Abstract
Background:
Postoperative oropharyngeal complications such as dysphagia after anterior cervical spine surgery are some of the least discussed surgery-related complications. The purpose of this retrospective study is to investigate the incidence and possible risk factors for 30-day postoperative dysphagia after anterior cervical discectomy and fusion (ACDF).
Materials and Methods:
This study included 152 consecutive patients who underwent 1- or 2-level ACDF using a rectangular titanium stand-alone cage in our institutes. Surgery-related dysphagia early after surgery was analyzed based on hospital charts. Radiological evaluation of prevertebral soft tissue swelling (PSTS) was performed by comparing plain lateral radiographs of the cervical spine before surgery with those after surgery. The percentage of PSTS (%PSTS) was defined by retropharyngeal soft tissue diameter divided by vertebral diameter. Positive %PSTS was determined when %PSTS exceeded its mean + 2 standard deviations.
Results:
Twelve patients (7.9%) demonstrated prolonged symptoms of dysphagia within 30-day postoperatively. All patients eventually demonstrated satisfactory or acceptable recovery late after surgery, except one case of hypoglossal nerve palsy. %PSTS was significantly highest early after surgery and returned to presurgical levels within 30 days after surgery. Statistical analysis suggested that the positive %PSTS at C3 or C4 level early after surgery was significantly associated with the occurrence of postoperative dysphagia.
Conclusions:
Although the possible reasons for postoperative dysphagia may not only be multifactorial but also be highly surgeon-dependent, such a complication is still underestimated and needs to be carefully resolved. %PSTS appeared to be easy and reliable index to judge the possible risk of postoperative dysphagia.
Keywords: Anterior cervical discectomy and fusion, dysphagia, oropharyngeal complication, prevertebral soft tissue swelling, rectangular titanium stand-alone cage
INTRODUCTION
Currently, titanium stand-alone cages are commonly used in anterior cervical discectomy and fusion (ACDF).[1,2,3,4,5,6,7] ACDF using a rectangular titanium stand-alone cage has been shown to be technically easy, safe, and effective. Cervical intervertebral disc replacement using a titanium stand-alone cage can restore physiologic disc height and provide immediate load-bearing support to the anterior column, and it may promote arthrodesis. However, oropharyngeal complications after ACDF such as dysphagia, hoarseness, or esophageal perforation may be still underestimated although they have been discussed in the literature.[8,9,10,11,12,13,14,15,16,17,18,19,20,21] Their frequency, severity, and possible eventual recovery after surgery need to be carefully considered. The purpose of this study is to investigate the incidence, severity, and possible risk factors for postoperative dysphagia recognized within 30 days after 1- or 2-level ACDF using a rectangular titanium stand-alone cage.
MATERIALS AND METHODS
Patients
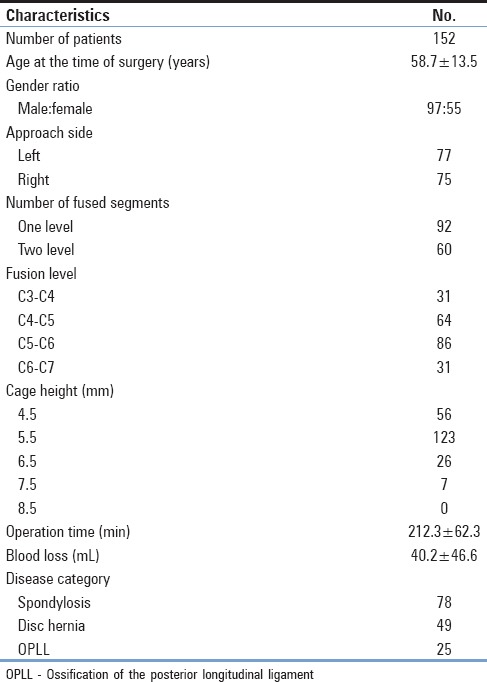
This retrospective study included 152 consecutive patients who underwent 1- or 2-level ACDF with placement of a rectangular titanium stand-alone cage in our institutes from 2008 to 2015. All patients presented with radiculopathy and/or myelopathy due to disc herniation, osteophyte formation, or ossification of the posterior longitudinal ligament. Patients with trauma or who were previously treated by anterior or posterior cervical spine surgery were excluded from the study. Surgery-related oropharyngeal complications early after surgery were carefully analyzed retrospectively based on hospital charts. Records were reviewed for patient age and sex, primary disease of the cervical spine, right- or left-sided anterior approach, fusion level, number of fusions, operative time, estimated blood loss during surgery, and radiological examinations. Patient characteristics are summarized in Table 1.
