Abstract
Background:
Current knowledge indicates that oxidative damage and the following inflammation is pivotal pathway for myocardial cell death. In recent decades, hydrogen sulfide (H2S) has been identified as a novel endogenous vasodilator and neuromodulator due to its antioxidation capacity. However, whether H2S pretreatment in neonatal mouse cardiomyocytes is a protection effect against oxidative stress remains elusive.
Methods:
Primary neonatal mouse cardiomyocytes were isolated and cultured, subsequently, pretreated with the H2S donor, sodium hydrosulfide (NaHS). Cell viability, lactate dehydrogenase (LDH) release, and reactive oxygen species (ROS) production are evaluated. The levels of superoxide dismutase (Sod2) and Sirtuin 1 (Sirt1), a deacetylation enzyme, were detected by Western blotting. The statistics was performed using independent-sample t-test.
Results:
NaHS (100 μmol/L) had no toxicity to primary neonatal mouse cardiomyocytes. Furthermore, NaHS pretreatment significantly improved neonatal mouse cardiomyocytes survival after H2O2-induced cell death, indicated by the decrease in LDH release (40.00 ± 2.65% vs. 65.33 ± 4.33%, P < 0.01) and ROS production (1.90 ± 0.33 vs. 4.56 ± 0.56, P < 0.05), and that the salubrious effect was accompanied by the upregulation of Sod2 expression. In addition, the study showed that NaHS pretreatment improved mitochondrial DNA number in neonatal mouse cardiomyocyte. Furthermore, the result demonstrated NaHS increased the expression of Sirt1 in neonatal mouse cardiomyocyte. Ex 527, an inhibitor of Sirt1, could attenuate these effects of NaHS-induced Sod2 expression and mtDNA number increase, furthermore, abrogate the cytoprotective effects of NaHS for neonatal mouse cardiomyocytes.
Conclusion:
Sirt1 mediated H2S-induced cytoprotection effects in neonatal mouse cardiomyocytes.
Keywords: Hydrogen Sulfide, Neonatal Mouse Cardiomyocyte, Oxidative Stress, Sirtuin 1
INTRODUCTION
Ischemia-reperfusion (I/R) injury is an important clinical problem for some organs, including brain, kidney, liver, and heart. The restoration of blood supply after a certain period of no flow ischemia would result in parenchymal damage to organs. In heart, reperfusion may lead to further damage such as myocardial infarction, cardiac arrhythmias, and contractile dysfunction.[1] Increasing experimental evidence have implicated that myocardial cell death due to I/R is a major cause of morbidity and mortality. Although the mechanisms about I/R injury are complicated, the current knowledge indicates that oxidative damage and the following inflammation are pivotal pathway for cellular death and mucosal injury.[2]
Oxidative stress is an important factor in many pathological conditions including inflammation, cancer, aging, and organ response to I/R. Tissues would adapt to anaerobic metabolism when an organ is in a state of ischemia. However, after the blood supply is restored, the cells will produce an excess of super oxide radicals, also referred as reactive oxygen species (ROS). An excess of ROS causes oxidative stress.[3] ROS is the key initiators of reperfusion injury, which leads to endothelial injury and further release of pro-inflammatory cytokines.[3] Accordingly, targeting the generation of ROS with various antioxidants has been shown to reduce the deleterious effects of oxidative stress, and improve recovery from I/R injury.
Hydrogen sulfide (H2S) is generated in mammalian tissues from homocysteine and cysteine catalyzed by cystathionine γ-synthase and cystathionine c-lyase, the key enzymes of the transsulfuration pathway of methionine metabolism.[4] Although H2S is most notably recognized for its toxicity, it has been identified as a novel endogenous vasodilator and neuromodulator in recent decades. One of the most important mechanisms responsible for H2S protection is antioxidation.[5] Previous reports also have proved that H2S has a potential effect on I/R injury by decreasing oxidative stress and anti-inflammatory effect.[6,7,8] In addition, H2S has been proposed to have a role in the physiology and pathophysiology of the cardiovascular system. The role of H2S in the pathophysiology of I/R injury of the heart is an emerging field. Exogenous administration of the H2S donor sodium hydrosulfide (NaHS) attenuates isoproterenol-induced cardiac injury and dysfunction.[9,10,11]
Sirtuin 1 (Sirt1), the ortholog of the yeast Sir2 protein, plays a crucial role in the DNA damage response, carcinogenesis, and regulation of lifespan, which are mediated by its nuclear nicotinamide adenice dinucleotide-dependent deacetylase activity.[12] Previous study reported that moderate overexpression of Sirt1 protected the heart from oxidative stress induced by paraquat, with increased the expression of antioxidants.[13] The aim of the present study was to assess whether H2S pretreatment in neonatal mouse cardiomyocytes is a protection effect against oxidative stress. The results showed that NaHS prevented H2O2-induced injury to neonatal mouse cardiomyocytes, which was mediated by increasing the expression of Sirt1, furthermore, improving mitochondrial function and reducing the deleterious effects of oxidative stress.
METHODS
Ethical approval
All animals received humane care in compliance with the Guide for the Care and Use of Laboratory Animal published by the National Institutes of Health (NIH Publication 83-23, revised 1985). The study protocol was approved by the Laboratory Animal Ethics Committee of Beijing Anzhen Hospital, Capital Medical University.
