Abstract
Background:
Numerous studies have demonstrated that patients with Parkinson's disease (PD) have a higher prevalence of substantia nigra (SN) hyperechogenicity compared with controls. Our aim was to explore the neuroimaging characteristics of transcranial sonography (TCS) of patients with PD and those with PD with dementia (PDD). The correlation between the echogenicity of the SN and clinical symptoms in Chinese patients with PDD was also assessed.
Methods:
The ratios of SN hyperechogenicity (SN+), maximum sizes of SN+, and widths of third ventricle (TV) were measured using TCS for all the recruited patients. Data were analyzed using one-way analysis of variance, rank-sum test, Chi-square test, and receiver-operating characteristic (ROC) curve analysis.
Results:
The final statistical analysis included 46 PDD patients, 52 PD patients, and 40 controls. There were no significant differences in ratios of SN+ and maximum sizes of SN+ between PDD and PD groups (P > 0.05). TV widths were significantly larger in PDD group (7.1 ± 1.9 mm) than in PD group (6.0 ± 2.0 mm) and controls (5.9 ± 1.5 mm, P < 0.05); however, the ratios of enlarged TV did not differ among the three groups (P = 0.059). When cutoff value was set at 6.8 mm, the TV width had a relatively high sensitivity and specificity in discriminating between PDD and PD groups (P = 0.030) and between PDD group and controls (P = 0.003), based on ROC curve analysis. In PDD patients, SN+ was more frequently detected in akinetic-rigid subgroup, and patients with SN+ showed significantly higher Hoehn and Yahr stage and Nonmotor Symptoms Questionnaire scores (P < 0.05).
Conclusions:
Compared to Chinese patients with PD, patients with PDD had a wider TV, altered SN sonographic features, and more severe clinical symptoms. Our findings suggest that TCS can be used to assess brain atrophy in PD and may be useful in discriminating between PD with and without dementia.
Keywords: Dementia, Parkinson's Disease, Substantia Nigra, Third Ventricle, Transcranial Sonography
INTRODUCTION
Parkinson's disease (PD) is a chronic progressive neurodegenerative disorder with a number of motor and nonmotor features that include cognitive impairment and dementia. PD patients have a 2- to 6-fold increased risk of developing dementia compared with the general population.[1] However, the actual prevalence of PD with dementia (PDD) ranges from 10–80% and is controversial due to heterogeneous samples and the use of alternate clinical and neuropsychological criteria.[2,3] Increasing age, being male, a longer disease duration, higher motor scores on the Unified Parkinson's Disease Rating Scale (UPDRS-III), and higher Hoehn and Yahr (H-Y) stage grading are associated with a higher risk of dementia in PD.[2,4] Cognitive dysfunction in PD patients can lead to a decline in the quality of life, as well as increased caregiver burden, economic burden, and mortality. As a novel neuroimaging technology, transcranial sonography (TCS) of PD patients reveals characteristic substantia nigra (SN) hyperechogenicity, which may be related to iron accumulation and functional impairment of nigrostriatal pathways.[5,6] TCS has primarily been used to analyze motor symptoms in PD patients.[7,8] Nevertheless, the limited cognitive assessment available from PD patients with TCS data demonstrated that the width of the third ventricle (TV) in PDD patients was larger than in PD patients.[9,10,11,12] However, these data have not been validated in the Chinese population. This study analyzed the characteristics of TCS in PDD and PD patients and assessed the correlation between TCS features and clinical symptoms in Chinese patients.
METHODS
Ethical approval
This study was approved by the Ethics Committee of The Second Affiliated Hospital of Soochow University and oral informed consent was obtained from all patients.
Participants
Sixty PDD patients, sixty PD patients, and forty neurologically normal controls at The Second Affiliated Hospital of Soochow University, between February 2015 and December 2016, were enrolled in this study. All PD patients fulfilled the criteria of the UK Parkinson's Disease Society Brain Bank.[13] The diagnosis of PDD was based on clinical diagnostic criteria for dementia associated with PD.[14] To assess motor symptoms and disease severity, the UPDRS-III score and H-Y stage were determined for all patients during the “off” stage. Cognitive function of all patients was evaluated by a full neuropsychological examination, which included a Mini-Mental State Examination (MMSE) and Montreal Cognitive Assessment (MoCA, Beijing version). Nonmotor symptoms were assessed by the Nonmotor Symptoms Questionnaire (NMSQ).
