Abstract
The toxicity effect of Concanavalin A ( Canavalia ensiformis lectin, ConA) to bird cherry-oat aphid, Rhopalosiphum padi L. (Hemiptera: Aphididae), was investigated in the laboratory by using artificial diets containing ConA concentrations. Bird cherry-oat aphid performance was affected by the presence of Con A in artificial diets. The lectin added into the liquid diet increased the prereproductive period, mortality, and the average time of generation development ( T ) and decreased fecundity and the intrinsic rate of natural increase ( rm ). In attempt to unravel the mode of action of ConA, the interaction of the lectin with insect gut and the effect of ConA on feeding behavior were investigated. Extract of gut of treated grain aphid demonstrated DNA fragmentation, and this was accompanied with an increase in caspase 3 activity. Moreover, addition of ConA to the sucrose–agarose gels reduced salivation and passive ingestion of fluids from the gel. The results indicate that the insecticidal activity of ConA on R. padi may involve effects on death of the gut epithelial cells and effects on feeding behavior. This can be employed to create plants that are resistant to aphids.
Keywords: lectin, EPG, antifeeding, apoptosis, caspase 3
Aphids are an extremely successful group that occurs throughout the world, and they are pests of vegetables, grains, tree crops, flowers, and ornamentals. Aphids weaken their host plant diverse ways. First, as phloem feeders, they are diverting for their own profit the nutrients necessary to plant growth and reproduction. Second, during the feeding phase, they inject saliva that could be phytotoxic. Third, sooty molds frequently grow on aphids’ honeydew and hinder photosynthetic activity. Finally, aphids are responsible for transmitting ∼50% of insect-borne plant viruses, many of which cause diseases of major economic importance in crops ( Quiros et al. 2006 , Dedryver et al. 2010 ). Among the 14 most agricultural importance worldwide, aphid pest is the bird cherry-oat aphid, Rhopalosiphum padi L. (Hemiptera: Aphididae). R. padi is one of the main species from the family Aphididae characterized, in part, by phytophagous phloem feeders with rapid turnover of generations. Moreover, R. padi is highly polyphagous with innumerable hosts from the family Poaceae including all the major cereals ( Blackman and Eastop 2000 , Finlay and Luck 2011 ). The insect causes the most damage by transmitting a number of viruses, especially Barley yellow dwarf virus, for which it is the most important vector ( Oswald and Houston 1951 , Descamps and Chopa 2011 ).
Although plant chemicals can be used as biopesticides to control insect pests, aphids are difficult to control because of their unique feeding habits and fast multiplication rates ( Majumder et al. 2004 ). As a consequence, researchers are developing a biotechnological control in which novel genes from plant sources are introduced into plant genomes to enhance the resistance of crop plants to phloem-feeding insects. Several different classes of proteins including lectins, ribosome-inactivating proteins, and protease inhibitors have been tested as alternative resistance factors ( Jaber et al. 2010 ).
Plant lectins are a class of carbohydrate-binding nonimmune origin proteins ubiquitously distributed in a variety of plant species and play different roles and functions in biological processes such as recognition molecules within the immune system in animals ( Kilpatrick 2002 ) and as storage proteins or in defence mechanisms against pest and pathogens in plants. The insecticidal activity of plant lectins against many important insects has been well documented to show their ability to be used as biopesticides ( Carlini and Grossi-de-Sá 2002 , Jaber et al. 2010 ). These proteins are particularly important as potential control agents for hemipteran pest/aphids because these insects are not susceptible to any known Bt toxins, and so cannot be controlled by existing plant genetic engineering technologies using Bt toxin genes ( Fitches et al. 2008 ). Previous work showed that mannose and glucose lectins induced the most interesting toxic effects from all classes of lectins tested, snowdrop lectin (GNA) and Concanavalin A (ConA) in particular ( Sauvion et al. 2004a ). ConA, the first plant lectin with a mannose/glucose-binding specificity, was found in the jack bean ( Canavalia ensiformis ). ConA exists as a homotetramer at physiological pH with molecular mass of 102.5 kDa. Each monomer (non-glycosylated subunits, each of ∼27 kDa) consists of Mn 2+ , Ca 2+, and carbohydrate-binding sites ( Rűdiger and Gabius 2001 , Sridhar and Seena 2006 ). Bioassay has demonstrated that ConA affected survival and delayed development durations, larval weight, and mortality of several aphid species: Acyrthosiphon pisum (Harris), Macrosiphon albifrons (Essig), Aphis gossypii (Glover), Myzus persicae (Sulzer), Macrosiphum euphorbiae (Thomas) , and Aulacorthum solani (Kaltenbach) (Hemiptera: Aphididae). What perhaps should be noted here is the fact that the effect of ConA consumption on these species may fluctuate, because some species are highly susceptible to ConA, and the effect on other be less or moderate ( Sauvion et al. 2004a , Jaber et al. 2010 ). Despite current interest in the insecticidal properties of plant lectins, there is no consensus for the mode of action of these proteins toward insects. Three possible modes of action have been suggested. Binding lectins to insect gut structure causes morphological and physiological modifications in the insect intestine ( Sauvion et al. 2004b ). Another possibility is that the lectins can interfere with digestive enzymes and assimilatory proteins, thereby inhibiting food ingestion ( Singh et al. 2006 , Sprawka et al. 2012 ). Moreover, binding of lectins to the carbohydrate moieties associated with membrane proteins of chemosensory sensillae in mouthparts could block the access of chemical signals to receptor proteins, leading to an antifeedant effect ( Pyati et al. 2012 ).
In this article, we have studied the toxicity and the mechanisms of toxicity of ConA in bird cherry-oat aphid. First, we carried out experiments to determine the effects of ConA on development, fecundity, and mortality of the bird cherry-oat aphid. Because a recent report that analyzed the action mechanism of lectins at the cellular levels illustrated that effects of binding of lectins to the midgut epithelium may lead to severe anatomical abnormalities with pathological consequences such as apoptosis in insect’s epithelial cells ( Hamshou et al. 2010 , Shahidi-Noghabi et al. 2010a ), the occurrence of apoptosis in the treated insects was investigated. Moreover, to better understand the mechanisms of the interactions between Con A and aphids, we have developed a bioassay to screen for the effect of ConA on R. padi feeding behavior.
