Abstract
The rhinoceros borer Oryctes agamemnon Burmeister (Coleoptera: Scarabaeidae) is a date palm insect pest that causes damage to trunk and roots of palm trees in several countries, including Tunisia, the United Arab Emirates (UAE), Oman, and Saudi Arabia. The aim of this study was to monitor the seasonal and nocturnal activities of this beetle. Experiments were performed on a date palm of Rjim Maatoug during a 6-yr period (2004–2007, 2009–2010). Field survey using light traps shows that O. agamemnon is a univoltine, with a single population peak. Adults appear in the field around late May–early June and the population continued to build until maximum numbers are reached between the end of July and the beginning of August in the same year. No adults were found after first 10 d of November. This peak was characterized by female dominance in number. The monitoring of nocturnal activity showed that it starts its activities roughly 40 min after the sundown and continues until approximately 1 h before sunrise. The highest number of trapped beetles was remarked in the two first hours of flight activity, with a dominance of female in the first hour and a dominance of male in the second hour. We remarked that the sex ratio (female:male) of the cumulated number of trapped adults in the different years and nights of survey was in favor of females.
Keywords: date palm, Oryctes agamemnon, flight pattern, peak, sex ratio
Palms ( Phoenix ) constitute one of the important botanical families, and include some of the world’s most important economic plants. Date palm ( Phoenix dactylifera L.) has long been one of the most important fruit crops in the arid regions of the Arabian Peninsula, North Africa, and the Middle East ( Chao and Krueger 2007 , El Hadrami and Al-Khayri 2012 ). It has been cultivated in North Africa and the Middle East for at least 5,000 yr ( Zohary and Hopf 2000 ). It plays by now an undeniable role in maintaining human populations in arid regions where natural resources are limited and living conditions are difficult. So, it is a stabilizer of economy in these regions, where production of dates provides jobs for estimated 50 million people, 35% of which are located in the North Africa ( El Hadrami and El Hadrami 2009 ). In Tunisia, particularly in southern regions, the culture of date palms plays an important role, both at socioeconomic and ecological levels ( El-Juhany 2010 ). So, there are about 10% of the population who earn their living from the palm dates’ sector ( Karim et al. 2010 ).
Date palms are afflicted with many diseases and pests ( Howard 2001 , El-Shafie 2012 ), and the nature and severity of the problems vary with cultivar, location, weather, and cultural practices ( Carpenter and Elmer 1978 , Zaid et al. 2002 ). For example, in their review of pests and diseases of the date palm, Carpenter and Elmer (1978) reported more than 50 species of insects and mites as pests of date palms in various countries.
In North Africa, there are many species of insects and mites considered serious pests to palm, that have without any doubt negative impacts on their normal growth and production ( Haouel et al. 2010 ). The mite Oligonychus afrasiaticus McGregor (Acarina: Tetranychidae) affects essentially the fruit ( Been Chaabene and Chermiti 2010 , Chaaban et al. 2011 ). The white scale Parlatoria blanchardi Targ (Hemiptera : Diaspididae) colonizes all parts of the tree with the heaviest infestation at the base of the leaves and crown ( Khoualdia et al. 1993 ). The larva of the moth Ectomyelois ceratoniae Zeller (Lepidoptera: Pyralidae) infests the dates internally. An emerging pest of date palm declared since 1995 ( Khoualdia et al. 1997 ) is the Oryctes agamemnon Burmeister (Coleoptera: Scarabaeidae), which was accidentally introduced from the middle East into the oasis of Tozeur in south-western Tunisia ( Khoualdia et al. 1997 ), where it was spread to others oases in the same region, including the oases of Rjim Maatoug in the governorate of Kibili ( Soltani et al. 2008a , b , Ehsine et al. 2009 ) where this study was conducted. O. agamemnon also occurs in Egypt, Saudi Arabia, and the Persian Gulf of Iran ( Bedford 1980 , Hallet et al. 1995 , Rochat et al. 2004 , Bedford 2013 ). Oryctes larvae have strong mandibles and as a result they can cause severe damage mainly on the collar and the root system ( Soltani et al. 2008a , Ehsine et al. 2009 ). The adults can make a little damage on the petiole and the cluster ( Ehsine et al. 2009 ).