Exclusion criteria included: (1) atypical and secondary parkinsonism (i.e., multiple system atrophy, progressive supranuclear palsy, and brain injury), (2) dementia with Lewy bodies, Alzheimer's disease, major depression/anxiety, and other neuropsychiatric disorders, (3) reversible contributions to cognitive decline such as Vitamin B12 deficiency or thyroid disorders, (4) patients with insufficient temporal bone window.
Transcranial sonography
To ensure the reproducibility and reliability of measurements, TCS was performed by two sonologists with more than 5 years of experience. TCS data were acquired through the temporal acoustic window using an ultrasound system with a 2.5-MHz phased-array transducer (Sequoia 512, Siemens Medical Solutions Inc., Malvern, PA 19355, USA), a dynamic range of 45–55 dB, and penetration depth of 14–16 cm. The brightness and time gain compensation were adapted as needed.
The SN was scanned through the bilateral bone window in the axial plane. The SN hyperechoic sizes were encircled manually and measured automatically. Unilateral hyperechoic sizes of ≥0.20 cm2 were classified as SN hyperechogenicity (SN+) and the maximum SN+ size was defined as the greater value across bilateral SN regions.[10,15,16] SN echogenicity is shown in Figure 1. The width of the TV [Figure 2] was calculated by measuring the distance between the inner boundaries of both hyperechoic lines of ependyma at the thalamus level.[17] A TV width >7/10 mm in patients under/over 60 years old was regarded as an enlarged ventricle.[18] To exclude bias, TV values were independently calculated by a second sonologist who was blinded to the patient diagnosis. Patients with disagreement in the evaluation of TCS images by the two sonologists were excluded from the study.
Figure 1.
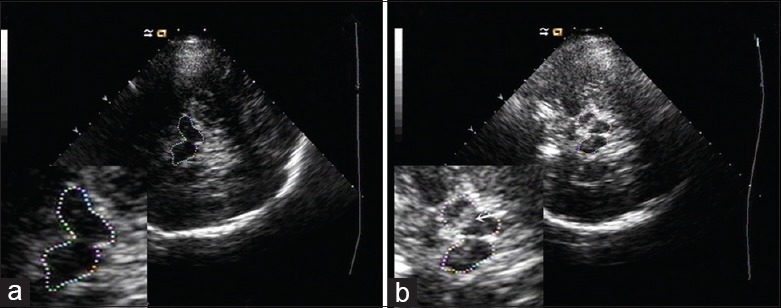
Transcranial sonography images of the substantia nigra at the midbrain level. Zoomed images of the butterfly-shaped midbrain are shown in the bottom left corner. (a) Normal substantia nigra echogenicity. (b) Substantia nigra hyperechogenicity (white arrow).
Figure 2.
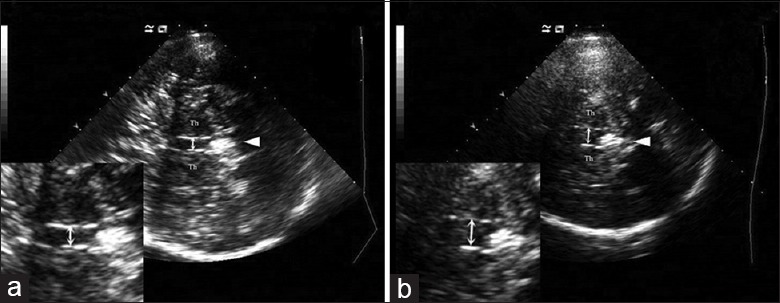
Transcranial sonography images of the third ventricle at the thalamus level. Th: Thalamus; arrowhead: Pineal gland; double arrow: Width of third ventricle. (a) Normal width of third ventricle. (b) Dilation of the third ventricle.