Materials and Methods
Aphid Culture
The bird cherry-oat aphid ( R. padi L.) used in this study were obtained from a stock culture kept at the Siedlce University of Natural Sciences and Humanities. The stock culture was maintained on winter wheat ( Triticum aestivum L. cv. Tonacja) in an environmental chamber at 21 ± 1°C, 16:8 (L:D) h photoperiod, and 70% relative humidity (RH). Wingless females were used in all experiments.
Chemicals
Lectin Con A were purchased from MP Biomedicals (Santa Ana, California, USA) (CN.150710). Genomic DNA was extracted with the application of Genomic Mini AX Tissue kit (A&A Biotechnology, Gdynia, Poland, www.aabiot.com ). All dietary components and other chemical reagents were obtained from Sigma (Sigma Chemical Co., Poznań, Poland) and were of analytical or best available grade.
Aphid Feeding Bioassays
The liquid diet used for aphid feeding bioassays was prepared as described by Kieckhefer and Derr (1976) . The prepared diets (500 mm 3 ) were introduced between two layers of parafilm (Sigma Chemical Co., Poznań, Poland) (sandwich layers), which were placed on plastic rings ( h = 1.5 cm 3 , Ø = 3.5 cm 3 ). Con A was incorporated into diet at four concentrations 50, 500, 1,000, and 1,500 µg/cm 3 . Control diets (without ConA) were also included. The diets were sterilized by filtration through 0.45-µm millipore filters. For assays wingless females of R. padi were placed on artificial diet and left to produce nymphs overnight. Adults were then removed, and the nymphs were maintained on the control diet for a further 24 h. After 24 h, nymphs were transferred to new feeding chambers (five insects per dish) containing sachets with test diets. Ten replicates were set up for each treatment for the controls. Ten replicates (five insects per replicate) were used for each treatment for the bioassays. Feeding chambers were kept at 21 ± 1°C, 16:8 (L:D) h photoperiod, and 70% RH, and fresh diet sachets (sandwich layers) were provided every 3 d to avoid fungal and bacterial contamination. Larval development time (prereproductive period), daily fecundity, and mortality of the aphids were monitored daily for 15 d. Population parameters were used to determine the influence of Con A on bird cherry-oat aphid population growth potential. The average time of generation development ( T ) and the intrinsic rate of natural increase ( rm ) were calculated using equations of Wyatt and White (1977) :
were d is the length of prereproductive period , Md the number of larvae born during the reproduction period, which equals the d period, and 0.74, the correction factor.
Lectin-Induced Apoptosis
In the next experiment, R.padi adult aphids were placed on artificial control diet (without phytohemagglutinin [PHA]) and diet containing 1,500 µg/cm 3 of ConA as described above. For feeding chamber, 30 apterae morphs were placed on feeding sachets, and the experiment was repeated three times. After 48 h of diet probing, aphids were collected. Next, the entire guts of adult aphids were dissected under the binocular and analyzed both for DNA fragmentation and caspase-3 - like activity.
DNA Fragmentation Assay
The dissected aphid guts (60 guts) were collected in sterile deionized water. Genomic DNA was extracted from aphid guts with the application of Genomic Mini AX Tissue kit (A&A Biotechnology, www.aabiot.com ), following the manufacturer’s protocol. The quantification of DNA samples was conducted using an Epoch Microplate spectrophotometer (BioTek Instruments, Inc.). Additionally, A260/280 and A260/230 ratios were calculated to evaluate the sample integrity and contamination of proteins or other organic substances. DNA preparates of high integrity and purity were accepted for electrophoretic analysis. Separation of DNA samples (8 µg) was performed using a horizontal gel electrophoresis (2% agarose) under standard conditions. DNA fragments were detected using ethidium bromide staining and UV transillumination. The low-molecular weight marker (Genoplast Biochemicals, Poland, 50-bp ladder) was used as standard.
Caspase Activity Assay
Dissected gut tissues of R. padi adults were incubated in ice-cold lysis buffer (50 mM HEPES, pH 7.4, 5 mM CHAPS, and 5 mM DTT). The caspase 3 activity assay was carried out with the application of Caspase 3 Colorimetric Assay Kit (Sigma–Aldrich, Poznań, Poland, PC CASP-3-C), following the manufacturer’s protocol. This assay is based on the amount of p -nitroaniline released from hydrolysis of the peptide substrate acetyl-Asp-Glu-Val-Asp-pnitroanilide (Ac-DEVD-pNA) by caspase 3. The assay can be performed in 1 cm 3 volume and measured using a spectrophotometer. Activity of caspase-3 was expressed as nanomoles (nmol) of released p -nitroaniline per minute per cm 3 . Three insect sampling were made for each assay.
Effect of ConA on R. padi Feeding Behavior
The effect of lectin ConA on R. padi feeding behavior was investigated in vitro, using sucrose–agarose gels. Gels were prepared by incorporating 1.25% agarose (Sigma A-0169) into a 30% sucrose solution. After the mixtures were stirred, they were heated in a water bath (75°C for 15 min). ConA at 50, 500, 1,000, and 1,500 µg/cm 3 was added to the mixtures when they had cooled to 37°C; control gels without ConA was also prepared. The cool but molten mixtures were then poured into plastic rings (10 mm in height and 15 mm in diameter) covered with a stretched Parafilm M membrane. Transparent gels formed after 1–2 min and were offered to aphids for probing.
The experiment was investigated using the electrical penetration graph (EPG) technique that is frequently employed in insect probing and feeding behavior studies ( Tjallingii 1995 , Sauvion et al. 2004a , Pettersson et al. 2007 ). The EPG technique makes it possible to record different waveform patterns related to aphid activities and stylet locations during penetration, usually within the plant tissue ( Sauvion and Rahbe 1999 ). Apterous adults were collected between 6 and 7 a.m., attached to a golden wire electrode (2 cm in length, 20 µm in diameter) with conductive silver paint (Demetron, L2027, Darmstadt, Germany) and starved for 2 h prior to the experiment. After the aphids were starved probing, the feeding behavior was monitored for 4 h continuously with the two four-channel DC EPG recording equipment (Wageningen, Agricultural University, Entomology Department, The Netherlands). Each aphid was given access to a freshly prepared sucrose–agarose gel. The recordings of all replicates of a same treatment were performed during two consecutive days due to equipment limitations. All experiments were started at 9–10 a.m. Signals were saved on the IBM computer through a DAS 8 SCSI acquisition card (Keithley). During EPG recordings, aphids were in a Faraday cage in the laboratory (21 ± 1°C, 16:8 (L:D) h photoperiod, and 70% RH). EPG recordings were made for 10 aphids on gels without lectin (control) and for 10 aphids for each lectin concentration (50, 500, 1,000, 1,500 µg/cm 3 ).
