Abstract
Background:
Circulating endometrial cells (CECs) have been reported to be present in the peripheral blood of women with endometriosis (EM), providing clear and specific evidence of the presence of ectopic lesions. In this study, we established a method with a high detection rate of CECs, assessed the diagnostic value of CECs for EM and compared with serum CA125, and proposed a hypothesis for the pathogenesis of EM from the new perspective of CECs.
Methods:
The participants were enrolled prospectively from October 2015 to July 2016. The peripheral blood samples were collected from 59 participants, and the blood cells were isolated for immunofluorescence staining via microfluidic chips. The cells that were positive for vimentin/cytokeratin and estrogen/progesterone receptor and negative for CD45 were identified as CECs. The serum CA125 level was tested with electrochemiluminescence immunoassay.
Results:
The detection rate of CECs reached 89.5% (17/19) in the EM group, which was significantly higher than that of the control group (15.0% [6/40], P < 0.001) and was independent of menstrual cycle phases. Furthermore, a positive CEC assay detected 4/5 cases of Stage I–II EM. In contrast, a positive CA125 test had limited value in detecting EM (13/19, 68.4%) and detected only one case of Stage I–II EM.
Conclusion:
CECs are promising biomarkers for EM with great potential for a noninvasive diagnostic assay.
Keywords: Biomarker, Circulating Endometrial Cells, Clinical Diagnosis, Endometriosis, Pathogenesis
INTRODUCTION
Endometriosis (EM) is defined as the presence of endometrial glands and/or stroma in any location outside of the uterine cavity. The ectopic endometrial tissue can be present in the peritoneum, pelvic organs, and even in distant organs such as the lung and brain. EM typically causes infertility, pelvic pain, and ovarian masses.[1] Its prevalence among women of reproductive age is approximately 10%, which might be an underestimate because the gold standard diagnostic method is invasive laparoscopy, and in some patients, the clinical symptoms are not typical. Furthermore, in the absence of a reliable clinical marker, the diagnosis of EM is usually delayed by an average of 7–11 years.[2] The infertility, pelvic pain, and high medical expenses that patients experience during the lengthy process of seeking a diagnosis and treatment for EM severely influence their quality of life.[3] Therefore, diagnostic methods that are more convenient and less invasive are urgently needed.
In recent years, a novel noninvasive test that measures circulating tumor cells (CTCs) has received increasing attention in the field of cancer research. It has been extensively applied in studies of the mechanism of metastasis and in the early diagnosis and prediction of prognosis for a variety of cancers.[4,5] Although EM is a benign disease, it has many malignant features such as dissemination, implantation, and metastasis. The postoperative 5-year recurrence rate of EM is approximately 50%, and the malignant transformation rate is approximately 1%.[6,7] In addition, endometrial cells in EM lesions including primary cells[8,9] and immortalized cell lines[10] have similar invasive properties to tumor cells, thus making their entry into circulation possible. A recent study reported the presence of endometrial cells, referred as circulating endometrial cells (CECs), in the peripheral blood of EM patients; these cells provide clear and specific evidence for the presence of active ectopic lesions and could be used as a novel biomarker for EM.[11]
There are many theories regarding the pathogenic mechanism of EM, and the retrograde menstruation theory proposed by Sampson is the most recognized. This theory proposes that ectopic lesions develop through the implantation of endometrial fragments that retrogradely flow into the pelvic cavity via menstrual blood. A limitation of this theory is that the retrograde menstruation phenomenon is present in a majority of fertile women, but only approximately 10% of them may develop EM. The vascular metastasis theory proposes another route of metastasis and suggests that blood circulation transfers the endometrial tissue fragments.[12] Although this transfer mechanism is still poorly understood, CECs provide powerful evidence in support of this theory and are beneficial for elucidating the metastatic process, which could facilitate both basic and clinical research.
In contrast to CTCs, the use of CECs as a biomarker for EM is a comparatively new concept, and many aspects still require investigation. Its detection rate reported in the literature was relatively low (23.5%),[11] which limited the future applications. Therefore, by establishing a novel method with an improved detection rate for the identification of CECs, this study aimed to assess the suitability of CECs as a biomarker for EM and compared the diagnostic performance with another putative clinical marker, serum CA125.
METHODS
Ethical approval
This study was approved by the Ethics Committee of the Peking University People's Hospital, and informed consent was obtained from all the enrolled individuals.