Isolation and culture of primary neonatal mouse cardiomyocytes
Primary neonatal mouse cardiomyocytes were isolated and cultured according to the previous study.[14] In brief, the 1–3-day-old neonatal mice were sacrificed by cervical dislocation. Hearts were removed aseptically (retaining the ventricles only) and maintained in cold Hanks’ balanced salt solution (HBSS) without Ca2+ and Mg2+. The ventricles were washed with the same HBSS and minced into small fragments. The cells were dissociated at 37°C with 5% CO2 and 95% air for 15 min with an enzyme solution (0.25% w/v trypsin in HBSS without Ca2+ and Mg2+, pH 7.4). Cells from subsequent digestion were added to an equal volume of cold HBSS with Ca2+ and Mg2+, pH 7.4, until all cardiac cells were isolated. The resulting mixture was centrifuged for 8 min at 200 ×g, and the cells were resuspended in the fetal bovine serum (FBS)-modified Eagle's medium (MEM; MEM supplemented with 10% FBS, 100 U/ml penicillin, and 100 mg/ml streptomycin). To exclude nonmuscle cells, the isolated cells were first plated onto tissue culture dishes at 37°C for 2 h under a water-saturated atmosphere of 5% CO2 with 95% air based on the observation that nonmuscle cells attach to the substrata more rapidly. The suspended cells were then collected and plated at a density of 1.0 × 105 cells/cm2 and incubated under the same conditions as above.
Cell viability assay
Neonatal mouse cardiomyocytes were cultured in 96-well tissue culture plates (1 × 104 cells/well) with complete medium for 24 h. Then, the serum-free medium was used and cells were exposed to different concentrations of NaHS for 6, 12, and 24 h. Then, cells were washed twice with phosphate-buffered saline (PBS) and were changed with 10% FBS medium and were cultured for 3 days. Cell viability was measured by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; Roche Diagnostics, USA) incorporation assays.
Lactate dehydrogenase leakage
The cell viability is tested through measuring cellular lactate dehydrogenase (LDH) leakage in damaging cells according to the previous study.[15] Neonatal mouse cardiomyocytes were cultured in 96-well tissue culture plates (1 × 104 cells/well) with complete medium for 24 h. Then, the serum-free medium was used and cells were exposed to different concentrations of NaHS for indicated time. Then, cells were washed twice with PBS and were changed with complete medium and were cultured for 24 h. Following the manufacturer's instructions from the LDH assay kit (Takara, Japan), samples in different treatment groups were centrifuged at 250 ×g for 10 min, and 100 μl of the supernatant was then mixed with an equal volume of premixed reagent (catalyst/dye solution = 1:45) for 30 min at room temperature in a 96-well plate. The absorbance of each sample at 490 nm was measured using a microplate reader, and the percentage of LDH release for each sample was normalized according to the absorbance reading from samples treated with 0.5% Triton X-100.
Measurement of cellular reactive oxygen species accumulation
Superoxide production was detected using the fluorescent 2’,7’-dichlorofluorescein (DCF) obtained from vigorous as previously reported.[16] For this assay, neonatal mouse cardiomyocytes were plated in six-well plates. Cells were treated with Celastrol or dimethyl sulfoxide. After 24 h, cells were changed with serum-free medium and were exposed to 100 μmol/L NaHS for 12 h. Then, cells were washed twice with PBS and were changed with 10% FBS medium and were cultured for 24 h. Moreover, cells were trypsinized and harvested, then were trypsinized, harvested, and loaded with DCF for 10 min in the dark at 37°C. Next, cells were washed twice with PBS, and fluorescence was measured using flow cytometry. Data analysis was performed with c-flow software, and the mean fluorescence intensity is used to quantify the responses. A minimum of 10,000 cells was acquired for each sample.
Mitochondrial DNA copy number
The mitochondrial DNA (mtDNA) copy number was used as a marker for mitochondrial density using quantitative polymerase chain reaction (qPCR) as previously reported.[17] Briefly, total DNA was isolated from neonatal mouse cardiomyocytes using a Universal Genomic DNA Extraction Kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. The mtDNA copy number was calculated from the ratio of COX II (mitochondrial-encoded gene)/Cyclophilin A (nuclear-encoded gene). The primer sequences are, COX II, 5’-CAGGCCGACTAAATCAAGCAAC-3’, 5’-CTAGGACAATGGGCATAAAGCT-3’; Cyclophilin A, 5’-TTCCTCCTTTCACAGAATTATTCCA-3’, 5’-CCGCCAGTGCCATTATGG-3’.
Western blotting analysis and antibodies
Neonatal mouse cardiomyocytes were lysed in 100 ml of lysis buffer (50 mmol/L Tris, pH 7.4, 1 mmol/L ethylene diamine tetraacetic acid, 150 mmol/L NaCl, 0.25% sodium deoxycholate, and 1% NP40) containing protease inhibitors (1 mmol/L phenylmethylsulfonyl fluoride, 1 mg/ml aprotinin, 1 mg/ml leupeptin, and 1 mg/ml pepstatin; Roche). Samples were incubated at °C for 1 h and then centrifuged at 12,000 ×g for 30 min, and the supernatant was collected for analysis. Lysates were resolved by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and then transferred to poly (vinylidene fluoride) membranes. The membranes were blocked and incubated with specific antibodies against Sirt1 (Millipore, USA), superoxide dismutase (Sod2; Abclonal, USA) and α-tubulin (Abmart, China). Peroxidase-conjugated anti-mouse or anti-rabbit immunoglobulin secondary antibody was used. Proteins were visualized by enhanced chemiluminescence. Densitometry analysis of Western blots was performed using AlphaEaseFC software (Alpha Innotech, USA).
