Abstract
Background:
As a traditional Chinese medicine, Cordyceps sinensis (CS) possesses a variety of immunoregulatory properties. This study aimed to explore the therapeutic potential of CS in a mice model of multiple sclerosis (MS)-experimental autoimmune encephalomyelitis (EAE).
Methods:
Female C57BL/6 mice were immunized with myelin oligodendrocyte glycoprotein35–55 to induce EAE, followed by an instant intragastric feeding with a low dosage of CS (low-CS group, n = 5), high dosage of CS (high-CS group, n = 5), or the same volume of normal saline (control group, n = 5). All the mice were observed for clinical assessment. Over the 30 days of CS treatment, flow cytometry was used to detect the frequency of helper T-cell (Th) subsets, Th1 and Th17, and CD4+ CD25+ regulatory T cells in the spleen and lymph nodes. Meanwhile, pathological changes in brain were determined using both hematoxylin-eosin and luxol fast blue staining. Data were analyzed using the one-way analysis of variance (ANOVA).
Results:
Over the 15 and 30 days of CS treatment, the clinical assessment for EAE demonstrated that both high-CS group (2.51 ± 0.31 and 2.26 ± 0.39 scores, respectively) and low-CS group (2.99 ± 0.40 and 2.69 ± 0.46, respectively) had lower disease severity scores than those of control group (3.57 ± 0.53 and 3.29 ± 0.53, all P < 0.01, respectively). Meanwhile, after 15 and 30 days, the high-CS group (19.18 ± 1.34 g and 20.41 ± 1.56 g, respectively) and low-CS group (18.07 ± 1.18 g and 19.48 ± 1.69 g, respectively) had a lower body weight, as compared with control group (16.85 ± 1.15 g and 18.22 ± 1.63 g, all P < 0.01, respectively). At 30 days post-CS treatment, there was a lower Th1 frequency in the lymph nodes (2.85 ± 1.54% and 2.77 ± 1.07% vs. 5.35 ± 1.34%, respectively; P < 0.05) and spleens (3.96 ± 1.09% and 3.09 ± 0.84% vs. 5.07 ± 1.50%, respectively; P < 0.05) and less inflammatory infiltration and demyelination in the brain of CS-treated mice than that of control group.
Conclusions:
Our preliminary study demonstrated that CS efficiently alleviated EAE severity and EAE-related pathology damage and decreased the number of Th1s in the periphery, indicating its effectiveness in the treatment of murine EAE. Thus, our findings strongly support the therapeutic potential of this agent as a new traditional Chinese medicine approach in MS treatment.
Keywords: Autoimmune, Cordyceps, Encephalomyelitis, Experimental, Multiple Sclerosis
INTRODUCTION
As a well-known traditional Chinese medicine (TCM), Cordyceps sinensis (CS), also known as Ophiocordyceps sinensis fungi, exhibits multiple functions including immunomodulation, antitumor, hematopoietic facilitation, anti-inflammatory, bacteriostasis, and antiarrhythmic properties, with less adverse effects. So far, it has been clinically administrated for the treatment of various human diseases such as chronic bronchitis and chronic renal insufficiency.[1]
Multiple sclerosis (MS) is a demyelinating autoimmune disease of the central nervous system (CNS) and occurs predominantly in younger and middle-aged patients. The disease often has a relapsing-remitting course and causes severe neurological disability during the late stage. Currently, both immunosuppressive and immunomodulatory agents are the recommended options for the treatment of MS.[2] Immunosuppressants such as glucocorticoid and mitoxantrone produce significant side effects after long-term use, and the costly expense of immunomodulators such as interferon (IFN)-β and fingolimod (FTY720) becomes a financial burden for most Chinese patients. In contrast, increasing evidence has favored the use of TCM such as You-Gui pills and acupuncture in the treatment of MS.[3,4] Of note, CS is now attracting more attention as fingolimod, an extract of CS bioactive components, has been approved for the treatment of MS in most Western countries. However, the therapeutic efficacy of this TCM agent remains unclear on the treatment of MS. Therefore, in this work, a mice model of experimental autoimmune encephalomyelitis (EAE) was employed to observe the initial effect of this agent on neurologic deficits, CD4+ T-cell subsets in the spleen and lymph nodes, and EAE-related brain pathology to provide a new TCM approach in MS.