Study participants
The participants were enrolled prospectively from October 2015 to July 2016. The inclusion criteria consisted of women between 18 and 50 years old receiving surgical treatment due to the detection of ovarian masses by ultrasound. In addition, asymptomatic women with regular menstruation and normal transvaginal ultrasound results were recruited as healthy controls.
All enrolled individuals were not pregnant, had not undergone menopause, had no other malignant tumors, had no history of cancer, and had not used steroid hormones within 3 months before the study enrollment.
Specimen collection
Peripheral blood was collected from the patients in two blood collection tubes (367,983 and 367,861, BD Biosciences, USA) 1 day before surgery (4.5 ml/tube). The blood samples from healthy volunteers were collected on the 1st or 2nd day of the menstrual period. The first tube containing the silicone salt was centrifuged at 2500 ×g for 10 min at 4°C for serum separation. The serum was divided into 200 μl aliquots and stored at −80°C for the CA125 test. The second tube containing the anticoagulant K2 ethylenediaminetetraacetic acid (EDTA) was centrifuged at 500 ×g for 5 min at 4°C for the CEC assay. The supernatant was discarded, and the blood cells were resuspended in wash buffer (1X phosphate-buffered saline, 1% bovine serum, and 8 mM EDTA) (Wisent, Canada).
Detection of circulating endometrial cells
The cell suspension was injected into a microfluidic chip at a rate of 0.5 ml/h under the control of an injection pump, as described previously.[13] The cells were captured using the size-based microfluidic chip (CapitalBio, China) with patterned micropillars [Figure 1a]. The gap of the pillars ranged from 6 μ, which allowed most red blood cells and white blood cells to flow through,[13] to 25 μ to capture the endometrial cells (8–30 μ[11]). Immunofluorescence staining was used to identify the cells inside the chip. The captured cells were fixed for 20 min by injecting 4% paraformaldehyde and permeabilized with 0.1% Triton X-100 (Sigma, USA) for 10 min, followed by blocking for 20 min with 10% bovine serum (Wisent). The cells were then incubated with the primary antibodies and 4’,6-diamidino-2-phenylindole (DAPI) (Invitrogen, USA) overnight at 4°C and with secondary antibody at room temperature the next morning. The images were acquired with an inverted fluorescence microscope (DM IL LED, Leica, Germany). The primary antibodies included phycoerythrin (PE)-labeled anti-vimentin mouse monoclonal antibodies (1:100, Abcam, UK), PE-labeled anti-pan cytokeratin mouse monoclonal antibodies (1:100, Abcam), anti-estrogen receptor (ER) rabbit monoclonal antibodies (1:100, Abcam), and anti-progesterone receptor (PR) rabbit monoclonal antibodies (1:200, Abcam), which were used to identify the endometrial cells. Alexa Fluor 647-labeled anti-CD45 mouse monoclonal antibodies (1:20, BioLegend, USA) were used to exclude white blood cells. The secondary antibodies were Alexa Fluor 488-conjugated goat anti-rabbit antibodies (1:1000, Invitrogen).
Figure 1.
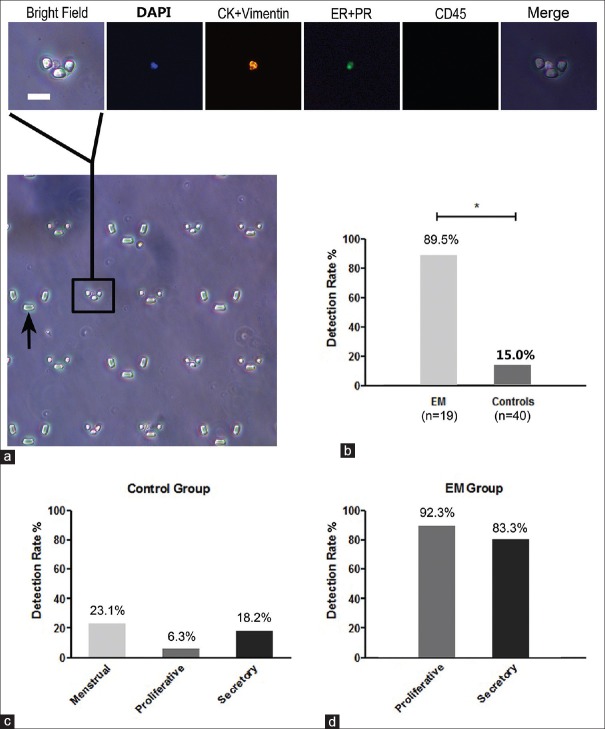
Measurement of circulating endometrial cells. (a) An example of circulating endometrial cell observed under a fluorescence microscope. The micropillars (black arrow) were used for capturing cells. A circulating endometrial cell was detected as the 4’,6-diamidino-2-phenylindole + cytokeratin (CK)/vimentin + estrogen receptor (ER)/progesterone receptor (PR) + CD45 - cell in four fields (Bar = 25 μm). (b) The detection rate in patients with endometriosis was 89.5% (17/19), which was significantly higher than that of the control group (6/40, 15.0%, P < 0.001). (c and d) No significant difference in the detection of circulating endometrial cells was seen across the cycle phases in controls (P = 0.425) and cases (P = 0.554).