Statistical analysis
Data are expressed as mean ± standard error (SE). Differences among means were analyzed by independent-sample t-test using the SPSS software version 18.0 (SPSS Inc., USA). A P < 0.05 was considered as statistically significant.
RESULTS
Sodium hydrosulfide is nontoxic to neonatal mouse cardiomyocytes
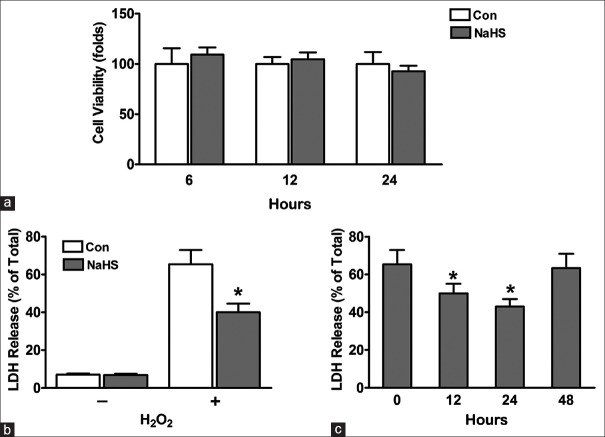
Previous studies have established that H2S is able to exert its protective effect for some organs, such as heart, kidney, and brain.[18,19,20] However, little is known about whether it is protective for mouse cardiomyocytes in vitro. First, we performed cell viability assays to determine the effect of NaHS treatment on neonatal mouse cardiomyocytes survival. Cardiomyocytes were treated without or with 100 μmol/L NaHS in free serum for indicated hours. As determined by the MTT viability assay, 100 μmol/L NaHS was found to be nontoxic to mouse cardiomyocytes within 24 h [Figure 1a].
Figure 1.
Protective effects of pretreated neonatal mouse cardiomyocytes with Hydrogen sulfide. (a) NaHS is nontoxic to neonatal mouse cardiomyocytes. Cardiomyocytes were plated in 96-well plates. After 24 h, cells were pretreated in free-serum medium with 100 μmol/L NaHS for different time. Then cells were washed twice with PBS and then were cultured with FBS-MEM for 3 days. Cell viability was measured by MTT incorporation assays. (b-c) LDH release. Neonatal mouse cardiomyocytes were plated in 96-well plates. After 24 h, cells were pretreated in free serum with 100 μmol/L NaHS for 12 h. Then cells were washed twice with PBS and were recovered by changing with FBS-MEM. And (b) cells were recovered for 24 h, followed by exposure to free-serum medium without or with 600 μmol/L H2O2 for another 4 h (*P < 0.01 vs. Con); (c) Cells were recovered for indicated time, followed by exposure to free-serum medium with 600 μmol/L H2O2 for another 4 h (*P < 0.05 vs. 0 h). The data shown are mean ± SE of three independent experiments. PBS: Phosphate-buffered saline; FBS: Fetal bovine serum; MEM: Modified Eagle's medium; MTT: 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; LDH: Lactate dehydrogenase; Con: Control; SE: Standard error; NaHS: Sodium hydrosulfide.
Protective effects of pretreated neonatal mouse cardiomyocytes with sodium hydrosulfide
To test the hypothesis that H2S is cytoprotective for neonatal mouse cardiomyocytes, we performed LDH release assays. We found that 100 μmol/L NaHS treatment for 12 h had no obvious effect on LDH release in without H2O2 induce state [Figure 1b]. Under oxidative stress conditions induced by 600 μmol/L H2O2, cardiomyocytes pretreated with 100 μmol/L NaHS for 12 h showed significant decreased LDH release (40.00 ± 2.65% vs. 65.33 ± 4.33%, P < 0.01) [Figure 1b], indicating greater survival after oxidative stress in cells pretreated with 100 μmol/L NaHS. To determine how long the cytoprotection of NaHS may last after cardiomyocytes pretreatment, pretreated cardiomyocytes were subjected to recovery for 12–48 h. We have observed that additional 12 h and 24 h recovery showed better cytoprotective effects (recovery 12 h: 50.00 ± 2.89 vs. 65.33 ± 4.33, P < 0.05; and recovery 24 h: 43.00 ± 2.31 vs. 65.33 ± 4.33, P < 0.05) [Figure 1c].