Chemicals, biological control, pheromone trapping, quarantine, and sanitation practices are used to control insect pests of date palms ( Howard 2001 ). Different control measures, that have been considered, have been only partially successful in controlling the spread of Rhynchophorus ferrugineus in Saudi Arabia ( Prabhu et al. 2010 ) as has been found also in other countries ( Abbas 2010 ). It has been reported that males of Oryctes elegans (Hallett et al. 1995) and Oryctes rhinoceros ( Rochat et al. 2004 ) produce aggregation pheromones that help conspecifics locate one another on palm trees. Understanding the bioecology of these species is essential in developing new control strategies.
Light traps have been introduced by many researchers as means of monitoring beetle populations, as well as a method of physical control ( Zaid et al. 2002 ). In this study, light trap was used as a monitoring tool to detect the time of appearance of adult O. agamemnon , in Rjim Maatoug date palm plantations and to provide data regarding the size of population, the number of generation, the seasonal and nocturnal activities, and the sex ratio.
Materials and Methods
Study Area
This study was carried out in the oases of Rjim Maatoug located in the Saharan region of the southwestern Tunisia (33° 19′ 266″ N and 7° 58′ 554″ E) ( Fig. 1 ). It is an area which lies within 120 km in the south west of Kebili. It is characterized by a continental Saharan climate, with an average rainfall <100 mm, an average temperature of 21°C with extremes of +55°C in summer in the shade and −7°C in winter, potential evapotranspiration = 2,200–4,300 mm. The survey was carried out in the experimental orchard of the Office of Development of Rjim Maatoug which has a mean planting space of 9 by 9 m. The principal vegetation was date palm trees with predominance of the Deglet Noor variety, associated with other crops species.
Fig. 1.
The geographic location of study area.
Seasonal Activity
Solar energy based light trap ( Fig. 2 ) was used during the period of this study. Except 2004 when the survey was not regular, the examination of trap was made weekly all along the years of survey with the aim to determine the starting time of flight activity, the duration of flight period, and the interruption of seasonal activity. Monitoring started since July 2004 date of installation of the trap on the oases and during 2005, 2006, 2007, 2009, and 2010. The trapped insects were numbered and sexed.
Fig. 2.
The light trap. The trap was designed and manufactured by ourselves with the help of private society specialized in solar energy. The trap is made up of an overcome support of a square funnel divided in four parts. The funnel itself is surmounted by a wooden carpentry which vertically supports four Plexiglas plates to facilitate fallen off insects in the funnel then they will be guided to the buckets of recuperation by means of plastic tube. All of these are supported with a framework of iron. This trap is equipped with a solar plate, a system which transforms solar energy into D.C. current, a battery to store the current, and a system which ensures the automatic starting of the light 10 min after sunset. A neon lamp with a white light color was used.
Nocturnal Activity
Night-time activity for this study was released by the same light traps ( Fig. 2 ). The duration of the flights was determined by sampling flying insects at 1 hr intervals that began from close to dusk and lasted until 1 h after dawn. The number and sex of beetles in each hour was recorded. The monitoring was done in a period of high trapping success during seven nights in August 2009.
Sex Ratio
The adults of Oryctes were sexed by examination of the clypeus; males have a cephalic horn that is larger than females ( Rochat et al. 2004 ). For the insects having a small size, the determination of sex is realized through the examination of the end of the abdomen ventral face ( Ehsine et al. 2009 ). For the male, the last segment is narrow with more or less parallel edges and with a notch in the middle of the posterior edge, whereas for the female the last segment is clearly triangular with a point in the middle of the posterior edge, and females have a smaller, triangular, red marking. We determined the ratio of female to male (female/male) of O. agamemnon collected during the period of monitoring to test whether equal numbers of males and females are attracted to the traps.