Statistical analysis
Data were analyzed using SPSS 19.0 (IBM Corp., Chicago, IL, USA). The data were presented as mean ± standard deviation (SD) when parameters were normally distributed and as median (Q1, Q3) when parameters were not normally distributed. Differences of age, MMSE score, MoCA score, SN+ maximum size, and TV width in PDD group, PD group, and controls were analyzed by one-way analysis of variance or Kruskal-Wallis H-test as appropriate, and comparisons of data between any two groups were made by the least significant difference test or Mann-Whitney U-test. Student's t-test and Mann–Whitney U-test were used to compare duration, UPDRS-III score, H-Y stage, NMSQ score between PDD and PD groups, and were also used to compare age, duration, UPDRS-III score, H-Y stage, and MMSE score between PDD with and without SN+. The categorical values (sex, dominant motor symptom, the ratio of SN+, and enlarged TV) were analyzed by Chi-square test or Fisher's exact test. The receiver-operating characteristic (ROC) curve was used to analyze the cutoff value of the width of TV between PDD and PD groups and between PDD group and controls. A P < 0.05 was used as the criterion for statistical significance.
RESULTS
Clinical characteristics
Fifteen participants (12.5%, 15/120, 9 PDD and 6 PD) were excluded from this study due to insufficient temporal bone window and seven additional participants (5.8%, 7/120, 5 PDD and 2 PD) were excluded due to disagreements in the evaluation of images by the two sonologists. A total of 46 PDD patients, 52 PD patients, and 40 controls were included in the final statistical analysis. Demographic and clinical characteristics of the study participants are summarized in Table 1. There were no significant differences among the three groups for age or sex. The MMSE and MoCA scores were lower in the PDD group than in the PD and control groups (P < 0.001), whereas the MMSE and MoCA scores did not differ significantly between PD group and controls (P = 0.087 and P = 0.389, respectively). Except for the MMSE and MoCA scores, the clinical characteristics of the patients with PDD and PD showed no significant differences.
Table 1.
Demographic and clinical characteristics of all participates in this study
| Items | PDD group (n = 46) | PD group (n = 52) | Controls (n = 40) | Statistics | P |
|---|---|---|---|---|---|
| Age (years) | 68.9 ± 9.9 | 66.9 ± 8.3 | 64.8 ± 9.9 | 2.081* | 0.129 |
| Male | 30 (65.2) | 38 (73.1) | 27 (67.5) | 0.750† | 0.687 |
| Duration (years) | 6.7 ± 2.5 | 6.0 ± 3.5 | – | 1.052‡ | 0.295 |
| Dominant motor symptom | –† | 0.086 | |||
| Tremor dominant | 12 (26.1) | 24 (46.2) | – | ||
| Akinetic rigid | 28 (60.9) | 25 (48.1) | – | ||
| Mixed type | 6 (13.0) | 3 (5.7) | – | ||
| UPDRS-III score | 23.8 ± 8.9 | 21.6 ± 7.2 | – | 1.383‡ | 0.170 |
| H-Y stage | 2.5 (2.0, 2.5) | 2.0 (1.5, 2.5) | – | −1.899§ | 0.058 |
| NMSQ | 8.6 ± 4.1 | 7.3 ± 3.9 | – | 1.660‡ | 0.100 |
| MMSE | 22.9 ± 2.3 | 28.2 ± 1.3 | 27.7 ± 1.4 | 93.325|| | <0.001 |
| MoCA | 18.0 ± 4.0 | 25.2 ± 3.4 | 26.8 ± 1.0 | 78.866|| | <0.001 |
The data are given as mean ± SD, median (Q1, Q3), or n (%). *One-way analysis of variance; †Chi-square test or Fisher's exact test; ‡t-test; §Mann-Whitney U-test; ||Kruskal-Wallis H-test. PDD: Parkinson's disease dementia; PD: Parkinson's disease; UPDRS-III: Unified Parkinson's Disease Rating Scale-III, motor part; H-Y stage: Hoehn and Yahr stage; NMSQ: Nonmotor Symptoms Questionnaire; MMSE: Mini-Mental State Examination; MoCA: Montreal Cognitive Assessment; SD: Standard deviation; –: Not available.