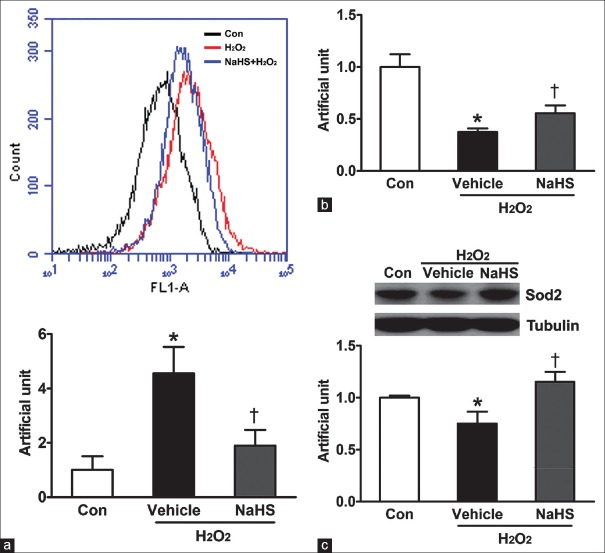
Sodium hydrosulfide ameliorates oxidative stress and improves mitochondrial biogenesis in neonatal mouse cardiomyocytes
It is well known that mitochondria are a major source of superoxide formed by the reaction of respiratory chain enzymes with molecular oxygen. Mitochondria functions play an important role in oxidative stress under both physiologic and pathologic conditions. The ROS generation for the activation of death is important by inhibitor and antioxidative drugs or enzymes studies. To detect the effect of NaHS on antagonizing oxidative stress, we investigated the ROS level of neonatal mouse cardiomyocytes. Our results showed that H2O2-induced substantial ROS production and pretreatment with NaHS obviously decreased H2O2-induced ROS production (4.56 ± 0.56 vs. 1.00 ± 0.29, P < 0.01; 1.90 ± 0.33 vs. 4.56 ± 0.56, P < 0.05) [Figure 2a]. We also evaluated the role of NaHS in mitochondrial biogenesis by measure mtDNA directly. We isolated total DNA and determined the relative copy number of mtDNA by a qPCR assay of the mtDNA-encoded COX II gene. Compared to the control, H2O2 reduced mtDNA content per cell, and NaHS pretreatment reversed the decrease of mtDNA content caused by H2O2 [Figure 2b]. Since Sod2 involves in mitochondrial biogenesis, the expression of Sod2 was tested by Western blotting. When neonatal mouse cardiomyocytes were stimulated with H2O2, the expression of Sod2 exhibited a significant decrease compare to the control [Figure 2c]. However, pretreatment with NaHS significantly ameliorated H2O2-induced decrease in the expression of Sod2 [Figure 2c].
Figure 2.
Hydrogen sulfide ameliorates oxidative stress and improves mitochondrial biogenesis in neonatal mouse cardiomyocytes. Neonatal mouse cardiomyocytes were pretreated in free-serum medium with 100 μmol/L NaHS for 12 h. Consequently, cells were washed twice with PBS and were recovered for 24 h by changing into FBS-MEM, followed by exposure to free-serum medium without or with 600 μmol/L H2O2 for another 4 h. (a) ROS levels were tested by flow cytometry (*P < 0.01 vs. Con, †P < 0.05 vs. vehicle). (b) Mitochondrial DNA copy number was tested by quantitative polymerase chain reaction (*P < 0.001 vs. Con, †P < 0.05 vs. vehicle).(c) Sod2 protein expression level is determined using Western blot analysis (*P < 0.05 vs. Con, †P < 0.01 vs. vehicle). The data shown are mean ± SE of three independent experiments. PBS: Phosphate-buffered saline; FBS: Fetal bovine serum; MEM: Modified Eagle's medium; ROS: Reactive oxygen species; Con: Control; SE: Standard error; NaHS: Sodium hydrosulfide; Sod2: Superoxide dismutase.
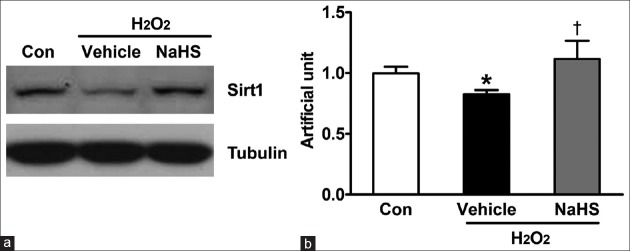
Sodium hydrosulfide increases Sirtuin 1 expression in neonatal mouse cardiomyocytes
Sirt1, as a deacetylation enzyme, has important role in oxidative stress and mitochondrial biogenesis. To study whether Sirt1 involve in H2S-induced anti-oxidative stress, we determined the effect of NaHS on Sirt1 expression. As shown in Figure 3, H2O2 treatment reduced the protein expression of Sirt1, pretreatment with NaHS significantly reversed H2O2-induced decrease in the expression of Sirt1.
Figure 3.
NaHS induces Sirt1 expression in neonatal mouse cardiomyocytes. Neonatal mouse cardiomyocytes were plated in six-well plates. After 24 h, cells were pretreated in free-serum medium with or without 100 μmol/L NaHS for 12 h. Consequently, cells were washed twice with PBS and were recovered for 24h by changing into FBS-MEM, followed by exposure to free-serum medium without or with 600 μmol/L H2O2 for another 4 h. (a) Cells were lysed and Sirt1 protein expression level is determined using Western blot analysis. (b) The bar charts indicating the different intensities of Sirt1 between groups. Values were normalized against the control values. The data shown are mean ± SE of three independent experiments (*P < 0.05 vs. Con, †P < 0.01 vs. vehicle). PBS: Phosphate-buffered saline; FBS: Fetal bovine serum; MEM: Modified Eagle's medium; SE: Standard error; Con: Control; NaHS: Sodium hydrosulfide; Sirt1: Sirtuin 1.