Result and Discussion
Seasonal Activity
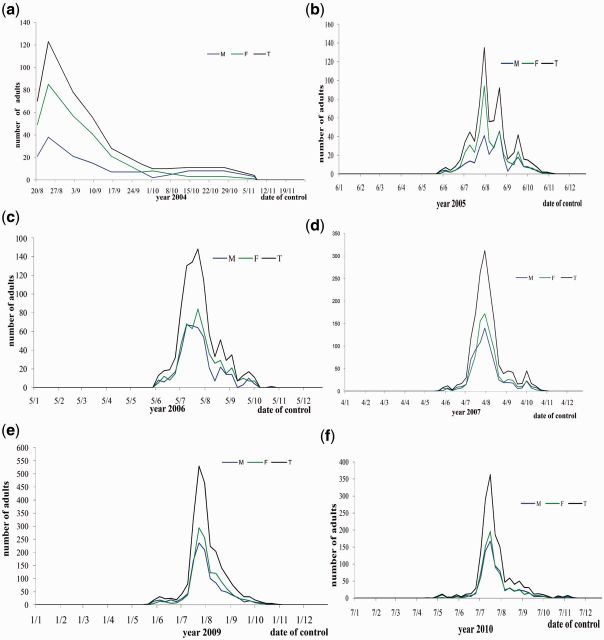
In total, 7,750 beetles were collected during the 5-yr survey. The number of the trapped insects shows a remarkable increase from one year to another. It is about five times between 2004 and 2009 and plus then three times between 2005 and 2009. Introduced years ago, probably that the insect found a favorable medium and climate for its proliferations, and the gradually increasing beetle capture over the years indicates increasing beetle population in that particular location.
In fact, except the year 2004 ( Fig. 3 a), when the survey was not regular more or less stable flight of O. agamemnon toward the light trap was recorded from later May to early June for the first 10-d period of November ( Fig. 3 a–f). With no beetle activity was observed during January, February, March, April, and December. Figure 3 b–f has much the similar flight activities of the beetle. Beetle emerges in early June, reaches its maximum flight activities in late July to early August, and sometime in early October to early November flight activities are ceased.
Fig. 3.
Seasonal activity of O. agamemnon . (a)Year 2004; (b) Year 2005; (c) Year 2006; (d) Year 2007; (e) Year 2009; (f) Year 2010.
The results show that the populations peaked once each year. The peaks were recorded in different years in late July to early August ( Fig. 3 b–f). After this date, the population records a gradual decrease in number which started in general about 1 wk after the date of peak to become nil at the first 10-d of November.
The data show O. agamemnon a univoltine pest with a single population peak. These results are consistent with Al-Deeb and Enan (2010) and Al-Deeb et al. (2012) . The peak of activity was generally recorded between the last week of July and the first week of August. The seasonal activity of this insect was night spread out over 5 mo it started from later May, earlier June until the first 10 d of November. This result corroborated the study of Al-Sayed and Al-Tamiemi (1999) , in the oasis of Sultanate Oman, while Al-Deeb and Enan (2010) pointed that adults showed from late March to till the end of September. The highest period of adult activity was during July and August, therefore, we have the possibility of application control program to adults in this period. High efficient of light trap in catch of adults to possibility will be used in integrate pest management programs to this pest.
Nocturnal Activity
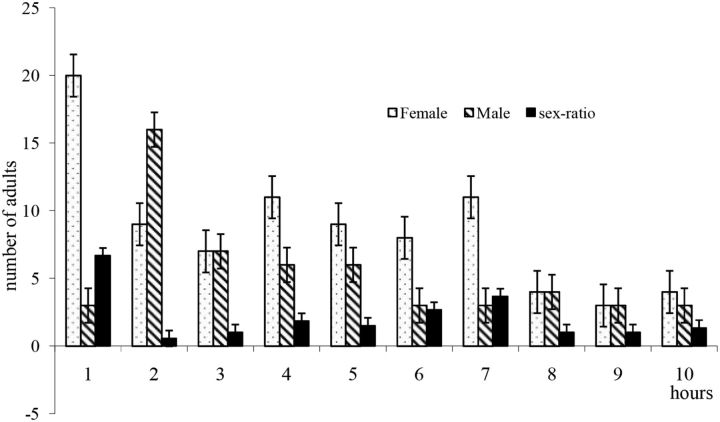
Although O. agamemnon is nocturnal beetle ( Ehsine et al. 2009 , Al-Deeb et al. 2012 ), no research has defined the timing and duration of night-time activity. The monitoring showed that there was a total of 140 adults of O. agamemnon arriving at luminous trap during the nocturnal periods (seven nights in August 2009), with an average of approximately 21 beetles by night. The cumulated number of adults in each hour during the period of survey ( Fig. 4 ) showed that beetles exhibited highest activity between the first and the second hours after starting of activity that began 40 min after the sunset. Females of O. agamemnon were more active than males in the first, fourth, fifth, sixth, seventh, and the last hours of activity ( Fig. 4 ). At the second hour, males were more attracted to the light then females. In the third, eighth, and ninth hours of nocturnal activity we registered an equal number between the two sexes.