EPGs were acquired and analyzed with STYLET 2.2 software provided by W.F. Tjallingii. Waveforms were identified by analogy to those waveforms found by others in artificial diets and sucrose–agarose gels ( Sauvion and Rahbe 1999 , Sauvion et al. 2004a , Goławska 2007 , Cid and Fereres 2010 , Sprawka and Goławska 2010 , Goławska and Łukasik 2012 ) as indicative of different feeding activities described for plants ( Tjallingii 1990 ) and called g-np, g-C, g-E1, g-E2, and g-G. In this study, for the probing and feeding behavior on gels, we used the same nomenclature with the prefix g to describe the feeding waveforms based on the first letter of the name gel (g—gel sucrose–agarose). The main waveforms generated by aphids probing and feeding on gels are interpreted to be analogous to or representative of the waveforms previously described for the probing and feeding of aphids on plants ( Tjallingii 1988 , 1990 , 1994 ). In waveform g-np, the aphids stylet is outside the gel (by analogy to the stylet being outside the plant, pattern np on plants). Waveform g-C indicates stylet activity in the gel (by analogy to the stylet penetrating the epidermis and masophyll, before salivation and ingestion, pattern C on plants). Waveform g-E1 indicates salivation into the gel (by analogy to the stylet salivating into phloem sieve tubes, pattern E1 on plants). Waveform g-E2 indicates passive ingestion of fluids from the gel (by analogy to the stylet passively ingesting phloem sap, pattern E2 on plants). Waveform g-G indicates active ingestion of the fluids from the gel (by analogy to the stylet actively ingesting xylem sap, pattern G on plants). EPG parameters were measured in each group and recalculated per one insect.
Statistical Analysis
Effect of the studied concentrations of ConA on bird cherry-oat aphid performance was subjected to two-tailed unpaired Student’s t test. Relationships between concentration of ConA and population parameters were calculated using Pearson correlation. The values of the EPG parameters were analyzed with the Kruskal–Wallis test followed by the post hoc multiple comparisons of mean ranks for all groups. All statistical analyses used Statistica for Windows v.7.0 ( StatSoft 2003 ).
Results
Effects of ConA on Bird Cherry-Oat Aphid Adult in Artificial Diet
In this study, the influence of ConA was demonstrated in significant prolongation of prereproductive period and thus in the average time of generation development ( T ) of this bird cherry-oat aphid. The prereproductive period increased from 6.60 d in control to 11.20 d at 1,500 µg/cm 3, i.e. increase of 4.6 d. Thus, the average time of generation development increased by 6.20 d ( Table 1 ). The fecundity and the intrinsic rate of natural increase ( rm ) was also deleteriously and significantly affected ( P < 0.01; P < 0.001, t test). As can be seen from table, the mean daily fecundity declined from 1.43 in control diets to 1.16 at 1,500 µg/cm 3 of ConA in the diet. The intrinsic rate of natural increase also showed a decline in dose-dependent manner being 0.17 at 1,500 µg/cm 3 when compared with control diet. Only for the 50 μg/cm 3 concentration of ConA significant differences were not found ( P > 0.05, t test). Wingless females of bird cherry-oat aphid on all concentrations of PHA showed a significant ( P < 0.001, P < 0.01 t test) mortality in comparison to insects, which fed on control diets ( Table 2 ).
Table 1.
Population parameters of R. padi as affected by ConA in the diet (values are means ± SD)
| Parameters | Control |
Lectin ConA
|
|||
|---|---|---|---|---|---|
| 50 (μg/cm3) | 500 (μg/cm 3 ) | 1,000 (μg/cm 3 ) | 1,500 (μg/cm 3 ) | ||
| Mean daily fecundity | 1.43 ± 0.08 | 1.39 ± 0.01 NS | 1.29 ± 0.007** | .23 ± 0.01** | 1.16 ± 0.02*** |
| Prereproductive period (d) | 6.60 ± 0.94 | 7.00 ± 0.00 NS | 7.80 ± 0.44** | 8.60 ± 0.54*** | 11.20 ± 0.44*** |
| Intrinsic rate of natural increase ( rm ) | 0.25 ± 0.01 | 0.24 ± 0.00 NS | 0.22 ± 0.005** | 0.20 ± 0.005*** | 0.17 ± 0.00*** |
| Average time of generation development ( T ) | 8.92 ± 0.79 | 9.46 ± 0.00 NS | 10.54 ± 0.60** | 11.62 ± 0.73*** | 15.13 ± 0.60*** |
NS, not significant.
Significance of differences from the control values **P < 0.01; ***P < 0.001 (Student’s t -test) ( n = 10).
Table 2.
Effect of ConA on mortality of R. padi (values are means ± SD)
| Concentration of lectin (µg/cm 3 ) | Mortality (%) |
|---|---|
| Control | 2.00 ± 4.47 |
| 50 | 20.00 ± 8.16* |
| 500 | 25.00 ± 5.77** |
| 1,000 | 45.11 ± 5.77** |
| 1,500 | 75.00 ± 5.77** |
Significance of differences from the control values ** P < 0.001; * P < 0.01 (Student’s t -test) ( n = 10).
However, there was found significant relationship between studied concentrations of Con A and population parameters: the aphid fecundity ( R = −0.92, P < 0.001, Pearson correlations), larval development ( R = 0.90, P < 0.001, Pearson correlations), average time of generation development ( R = 0.90, P < 0.001, Pearson correlations), intrinsic rates of natural increase ( R = −0.95, P < 0.001, Pearson correlations), and mortality ( R = 0.95, P < 0.001, Pearson correlations).