Measurement of serum CA125
The serum CA125 level was quantified by electrochemiluminescence immunoassay on a Cobas e411 automated analyzer system (RocheDiagnostics GmbH, Germany) in the Department of Clinical Laboratory of the Peking University People's Hospital according to manufacturer's protocols. The range of CA125 assay was 0.600–5000 U/ml and the cutoff value was 35 U/ml.
Statistical analysis
In our preliminary results, the detection rates of CECs in EM, other benign masses, and the healthy control group were 71.4%, 25%, and 12.5%, respectively. The sample size was calculated to be eight women per group, using the 2-sample noninferiority or superiority, with a power of 80% and an alpha of 5%. The statistical analysis was performed using SPSS software (version 22.0, IBM, USA). The normality of the data was examined using the Kolmogorov-Smirnov test. Age is expressed as mean ± standard deviation (SD), and parity is expressed as the median (range). The differences in the detection rates of CECs and serum CA125 among the groups were compared using the Chi-square test. A value of P < 0.05 was considered statistically significant.
RESULTS
Measurement of circulating endometrial cells
Participant characteristics
A total of 59 patients were recruited for this study, among which 44 patients had received surgical treatment with pathological diagnosis. Nineteen cases were confirmed to have EM, and 25 cases were absent of EM (including 16 cases of other benign ovarian masses, 4 cases of ovarian cancer, and 5 cases of other benign ovarian masses combined with adenomyosis of the uterus) [Table 1]. In the EM group, the mean age was 33.4 ± 7.8 years (22–45 years), and the mean BMI was 21.5 ± 2.8 kg/m2 (17.4–26.7 kg/m2). In total, 13 cases (68.4%) had dysmenorrhea, 13 cases (68.4%) were in the proliferative phase, and 6 cases (31.6%) were in the secretory phase. The EM stages were determined according to the revised American Fertility Society Classification, and there were 5 cases (26.3%) of Stage I–II and 14 cases (73.7%) of Stage III–IV. In addition, 15 asymptomatic women with regular menstruation and normal transvaginal ultrasound results were recruited as healthy controls. The characteristics of the control groups are shown in Table 1.
Table 1.
Characteristics of the study participants
| Characteristics | EM | Healthy controls | Other benign | Cancer | Adenomyosis |
|---|---|---|---|---|---|
| n | 19 | 15 | 16 | 4 | 5 |
| Age (years), mean ± SD | 33.4 ± 7.8 | 30.9 ± 7.4 | 33.1 ± 7.6 | 38.3 ± 9.7 | 46.0 ± 4.2 |
| BMI (kg/m2), mean ± SD | 21.5 ± 2.8 | 20.1 ± 2.2 | 22.5 ± 2.9 | 23.7 ± 1.3 | 26.1 ± 3.6 |
| Parity, median (range) | 0 (0–1) | 0 (0–3) | 0 (0–1) | 1 (1–2) | 1 (1–2) |
| Pelvic pain, n | 13 | 5 | 2 | 1 | 5 |
| Menstrual cycle stage, n | |||||
| Menstrual | 0 | 13 | 0 | 0 | 0 |
| Proliferative | 13 | 1 | 10 | 2 | 3 |
| Secretory | 6 | 1 | 6 | 2 | 2 |
| EM stage, n | |||||
| Stage I–II | 5 | NA | |||
| Stage III–IV | 14 | ||||
NA: Not applicable; BMI: Body mass index; EM: Endometriosis; SD: Standard deviation.