Fig. 4.
Nocturnal activity of O. agamemnon .
The Sex-Ratio
The number of female is 4,220 and the number of males is 3,530 with a sex ratio (female:male) of 1.195 ( Fig. 3 a–f). For the different years of survey, the sex ratio varied: 2.097 in 2004; 1.458 in 2005; 1.220 in 2007; 1.152 in 2007; 1.141 in 2009; and 1,042 in 2010.
At the first of seasonal activity and after the date of peak until the interruption of activity; for the different years of survey; we remarked that the number of trapped beetles fluctuated some time in favor of male other time in favor of female.
The peak is accompanied by a remarkable rising by the trapped females which exceeds the manpower of males ( Fig. 3 b–f). At this date, the sex ratio was, respectively, as follows: 1.436 in 2005, 1.312 in 2006, 1.228 in 2007, 1.224 in 2009, and 1.173 in 2010. This conducts us to say that probably period of peak is the period of mating and reproduction for this genus. So, probably it was similar to the other species of Oryctes that the male O. agamemnon secreted a sexual pheromone to attract the female ( Gries et al. 1994 , Hallet et al. 1995 , Rochat et al. 2002 , 2004 ). So that, females stimulated by the smell of the pheromone left their galleries to search the target which can increase attraction of females at the light trap.
Concerning the nocturnal activity, the sex ratio of the trapped adults during the survey was 1.59. In the first hour, females showed more activity than males with a sex ratio of 6.66. In the second hour, the sex ratio was 0.56 ( Fig. 4 ). In the fourth, fifth, sixth, and the seventh hours, the sex ratio became again in favor of females with a ratio of 1.83, 1.5, 2.66, and 3.66, respectively. In the third, eighth, and ninth hours, we registered the same number of adults whereas in the last hour of nocturnal activity there is a minor superiority of female against the male with a sex-ratio equal to 1.33.
In Tunisia, date palm culture is one of the important and strategic sectors in the national economy. In spite of this importance, date palm and their production are affected by many pests and diseases, from them the root-borer, O. agamemnon , which is an important insect pest on date palm trees in the south-west of Tunisia. The study revealed that the light trapping is an effective tool for the monitoring and forecasting of Oryctes infestations and it is useful in determining the flight pattern of the insect. The monitoring of the seasonal activity of the insect using a light trap revealed that the number of captured insect increases during the years of following, which indicates the increasing of beetle population in this region. The O. agamemnon is a univoltine species, their activity starts from late May to early June until the first 10 d of November. The populations peaked once each year generally between the last week of July and the first week of August, and the increasing of adults’ number during the peak is accompanied by a remarkable rising by the trapped females which exceeds males. The survey of nocturnal activity of the insect showed that the insects started their activity 40 min after the sundown until 1 h before the sun rise, and the maximum number of captured adults was obtained in the two first hours, with a superiority in favor of female during the first hour, and in favor of male in the second hour.
Acknowledgments
We gratefully acknowledge the Office of Development of Rjim Maatoug for kind cooperation.