The echogenicity of substantia nigra and width of third ventricle
The SN+ was detected in 82.6% (38/46) of PDD patients, 71.2% (37/52) of PD patients, and 12.5% (5/40) of controls (χ2 = 49.114, P < 0.001). SN+ was more frequently detected in PDD patients (82.6% vs. 12.5%, respectively, χ2 = 42.065, P < 0.001) and PD patients (71.2% vs. 12.5%, respectively, χ2 = 31.349, P < 0.001), compared with controls, and no significant difference in SN+ was detected between PDD and PD patients (82.6% vs. 71.3%, respectively, χ2 = 1.783, P = 0.182). Among these patients with SN+, the maximum SN hyperechoic sizes were 0.4 ± 0.1 cm2 in PDD group, 0.4 ± 0.1 cm2 in PD group, and 0.3 ± 0.1 cm2 in controls (F = 1.997, P = 0.143).
The TV widths in PDD, PD, and control groups were 7.1 ± 1.9 mm, 6.0 ± 2.0 mm, and 5.9 ± 1.5 mm, respectively (F = 5.732, P = 0.004). The TV was significantly wider in PDD group than in PD group (t = 2.945, P = 0.004) and controls (t = 2.913, P = 0.004), but there was no significant difference between PD group and controls (t = 0.159, P = 0.874). The proportion of enlarged TV was 21.7% (10/46) in PDD group, 7.7% (4/52) in PD group, and 7.5% (3/40) in controls, which were not significantly different (χ2 = 5.670, P = 0.059) among three groups. Based on the ROC curve, a TV width cutoff of 6.8 mm was relatively highly sensitive (69.6%, 69.6%) and specificity (61.5%, 67.5%) for discriminating between PDD and PD groups and between PDD group and controls (P = 0.030 and P = 0.003, respectively).
Correlation between substantia nigra and clinical symptoms in Parkinson's disease dementia patients
Forty-six cases of PDD patients were then divided into normal SN echogenicity (SN−) and SN+ subgroups [Table 2]. PDD patients with SN+ showed significantly higher H-Y stage and NMSQ scores compared with PDD patients with SN− (P < 0.05). No significant differences in SN+ subgroup were observed in tremor-dominant type (50.0% vs. 100.0%, respectively, P = 0.054) and akinetic-rigid type (92.9% vs. 100.0%, respectively, P = 1.000) compared with mixed type. SN+ was more frequently observed in akinetic-rigid type compared with tremor-dominant type (92.9% vs. 50.0%, respectively, P = 0.005), and in SN+, UPDRS-III scores in akinetic-rigid type were higher than those of tremor-dominant type (26.9 ± 9.5 vs. 17.8 ± 4.2, respectively, t = 2.265, P = 0.031).
Table 2.