Table 1.
Summary of patient characteristics
Surgicalprocedure
The surgical procedure was described in our previous publications.[5,7,22] Briefly, patients were placed in the supine position under general anesthesia. In cases of severe anterior compression, intubation was performed using a fiberoptic device. The endotracheal cuff was inflated during general anesthesia in all cases. The patient's head was mildly extended and secured under fluoroscopic guidance. The anterior cervical spine was approached from the symptomatic side, with reference to imaging studies. A standard anterior cervical approach was used. A self-retraction system (Trimline; Medtronic, Inc., Memphis, TN, USA) was preferentially used in the majority of cases. All disc tissue, including herniated disc fragments and osteophytes, was removed meticulously under a surgical microscope. During the decompression procedure, damage to the vertebral endplates was scrupulously avoided. The posterior longitudinal ligament was partially resected to confirm decompression of the neural structures. To accomplish interbody fusion, a rectangular titanium stand-alone cage (DePuy Synthes, Johnson & Johnson, USA) was used. Cage trials were used to determine the appropriate size. Cages with a curved shape were preferred. Graft material packed into the cage was either autologous cancellous bone, β-tricalcium phosphate granules, or self-organized hydroxyapatite-collagen composite. The cage was inserted into the disc space under interbody distraction or cervical traction. The cage position was aligned to the anterior vertical line as closely as possible, with careful fluoroscopic guidance. All patients were permitted to walk the next day, and they were kept in a soft neck collar for at least 2 weeks after surgery.
Swallowing evaluation
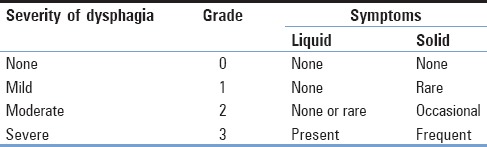
Swallowing difficulty was evaluated on 2 or 3, 7, and 30 days after surgery and was further diagnosed by an otolaryngologist if necessary. Oropharyngeal dysphagia was graded into 4 categories based on the patient's symptoms [Table 2].[9] Severity was graded as none, mild, moderate, or severe dysphagia. Patients who had no episodes of swallowing difficulty were graded as 0. Those who had only a rare episode of swallowing difficulty were graded as 1. Those who had an occasional episode of swallowing difficulty only with specific solid foods were graded as 2. Those who had frequent episodes of swallowing difficulty with liquid or the majority of solid foods were graded as 3.
Table 2.
Bazaz dysphagia grade
Radiological evaluation
Radiological evaluation of prevertebral soft tissue swelling (PSTS) was performed by comparing plain lateral radiographs of the cervical spine obtained before surgery to those at 2 or 3, 7, 30, and 60–90 days after surgery.[12] The percentage of PSTS (%PSTS) was calculated for each level of the cervical spine from C3 to C7 using the following formula as demonstrated in Figure 1: %PSTS = retropharyngeal soft tissue diameter/vertebral diameter × 100. Positive %PSTS was determined when %PSTS exceeded its mean + 2 standard deviations.
Figure 1.
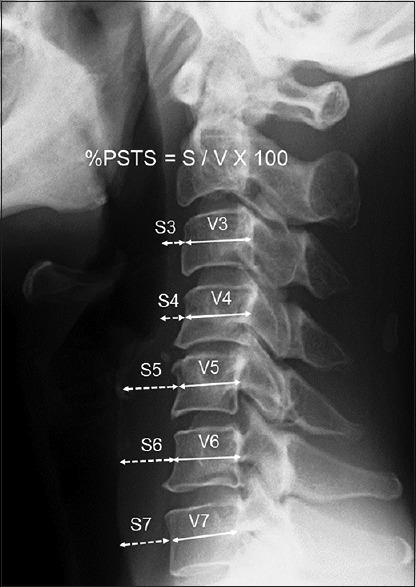
Radiological evaluation of prevertebral soft tissue swelling. The percentage of prevertebral soft tissue swelling was calculated for each level of the cervical spine from C3 to C7. Positive percentage of prevertebral soft tissue swelling was determined when percentage of prevertebral soft tissue swelling exceeded its mean + 2 standard deviations
Statistical analysis
All data are expressed as means ± standard deviation. JMP version 9.0 software (SAS Institute, Cary, NC, USA) was used for all statistical analyses in the present study. Univariate analysis was performed to examine for the presence of an association between study variables and dysphagia after surgery. The Chi-square test was used to test the relationship between them. Multivariate analysis was performed using a logistic regression model. The relationship between each factor and dysphagia after surgery is presented in terms of an odds ratio and a 95% confidence interval. P < 0.05 was considered significant. The sensitivity and specificity of %PSTS were determined by receiver operating characteristic (ROC) curves, and the areas under the curve were calculated.[23]
Statement of ethics
The authors certify that all applicable institutional and governmental regulations concerning the ethical use of clinical data were adhered to for the present study. This retrospective outcome analysis of spine surgery was approved by the Ethics Committee of Osaka City University Graduate School of Medicine.