References Cited
- Abbas M.S.T. 2010. . IPM of the red palm weevil, Rhynchophorus ferrugineus , pp. 209 – 233 , InCiancio A., Mukerji K. G. (eds.), Integrated management of arthropod pests and insect borne diseases. Integrated management of plant pests and diseases , vol. 5 . Springer; , New York: . [Google Scholar]
- Al-Deeb M. A., Enan M. R. . 2010. . First record of a phoretic astigmatid Mite Sancassania sp. (Acaridae: Astigmata) on Oryctes agamemnon (Coleoptera: Scarabaeidae) in UAE . Int. J. Agric. Biol. 12 : 157 – 160 . [Google Scholar]
- Al-Deeb M. A., Mahmoud S. T., Sharif E. M. . 2012. . Use of light traps and differing light color to investigate seasonal abundance of the date palm pest, Oryctes agamemnon arabicus (Coleoptera: Scarabaeidae) . J. Econ. Entomol. 105 : 2062 – 2067 . [DOI] [PubMed] [Google Scholar]
- Al-Sayed A. E., Al-Tamiemi S. S. . 1999. . Seasonal activity of the fruit stalk-borer, Oryctes agamemnon (Burm.) (Coleoptera: Scarabaeidae) in Sultanate of Oman . Egypt. J. Agric. Res. 77 : 1597 – 1605 . [Google Scholar]
- Bedford G. O. 1980. . Biology, ecology and control of palm rhinoceros beetles . Annu. Rev. Entomol. 25 : 309 – 339 . [Google Scholar]
- Bedford G. O. 2013. . Biology and management of palm dynastid beetles: recent advances . Annu. Rev. Entomol. 58 : 353 – 372 . [DOI] [PubMed] [Google Scholar]
- Been Chaabene S., Chermiti B. . 2010. . Oligonychus afrasiaticus. Seasonal abundance and life history of the old world mite in various date palm cultivars in Segdoud Oasis, south Tunisia . African J. Plant Sci. Biotechnol. 4 : 59 – 63 . [Google Scholar]
- Carpenter J. B., Elmer H. S. . 1978. . Pests and diseases of the date palm. United States Department of Agriculture . Agric. Hand Book 527 : 1 – 42 . [Google Scholar]
- Chaaban S. B., Chermiti B., Kreiter S. . 2011. . Comparative demography of the spider mite, Oligonychus afrasiaticus , on four date palm varieties in southwestern Tunisia . J. Insect Sci. 11 : 136. ( www.insectscience.org/11.136 ) . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao C.C.T., Krueger. R. R. 2007. . The date palm ( Phoenix dactylifera L.): overview of biology, uses and cultivation . Hortic. Sci. 42 : 1077 – 1082 . [Google Scholar]
- Ehsine M., Belkadhi M. S., Chaieb. M. 2009. . Bio-ecologic observations on rhinoceros beetle Oryctes agamemnon (Burmeister 1847) on the palm dates Oasis of Rjim Maatoug in South-Western Tunisia . J. Arid Land Stud. 19 : 379 – 382 . [Google Scholar]
- El Hadrami A., Al-Khayri. J. M. 2012. . Socioeconomic and traditional importance of date palm . Emir. J. Food Agric. 24 : 371 – 385 . [Google Scholar]
- El Hadrami E., El Hadrami A. . 2009. . Breeding date palm , pp. 191 – 216 . InJain S. M., Priyadarshan P. M. (eds.), Breeding plantation tree crops: tropical species . Springer; , New York: . [Google Scholar]
- El-Juhany L. I. 2010. . Degradation of date palm trees and date production in Arab countries: causes and potential rehabilitation . Aust. J. Basic Appl. Sci. 4 : 3998 – 4010 . [Google Scholar]
- El-Shafie H.A.F. 2012. . Review: list of arthropod pests and their natural enemies identified worldwide on date palm, Phoenix dactylifera L . Agric. Biol. J. N. Am. 3 : 516 – 524 . [Google Scholar]
- Gries G., Gries R., Perez A. L., Oehlschager A. C., Gonzalez L. M., Pierce H. D., Jr., Zebeyou M., Kouame B. . 1994. . Aggregation pheromone of the African rhinoceros beetles, Oryctes monoceros (Olivier) (Coleoptera, Dynastidae) . Zeitschrift fur Naturforschung C: A J. Biosci. 49 : 363 – 366 . [Google Scholar]