METHODS
Ethical approval
The study was approved by the Ethics Committee of Peking University People's Hospital. The treatments for animal were in accordance with the National Institute of Health's Guide for the Care and Use of Laboratory Animals. We have taken all steps to minimize the animal's pain and suffering.
Animal preparation
Female C57BL/6 wild-type (WT) mice were purchased from Beijing Vital River Company (Beijing, China). All experimental mice were bred and maintained under specific pathogen-free conditions in the animal facility of Peking University People's Hospital (Beijing, China).
Establishment of autoimmune encephalomyelitis model
According to the method of Lalive et al.,[5] 21 C57BL/6 WT mice (7–8 weeks old) were immunized subcutaneously with 300 μg of myelin oligodendrocyte glycoprotein (MOG) peptide35–55(Beijing SBS Genetech Co., Ltd., China) in complete Freund's adjuvant (Sigma-Aldrich, St. Louis, MO, USA). Each mouse was intraperitoneally administered 400 ng of pertussis toxin (Calbiochem, Merck, Darmstadt, Germany) twice on the day of immunization and 48 h later. As a result, among all experimental mice (n = 21), 17 mice presented with disease, while for the remaining four mice, two died and another two were not diseased. Clinical assessment for EAE was performed daily after disease induction, and disease severity was scored using the following criteria:[6] (1) 0 = no clinical symptoms; (2) 1 = tail paralysis; (3) 2 = hindlimb weakness or partial paralysis; (4) 3 = complete paralysis of two hindlimbs; (5) 4 = paralysis of both forelimbs and hindlimbs; and (6) 5 = moribund or dead.
Cordyceps sinensis treatment
After EAE model was established, CS-treated mice were intragastrically administered 0.4 ml of solution per mouse on the day of paralytic symptom appearance. According to the completely randomized block design, 15 mice were randomly chosen and divided into three groups (n = 5 for each group) using the stochastic indicator method: low dosage of CS group (low-CS group; CS: 1 g/kg, 0.05 g/ml), high dosage of CS group (high-CS group; CS: 5 g/kg, 0.25 g/ml), and the control group which was administered with 0.4 ml normal saline. In all experimental mice, the intragastric administration lasted for 30 days consecutively. At 15 days and 30 days post-CS treatment, all the mice were observed for clinical assessment of EAE. All mice were sacrificed 30 days after the administration for additional flow cytometry and histological analyses.
Flow cytometry
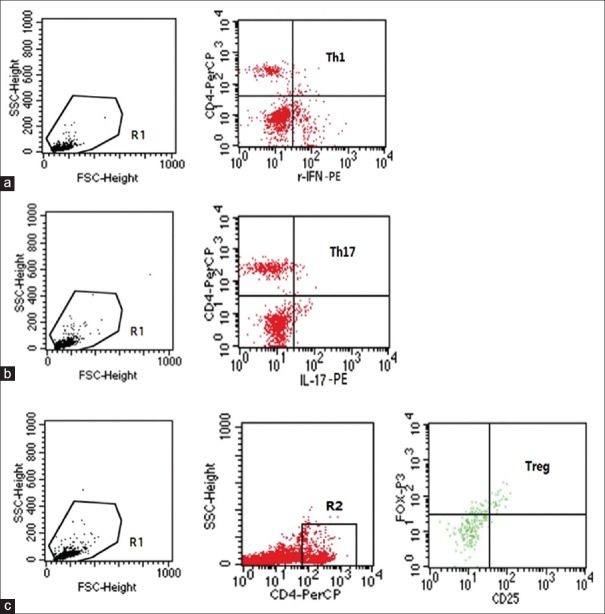
The spleen and lymph nodes were mechanically separated, followed by a step-wise filtration of tissues through 70 μm Cell Strainer. After collection of the floating cells, mononuclear cells were obtained by Percoll gradient centrifugation and further characterized by the frequency of T-cell subsets by three-color direct immunofluorescence and flow cytometry using a FACScan (Becton Dickinson, CA, USA). Cells were washed with phosphate-buffered saline (PBS; pH 7.2) and resuspended in PBS buffer. For intracellular staining, 1 × 106 cells in a 25-μl cell suspension were prepared and surface stained with peridinin chlorophyll protein-labeled anti-CD4 antibody or fluorescein isothiocyanate-labeled anti-CD25 antibody (eBioscience, San Diego, CA, USA). After 30-min incubation at 4°C, cells were washed twice with staining buffer and then fixed and permeabilized using a fixation/permeabilization kit (BD Biosciences, San Jose, CA, USA). Subsequently, the cells were washed and stained with phycoerythrin (PE)-labeled anti-interleukin (IL)-17 antibody, PE-labeled anti-IFN-γ antibody, or PE-labeled anti-forkhead box P3 (FoxP3) antibody (eBioscience, San Diego, CA, USA). After 30-min incubation at 4°C, the cells were washed twice with staining buffer and analyzed using FACScan (BD Biosciences, San Jose, CA, USA) with BD CellQuest™ Pro software (© 2002, BD Biosciences, San Jose, CA, USA) [Figure 1].