Detection rate of circulating endometrial cells
The presence of CECs was assessed in all participants. The cells that were positive for DAPI, vimentin/pan-CK, and ER/PR and negative for CD45 were identified as CECs [Figure 1a]. Among the 19 EM patients, 17 cases had detectable CECs, and the detection rate reached 89.5%, which was significantly higher than that of the control group (6/40, 15.0%, P < 0.001) [Figure 1b]. Of the 40 controls, CECs were detected in 3/15 of the healthy controls, 2/16 of the patients with other benign masses, 0/4 of the patients with ovarian cancer, and 1/5 of the patients with adenomyosis.
Circulating endometrial cells and menstrual cycle
We analyzed the CEC detection rates during different phases of the menstrual cycles of the control group and the EM group to examine the relationship between CECs and the menstrual cycle phases. In the control group, CECs were detected in 3 of 13 cases during the menstrual phase, 1 of 16 cases during the proliferative phase, and 2 of 11 cases during the secretory phase, and the corresponding detection rates were 23.1%, 6.3%, and 18.2%, respectively, which were not significantly different (P = 0.425) [Figure 1c]. In the EM group, CECs were detected in 12 of 13 cases during the proliferative phase and 5 of 6 cases during the secretory phase, and the corresponding detection rates were 92.3% and 83.3%, respectively, which were not significantly different (P = 0.554) [Figure 1d].
Diagnostic performance of circulating endometrial cells
Circulating endometrial cells, serum CA125, and endometriosis
Employing a clinical cutoff of >35 U/ml, CA125 tests were positive in 13 of 19 patients with EM (68.4%), which was also higher than that of the control group (12/40, 30.0%; P = 0.005). Of the six patients who were negative for the CA125 test, five were positive for the CEC assay. Conversely, of the two patients who were negative for the CEC assay, one was positive for the CA125 test [Table 2]. The CECs sensitivity was 17/19 (89.5%) and CA125 sensitivity was 13/19 (68.4).
Table 2.
Comparison of CECs and serum CA125 levels as biomarkers in EM
| CEC, n | |||
|---|---|---|---|
| CA125, n | Positive | Negative | Total |
| Positive | 12 | 1 | 13 |
| Negative | 5 | 1 | 6 |
| Total | 17 | 2 | 19 |
CECs: Circulating endometrial cells; EM: Endometriosis.
Circulating endometrial cells, serum CA125, and stage of disease
We also evaluated the relationship between CECs, serum CA125, and stage of disease [Figure 2]. The positive rates of the CEC assay for Stage I–II and Stage III–IV EM were 80.0% (4/5) and 92.9% (13/14), respectively, and they were not significantly different (P = 0.468). Compared with the other benign ovarian masses and healthy controls, there was still a significant difference (P < 0.001). However, the difference in the positive rates of the CA125 assay between Stage I–II and Stage III–IV EM was significant (P = 0.017). The positive rate for Stage I–II EM was only 20.0% (1/5), which was significantly lower than that for Stage III–IV EM (85.7%, 12/14), but it was not significantly different from the positive rates for the other benign ovarian masses and healthy controls (P = 0.609).
Figure 2.

The positive rates of circulating endometrial cell assay and CA125 assay for other benign, healthy control, Stage I–II and Stage III–IV endometriosis patients.
Diagnostic performance
The diagnostic performance of the CEC assay and CA125 for EM was examined [Table 3]. Among the 35 patients with EM and other benign ovarian masses, the CEC assay had 89.5% sensitivity and 87.5% specificity, while the CA125 assay had 68.4% sensitivity and 87.5% specificity. In distinguishing EM from healthy controls, the CEC assay had 89.5% sensitivity and 80.0% specificity, while the CA125 assay had 68.4% sensitivity and 73.3% specificity.
Table 3.
Comparison of diagnostic performance of CEC assay and CA125 assay for EM
| Items | Sensitivity | Specificity | PPV | NPV | LR |
|---|---|---|---|---|---|
| EM versus other benign, n (%) | |||||
| CECs | 17/19 (89.5) | 14/16 (87.5) | 17/19 (89.5) | 14/16 (87.5) | 7.2 |
| CA125 | 13/19 (68.4) | 14/16 (87.5) | 13/15 (86.7) | 14/20 (70.0) | 5.5 |
| EM versus healthy control, n (%) | |||||
| CECs | 17/19 (89.5) | 12/15 (80.0) | 17/20 (85.0) | 12/14 (85.7) | 4.5 |
| CA125 | 13/19 (68.4) | 11/15 (73.3) | 13/17 (76.5) | 11/17 (64.7) | 2.6 |
CECs: Circulating endometrial cells; EM: Endometriosis; NPV: Negative predicative value; PPV: Positive predicative value; LR: Likelihood ratio.