DNA Fragmentation and Caspase 3 Activity in Gut Tissues of R. padi Upon Exposure to ConA
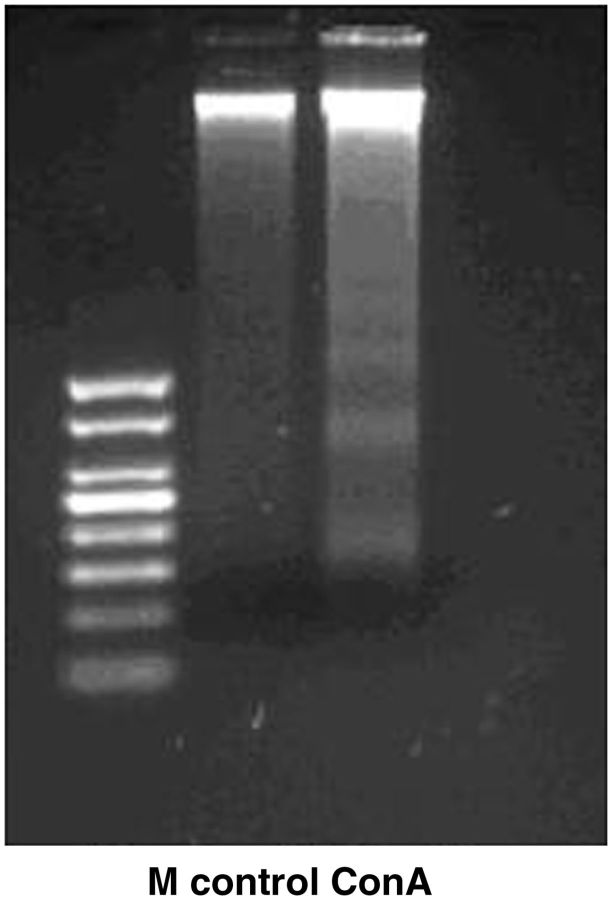
Analysis of DNA extracted from gut cells after 48 h of feeding of insects on diets with ConA showed a clear ladder pattern, whereas no DNA fragmentation was observed in the control samples (DNA extracted from gut cells after 48 h of feeding of insects on diets without ConA) ( Fig. 1 ).
Fig. 1.
Electrophoretic detection of DNA fragmentation in the total DNA isolated from gut cells after 48 h of feeding of insects on diets with ConA (ConA) and without ConA (control). M (low-molecular-weight DNA marker: 50,100, 150, 200, 250, 300, 400, and 500 bp).
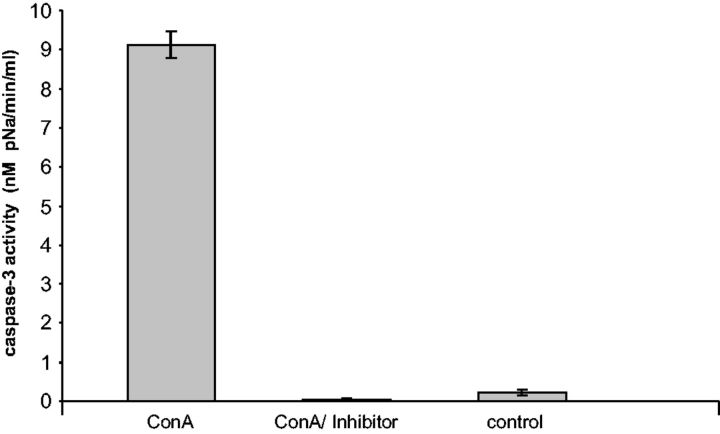
Moreover, in adults of R.padi fed on a diet containing 1,500 µg/cm 3 of ConA, increase in the caspase-3 activity was observed in the gut samples ( Fig. 2 ). In contrast, no enzymatic activity was detected in samples from aphids treated with ConA in the presence 2 mM of the specific caspase-3 inhibitor Ac-DEVD-CHO as was the case with control samples (samples of gut cells after 48 h of feeding of insects on diets without ConA).
Fig. 2.
Caspase 3 activity in the gut tissues of R. padi adults. Caspase-3 activity in R. padi gut extracted from adults after 48 h of feeding on artificial diet containing 1,500 µg/cm 3 of ConA (ConA) or diet without ConA (control). The addition of 2 mM Ac-DEVD-CHO blocked caspase-3 activity (ConA/inhibitor). Values are presented as mean (±SD) based on three individual repetitions.
Bird Cherry-Oat Aphid Feeding Behavior on Gels With ConA
EPG recordings indicated that the addition of the lectin ConA to the sucrose–agarose gel clearly affected the probing and feeding behavior of R. padi and that the effect depended on lectin concentration. Lectin ConA statistically affected stylet activity in the gel (g-C waveform), salivation into the gel (g-E1 waveform), passive ingestion from the gel (g-E2 waveform), and active ingestion from the gel (g-E2 waveform) (Kruskal–Wallis test; P < 0.001 in all cases). Although aphids probed the gel in the controls and in all lectin treatments (as indicated by the presence of g-C waveform), aphids did not exhibit salivation and passive ingestion (as indicated by the absence of g-E1 and g-E2 waveforms) with gels containing 1,500 µg/cm 3 of lectin. All EPG waveforms were observed on gels that contained ≤1,000 µg/cm 3 of lectin ConA ( Table 3 ). The average time of probing tended to be higher with addition of the lectin ConA, but the effect was statistically significant for lectin at 1,000 and 1,500 µg/cm 3 ( Table 3 ). On gels that contained ≤500 µg/cm 3 of lectin ConA, the duration of salivation into the gels tended to be prolonged but without statistical significance ( Table 3 ). Lectin ConA at 1,000 µg/cm 3 significantly reduced the total time waveform g-E1, and lectin ConA at 1,500 µg/cm 3 reduced to 0 the total time that aphids salivated into the gels. The higher concentrations of lectin ConA (≤500 µg/cm 3 ) reduced or completely inhibited aphid passive ingestion (waveform g-E2) ( Table 3 ). Lectin ConA at 500 µg/cm 3 reduced the duration of passive ingestion up to 9 times, at 1,000 µg/cm 3 up to 60 times, and no passive ingestion occurred with ConA at 1,500 µg/cm 3 ( Table 3 ). As indicated by the g-G waveform, the lectin ConA tended to shorten or prolong, active ingestion, but the effect was not statistically significant ( Table 3 ).
Table 3.