Figure 1.
Flow cytometry. Region 1 (R1) was selected to set the mononuclear cell gate according to the forward light scatter and side light scatter properties for analysis of the Th1 (a) and Th17 (b) frequency. Region 2 (R2) was used to set the second gate to separate CD4+ T-cells to analyze the frequency of CD4+ CD25+ FoxP3+ Treg (c). Staining with isotype control antibodies was used as a control to define the gate.
Histological analysis
For histological analyses, the mice were anesthetized with pentobarbital and then perfused through the left ventricle, first with normal saline to eliminate the blood and then with buffered 4% paraformaldehyde. The mice brain tissue was embedded in paraffin, and 5-μm-thick sections that had been deparaffinized and rehydrated were stained with hematoxylin and eosin (H & E) or luxol fast blue (LFB). Inflammation and demyelination were analyzed to clarify the influence of CS treatment on the pathogenesis of EAE.
Statistical analysis
The statistical analysis was achieved by the SPSS 18.0 statistical software (SPSS Inc., Chicago, IL, USA). Data were presented as mean ± standard deviation (clinical scores, body weight, and the frequency of T-cell subsets). Data with a normal distribution were analyzed using the one-way analysis of variance (ANOVA), followed by the Student-Newman-Keul's post hoc test for all pairwise comparisons. A value of P <0.05 was considered statistically significant.
RESULTS
Cordyceps sinensis treatment for experimental autoimmune encephalomyelitis symptoms
C57/BL mice presented with diseases about 14 days after the MOG35–55 peptide injection, and their symptoms peaked 20 days after the injection. The clinical assessment for EAE severity on 15 days and 30 days after CS treatment demonstrated that both high-CS group (2.51 ± 0.30 and 2.26 ± 0.39, respectively) and low-CS group (2.99 ± 0.40 and 2.69 ± 0.46 scores, respectively) had lower disease severity scores than control group (3.57 ± 0.53 and 3.29 ± 0.53; F = 23.68, P = 0.0001 and F = 37.68, P = 0.0001, respectively), in which mice began to experience EAE on days 13–14, and the maximum score was around 4.00 on day 20 [Figure 2]. Meanwhile, the high-CS group (19.18 ± 1.34 g and 20.41 ± 1.56 g, respectively) and low-CS group (18.07 ± 1.18 g and 19.48 ± 1.69 g, respectively) had a lower bodyweight, as compared with the control group (16.85 ± 1.15 g and 18.22 ± 1.63 g; F = 13.53, P = 0.0001 and F = 13.62, P = 0.0001, respectively; Figure 3).
Figure 2.