DISCUSSION
In this study, we established an efficient detection method for CECs and validated the presence of CECs in the peripheral blood of EM patients. The present study evaluated for the first time the clinical significance of CECs in the diagnosis of EM. These results not only provide a basis for the establishment of a novel noninvasive diagnostic method for EM but also provide new insights into the pathogenesis of this enigmatic disease.
In the early 20th century, Sampson[14] histologically confirmed that there were endometrial tissue fragments in the uterine veins of four patients with either peritoneal/ovarian EM or adenomyosis during the menstrual phase. In our study, CECs were detected in 89.5% of patients with ovarian EM, indicating that the endometrial tissue could enter not only the confined uterine vessels but also the peripheral circulation in the form of CECs. These results suggested that vascular metastasis may also play an important role in the development of intrapelvic EM. Our results also suggested that CECs could survive in circulation regardless of menstrual cycle phases. It has been reported that endometrial cells in EM lesions have invasive properties that are similar to tumor cells[8,9,10] and that the local immune cell subpopulation is dysregulated.[15]
Hence, we propose the following hypothesis for the pathogenesis of EM from the new perspective of CECs: endometrial cells might enter circulation, escape immune attack, and survive to be transferred for ectopic implantation in a suitable microenvironment and develop into EM. This hypothesis could be an important supplement to the retrograde menstruation theory, which is generally thought to better explain the development of extrapelvic EM, and it could also explain the development of EM in rare locations such as in the nasal cavity and lung.
It is worth noting that only one case had detectable CECs among the five adenomyosis patients. As another type of EM, adenomyosis involves the invasion of endometrial glands and stroma into the myometrium. However, the pathogenesis of adenomyosis differs from EM and is considered a result of the invagination and abnormal in-growth of the basal endometrium into the myometrium.[16] Our results supported the differences in the pathogenesis of these two diseases but still suggested the possibility that a minority of cases of adenomyosis may be a consequence of CECs implanting in the myometrium.
Currently, few studies have investigated the role of CECs in either EM or adenomyosis, and one major reason for this may be the relatively low CEC detection rate, which is restricted by detection methods and limits further applications of CECs. Bobek et al.[11] isolated CECs from the peripheral blood of EM patients through the use of porous polycarbonate membranes (pores with an 8-μm diameter). After in vitro culturing, the viable CECs were then identified by immunohistochemistry (IHC) with antibodies against the surface molecules of the epithelial and stromal cells, including pan-CK, CD10, and vimentin. Because of the limitations of the pore size of the membrane and in vitro culturing, only four cases had detectable CECs among the 17 EM patients, and the detection rate was 23.5%.
The CEC detection method established in this study not only significantly increased the sensitivity but also had a high specificity. Microfluidic chips, which have been extensively applied in CTC research, were employed for the isolation of CECs.[17] The micro-columns of the microfluidic chips that we used were arranged in a gradient and have been proven to be an effective means of isolation (>90%), resulting in a high purity (approximately 90%) of recovered CTCs.[13] Both small epithelial cells and large stromal cells could be captured using this chip, which increased the sensitivity, and the staining could be conveniently and directly performed without detaching and recovering the cells. Furthermore, our staining strategy had a high specificity for endometrial cells. Pan-CK/vimentin are surface markers for epithelial/stromal cells, and ERs/PRs are expressed on over 90% of epithelial and stromal cells in the endometrium.[18,19] CD45 is expressed only on the surface of white blood cells[20] and is widely used for the exclusion of white blood cells from CTCs.[21] Our results showed that the CEC detection rate in EM patients reached 89.5%, whereas none of the patients diagnosed with ovarian cancer were positive for CECs. Considering that approximately 30–60% of ovarian cancer lesions also expressed ERs/PRs,[22] each case of ovarian cancer was analyzed by IHC and showed either negative or weakly positive staining (<10%) for ER and PR, which validated the results of the CEC assay.
This improved detection method for CECs makes its application to clinical diagnosis possible. Our results showed that CEC assay had good diagnostic characteristics for ovarian EM. Compared with CA125, CECs had better discriminative ability in Stage I–II EM patients. The detection of CECs was not influenced by the disease severity, which may provide an option for early diagnosis and monitoring of recurrences. A meta-analysis including 22 studies revealed that the sensitivity of CA125 for Stage I–II EM was only ~24.8%, which was significantly lower than that for Stage III–IV EM (~63.1%). Therefore, negative CA125 tests cannot exclude the presence of light-mild EM,[23] whereas the circulating micro RNAs,[24] biglycan/leptin ratio[25] and plasma brain-derived neurotrophic factor levels[26] were reported to be able to distinguish Stage I–II EM patients from the controls.