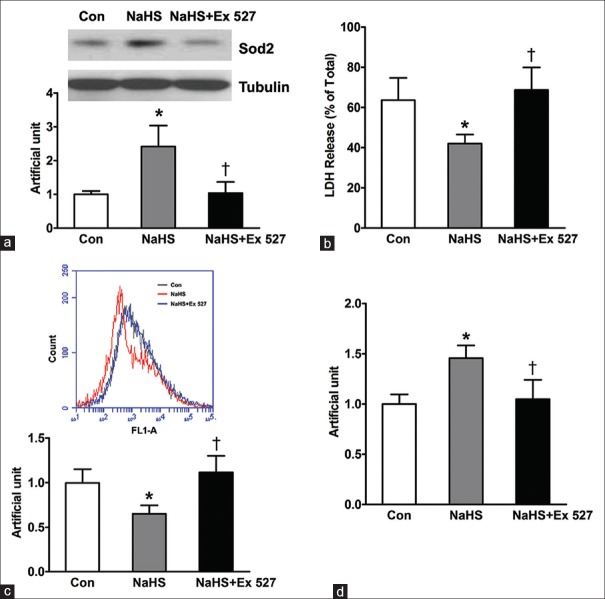
Sirtuin 1 mediates sodium hydrosulfide-induced cytoprotection effects
Since NaHS increased the expression of Sirt1, we further assess whether the inhibitor of Sirt1, Ex 527, abrogates the protective effect of NaHS for neonatal mouse cardiomyocytes. Because NaHS significantly ameliorated H2O2-induced decrease in the expression of Sod2, we first tested the effect of Ex 527 on NaHS-induced Sod2 expression. As shown in Figure 4a, Ex 527 inhibited NaHS-induced Sod2 expression increase. Furthermore, our study showed that the cytoprotective effect of NaHS was also diminished after Ex 527 treatment, as indicated by the significant increase of LDH release (NaHS + Ex 527 vs. NaSH: 68.67 ± 6.49 vs. 42.00 ± 2.65; NaSH + Ex 527 vs. control [Con]: 68.67 ± 6.49 vs. 63.67 ± 6.33, P = 0.611) and mitochondrial ROS production (NaHS + Ex 527 vs. NaSH: 1.12 ± 0.11 vs. 0.65 ± 0.06; NaSH + Ex 527 vs. Con: 1.12 ± 0.11 vs. 1.00 ± 0.09, P = 0.942) following 12-h NaHS pretreatment [Figure 4b and 4c]. In addition, NaHS-induced mtDNA increase was also canceled after Ex 527 treatment [Figure 4d]. These data indicate that Sirt1 plays an essential role in enhancing cardiomyocytes survival following pretreatment with NaHS.
Figure 4.
Sirt1 mediates hydrogen sulfide-induced cytoprotection effect in neonatal mouse cardiomyocytes. Neonatal mouse cardiomyocytes were pretreated in free-serum medium with or without 100 μmol/L NaHS and 10 μmol/L Ex 527 for 12 h as indicated group. Consequently, cells were washed twice with PBS and were recovered for 24h by changing into FBS-MEM, followed by exposure to free-serum medium with 600 μmol/L H2O2 for another 4 h. (a) Sod2 protein expression level is determined using Western blot analysis. (b) LDH release was detected. (c) ROS production was tested by flow cytometry. (d) Mitochondrial DNA number was detected by quantitative polymerase chain reaction. The data shown are mean ± SE of three independent experiments (*P < 0.05 vs. Con, †P < 0.05 vs. NaSH). FBS: Fetal bovine serum; MEM: Modified Eagle's medium; LDH: Lactate dehydrogenase, ROS: Reactive oxygen species; SE: Standard error; Con: Control; NaHS: Sodium hydrosulfide; Sod2: Superoxide dismutase; Sirt1: Sirtuin 1.
DISCUSSION
Pretreatment of myocardium is a well-adaptive response and markedly enhances the ability of the heart resistance to ischemic insult.[1] Many pretreatment strategies have been tested to enhance the survival of cardiomyocytes, including exposure to adenosine, hypoxia or anoxia, and anti-inflammatory or immune treatment.[21,22,23,24] In this study, we have shown that exposure of neonatal mouse cardiomyocytes to NaHS increases the resistance of these cells to H2O2-induced oxidative stress damage, which may be due to the induction of Sirt1 expression and the consequent upregulation of antioxidant enzyme, Sod2.