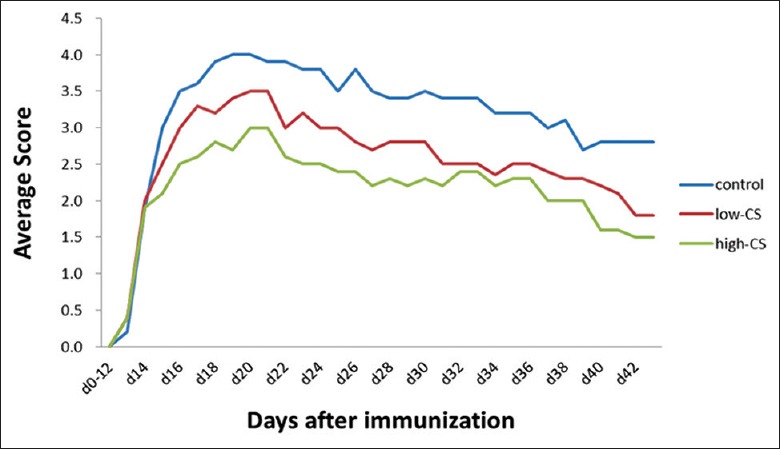
Changes in mean disease severity scores in the control (EAE; normal saline), low-CS (1 g·kg-1·d-1), and high-CS groups (5 g·kg-1·d-1). EAE: Experimental autoimmune encephalomyelitis; CS: Cordyceps sinensis.
Figure 3.
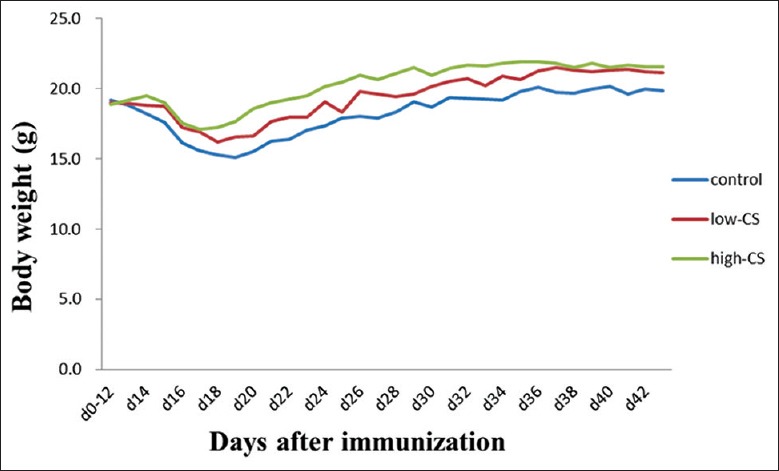
Changes in body weight in the control (EAE; normal saline), low-CS (1 g·kg-1·d-1), and high-CS groups (5 g·kg-1·d-1). EAE: Experimental autoimmune encephalomyelitis, CS: Cordyceps sinensis.
Frequency of T-cell subsets
Thirty days after CS treatment, both low-CS and high-CS groups experienced a decrease in Th1 frequency in the lymph nodes (2.85 ± 1.54% and 2.77 ± 1.07% vs. 5.35 ± 1.34%; P < 0.05, respectively) and spleens (3.96 ± 1.09% and 3.09 ± 0.84% vs. 5.07 ± 1.50%; P < 0.05, respectively) as compared to control group. In addition, the high-CS group had a significantly lower Th17 frequency in the lymph nodes than control group (1.79 ± 0.48% vs. 2.41 ± 0.50%, P < 0.05, respectively), in contrast to a slightly higher Th17 frequency in low-CS group (3.56 ± 1.48%, F = 5.20, P = 0.02). No significant differences in CD4+ CD25+ regulatory T cells (Treg) frequency in either the lymph nodes or spleen were found between CS-treated mice and control group [Table 1].
Table 1.