Clinical data of PDD patients subdivided by echogenicity of SN
| Items | SN+ subgroup (n = 38) | SN− subgroup (n = 8) | Statistics | P |
|---|---|---|---|---|
| Age (years) | 68.9 ± 10.5 | 68.5 ± 6.8 | 0.115* | 0.909 |
| Male | 26 (68.4) | 4 (50.0) | –† | 0.421 |
| Duration (years) | 6.9 ± 2.5 | 5.8 ± 2.7 | 1.181* | 0.244 |
| Dominant motor symptom | –† | 0.006 | ||
| Tremor dominant | 6 (15.8) | 6 (75.0) | ||
| Akinetic rigid | 26 (68.4) | 2 (25.0) | ||
| Mixed type | 6 (68.4) | 0 (0.0) | ||
| UPDRS-III score | 24.6 ± 9.4 | 20.4 ± 4.1 | −1.366‡ | 0.181 |
| H-Y stage | 2.5 (2.0, 2.5) | 1.5 (1.1, 2.3) | −2.918‡ | 0.004 |
| NMSQ score | 9.2 ± 3.8 | 5.6 ± 4.1 | 2.375* | 0.022 |
| MMSE score | 22.7 ± 2.5 | 23.8 ± 0.9 | −0.772‡ | 0.467 |
| MoCA score | 18.2 ± 3.9 | 17.3 ± 4.6 | 0.576* | 0.568 |
| Width of TV (mm) | 7.2 ± 2.0 | 6.6 ± 1.6 | 0.759* | 0.452 |
The data are given as mean ± SD, median (Q1, Q3), or n (%). *t-test; †Fisher's exact test; ‡Mann-Whitney U-test. SN: Substantia nigra; SN+: Substantia nigra hyperechogenicity; SN−: Normal substantia nigra echogenicity; TV: Third ventricle; UPDRS-III: Unified Parkinson's Disease Rating Scale-III, motor part; H-Y stage: Hoehn and Yahr stage; NMSQ: Nonmotor Symptoms Questionnaire; MMSE: Mini-Mental State Examination; MoCA: Montreal Cognitive Assessment; PDD: Parkinson's disease dementia; SD: Standard deviation; –: Not available.
DISCUSSION
This study demonstrates a higher prevalence of SN+ in PD patients compared with controls, which is consistent with previous studies using TCS.[6,8] The exact reason for SN+ in PD-related disorders is still unknown. However, SN+ may reflect the alteration of dopaminergic cells and could be related to increased iron content in the SN.[11,19] Previous research has suggested that SN+ remained stable throughout the course of the disease and was not correlated with age, motor severity, or disease stages.[20,21] In contrast, other studies found positive associations between age at onset, disease severity, different motor symptoms, and SN+.[11,21] Currently, there is no consensus in the field regarding SN+ progression in PD.
Previous reports found that elderly individuals with SN+ performed worse in neuropsychological tests than individuals with normal SN echogenicity.[22,23] In contrast, we found no significant differences in the ratios or maximum sizes of SN+ in PD patients with or without dementia, suggesting that the SN plays a less important role in cognitive function for PD patients. In support of this conclusion, cognitive function in PD has been shown to be associated with other neural networks, including the fronto-striatal and mesocortical dopamine networks.[9,24]
Our study demonstrated that the TV was significantly wider in PDD group than in PD group, which was consistent with previous studies.[9,10,11,12] At the pathological level, dementia in PD is thought to be a consequence of Lewy body accumulation in the neocortical and limbic system. In addition, pathological changes normally associated with Alzheimer's disease, such as abnormal deposition of β-amyloid and neurofibrillary tangles, may also contribute to dementia in some PD patients.[25,26] Accumulation of these abnormal proteins in PD patients could result in more widespread cortical and subcortical atrophy, leading to mild cognitive impairment.[24,27,28] Some hypothesized that abnormal enlargement of TV in PDD patients might be related to AD pathology.[7] Through the ROC curve analysis with a TV width cutoff of 6.8 mm, the TV size could be used to discriminate between PDD and PD groups or PDD group and controls. This finding suggests that TCS could be used as a simple, low-cost, and reproducible tool to detect changes in TV width. Moreover, TV widths as measured by TCS were consistent with computed tomography and magnetic resonance findings.[10,12] Our results suggest that measurements of TV width by TCS could be used to identify atrophy in surrounding structures and may predict cognitive decline in PD patients.
In PDD patients, our data showed that the percentage of SN+ and the UPDRS-III scores in tremor-dominant type patients was lower than that in akinetic-rigid-type patients. Therefore, the increase in SN+ for different motor subtype patients could contribute to higher UPDRS-III scores. In addition, we found that H-Y stages and NMSQ scores in PDD patients with SN+ were higher than in patients with SN−. However, as advanced H-Y stages are accompanied by an increase in nonmotor symptoms,[29] we cannot determine whether the difference in NMSQ scores between PDD with and without SN+ was induced by differences in the H-Y stages or was a consequence of increased nonmotor symptoms in PDD patients with SN+. Additional studies will be needed to understand the relationships between H-Y stages, NMSQ scores, and SN+ in PDD patients.