There are many practical aspects that favor the use of CECs as a clinical marker for EM. These cells are isolated from peripheral blood, samples of which can be easily collected with minimal invasiveness. Furthermore, the detection rate is not influenced by menstrual cycle phases; therefore, patients can be tested on the day they present to the clinic and do not have to wait for a specific cycle phase. More importantly, the current reported EM biomarkers are mainly molecules such as glycoproteins, cytokines, and RNAs, which may only reflect a single aspect of the disease.[27] As intact cells, CECs contain comprehensive information of genome, transcriptome, and the proteome and could reflect certain histological characteristics without invasive surgery. Therefore, through further studies such as omics analysis, quantitative counting, and gene sequencing, CECs are expected to become a target of liquid biopsies for EM, which could provide real-time and repeated “biopsies” for targeted drug screening, recurrence monitoring, and malignancy prediction to achieve precision medicine.
However, there are several limitations of the current detection method. First, determining the absolute quantity of the CECs captured by the microfluidic chip was unachievable because of certain technological drawbacks of the current detection system, which we will try to improve by automating the process. Second, malignant tumors such as ER/PR-positive breast cancers may inevitably interfere with this CEC detection method.[28] Furthermore, the shedding of vascular endothelial cells into the tubes during blood collection is also a confounding factor of this assay because these cells also express CK and ER/PR.[29,30,31] Although the second tube of blood was used for the CEC detection of each participant, there was still a possibility of contamination, which may explain the false positive cases in this study. Single-cell sorting and sequencing of the captured cells may be helpful to define more specific biomarkers of CECs.
In conclusion, CECs may be a promising biomarker for EM with great potential for the development of an early, noninvasive diagnostic assay, although our discovery phase study requires validation in a larger, multicenter study population. Further studies are needed to evaluate the relationship among the quality of CECs, characteristics of the ectopic lesions, and disease recurrence.
Financial support and sponsorship
This study was supported by grants from the National Natural Science Foundation of China (No. 81671431) and the Peking University People's Hospital Scientific Research Development Funds (No. RDY2016-12).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Yi Cui
REFERENCES
- 1.Buck Louis GM, Hediger ML, Peterson CM, Croughan M, Sundaram R, Stanford J, et al. Incidence of endometriosis by study population and diagnostic method: The ENDO study. Fertil Steril. 2011;96:360–5. doi: 10.1016/j.fertnstert.2011.05.087. doi: 10.1016/j.fertnstert.2011.05.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Riazi H, Tehranian N, Ziaei S, Mohammadi E, Hajizadeh E, Montazeri A, et al. Clinical diagnosis of pelvic endometriosis: A scoping review. BMC Women's Health. 2015;15:39. doi: 10.1186/s12905-015-0196-z. doi: 10.1186/s12905-015-0196-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simoens S, Dunselman G, Dirksen C, Hummelshoj L, Bokor A, Brandes I, et al. The burden of endometriosis: Costs and quality of life of women with endometriosis and treated in referral centres. Hum Reprod. 2012;27:1292–9. doi: 10.1093/humrep/des073. doi: 10.1093/humrep/des073. [DOI] [PubMed] [Google Scholar]