Comparison of frequency of Th subsets in the spleen and lymph nodes in mice model of experimental autoimmune encephalomyelitis with different treatments (n = 5)
| Items | High-CS group | Low-CS group | Control group | F | P* |
|---|---|---|---|---|---|
| Lymph nodes (%) | |||||
| Th1 | 2.77 ± 1.07† | 2.85 ± 1.54† | 5.35 ± 1.34 | 6.48 | 0.0100 |
| Th17 | 1.79 ± 0.48† | 3.56 ± 1.48 | 2.41 ± 0.50 | 5.20 | 0.0200 |
| Treg | 6.41 ± 4.40 | 4.64 ± 3.84 | 2.20 ± 1.12 | 1.95 | 0.1800 |
| Spleen (%) | |||||
| Th1 | 3.09 ± 0.84† | 3.96 ± 1.09† | 5.07 ± 1.50 | 3.96 | 0.0400 |
| Th17 | 3.21 ± 1.42 | 2.23 ± 1.02 | 3.98 ± 1.15 | 2.55 | 0.1100 |
| Treg | 12.82 ± 14.68 | 11.40 ± 8.89 | 4.05 ± 2.59 | 1.06 | 0.3700 |
Data were expressed by mean ± SD. *Comparison of frequency of Th subsets among the three groups (high-CS, low-CS, and control groups) was performed using the one-way ANOVA, and P<0.05 was considered statistically significant. †P<0.05 for post hoc comparison with control group. CS: Cordyceps sinensis dose; Treg: Regulatory T-cells; Th: Helper T-cells; SD: Standard deviation; ANOVA: Analysis of variance.
Histopathology
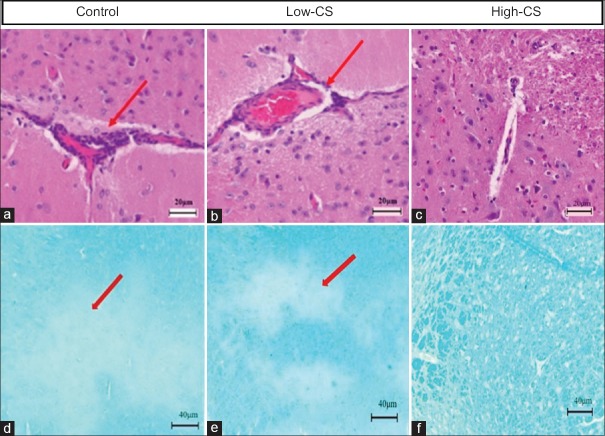
At 30 days post-CS treatment, in both the high-CS group and low-CS group, H & E staining displayed less inflammatory cell infiltration in perivascular region and brain parenchyma than controls. In either of these two CS-treated groups, LFB staining showed significant decrease of demyelination in brain parenchyma compared with control group [Figure 4a–4f].
Figure 4.
Histological analysis. Degree of inflammatory cell infiltration (H & E, ×100) and demyelination (LFB, ×50) in the corpus striatum in the control (a and d), low-CS (b and e), and high-CS (c and f) groups. Less inflammatory infiltration (long red arrow) or demyelination (short red arrow) was present in both high-CS and low-CS groups than control group. CS: Cordyceps sinensis.
DISCUSSION
Although the etiology of MS remains unclear, it is generally believed that a T-cell-mediated autoimmune response against CNS myelin underlines the pathogenesis of the disease.[7] Recent studies have provided strong evidence that, in addition to CD4+ Th1, a variety of other immune cells such as CD4+ Th17 and CD4+ CD25+ Treg seem to be involved in disease pathogenesis by inducing or controlling immune response in MS.[8,9] In a cytokine milieu, with TCR activation, naïve CD4 T-cells may differentiate into one of the several lineages of Th cells including Th1, Th2, Th17, and CD4+ CD25+ Treg, as defined by their pattern of cytokine production and function.[10] So far, it remains controversial in terms of the relative importance of TH1 cells versus Th17 cells in EAE pathogenesis. IFN-γ-secreting Th1 cells are considered the primary effect or of T-cells in EAE pathology, but EAE can occur in IFN-γ knockout mice.[11] Subsequent studies have shown that Th17 cells that secrete IL-17 and IL-23 are also essential in the development of EAE.[12] As a master regulator of immune responses, CD4+ CD25+ Treg expressing FoxP3 maintain homeostasis between immune activation and suppression.[13,14] Animal studies have shown that the symptoms of EAE, an animal model of MS, were remarkably alleviated after the adoptive transfer of CD4+ CD25+ Treg in mice.[15] However, marked disease deterioration was observed in EAE mice if CD4+ CD25+ Tregs were removed,[16] especially in the mouse model of EAE induced by myelin as a self-antigen.[17] Intriguingly, it is believed that an imbalance between Th17 and Treg is associated with MS because of the important role that this imbalance has in the induction of inflammatory immune responses.[18]