H2S is a colorless, flammable, and water-soluble gas and has long been regarded as only an environmental hazard for more than 300 years. Now, H2S is also thought to be the third gaseous signaling molecule after nitric oxide and carbon monoxide. H2S has multiple biological actions including the regulation of vasodilation, angiogenesis, inflammation, oxidative stress, and apoptosis.[9] Collective evidence indicated that H2S can regulate cardiovascular functions and has an important role in the pathogenesis and development of cardiovascular diseases by a number of mechanisms such as vasorelaxation, inhibition of cardiovascular remodeling, and resistance to form foam cells.[25] The previous study indicates that tissue content of H2S in the brain has been determined to be between 50 and 160 μmol/L under physiological conditions.[26] In this study, 100 μmol/L NaHS pretreatment had no toxicity to neonatal mouse cardiomyocytes. Our data showed that NaHS pretreatment significantly improved mouse cardiomyocytes survival after H2O2-induced cell death, indicated by the decrease in LDH release and ROS production and that the salubrious effect was accompanied by the up-regulation of Sod2 expression. It is well known that oxidation caused by ROS is a major cause of cellular damage and death and has been implicated in cancer, neurodegenerative, and cardiovascular diseases.[27] ROS formed during oxidative stress can initiate lipid peroxidation, oxidize proteins to inactive states and cause DNA strand breaks, all potentially damaging to normal cellular function. In normal physiological condition, ROS production is usually homeostatically controlled by endogenous free radical scavengers such as Sod, catalase, and the glutathione peroxidase.[28] Sod2 is a key antioxidant enzyme that catalyzes the conversion of superoxide to hydrogen peroxide and molecular oxygen. Sod2 is located within the mitochondrial matrix, the main site of free radical production from the electron transport chain.[29] Mitochondrial superoxide levels are normally controlled by Sod2. Previous studies indicate that Sod2 involves in NaHS-exhibited protection effect on cardiovascular disease, neurodegenerative disorders, chronic kidney disease, etc.[19,29,30,31] The numerous studies have shown that Sod2 has an important role in protection against ROS.[32] Moreover, the decrease in Sod2 activity is associated with increased mitochondrial oxidative damage.[33] Mitochondria are a major source of superoxide and mitochondrial oxidative damage contributes to a wide range of pathologies, including cardiovascular disorders and neurodegenerative diseases. Therefore, protecting mitochondria from oxidative damage should be an effective therapeutic strategy. H2S exerts protective effects on mitochondrial function and respiration. The study showed that pretreatment NaHS improved mtDNA number in mouse cardiomyocyte.
The previous study indicates that Sirt1 could enhance the biosynthesis and degradation of mitochondria, thereby replenishing and improving mitochondrial function.[34] Our result demonstrated NaHS increased the expression of Sirt1 in neonatal mouse cardiomyocyte. Moreover, Ex 527, the inhibitor of Sirt1, attenuated these effects of NaHS-induced Sod2 expression and mtDNA number increase, eventually, and abrogated the cytoprotective effect of NaHS for neonatal mouse cardiomyocytes. Recent studies also showed that H2S prevents H2O2-induced cell damage through Sirt1 activation in human umbilical vein endothelial cells and H9c2 cardiomyocytes.[35,36] In addition, Sirt1 could retard aging and confer stress resistance to the heart in vivo.[13]
All in all, novel approaches for protection and salvage injured heart tissue would have important prospects in the therapy of I/R myocardial injury. In this study, our data show NaHS may prevent H2O2-induced injury to neonatal mouse cardiomyocytes, which is mediated by increasing the expression of Sirt1, furthermore improving mitochondrial function and reducing the deleterious effects of oxidative stress.
Financial support and sponsorship
This study was supported by grants from the National Natural Science Foundation of China (No. 81570443) and the Foundation of Beijing Anzhen Hospital, Capital Medical University (No. 2016P04).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Qiang Shi
REFERENCES
- 1.Ferdinandy P, Schulz R, Baxter GF. Interaction of cardiovascular risk factors with myocardial ischemia/reperfusion injury, preconditioning, and postconditioning. Pharmacol Rev. 2007;59:418–58. doi: 10.1124/pr.107.06002. doi: 10.1124/pr.107.06002. [DOI] [PubMed] [Google Scholar]