- 4.Gold B, Cankovic M, Furtado LV, Meier F, Gocke CD. Do circulating tumor cells, exosomes, and circulating tumor nucleic acids have clinical utility. A report of the association for molecular pathology? J Mol Diagn. 2015;17:209–24. doi: 10.1016/j.jmoldx.2015.02.001. doi: 10.1016/j.jmoldx.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pearl ML, Zhao Q, Yang J, Dong H, Tulley S, Zhang Q, et al. Prognostic analysis of invasive circulating tumor cells (iCTCs) in epithelial ovarian cancer. Gynecol Oncol. 2014;134:581–90. doi: 10.1016/j.ygyno.2014.06.013. doi: 10.1016/j.ygyno.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koga K, Takamura M, Fujii T, Osuga Y. Prevention of the recurrence of symptom and lesions after conservative surgery for endometriosis. Fertil Steril. 2015;104:793–801. doi: 10.1016/j.fertnstert.2015.08.026. doi: 10.1016/j.fertnstert.2015.08.026. [DOI] [PubMed] [Google Scholar]
- 7.Zafrakas M, Grimbizis G, Timologou A, Tarlatzis BC. Endometriosis and ovarian cancer risk: A systematic review of epidemiological studies. Front Surg. 2014;1:14. doi: 10.3389/fsurg.2014.00014. doi: 10.3389/fsurg.20000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guan YT, Huang YQ, Wu JB, Deng ZQ, Wang Y, Lai ZY, et al. Overexpression of chloride channel-3 is associated with the increased migration and invasion ability of ectopic endometrial cells from patients with endometriosis. Hum Reprod. 2016;31:986–98. doi: 10.1093/humrep/dew034. doi: 10.1093/humrep/dew034. [DOI] [PubMed] [Google Scholar]
- 9.Renner SP, Strissel PL, Beckmann MW, Lermann J, Burghaus S, Hackl J, et al. Inhibition of adhesion, proliferation, and invasion of primary endometriosis and endometrial stromal and ovarian carcinoma cells by a nonhyaluronan adhesion barrier gel. Biomed Res Int. 2015;2015:450468. doi: 10.1155/2015/450468. doi: 10.1155/2015/450468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zeitvogel A, Baumann R, Starzinski-Powitz A. Identification of an invasive, N-cadherin-expressing epithelial cell type in endometriosis using a new cell culture model. Am J Pathol. 2001;159:1839–52. doi: 10.1016/S0002-9440(10)63030-1. doi: 10.1016/S0002-9440(10)63030-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bobek V, Kolostova K, Kucera E. Circulating endometrial cells in peripheral blood. Eur J Obstet Gynecol Reprod Biol. 2014;181:267–74. doi: 10.1016/j.ejogrb.2014.07.037. doi: 10.1016/j.ejogrb.2014.07.037. [DOI] [PubMed] [Google Scholar]
- 12.Maruyama T, Yoshimura Y. Stem cell theory for the pathogenesis of endometriosis. Front Biosci. 2012;4:2854–63. doi: 10.2741/e589. doi: 10.2741/589. [DOI] [PubMed] [Google Scholar]
- 13.Lv P, Tang Z, Liang X, Guo M, Han RP. Spatially gradated segregation and recovery of circulating tumor cells from peripheral blood of cancer patients. Biomicrofluidics. 2013;7:34109. doi: 10.1063/1.4808456. doi: 10.1063/1.4808456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sampson JA. Metastatic or embolic endometriosis, due to the menstrual dissemination of endometrial tissue into the venous circulation. Am J Pathol. 1927;3:93–110.43. [PMC free article] [PubMed] [Google Scholar]
- 15.Herington JL, Bruner-Tran KL, Lucas JA, Osteen KG. Immune interactions in endometriosis. Expert Rev Clin Immunol. 2011;7:611–26. doi: 10.1586/eci.11.53. doi: 10.1586/eci.11.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benagiano G, Habiba M, Brosens I. The pathophysiology of uterine adenomyosis: An update. Fertil Steril. 2012;98:572–9. doi: 10.1016/j.fertnstert.2012.06.044. doi: 10.1016/j.fertnstert.2012.06.044. [DOI] [PubMed] [Google Scholar]
- 17.Dong Y, Skelley AM, Merdek KD, Sprott KM, Jiang C, Pierceall WE, et al. Microfluidics and circulating tumor cells. J Mol Diagn. 2013;15:149–57. doi: 10.1016/j.jmoldx.2012.09.004. doi: 10.1016/j.jmoldx.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 18.Engemise SL, Willets JM, Taylor AH, Emembolu JO, Konje JC. Changes in glandular and stromal estrogen and progesterone receptor isoform expression in eutopic and ectopic endometrium following treatment with the levonorgestrel-releasing intrauterine system. Eur J Obstet Gynecol Reprod Biol. 2011;157:101–6. doi: 10.1016/j.ejogrb.2011.02.013. doi: 10.1016/j.ejogrb.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 19.Mehasseb MK, Panchal R, Taylor AH, Brown L, Bell SC, Habiba M, et al. Estrogen and progesterone receptor isoform distribution through the menstrual cycle in uteri with and without adenomyosis. Fertil Steril. 2011;95:2228–35. doi: 10.1016/j.fertnstert.2011.02.051. 2235.e1. doi: 10.1016/j.fertnstert.2011.02.051. [DOI] [PubMed] [Google Scholar]