Prior literature on TCS measurements of SN echogenicity in PDD patients is limited, especially for the Chinese population. This was the first study to analyze the clinical data of PDD patients with hyperechoic or normal SN in China.[7,10,11] Compared with previous reports, the disease duration reported here was relatively short, UPDRS-III scores were relatively low, and the majority of patients were in earlier stages of the disease. However, our results were similar to previous studies, suggesting that TCS measurements of TV widths and SN echogenicity could be an effective tool for distinguishing between PD and PDD patients, even at early stages of disease progression.
The main limitation of this study was the small sample size in PDD patients with SN- In addition, about 12.5% (15/120) of patients could not receive TCS examination due to insufficient temporal bone window. Despite our small sample size, when the cutoff value for TV widths was set at 6.8 mm, the ROC curve could be used to discriminate between PDD and PD patients. However, the difference between groups was only around 1 mm, so further research with a larger sample size will be necessary to validate these results.
In conclusion, SN+ was more frequently detected in akinetic-rigid-type PDD patients, and PDD patients with SN+ showed higher H-Y stage and NMSQ scores. This study determined that PDD and PD could not be distinguished by TCS measurements of SN+ or maximum sizes of SN, but TCS measurement of the TV width may be a useful prognostic tool.
Financial support and sponsorship
This work was supported by grants from Jiangsu Provincial Special Program of Medical Science (BL2014042), Jiangsu Provincial Medical Key Discipline Project, and SuZhou Clinical Research Center of Neurological Disease (Szzx201503). This was also partly supported by the priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Li-Min Chen
REFERENCES
- 1.Buter TC, van den Hout A, Matthews FE, Larsen JP, Brayne C, Aarsland D. Dementia and survival in Parkinson disease: A 12-year population study. Neurology. 2008;70:1017–22. doi: 10.1212/01.wnl.0000306632.43729.24. doi: 10.1212/01.wnl.0000306632.43729.24. [DOI] [PubMed] [Google Scholar]
- 2.Hely MA, Reid WG, Adena MA, Halliday GM, Morris JG. The Sydney multicenter study of Parkinson's disease: The inevitability of dementia at 20 years. Mov Disord. 2008;23:837–44. doi: 10.1002/mds.21956. doi: 10.1002/mds.21956. [DOI] [PubMed] [Google Scholar]
- 3.Sandoval-Rincon M, Sáenz-Farret M, Miguel-Puga A, Micheli F, Arias-Carrión O. Rational pharmacological approaches for cognitive dysfunction and depression in Parkinson's disease. Front Neurol. 2015;6:71–80. doi: 10.3389/fneur.2015.00071. doi: 10.3389/fneur.2015.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poewe W, Gauthier S, Aarsland D, Leverenz JB, Barone P, Weintraub D, et al. Diagnosis and management of Parkinson's disease dementia. Int J Clin Pract. 2008;62:1581–7. doi: 10.1111/j.1742-1241.2008.01869.x. doi: 10.1111/j.1742-1241.2008.01869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Toomsoo T, Liepelt-Scarfone I, Kerner R, Kadastik-Eerme L, Asser T, Rubanovits I, et al. Substantia nigra hyperechogenicity: Validation of transcranial sonography for Parkinson disease diagnosis in a large Estonian cohort. J Ultrasound Med. 2016;35:17–23. doi: 10.7863/ultra.14.12069. doi: 10.7863/ultra.14.12069. [DOI] [PubMed] [Google Scholar]