- 2.Guo C, Liang F, Shah Masood W, Yan X. Hydrogen sulfide protected gastric epithelial cell from ischemia/reperfusion injury by Keap1 s-sulfhydration, MAPK dependent anti-apoptosis and NF-κB dependent anti-inflammation pathway. Eur J Pharmacol. 2014;725:70–8. doi: 10.1016/j.ejphar.2014.01.009. doi: 10.1016/j.ejphar.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 3.Tapuria N, Kumar Y, Habib MM, Abu Amara M, Seifalian AM, Davidson BR. Remote ischemic preconditioning: A novel protective method from ischemia reperfusion injury – A review. J Surg Res. 2008;150:304–30. doi: 10.1016/j.jss.2007.12.747. doi: 10.1016/j.jss.2007.12.747. [DOI] [PubMed] [Google Scholar]
- 4.Yan SK, Chang T, Wang H, Wu L, Wang R, Meng QH. Effects of hydrogen sulfide on homocysteine-induced oxidative stress in vascular smooth muscle cells. Biochem Biophys Res Commun. 2006;351:485–91. doi: 10.1016/j.bbrc.2006.10.058. doi: 10.1016/j.bbrc.2006.10.058. [DOI] [PubMed] [Google Scholar]
- 5.Ju Y, Zhang W, Pei Y, Yang G. H(2)S signaling in redox regulation of cellular functions. Can J Physiol Pharmacol. 2013;91:8–14. doi: 10.1139/cjpp-2012-0293. doi: 10.1139/cjpp-2012-0293. [DOI] [PubMed] [Google Scholar]
- 6.Theissen U, Hoffmeister M, Grieshaber M, Martin W. Single eubacterial origin of eukaryotic sulfide: Quinone oxidoreductase, a mitochondrial enzyme conserved from the early evolution of eukaryotes during anoxic and sulfidic times. Mol Biol Evol. 2003;20:1564–74. doi: 10.1093/molbev/msg174. doi: 10.1093/molbev/msg174. [DOI] [PubMed] [Google Scholar]
- 7.Li L, Rossoni G, Sparatore A, Lee LC, Del Soldato P, Moore PK. Anti-inflammatory and gastrointestinal effects of a novel diclofenac derivative. Free Radic Biol Med. 2007;42:706–19. doi: 10.1016/j.freeradbiomed.2006.12.011. doi: 10.1016/j.freeradbiomed.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 8.Lagoutte E, Mimoun S, Andriamihaja M, Chaumontet C, Blachier F, Bouillaud F. Oxidation of hydrogen sulfide remains a priority in mammalian cells and causes reverse electron transfer in colonocytes. Biochim Biophys Acta. 2010;1797:1500–11. doi: 10.1016/j.bbabio.2010.04.004. doi: 10.1016/j.bbabio.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 9.Yu XH, Cui LB, Wu K, Zheng XL, Cayabyab FS, Chen ZW, et al. Hydrogen sulfide as a potent cardiovascular protective agent. Clin Chim Acta. 2014;437:78–87. doi: 10.1016/j.cca.2014.07.012. doi: 10.1016/j.cca.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 10.Sivarajah A, McDonald MC, Thiemermann C. The production of hydrogen sulfide limits myocardial ischemia and reperfusion injury and contributes to the cardioprotective effects of preconditioning with endotoxin, but not ischemia in the rat. Shock. 2006;26:154–61. doi: 10.1097/01.shk.0000225722.56681.64. doi: 10.1097/01.shk.0000225722.56681.64. [DOI] [PubMed] [Google Scholar]
- 11.Zhu YZ, Wang ZJ, Ho P, Loke YY, Zhu YC, Huang SH, et al. Hydrogen sulfide and its possible roles in myocardial ischemia in experimental rats. J Appl Physiol. 2007;102:261–8. doi: 10.1152/japplphysiol.00096.2006. doi: 10.1152/japplphysiol.00096.2006. [DOI] [PubMed] [Google Scholar]
- 12.Zschoernig B, Mahlknecht U. SIRTUIN 1: Regulating the regulator. Biochem Biophys Res Commun. 2008;376:251–5. doi: 10.1016/j.bbrc.2008.08.137. doi: 10.1016/j.bbrc.2008.08.137. [DOI] [PubMed] [Google Scholar]
- 13.Alcendor RR, Gao S, Zhai P, Zablocki D, Holle E, Yu X, et al. Sirt1 regulates aging and resistance to oxidative stress in the heart. Circ Res. 2007;100:1512–21. doi: 10.1161/01.RES.0000267723.65696.4a. doi: 10.1161/01.RES.0000267723.65696.4a. [DOI] [PubMed] [Google Scholar]
- 14.Wang GW, Kang YJ. Inhibition of doxorubicin toxicity in cultured neonatal mouse cardiomyocytes with elevated metallothionein levels. J Pharmacol Exp Ther. 1999;288:938–44. [PubMed] [Google Scholar]
- 15.Cai C, Teng L, Vu D, He JQ, Guo Y, Li Q, et al. The heme oxygenase 1 inducer (CoPP) protects human cardiac stem cells against apoptosis through activation of the extracellular signal-regulated kinase (ERK)/NRF2 signaling pathway and cytokine release. J Biol Chem. 2012;287:33720–32. doi: 10.1074/jbc.M112.385542. doi: 10.1074/jbc.M112.385542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eruslanov E, Kusmartsev S. Identification of ROS using oxidized DCFDA and flow-cytometry. Methods Mol Biol. 2010;594:57–72. doi: 10.1007/978-1-60761-411-1_4. doi: 10.1007/978-1-60761-411-1_4. [DOI] [PubMed] [Google Scholar]
- 17.Kong X, Wang R, Xue Y, Liu X, Zhang H, Chen Y, et al. Sirtuin 3, a new target of PGC-1alpha, plays an important role in the suppression of ROS and mitochondrial biogenesis. PLoS One. 2010;5:e11707. doi: 10.1371/journal.pone.0011707. doi: 10.1371/journal.pone.0011707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sivarajah A, Collino M, Yasin M, Benetti E, Gallicchio M, Mazzon E, et al. Anti-apoptotic and anti-inflammatory effects of hydrogen sulfide in a rat model of regional myocardial I/R. Shock. 2009;31:267–74. doi: 10.1097/SHK.0b013e318180ff89. doi: 10.1097/SHK.0b013e318180ff89. [DOI] [PubMed] [Google Scholar]
- 19.Han SJ, Kim JI, Park JW, Park KM. Hydrogen sulfide accelerates the recovery of kidney tubules after renal ischemia/reperfusion injury. Nephrol Dial Transplant. 2015;30:1497–506. doi: 10.1093/ndt/gfv226. doi: 10.1093/ndt/gfv226. [DOI] [PubMed] [Google Scholar]