- 20.Fehm T, Solomayer EF, Meng S, Tucker T, Lane N, Wang J, et al. Methods for isolating circulating epithelial cells and criteria for their classification as carcinoma cells. Cytotherapy. 2005;7:171–85. doi: 10.1080/14653240510027082. doi: 10.1080/14653240510027082. [DOI] [PubMed] [Google Scholar]
- 21.Attard G, Crespo M, Lim AC, Pope L, Zivi A, de Bono JS. Reporting the capture efficiency of a filter-based microdevice: A CTC is not a CTC unless it is CD45 negative – Letter. Clin Cancer Res. 2011;17:3048–9. doi: 10.1158/1078-0432.CCR-10-3234. doi: 10.1158/1078-0432.CCR-10-3234. [DOI] [PubMed] [Google Scholar]
- 22.Sieh W, Köbel M, Longacre TA, Bowtell DD, deFazio A, Goodman MT, et al. Hormone-receptor expression and ovarian cancer survival: An Ovarian Tumor Tissue Analysis consortium study. Lancet Oncol. 2013;14:853–62. doi: 10.1016/S1470-2045(13)70253-5. doi: 10.1016/S1470-2045(13)70253-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hirsch M, Duffy J, Davis CJ, Nieves Plana M, Khan KS. Diagnostic accuracy of cancer antigen 125 for endometriosis: A systematic review and meta-analysis. BJOG. 2016;123:1761–8. doi: 10.1111/1471-0528.14055. doi: 10.1111/1471-0528.14055. [DOI] [PubMed] [Google Scholar]
- 24.Wang L, Huang W, Ren C, Zhao M, Jiang X, Fang X, et al. Analysis of serum microRNA profile by solexa sequencing in women with endometriosis. Reprod Sci. 2016;23:1359–70. doi: 10.1177/1933719116641761. doi: 10.1177/1933719116641761. [DOI] [PubMed] [Google Scholar]
- 25.Kocbek V, Vouk K, Bersinger NA, Mueller MD, Lanišnik Rižner T. Panels of cytokines and other secretory proteins as potential biomarkers of ovarian endometriosis. J Mol Diagn. 2015;17:325–34. doi: 10.1016/j.jmoldx.2015.01.006. doi: 10.1016/j.jmoldx.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 26.Wessels JM, Kay VR, Leyland NA, Agarwal SK, Foster WG. Assessing brain-derived neurotrophic factor as a novel clinical marker of endometriosis. Fertil Steril. 2016;105:119–28.e1. doi: 10.1016/j.fertnstert.2015.09.003. doi: 10.1016/j.fertnstert.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 27.Fassbender A, Burney RO, O DF, D’Hooghe T, Giudice L. Update on biomarkers for the detection of endometriosis. Biomed Res Int 2015. 2015:130854. doi: 10.1155/2015/130854. doi: 10.1155/2015/130854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aktas B, Kasimir-Bauer S, Müller V, Janni W, Fehm T, Wallwiener D, et al. Comparison of the HER2, estrogen and progesterone receptor expression profile of primary tumor, metastases and circulating tumor cells in metastatic breast cancer patients. BMC Cancer. 2016;16:522. doi: 10.1186/s12885-016-2587-4. doi: 10.1186/s12885-016-2587-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saavedra P, Girona J, Bosquet A, Guaita S, Canela N, Aragonès G, et al. New insights into circulating FABP4: Interaction with cytokeratin 1 on endothelial cell membranes. Biochim Biophys Acta. 2015;1853:2966–74. doi: 10.1016/j.bbamcr.2015.09.002. doi: 10.1016/j.bbamcr.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 30.Jobe SO, Ramadoss J, Wargin AJ, Magness RR. Estradiol-17β and its cytochrome P450- and catechol-O-methyltransferase-derived metabolites selectively stimulate production of prostacyclin in uterine artery endothelial cells: Role of estrogen receptor-α versus estrogen receptor-β. Hypertension. 2013;61:509–18. doi: 10.1161/HYPERTENSIONAHA.112.200717. doi: 10.1161/HYPERTENSIONAHA.112.200717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pang Y, Dong J, Thomas P. Progesterone increases nitric oxide synthesis in human vascular endothelial cells through activation of membrane progesterone receptor-α. Am J Physiol Endocrinol Metab. 2015;308:E899–911. doi: 10.1152/ajpendo.00527.2014. doi: 10.1152/ajpendo.00527.2014. [DOI] [PubMed] [Google Scholar]