Effect of lectin ConA added into sucrose–agarose gels, on R. padi feeding activity
| Aphid activity (min) | Control |
Lectin ConA (μg/cm
3
)
|
|||
|---|---|---|---|---|---|
| 50 | 500 | 1,000 | 1,500 | ||
| No probing (g-np) | 32.80 ± 13.86b | 24.59 ± 26.56b | 103.23 ± 23.61a | 104.54 ± 26.33a | 97.36 ± 15.27a |
| Pathways (g-C) | 22.31 ± 18.49b | 24.98 ± 25.06b | 55.29 ± 42.71ab | 78.22 ± 25.07a | 90.33 ± 45.39a |
| Salivation into sieve elements (g-E1) | 9.88 ± 5.64ab | 65.60 ± 23.19a | 46.94 ± 24.19a | 4.56 ± 6.67b | 0.00 ± 0.00b |
| Phloem sap ingestion (g-E2) | 97.47 ± 32.64a | 15.75 ± 10.76ab | 10.38 ± 13.48bc | 1.61 ± 3.27bc | 0.00 ± 0.00c |
| Xylem sap ingestion (g-G) | 73.23 ± 40.94ab | 110.74 ± 56.07a | 24.17 ± 29.91b | 48.98 ± 22.68ab | 52.21 ± 42.75ab |
Values were derived from 4-h EPG recordings and are presented as means ± SD; n = 10. Means in rows followed by different letters are different at P < 0.001 (Kruskal–Wallis test).
Discussion
Lectins incorporated in artificial diets or expressed in different transgenic plants have been shown to reduce the growth and development of several insect species ( Carlini and Grossi-de-Sá 2002 , Vanderborre et al. 2009 ). It is particularly pertinent to the control of homopteran insects pests where no effective Bt strains have as yet been identified for control of insects within this order. In this study, the effects of ConA in artificial diet on survival, development, and fecundity of R. padi were investigated. In general, bird cherry-oat aphid performance was affected by the presence of Con A in artificial diets. When R. padi was fed, an artificial diet containing the lectin ConA, its fecundity was reduced, its prereproductive period and generation time were prolonged, and its morality was increased.
The mannose/glucose-specific lectin ConA has been used a lot in feeding experiments on pest herbivores. When fed to larvae of tomato moth, Lacanobia oleracea L. in artificial diet, or on ConA-expressing potato plants, larval development was retarded, 90% mortality was scored, and also showed a decrease in larval weight ( Gatehouse et al. 1999 ). Toxicity of ConA to Meligethes aeneus F. was also reported by Melander et al. (2003) . Similarly, when ConA was added to artificial diets and fed to tara planthopper, Tarophagous Proserpina Kirkaldy, a corrected mortality of 93 % was noted ( Powell 2001 ). Moreover, artificial diet studies have shown that this lectin has insecticidal activity, particularly toward Homoptera including the rice brown planthopper, Nilaparvata lugens Stål ( Powell et al. 1993 ), and the pea aphid, A. pisum ( Rahbe and Febvay 1993 , Rahbe et al. 1995 ). The effects of ConA on the peach-potato aphid, M.persicae, also show that the lectin has deleterious effects on growth and development in this insect when administrated in artificial diet, resulting in decreased fecundity. In agreement with the diet bioassay results, ConA-expressing potatoes decreased the fecundity of M. persicae by up to 45% ( Sauvion et al. 1996 , Gatehouse et al. 1999 ).
Although the biochemical properties and insecticidal activity of lectins are well studied, but our knowledge about the exact mechanism of action of lectins is still limited. The insecticidal activity of plant lectins is to be related to the sugar-binding capacity of these proteins. When plant material is ingested by insect herbivores, the primary site of action for lectins is the insect digestive tract. Because most of the digestive enzymes or transport proteins secreted in the gut of insects or proteins embedded in the epithelial cell membrane contain glycan structures, these glycoproteins are all potential targets for plant lectins ( Vandenborre et al. 2011 ). Ultrastructural studies have shown insecticidal lectins to be bound to suitable glycosylated targets in the insect gut ( Habibi et al. 2000 , Fitches et al. 2001 , Majumder et al. 2004 ). Immunolocalization studies showed that ConA interacts with glycosylated receptor present at the cell surface or within the midgut epithelium cells. Moreover, immunohistochemical and electron microscopy studies revealed that ConA induced severe cellular swelling of epithelial cells, accompanied by hypersecretion and progressive detachment of apical membrane ( Sauvion et al. 2004b ). A recent report that analyzed the lectins interaction with specific carbohydrate moieties on the midgut epithelial cells illustrated that it is an important step in lectin-mediated cell killing. Moreover, it was shown that the cytotoxicity of the lectin is mediated via induction of apoptosis ( Hamshou et al. 2010 , Shahidi-Noghabi et al. 2010a ). The results obtained here confirm this hypothesis. Samples of gut of R. padi showed two main characteristics of cell death or apoptosis. We showed a clear DNA fragmentation in R. padi guts. In addition, we also demonstrated that caspase 3 activity is induced in R.padi tissue, suggesting the involvement of this enzyme in the entomotoxic activity of ConA. Therefore, it can be suggested that caspase 3 is causing DNA fragmentation in the gut epithelium, and this in turn is responsible for the toxicity of ConA in bird cherry-oat aphid. Little research has been done to investigate the induction of apoptosis by plant lectins at insect level. The exposure of insect midgut CF-203 cells to Sclerotinia sclerotinum agglutinin resulted in DNA fragmentation, but the effect was caspase-3 independent ( Hamshou et al. 2010 ). But Shahidi-Noghabi et al. (2010a) reported that the samples of the (mid)gut of A.pisum and Spodoptera exigua Hübner upon feeding on the diet containing Sambucus nigra agglutinins (SNA) showed two main characteristics of apoptosis: a clear DNA fragmentations and the induction of caspase-3-like activity. Similarly, both SNA-I and SNA-II induced caspase-dependent apoptosis, leading to typical symptoms of cell death in midgut CF-203 cells ( Shahidi-Noghabi et al. 2010b ). However, the induction of apoptosis in mammalian cells under the influence of plant lectin has been studied intensively because lectins elicit apoptosis in different cancer cell lines ( Fu et al. 2011 ). Hitherto, several plant lectins such as wheat germ agglutinin, ricin, abrin, and ConA and PHA have been well studied to possess antiproliferative and apoptosis-inducing activities toward normal or cancer cells. But the mechanisms of apoptotic effects of lectins remain mostly unknown. Apoptosis can be mediated by death receptors initiated by lectins. Fas receptor (apoptosis antigen 1: APO-1 or APT) is the receptor with which lectins often interact. The interaction is probably by protein–protein interaction. Although the apoptotic pathways look different, activation of different caspases is usually involved. Caspase 3 plays a central role in apoptosis. It interacts with caspase 8 and caspase 9. Therefore, caspase 3 is usually investigated in apoptotic pathways ( Lam and Ng 2011 ).