- 20.Tay AS, Hu LF, Lu M, Wong PT, Bian JS. Hydrogen sulfide protects neurons against hypoxic injury via stimulation of ATP-sensitive potassium channel/protein kinase C/extracellular signal-regulated kinase/heat shock protein 90 pathway. Neuroscience. 2010;167:277–86. doi: 10.1016/j.neuroscience.2010.02.006. doi: 10.1016/j.neuroscience.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 21.Ikonomidis JS, Shirai T, Weisel RD, Derylo B, Rao V, Whiteside CI, et al. Preconditioning cultured human pediatric myocytes requires adenosine and protein kinase C. Am J Physiol. 1997;272(3 Pt 2):H1220–30. doi: 10.1152/ajpheart.1997.272.3.H1220. [DOI] [PubMed] [Google Scholar]
- 22.Verma S, Rao V, Weisel RD, Li SH, Fedak PW, Miriuka S, et al. Novel cardioprotective effects of pravastatin in human ventricular cardiomyocytes subjected to hypoxia and reoxygenation: Beneficial effects of statins independent of endothelial cells. J Surg Res. 2004;119:66–71. doi: 10.1016/j.jss.2003.10.011. doi: 10.1016/j.jss.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 23.Smith RM, Lecour S, Sack MN. Innate immunity and cardiac preconditioning: A putative intrinsic cardioprotective program. Cardiovasc Res. 2002;55:474–82. doi: 10.1016/s0008-6363(02)00288-2. doi: 10.1016/S0008-6363(02)00288-2. [DOI] [PubMed] [Google Scholar]
- 24.Hiasa G, Hamada M, Ikeda S, Hiwada K. Ischemic preconditioning and lipopolysaccharide attenuate nuclear factor-kappaB activation and gene expression of inflammatory cytokines in the ischemia-reperfused rat heart. Jpn Circ J. 2001;65:984–90. doi: 10.1253/jcj.65.984. doi: 10.1253/jcj.65.984. [DOI] [PubMed] [Google Scholar]
- 25.Polhemus DJ, Lefer DJ. Emergence of hydrogen sulfide as an endogenous gaseous signaling molecule in cardiovascular disease. Circ Res. 2014;114:730–7. doi: 10.1161/CIRCRESAHA.114.300505. doi: 10.1161/CIRCRESAHA.114.300505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abe K, Kimura H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J Neurosci. 1996;16:1066–71. doi: 10.1523/JNEUROSCI.16-03-01066.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Waris G, Ahsan H. Reactive oxygen species: Role in the development of cancer and various chronic conditions. J Carcinog. 2006;5:14. doi: 10.1186/1477-3163-5-14. doi: 10.1186/1477.3163.5.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Venardos KM, Perkins A, Headrick J, Kaye DM. Myocardial ischemia-reperfusion injury, antioxidant enzyme systems, and selenium: A review. Curr Med Chem. 2007;14:1539–49. doi: 10.2174/092986707780831078. doi: 10.2174/092986707780831078. [DOI] [PubMed] [Google Scholar]
- 29.Flynn JM, Melov S. SOD2 in mitochondrial dysfunction and neurodegeneration. Free Radic Biol Med. 2013;62:4–12. doi: 10.1016/j.freeradbiomed.2013.05.027. doi: 10.1016/j.freeradbiomed.2013.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jin ZQ, Zhou HZ, Cecchini G, Gray MO, Karliner JS. MnSOD in mouse heart: Acute responses to ischemic preconditioning and ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2005;288:H2986–94. doi: 10.1152/ajpheart.01144.2004. doi: 10.1152/ajpheart.01144.2004. [DOI] [PubMed] [Google Scholar]
- 31.Wen YD, Wang H, Kho SH, Rinkiko S, Sheng X, Shen HM, et al. Hydrogen sulfide protects HUVECs against hydrogen peroxide induced mitochondrial dysfunction and oxidative stress. PLoS One. 2013;8:e53147. doi: 10.1371/journal.pone.0053147. doi: 10.1371/journal.pone.0053147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zelko IN, Mariani TJ, Folz RJ. Superoxide dismutase multigene family: A comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radic Biol Med. 2002;33:337–49. doi: 10.1016/s0891-5849(02)00905-x. doi: 10.1016/S0891-5849(02)00905-X. [DOI] [PubMed] [Google Scholar]
- 33.Van Remmen H, Williams MD, Guo Z, Estlack L, Yang H, Carlson EJ, et al. Knockout mice heterozygous for Sod2 show alterations in cardiac mitochondrial function and apoptosis. Am J Physiol Heart Circ Physiol. 2001;281:H1422–32. doi: 10.1152/ajpheart.2001.281.3.H1422. [DOI] [PubMed] [Google Scholar]
- 34.Sato D, Itami N, Tasaki H, Takeo S, Kuwayama T, Iwata H. Relationship between mitochondrial DNA copy number and SIRT1 expression in porcine oocytes. PLoS One. 2014;9:e94488. doi: 10.1371/journal.pone.0094488. doi: 10.1371/journal.pone.0094488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suo R, Zhao ZZ, Tang ZH, Ren Z, Liu X, Liu LS, et al. Hydrogen sulfide prevents H2O2-induced senescence in human umbilical vein endothelial cells through SIRT1 activation. Mol Med Rep. 2013;7:1865–70. doi: 10.3892/mmr.2013.1417. doi: 10.3892/mmr.2013.1417. [DOI] [PubMed] [Google Scholar]
- 36.Wu D, Hu Q, Liu X, Pan L, Xiong Q, Zhu YZ. Hydrogen sulfide protects against apoptosis under oxidative stress through SIRT1 pathway in H9c2 cardiomyocytes. Nitric Oxide. 2015;46:204–12. doi: 10.1016/j.niox.2014.11.006. doi: 10.1016/j.niox.2014.11.006. [DOI] [PubMed] [Google Scholar]