- 6.Luo WF, Zhang YC, Sheng YJ, Fang JC, Liu CF. Transcranial sonography on Parkinson's disease and essential tremor in a Chinese population. Neurol Sci. 2012;33:1005–9. doi: 10.1007/s10072-011-0876-x. doi: 10.1007/s10072-011-0876-x. [DOI] [PubMed] [Google Scholar]
- 7.Zhou HY, Sun Q, Tan YY, Hu YY, Zhan WW, Li DH, et al. Substantia nigra echogenicity correlated with clinical features of Parkinson's disease. Parkinsonism Relat Disord. 2016;24:28–33. doi: 10.1016/j.parkreldis.2016.01.021. doi: 10.1016/j.parkreldis.2016.01.021. [DOI] [PubMed] [Google Scholar]
- 8.Lauckaite K, Rastenyte D, Šurkiene D, Vaitkus A, Sakalauskas A, Lukoševicius A, et al. Specificity of transcranial sonography in Parkinson spectrum disorders in comparison to degenerative cognitive syndromes. BMC Neurol. 2012;12:12. doi: 10.1186/1471-2377-12-12. doi: 10.1186/1471-2377-12-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bouwmans AE, Leentjens AF, Mess WH, Weber WE. Abnormal echogenicity of the substantia nigra, raphe nuclei, and third-ventricle width as markers of cognitive impairment in Parkinsonian disorders: A cross-sectional study. Parkinsons Dis. 2016;2016:4058580. doi: 10.1155/2016/4058580. doi: 10.1155/2016/4058580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walter U, Dressler D, Wolters A, Wittstock M, Greim B, Benecke R. Sonographic discrimination of dementia with Lewy bodies and Parkinson's disease with dementia. J Neurol. 2006;253:448–54. doi: 10.1007/s00415-005-0023-9. doi: 10.1007/s00415-005-0023-9. [DOI] [PubMed] [Google Scholar]
- 11.Walter U, Dressler D, Wolters A, Wittstock M, Benecke R. Transcranial brain sonography findings in clinical subgroups of idiopathic Parkinson's disease. Mov Disord. 2007;22:48–54. doi: 10.1002/mds.21197. doi: 10.1002/mds.21197. [DOI] [PubMed] [Google Scholar]
- 12.Walter U, Skoloudík D, Berg D. Transcranial sonography findings related to non-motor features of Parkinson's disease. J Neurol Sci. 2010;289:123–7. doi: 10.1016/j.jns.2009.08.027. doi: 10.1016/j.jns.2009.08.027. [DOI] [PubMed] [Google Scholar]
- 13.Gelb DJ, Oliver E, Gilman S. Diagnostic criteria for Parkinson disease. Arch Neurol. 1999;56:33–9. doi: 10.1001/archneur.56.1.33. doi: 10.1001/archneur.56.1.33. [DOI] [PubMed] [Google Scholar]
- 14.Emre M, Aarsland D, Brown R, Burn DJ, Duyckaerts C, Mizuno Y, et al. Clinical diagnostic criteria for dementia associated with Parkinson's disease. Mov Disord. 2007;22:1689–707. doi: 10.1002/mds.21507. doi: 10.1002/mds.21507. [DOI] [PubMed] [Google Scholar]
- 15.Walter U. How to measure substantia nigra hyperechogenicity in Parkinson disease: Detailed guide with video. J Ultrasound Med. 2013;32:1837–43. doi: 10.7863/ultra.32.10.1837. doi: 10.7863/ultra.32.10.1837. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y, Zhang YC, Sheng YJ, Chen XF, Wang CS, Ma Q, et al. Sonographic alteration of basal ganglia in different forms of primary focal dystonia: A cross-sectional study. Chin Med J. 2016;129:942–5. doi: 10.4103/0366-6999.179792. doi: 10.4103/0366-6999.179792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walter U, Školoudík D. Transcranial sonography (TCS) of brain parenchyma in movement disorders: Quality standards, diagnostic applications and novel technologies. Ultraschall Med. 2014;35:322–31. doi: 10.1055/s-0033-1356415. doi: 10.1055/s-0033-1356415. [DOI] [PubMed] [Google Scholar]