Because plant lectins can cause increased mortality or adverse effects on development or fecundity on insect, their effects on feeding behavior have received some attention over the past 10 years. Another argument for the investigation of the effect of lectins on feeding behavior is the taste and the olfactory receptors, being integral membrane-spanning proteins, are most likely glycosylated. So, lectins can interfere with these glycosylated receptors and thus hamper their proper functions or even initiate false signals to nervous system ( Michiels et al. 2010 ). Frazier and Chyb (1995) suggested that insect feeding can be inhibited at the preingestional level (associated with host finding and host selection processes involving gustatory receptors), at the ingestional level (related to food transport and the production and release of salivary enzymes), and at the postingestional level (involving long-term effects resulting from various aspects of food digestion and absorption). Aphids differ from chewing herbivores with respect to the location of their main gustatory receptors. Mouthparts lack external chemoreceptors, and the taste organ is located in the hypopharynx, i.e., the hypopharyngeal gustatory organ; hence, the ingestion of sap is crucial for recognition and acceptance of a host plant. Before reaching the phloem vessels of host food plants, aphid ingests small samples of parenchyma cell contents for gustatory purposes ( Dancewicz et al. 2008 ). Because aphid-probing behavior cannot be observed directly, the parameters derived from EPG recordings as used as reliable indicators of preingestional and ingestional factors that affect feeding ( Gabryś and Tjallingi 2002 ). This study used the EPG method to monitor the probing and feeding behavior of black cherry-oat aphids exposed to the lectin ConA in sucrose–agarose gel. No previous study has examined the effect of this lectin on the feeding behavior of the black cherry-oat aphid. The EPG-recorded behavior of R. padi on sucrose–agarose gel showed differences in crucial aspects on probing and feeding behavior. Addition of lectin ConA to the gels generally prolonged the period of stylet penetrations (as indicated by EPG waveform g-C), reduced salivation (as indicated as EPG waveform g-E1), and passive ingestion (as indicated as EPG waveform g-E2) by adult aphids. But at higher concentrations (≥1,500 µg/cm 3 ), lectin ConA completely stopped salivation into the gel and passive ingestion from the gel.
The current findings demonstrate detrimental effects of the lectin ConA on the feeding behavior of the R. padi . Aphid behavior on sucrose–agarose gel with lectin ConA was seriously altered when compared with aphids on sucrose–agarose gel without lectin ConA. Extending the whole of the probing and feeding periods might indicate the existence of antifeedant factors. This study of aphid behavior on sucrose–agarose gels showed that lectin ConA reduced salivation into the gel and passive ingestion of fluids from the gel. We interpret these data from gels to indicate that when feeding on plants containing ConA, aphids would spend longer times in penetrating the mesophyll, aphids would be delayed in their initiation of salivation and ingestion, and would spend less time in passive ingestion of phloem sap. Similar tendency was observed by Sauvion et al. (2004a) who also studied the effect of ConA on feeding behavior of the A.pisum . ConA caused some alterations in pea aphid ingestion within the first 4 h. At this stage, ConA did not alter the frequency of behaviors but significantly affected the duration of behaviors: in the presence of ConA, nonprobing and salivation bouts were longer, whereas ingestion phases were significantly shorter. In another study, the mannose-binding snowdrop lectin (GNA) influenced the feeding behavior of the N. lugens ; addition of GNA to the diet-reduced diet ingestion ( Powell and Gatehouse 1996 ). Moreover, Sprawka et al. (2013) showed that when the grain aphid, Sitobion avenae, fed on artificial diet with PHA ( Phaseolus vulgaris lectin), duration of pathways was increased by higher concentrations of PHA and the number of penetrations was reduced. Detailed understanding of how lectins modulate behavior especially feeding behavior remains unknown. Most studies agree that the effects of plant lectins on insect behavior are a consequence of intoxication rather than a direct sensory-mediated response of the insect ( Sauvion et al. 2004a ). Alternatively, binding of the lectin to the carbohydrate moieties associated with membrane proteins of chemosensory sensillae in mouthparts could block the access of chemical signals to receptor proteins, leading to an antifeedant effect ( Pyati et al. 2012 ).
In conclusion, when ingested in an artificial diet, ConA had dose-dependent detrimental effects on growth, fecundity, and survival of bird cherry-oat aphid. These detrimental effects were associated with the death of the gut epithelial cells and with feeding suppression. Future studies will be necessary to identify and characterize the exact binding target receptor(s) (carbohydrate-specific binding proteins and their glycosylation pattern) for ConA in the cell membrane at the aphid’s epithelial gut cells, taste, and olfactory receptors.
References Cited
- Blackman R. L., Eastop V. F. . 2000. . Aphids on the world’s crops, pp. 414–466. An identification guide, 2nd ed. John Wiley & Sons, Chichester, United Kingdom . [Google Scholar]
- Carlini C. R., Grossi-de-Sá M. F. . 2002. . Plant toxic proteins with insecticidal properties. A review on their potentialities as bioinsecticides . Toxicon 40 : 1515 – 1539 . [DOI] [PubMed] [Google Scholar]
- Cid M., Fereres A. . 2010. . Characterization of the probing and feeding behavior of Planococcus citri (Hemiptera: Pseudococcidae) on Grapevine . Ann. Entomol. Soc. Am. 103 : 404 – 417 . [Google Scholar]