In the present study, we found that, although the symptoms in all EAE mice spontaneously remitted in the absence of CS intervention, CS-treated mice demonstrated a remarkable delay in peak symptoms and less disease severity, as compared with the controls without CS treatment. In agreement with our pathological studies revealing less inflammatory infiltration and demyelination in CS-treated mice than controls, these results indicated the therapeutic potential of this agent in the treatment of MS. To clarify the mechanism of this agent, we further detected the frequency of peripheral Th1, Th17, and CD4+ CD25+ Treg. CS-treated mice had a lower frequency of peripheral Th1 in the presence of either a high-CS or low-CS group than control group, suggesting that CS can alleviate CNS demyelination at least partially through an immunoregulatory mechanism by reducing pathogenic Th1 infiltration into the brain. Interestingly, we also found that, as compared with control group, high-CS and low-CS groups had a decreased and increased Th17 frequency, respectively, in the lymph nodes. This is noteworthy as one previous study by Wang et al.[19] reported that different dosages of CS demonstrated distinct immunoregulatory effects; with increasing dosages, the immunosuppressive effects were more apparent. Thus, we speculate that CS may modulate the differentiation and proliferation of encephalitogenic T-cell subsets such as Th1 and Th17 in a dose-dependent manner. Nevertheless, more efforts, such as increase of sample size and study on the influence of CS on inflammatory immune responses within the CNS, should be made to elucidate the possible mechanism of this TCM agent in the treatment of EAE.
Our preliminary study demonstrated that CS efficiently alleviated EAE severity and EAE-related pathology and resulted in a lower Th1 number in the periphery, indicating its effectiveness in the treatment of murine EAE. Thus, our findings strongly support the therapeutic potential of this agent as a new TCM approach for MS. However, this study had some limitations: an obvious limitation of this study was the limited sample size, and this study did not elucidate the possible mechanism of CS in EAE treatment. Future investigations using a larger sample size or even conducting a clinical trial would help to gain more insight into the therapeutic potential of this agent in the treatment of MS.
Financial support and sponsorship
This study was supported by a grant from Jolly Pharmaceutical Co., Ltd. (No: 2119000260).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Ning-Ning Wang
REFERENCES
- 1.Xu J, Huang Y, Chen XX, Zheng SC, Chen P, Mo MH, et al. The mechanisms of pharmacological activities of Ophiocordyceps sinensis fungi. Phytother Res. 2016;30:1572–83. doi: 10.1002/ptr.5673. doi: 10.1002/ptr.5673. [DOI] [PubMed] [Google Scholar]
- 2.Harrison DM. In the clinic. Multiple sclerosis. Ann Intern Med. 2014;160:ITC4. doi: 10.7326/0003-4819-160-7-201404010-01004. doi: 10.7326/0003-4819-160-7-201404010-01004. [DOI] [PubMed] [Google Scholar]
- 3.Criado MB, Santos MJ, Machado J, Gonçalves AM, Greten HJ. Effects of acupuncture on gait of patients with multiple sclerosis. J Altern Complement Med. 2017 doi: 10.1089/acm.2016.0355. [Epub ahead of print] doi: 10.1089/acm.2016.0355. [DOI] [PubMed] [Google Scholar]
- 4.Ji X, Liu H, An C, Wang Y, Zhao H, Zhang Q, et al. You-Gui pills promote nerve regeneration by regulating netrin1, DCC and rho family GTPases RhoA, Racl, Cdc42 in C57BL/6 mice with experimental autoimmune encephalomyelitis. J Ethnopharmacol. 2016;187:123–33. doi: 10.1016/j.jep.2016.04.025. doi: 10.1016/j.jep.2016.04.025. [DOI] [PubMed] [Google Scholar]
