- and Q-biotype Bemisia tabaci to anthranilic diamides in Arizona . Pest Manag. Sci. 68 : 83 – 81 . [DOI] [PubMed] [Google Scholar]
- Liu B. M., Yan F. M., Chu D., Pan H. P., Jiao X. G., Xie W., Wu Q. J., Wang S. L., Xu B., Zhou X., et al. . 2012. . Difference in feeding behaviors of two invasive whiteflies on host plants with different suitability: implication for competitive displacement . Int. J. Biol. Sci. 8 : 697 – 706 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo C., Yao Y., Wang R. J., Yan F. M., Hu D. X., Zhang Z. L. . 2002. . The use of mitochondrial cytochrome oxidase mtCOI gene sequences for the identification of biotypes of Bemisia tabaci (Gennadius) in China . Acta Entomologica Sinica 45 : 759 – 763 . [Google Scholar]
- Luo C., Jones C. M., Zhang F., Denholm I., Gorman K. . 2010. . Insecticide resistance in Bemisia tabaci biotype Q (Hemiptera: Aleyrodidae) from China . Crop Prot. 29 : 429 – 434 . [Google Scholar]
- Ma D., Gormanc K., Devine G., Luo W., Denholm I. . 2007. . The biotype and insecticide-resistance status of whiteflies, Bemisia tabaci (Hemiptera: Aleyrodidae), invading cropping systems in Xinjiang Uygur autonomous region, northwestern China . Crop Prot. 26 : 612 – 617 . [Google Scholar]
- Nauen R., Denholm I. . 2005. . Resistance of insect pests to neonicotinoid insecticides: current status and future prospects . Arch. Insect Biochem. Physiol. 58 : 200 – 215 . [DOI] [PubMed] [Google Scholar]
- Pan H. P., Ge D. Q., Wang S. L., Wu Q. J., Xu B. Y., Xie W., Zhang Y. J. . 2010. . Replacement of B biotype Bemisia tabaci by Q biotype B. tabaci in some areas of Beijing and Hebei . Plant Prot. 36 : 40 – 44 . [Google Scholar]
- Pan H. P., Chu D., Ge D. Q., Wang S. L., Wu Q. J., Xie W., Jiao X. G., Liu B. M., Yang X., Yang N., et al. . 2011. . Further spread of and domination by Bemisia tabaci biotype Q on field crops in China . J. Econ. Entomol. 104 : 978 – 985 . [DOI] [PubMed] [Google Scholar]
- Pan H. P., Chu D., Yan W. Q., Su Q., Liu B. M., Xie W., Jiao X. G., Li R. M., Yang N., Yang X., et al. . 2012. . Rapid spread of tomato yellow leaf curl virus in China is aided differentially by two invasive whiteflies . PLoS One 7 : e34817 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perring T. M. 2001. . The Bemisia tabaci species complex . Crop Prot. 20 : 725 – 737 . [Google Scholar]
- Rao Q., Xu Y. H., Luo C., Zhang H. Y., Jones C. M., Devine G. J., Gorman K., Denholm I. . 2012. . Characterisation of neonicotinoid and pymetrozine resistance in strains of Bemisia tabaci (Hemiptera: Aleyrodidae) from China . J. Integr. Agric. 11 : 321 – 326 . [Google Scholar]
- Reitz S. R., Trumble J. T. . 2002. . Competitive displacement among insects and arachnids . Annu. Rev. Entomol. 47 : 435 – 465 . [DOI] [PubMed] [Google Scholar]
- Roditakis E., Roditakis N. E., Tsagkarakou A. . 2005. . Insecticide resistance in Bemisia tabaci (Homoptera: Aleyrodidae) populations from Crete . Pest Manag. Sci. 61 : 577 – 582 . [DOI] [PubMed] [Google Scholar]
- Roditakis E., Grispou M., Morou E., Kristoffersen J. B., Roditakis N., Nauen R., Vontasb J., Tsagkarakou A. . 2009. . Current status of insecticide resistance in Q biotype Bemisia tabaci populations from Crete . Pest Manag. Sci. 65 : 313 – 322 . [DOI] [PubMed] [Google Scholar]
- Shatters R. G., Powell C. A., Boykin L. M., He L. S., McKenzie C. L. . 2009. . Improved DNA barcoding method for Bemisia tabaci and related Aleyrodidae: development of universal and Bemisa tabaci biotype-specific mitochondrial cytochrome coxidase I polymerase chain reaction primers . J. Econ. Entomol. 102 : 750 – 758 . [DOI] [PubMed] [Google Scholar]
- Sun D. B., Liu Y. Q., Qin L., Xu J., Li F. F., Liu S. S. . 2013. . Competitive displacement between two invasive whiteflies: insecticide application and host plant effects . Bull. Entomol. Res. 103 : 344 – 353 . [DOI] [PubMed] [Google Scholar]
- Teng X., Wan F. H., Chu D. . 2010. . Bemisia tabaci biotype Q dominates other biotypes across China . Fla. Entomol. 93 : 363 – 368 . [Google Scholar]
- Vassiliou V., Emmanouilidou M., Perrakis A., Morou E., Vontas J., Tsagkarakou A., Roditakis E. . 2011. . Insecticide resistance in Bemisia tabaci from Cyprus . Insect Sci. 18 : 30 – 39 . [Google Scholar]
- Wang Z. Y., Yan H. F., Yang Y. H., Wu Y. D. . 2010. . Biotype and insecticide resistance status of the whitefly Bemisia tabaci from China . Pest Manag. Sci. 66 : 1360 – 1366 . [DOI] [PubMed] [Google Scholar]
- Xie W., Wang S. L., Wu Q. J., Feng Y. T., Pan H. P., Jiao X. G., Zhou L., Yang X., Fu W., Teng H. Y., et al. . 2011. . Induction effects of host plants on insecticide susceptibility and detoxification enzymes of Bemisia tabaci (Hemiptera: Aleyrodidae) . Pest Manag. Sci. 67 : 87 – 93 . [DOI] [PubMed] [Google Scholar]
- Zhang L. P., Zhang Y. J., Zhang W. J., Wu Q. J., Xu B. Y., Chu D. . 2005. . Analysis of genetic diversity among different geographical populations and determination of biotypes of Bemisia tabaci in China . J. Appl. Entomol. 129 : 121 – 128 . [Google Scholar]
- Zhou Y. 1949. . A list of aleyrodidae from China . China Entomol. 3 : 1 – 18 . [Google Scholar]