- Dancewicz K., Gabryś B., Dams I., Wawrzeńczyk C. . 2008. . Enantiospecific effect of pulegone and pulegone-derived lactones on Myzus persicae (Sulz.) settling and feeding . J. Chem. Ecol. 34 : 530 – 538 . [DOI] [PubMed] [Google Scholar]
- Dedryver C. H.-A., Le Ralec A., Fabre F. . 2010. . The conflicting relationships between aphids and men: a review of aphid damage and control strategies . Comptes Rendus Biologies 333 : 539 – 553 . [DOI] [PubMed] [Google Scholar]
- Descamps L. R., Chopa C. S. . 2011. . Population growth of Rhopalosiphum padi L (Homoptera: Aphididae) on different cereal crops from the semiarid Pampas of Argentina under laboratory conditions . Chilean J. Agric. Res. 71 : 390 – 394 . [Google Scholar]
- Finlay K. J., Luck J. E. . 2011. . Response of the bird cherry-oat aphid ( Rhopalosiphum padi ) to climate change in relation to its pest status, vectoring potential and function in a crop–vector–virus pathosystem . Agric. Ecosyst. Environ. 144 : 405 – 421 . [Google Scholar]
- Fitches E., Woodhouse S. D., Edwards J. P., Gatehouse J. A. . 2001. . In vitro and in vivo binding of snowdrop in vitro and in vivo binding of snowdrop ( Galanthus nivalis agglutinin; GNA) and jackbean ( Canavalia ensiformis ; Con A) lectins within tomato moth ( Lacanobia oleracea ) larvae; mechanisms of insecticidal action . J. Insect Physiol. 47 : 777 – 787 . [DOI] [PubMed] [Google Scholar]
- Fitches E., Wiles D., Douglas A. E., Hinchlffe G., Audsley N., Gatehouse J. A. . 2008. . The insecticidal activity of recombinant garlic lectins towards aphids . Insect Biochem. Mol. Biol. 38 : 905 – 915 . [DOI] [PubMed] [Google Scholar]
- Frazier J. L., Chyb S. . 1995. . Use of feeding inhibitors in insect control, pp. 364-381 . InChapman R. F., Boer G. (eds.), Regulatory mechanisms in insect feeding . Chapman & Hall; , New York, NY: . [Google Scholar]
- Fu L., Zhou C., Yao S., Yu J., Liu B., Bao J. . 2011. . Plant lectins: targeting programmed cell death pathways as antitumor agents . Int. J. Biochem. Cell Biol. 43 : 1442 – 1449 . [DOI] [PubMed] [Google Scholar]
- Gabryś B., Tjallingii W. F. . 2002. . The role of sinigrin in host plant recognition by aphids during initial plant penetration . Entomologia Experimentalis et Applicata 104 : 89 – 93 . [Google Scholar]
- Gatehouse A. M. R., Dawidson G. M., Stewart J. N., Gatehouse L. N., Kumar A., Geoghegan I. E., Birch A. N. E., Gatehouse J. A. . 1999. . Concanavalin A inhibits development of tomato moth ( Lancanobia oleracea ) and peach-potato aphid ( Myzus persicae ) when expressed in transgenic potato plants . Mol. Breeding 5 : 153 – 165 . [Google Scholar]
- Goławska S. 2007. . Deterrence and toxicity of plant saponins for the pea aphid Acyrthosiphon pisum Harris . J. Chem. Ecol. 33 : 1598 – 1606 . [DOI] [PubMed] [Google Scholar]
- Goławska S., Łukasik I. . 2012. . Anifeedant activity of luteolin and genistein against the pea aphid, Acyrthosiphon pisum .J. Pest Sci. 85 : 443 – 450 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habibi J. E., Backus E. A., Huesing J. E. . 2000. . Effects of phytohemagglutinin (PHA) on the structure of midgut epithelial cells and localization of its binding sites in western tarnished plant bug, Lygus hesperus Knight . J. Insect Physiol. 46 : 611 – 619 . [DOI] [PubMed] [Google Scholar]
- Hamshou M., Smagghe G., Shahidi-Noghabi S., De Geyter E., Lannoo N., Van Damme E. J. M. . 2010. . Insecticidal properties of Sclerotinia sclerotiorum agglutinin and its interaction with insect tissues and cells . Insect Biochem. Mol. Biol. 40 : 883 – 890 . [DOI] [PubMed] [Google Scholar]
- Jaber K., Haubruge É., Francis F. . 2010. . Development of entomotoxic molecules as control agents: illustration of some protein potential uses and limits of lectins (Review) . Biotechnol. Agronomy Soc. Environ. 14 : 225 – 241 . [Google Scholar]
- Kieckhefer R. W., Derr R. F. . 1976. . Rearing three species of cereal aphids on artificial diets . J. Econ. Entomol. 60 : 663 – 665 . [Google Scholar]
- Kilpatrick D. C. 2002. . Mannan-binding lectin and its role in innate immunity . Transfus. Med. 12 : 335 – 352 . [DOI] [PubMed] [Google Scholar]
- Lam S. K., Ng T. Z. . 2011. . Lectins: production and practical applications . Appl. Microbiol. Biotechnol. 89 : 45 – 55 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumder P., Santanu B., Sampa D. . 2004. . Identification of receptors responsible for binding of the mannose specific lectin to the gut epithelial membrane of the target insects . Glycoconj. J. 20 : 525 – 530 . [DOI] [PubMed] [Google Scholar]
- Melander M., Ahman I., Kamnert I., Strömdahl. A.-C. 2003. . Pea lectin expressed transgenically in oilseed rape reduces growth rate of pollen beetle larvae . Transgenic Res. 12 : 555 – 567 . [DOI] [PubMed] [Google Scholar]
- Michiels K., Van Damme E. J. M., Smagghe G. . 2010. . Plant-insect interactions: what can we learn from plant lectins? Arch . Insect Biochem. Physiol . 4 : 193 – 212 . [DOI] [PubMed] [Google Scholar]
- Oswald J. W., Houston B. R. . 1951. . A new virus disease of cereals transmissible by aphids . Plant Dis. Rep. 35 : 471 – 475 . [Google Scholar]
- Pettersson J., Tjallingii W. F., Hardie J. . 2007. . Host plant selection and feeding, pp. 87-114 . InVan Emden H. F., Harrington R. (eds.), Aphids as crop pests . CAB International; , Wallingford, Oxfordshire, UK: . [Google Scholar]
- Powell K. S. 2001. . Antimetabolic effects of plants lectins towards nymphal stages of the planthoppers Tarophagous proserpina and Nilaparvata lugens . Entomologia Experimentalis et Applicata 99 : 71 – 77 . [Google Scholar]
- Powell K. S., Gatehouse J. A. . 1996. . Mechanism of mannose-binding snowdrop lectin for use against brown planthopper in rice, pp. 753-758 . In Khush G. S. (ed.), Proceedings of the third international rice genetics symposium . IRRI; , Manila, Philippines: . [Google Scholar]