- 5.Lalive PH, Molnarfi N, Benkhoucha M, Weber MS, Santiago-Raber ML. Antibody response in MOG(35-55) induced EAE. J Neuroimmunol. 2011;240-241:28–33. doi: 10.1016/j.jneuroim.2011.09.005. doi: 10.1016/j.jneuroim.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 6.Urban JL, Kumar V, Kono DH, Gomez C, Horvath SJ, Clayton J, et al. Restricted use of T cell receptor V genes in murine autoimmune encephalomyelitis raises possibilities for antibody therapy. Cell. 1988;54:577–92. doi: 10.1016/0092-8674(88)90079-7. doi: 10.1016/0092-8674(88)90079-7. [DOI] [PubMed] [Google Scholar]
- 7.Markovic-Plese S, McFarland HF. Immunopathogenesis of the multiple sclerosis lesion. Curr Neurol Neurosci Rep. 2001;1:257–62. doi: 10.1007/s11910-001-0028-4. doi: 10.1007/s11910-001-0028-4. [DOI] [PubMed] [Google Scholar]
- 8.Dendrou CA, Fugger L, Friese MA. Immunopathology of multiple sclerosis. Nat Rev Immunol. 2015;15:545–58. doi: 10.1038/nri3871. doi: 10.1038/nri3871. [DOI] [PubMed] [Google Scholar]
- 9.Bhise V, Dhib-Jalbut S. Further understanding of the immunopathology of multiple sclerosis: Impact on future treatments. Expert Rev Clin Immunol. 2016;12:1069–89. doi: 10.1080/1744666X.2016.1191351. doi: 10.1080/1744666X.2016.1191351. [DOI] [PubMed] [Google Scholar]
- 10.Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations (*) Annu Rev Immunol. 2010;28:445–89. doi: 10.1146/annurev-immunol-030409-101212. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferber IA, Brocke S, Taylor-Edwards C, Ridgway W, Dinisco C, Steinman L, et al. Mice with a disrupted IFN-gamma gene are susceptible to the induction of experimental autoimmune encephalomyelitis (EAE) J Immunol. 1996;156:5–7. [PubMed] [Google Scholar]
- 12.Kebir H, Kreymborg K, Ifergan I, Dodelet-Devillers A, Cayrol R, Bernard M, et al. Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat Med. 2007;13:1173–5. doi: 10.1038/nm1651. doi: 10.1038/nm1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coffer PJ, Burgering BM. Forkhead-box transcription factors and their role in the immune system. Nat Rev Immunol. 2004;4:889–99. doi: 10.1038/nri1488. doi: 10.1038/nri1488. [DOI] [PubMed] [Google Scholar]
- 14.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor foxp3. Science. 2003;299:1057–61. doi: 10.1126/science.1079490. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 15.Beyersdorf N, Gaupp S, Balbach K, Schmidt J, Toyka KV, Lin CH, et al. Selective targeting of regulatory T cells with CD28 superagonists allows effective therapy of experimental autoimmune encephalomyelitis. J Exp Med. 2005;202:445–55. doi: 10.1084/jem.20051060. doi: 10.1084/jem.20051060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McGeachy MJ, Stephens LA, Anderton SM. Natural recovery and protection from autoimmune encephalomyelitis: Contribution of CD4+CD25+ regulatory cells within the central nervous system. J Immunol. 2005;175:3025–32. doi: 10.4049/jimmunol.175.5.3025. doi: 10.4049/jimmunol.175.5.3025. [DOI] [PubMed] [Google Scholar]
- 17.Akirav EM, Bergman CM, Hill M, Ruddle NH. Depletion of CD4(+)CD25(+) T cells exacerbates experimental autoimmune encephalomyelitis induced by mouse, but not rat, antigens. J Neurosci Res. 2009;87:3511–9. doi: 10.1002/jnr.21981. doi: 10.1002/jnr.21981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jamshidian A, Shaygannejad V, Pourazar A, Zarkesh-Esfahani SH, Gharagozloo M. Biased Treg/Th17 balance away from regulatory toward inflammatory phenotype in relapsed multiple sclerosis and its correlation with severity of symptoms. J Neuroimmunol. 2013;262:106–12. doi: 10.1016/j.jneuroim.2013.06.007. doi: 10.1016/j.jneuroim.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 19.Wang XD, Zhou Y, Zhang L, Wang JF, Ge DY, Li L. The immunologic function of Cordyceps sinensis on the mice (In Chinese) J Beijing Univ Tradit Chin Med. 1998;21:34–6. doi: 10.3321/j.issn:1006-2157.1998.06.009. [Google Scholar]