- 18.Walter U, Behnke S, Eyding J, Niehaus L, Postert T, Seidel G, et al. Transcranial brain parenchyma sonography in movement disorders: State of the art. Ultrasound Med Biol. 2007;33:15–25. doi: 10.1016/j.ultrasmedbio.2006.07.021. doi: 10.1016/j.ultrasmedbio.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 19.Berg D, Roggendorf W, Schröder U, Klein R, Tatschner T, Benz P, et al. Echogenicity of the substantia nigra: Association with increased iron content and marker for susceptibility to nigrostriatal injury. Arch Neurol. 2002;59:999–1005. doi: 10.1001/archneur.59.6.999. doi: 10.1001/archneur.59.6.999. [DOI] [PubMed] [Google Scholar]
- 20.Alonso-Cánovas A, López-Sendón JL, Buisán J, deFelipe-Mimbrera A, Guillán M, García-Barragán N, et al. Sonography for diagnosis of Parkinson disease-from theory to practice: A study on 300 participants. J Ultrasound Med. 2014;33:2069–74. doi: 10.7863/ultra.33.12.2069. doi: 10.7863/ultra.33.12.2069. [DOI] [PubMed] [Google Scholar]
- 21.Pilotto A, Yilmaz R, Berg D. Developments in the role of transcranial sonography for the differential diagnosis of Parkinsonism. Curr Neurol Neurosci Rep. 2015;15:43. doi: 10.1007/s11910-015-0566-9. doi: 10.1007/s11910-015-0566-9. [DOI] [PubMed] [Google Scholar]
- 22.Yilmaz R, Behnke S, Liepelt-Scarfone I, Roeben B, Pausch C, Runkel A, et al. Substantia nigra hyperechogenicity is related to decline in verbal memory in healthy elderly adults. Eur J Neurol. 2016;23:973–8. doi: 10.1111/ene.12974. doi: 10.1111/ene.12974. [DOI] [PubMed] [Google Scholar]
- 23.Liepelt I, Wendt A, Schweitzer KJ, Wolf B, Godau J, Gaenslen A, et al. Substantia nigra hyperechogenicity assessed by transcranial sonography is related to neuropsychological impairment in the elderly population. J Neural Transm (Vienna) 2008;115:993–9. doi: 10.1007/s00702-008-0043-6. doi: 10.1007/s00702-008-0043-6. [DOI] [PubMed] [Google Scholar]
- 24.Gratwicke J, Jahanshahi M, Foltynie T. Parkinson's disease dementia: A neural networks perspective. Brain. 2015;138(Pt 6):1454–76. doi: 10.1093/brain/awv104. doi: 10.1093/brain/awv104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lucero C, Campbell MC, Flores H, Maiti B, Perlmutter JS, Foster ER. Cognitive reserve and ß-amyloid pathology in Parkinson disease. Parkinsonism Relat Disord. 2015;21:899–904. doi: 10.1016/j.parkreldis.2015.05.020. doi: 10.1016/j.parkreldis.2015.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pagonabarraga J, Kulisevsky J. Cognitive impairment and dementia in Parkinson's disease. Neurobiol Dis. 2012;46:590–6. doi: 10.1016/j.nbd.2012.03.029. doi: 10.1016/j.nbd.2012.03.029. [DOI] [PubMed] [Google Scholar]
- 27.Armstrong RA. Laminar degeneration of frontal and temporal cortex in Parkinson disease dementia. Neurol Sci. 2017;38:667–71. doi: 10.1007/s10072-017-2828-6. doi: 10.1007/s10072-017-2828-6. [DOI] [PubMed] [Google Scholar]
- 28.Donaghy P, Thomas AJ, O’Brien JT. Amyloid PET imaging in Lewy body disorders. Am J Geriatr Psychiatry. 2015;23:23–37. doi: 10.1016/j.jagp.2013.03.001. doi: 10.1016/j.jagp.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 29.Chaudhuri KR, Martinez-Martin P, Brown RG, Sethi K, Stocchi F, Odin P, et al. The metric properties of a novel non-motor symptoms scale for Parkinson's disease: Results from an international pilot study. Mov Disord. 2007;22:1901–11. doi: 10.1002/mds.21596. doi: 10.1002/mds.21596. [DOI] [PubMed] [Google Scholar]