- Powell K. S., Gatehouse A. M. R., Hilder V. A., Gatehouse J. A. . 1993. . Antimetabolic effects of plants lectins and plant and fungal enzymes on the nymphal stages of two important rice pests, Nilaparvata lugens and Nephotettix cinciteps . Entomologia Experimentalis et Applicata 66 : 119 – 126 . [Google Scholar]
- Pyati P., Cellamuthu A., Gatehouse A. M. R., Fitches E., Gatehouse J. A. . 2012. . Insecticidal activity of wheat Hessian fly responsive proteins HFR-1 and HFR-3 towards a non-target wheat pest, cereal aphid ( Sitobion avenae F.) . J. Insect Physiol. 58 : 991 – 999 . [DOI] [PubMed] [Google Scholar]
- Quiros D. I., Emmen D. A., Dominguez E., Heller M. V., Coley P. D., Kursar T. A. . 2006. . A rapid, efficient method for the bioassay of extracts, fractions and compounds for activity against tropical aphids . Int. J. Pest Manage. 52 : 333 – 342 . [Google Scholar]
- Rahbe Y., Febvay G. . 1993. . Protein toxicity to aphids: an in vitro test on Acyrthosiphon pisum . Entomologia Experimentalis et Applicata 67 : 149 – 160 . [Google Scholar]
- Rahbe Y., Sauvion N., Febvay G., Peumans W. J., Gatehouse A. M. R. . 1995. . Toxicity of lectins and processing of ingested proteins in the pea aphid Acyrthosiphon pisum . Entomologia Experimentalis et Applicata 76 : 143 – 155 . [Google Scholar]
- Rűdiger H., Gabius H.-J. . 2001. . Plant lectins: occurrence, biochemistry, functions and applications . Glycoconj. J. 18 : 598 – 613 [DOI] [PubMed] [Google Scholar]
- Sauvion N., Rahbe Y. . 1999. . Recording feeding behaviour of Hemiptera with the EPG method: a review. Annales de la Société Entomologique de France 35: 175–183 . [Google Scholar]
- Sauvion N., Rahbe Y., Peumans W. J., Van Damme E. J. M., Gatehouse J. A., Gatehouse A. M. R. . 1996. . Effects of GNA and other mannose binding lectins on development and fecundity of the peach-potato aphid Myzus persicae . Entomologia Experimentalis et Applicata 79 : 285 – 293 . [Google Scholar]
- Sauvion N., Charles H., Febvay G., Rahbe Y. . 2004a. . Effects of jackbean lectin (ConA) on the feeding behaviour and kinetics of intoxication of the pea aphid, Acyrthosiphon pisum . Entomologia Experimentalis et Applicata 110 : 31 – 44 . [Google Scholar]
- Sauvion N., Nardon G., Febvay G., Gatehouse A. M. R., Rahbe Y. . 2004b. . Binding of the insecticidal lectin Concanavalin A in pea aphid, Acyrthosiphon pisum (Harris) and induced effects on the structure of midgut epithelial cells . J. Insect Physiol. 50 : 1137 – 1150 . [DOI] [PubMed] [Google Scholar]
- Shahidi-Noghabi S., Van Damme E. J. M., Mahdian K., Smagghe G. . 2010a. . Entomotoxic action of Sambucus nigra agglutinin in Acyrthosiphon pisum aphids and Spodoptera exigua caterpillars through caspase-3-like-dependent apoptosis . Arch. Insect Biochem. Physiol .3 : 207 – 210 [DOI] [PubMed] [Google Scholar]
- Shahidi-Noghabi S., Van Damme E. J. M., Masatoshi I., Smagghe G. . 2010b. . Exposure of insect midgut cells to Sambucus nigra L. agglutinins I and II causes cell death via caspase-dependent apoptosis . J. Insect Physiol. 56 : 1101 – 1107 . [DOI] [PubMed] [Google Scholar]
- Singh K., Kaur M., Rup M. K., Singh J. . 2006. . Exporation for anti-insect properties of lectin from seeds of soyabean, Glycine max L. using Bactrocera cucurbitae (Coquillett) as model . Phytoparasitica 34 : 463 – 473 . [Google Scholar]
- Sprawka I., Goławska S. . 2010. . Effect of the lectin PHA on the feeding behavior of the grain aphid . J. Pest Sci. 83 : 149 – 155 . [Google Scholar]
- Sprawka I., Goławska S., Goławski A., Czerniewicz P., Sytykiewicz H. . 2012. . Antimetabolic effect of phytohemagglutinin to the grain aphid Sitobion avenae Fabricius . Acta Biologica Hungarica 63 : 342 – 353 . [DOI] [PubMed] [Google Scholar]
- Sprawka I., Goławska S., Goławski A., Czerniewicz P. . 2013. . Toxic and deterrent effects of phytohemagglutinin on the grain aphid Sitobion avenae . Biologia 68 : 525 – 532 . [DOI] [PubMed] [Google Scholar]
- StatSoft Inc.2003. Statistica (Data Analysis Software System). Version 07. (cited 2012 June 15) Available from: www.statsoft.com .
- Sridhar K. R., Seena S. . 2006. . Nutritional and antinutritional significance of four unconventional legumes of the genus Canavalia —a comparative study . Food Chem. 99 : 267 – 288 . [Google Scholar]
- Tjallingii W. F. 1988. . Electrical recording of stylet penetration activities by aphids, pp. 89-99 . InCampbell R. K., Eikenbary R. D. (eds.), Aphid-plant genotype interactions . Elsevier, Amsterdam; , The Netherlands: . [Google Scholar]
- Tjallingii W. F. 1990. . Continuous recording of stylet penetration activities by aphids, pp. 88-89 . InCampbell R. K., Eikenbary R. D. (eds.), Aphid-plant genotype interactions . Elsevier, Amsterdam; , The Netherlands: . [Google Scholar]
- Tjallingii W. F. 1994. . Sieve element acceptance by aphids . Eur. J. Entomol. 91 : 47 – 52 . [Google Scholar]
- Tjallingii W. F. 1995. . Electrical signals from the depths of plant tissues: the electrical penetration graph (EPG), pp. 45-58 . InNiemeyer H. (ed.), Proceedings of IFS workshop in chemical ecology . Santiago; , Chile: . [Google Scholar]
- Vanderborre G., Smagghe G., Van Damme E. J. M. . 2009. . Natural products: plant lectins as important tools in controlling pest insects, pp. 163-187 . InIshaaya I., Horowitz A. R. (eds.), Biorational control of arthropod pests, Springer-Verlag, Dordrecht, Nederland . [Google Scholar]
- Vandenborre G., Smagghe G., Van Damme E. J. M. . 2011. . Plant lectins as defence protein against phytophagous insect . Phytochemistry 72 : 1538 – 1550 . [DOI] [PubMed] [Google Scholar]
- Wyatt I., White P. F. . 1977. . Simple estimation of intrinsic increase rates for aphids and tetranychid mites . J. Appl. Ecol. 14 : 757 – 776 . [Google Scholar]