- Hallet R. H., Perez A. L., Gries G., Gries R., Pierce H. D., Jr, Yue J., Oehlschlager A. C., Gonzalez L. M., Borden J. H. . 1995. . Aggregation pheromone of coconut rhinoceros beetle, Oryctes rhinoceros (L.) (Coleoptera: Scarabaeidae) . J. Chem. Ecol. 21 : 1549 – 1570 . [DOI] [PubMed] [Google Scholar]
- Haouel S., Mediouni-Ben Jemâa J., Khouja M. L. . 2010. . Postharvest control of the date moth Ectomyelois ceratoniae using eucalyptus essential oil fumigation . Tunis. J. Plant Protect. 5 ( 2 ): 201 – 212 . [Google Scholar]
- Howard F. W. 2001. . The animal class Insecta and the plant family Palmae , pp. 1 – 32 . InHoward F. W., Moore D., Giblin-Davis R. M., Abad R. G. (eds.), Insects on palms . CABI Publishing; , New York: . [Google Scholar]
- Karim K., Chokri B., Amel S., Wafa H., Rachid H., Nouredine D. . 2010. . Genetic diversity of Tunisian germplasm using ISSR markers . Int. J. Bot. 6 : 182 – 186 . [Google Scholar]
- Khoualdia O., Rhouma A., Hmidi M. S. . 1993. . Contribution to the bio-ecological study of the white scale Parlatoria blanchardi . Targ (Homoptera: Diaspididae) of date palm in Djerid (southern Tunisia) . Annales de l'Institut National de la Recherche Agronomique de Tunisie 66 : 89 – 108 . [Google Scholar]
- Khoualdia O., Rhouma A., Marro J. P., Brun J. . 1997. . Premières observations sur Oryctes agamemnon , ravageur du palmier dattier en Tunisie . Fruits 52 : 111 – 115. [Google Scholar]
- Prabhu S. T., Dongre T. K., Patil R. S. . 2010. . Effect of irradiation on the biological activities of red palm weevil, Rhynchophorus ferrugineus Olivier . Karnataka J. Agric. Sci. 23 : 186 – 188 . [Google Scholar]
- Rochat D., Morin J. P., Kakul T., Beaudoin-Ollivier L., Prior R., Renou M., Malosse I., Stathers T., Embupa S., Laup S. . 2002. . Activity of male pheromone of the Melanesian rhinoceros beetle Scapanes australis . J. Chem. Ecol. 28 : 479 – 500 . [DOI] [PubMed] [Google Scholar]
- Rochat D., Mohalladpoor K., Malosse C., Avand-Faghih A., Lettere M., Beauhaire J., Morin J. P., Pezier A., Renou M., Abdollahi G. A. . 2004. . Male aggregation pheromone of date palm fruit stalk borer Oryctes elegans . J. Chem. Ecol. 30 : 387 – 407 . [DOI] [PubMed] [Google Scholar]
- Soltani R., Chaieb I., Ben Hamouda M. H. . 2008a. . Descriptive study of damage caused by the rhinoceros beetle, Oryctes agamemnon , and its influence on date palm oases of Rjim Maatoug, Tunisia. 11pp . J Insect Sci. 8 : 57. ( www.insectscience.org/8.57 ) . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltani R., Chaieb I., Ben Hamouda M. H. . 2008b. . Life cycle of the root borer, Oryctes agamemnon , under laboratory conditions. 6pp . J. Insect Sci. 8 : 61. ( www.insectscience.org/8.61 ) . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaid A., De Wet P. F., Djerbi M., Oihabi A. . 2002. . Diseases and pests of date palm , pp. 227 – 242 . InZaid A. (ed.), Date palm cultivation. FAO Plant Production and Protection Paper no. 156 . Food and Agriculture Organisation of the United Nations; , Rome: . [Google Scholar]
- Zohary D., Hopf M. . 2000. . Domestication of plants in the old world: the origin and spread of cultivated plants in West Asia, Europe, and the Nile Valley . Oxford University Press; , Oxon, United Kingdom: . [Google Scholar]