RESULTS
In total, 12 of 152 patients (7.9%) demonstrated symptoms of mild, moderate, or severe dysphagia within 30 days postoperatively. Swallowing evaluation revealed mild dysphagia of Grade 1 in 5 patients, moderate dysphagia of Grade 2 in 5 patients, and severe dysphagia of Grade 3 in 2 patients. Statistical analysis indicated that none of the factors such as age at surgery, sex, approach side, operation time, or blood loss except the fusion level and number of fused segments affected the incidence of dysphagia within 30 postoperative days (PODs) [Table 3]. Although the possible reasons why these patients demonstrated symptoms of mild or moderate dysphagia appeared to be multifactorial and not certainly determined, one patient demonstrated prolonged symptoms of approach-sided hypoglossal nerve palsy [Figure 2]. No patients had esophageal perforation in the present study. All patients eventually demonstrated satisfactory or acceptable recovery within 30 PODs except the case of hypoglossal nerve palsy.
Table 3.
Univariate analysis of factors related to occurrence of dysphagia within 30 postoperative days
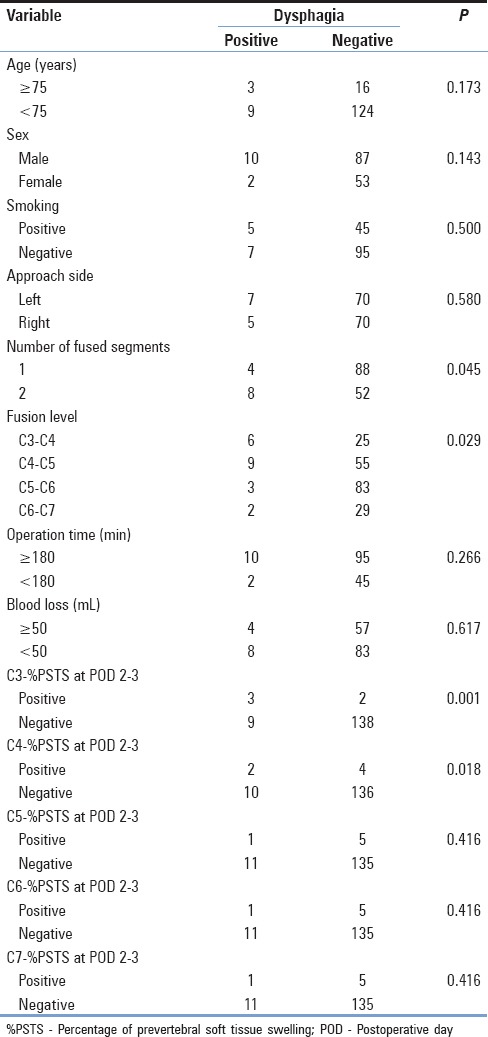
Figure 2.
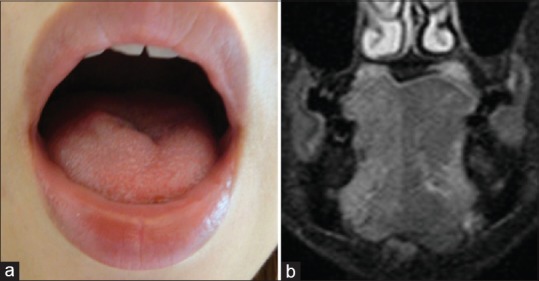
Case of hypoglossal nerve palsy after surgery, (a) photograph taken early after surgery demonstrating the hypoglossal nerve palsy on the right side. (b) Short-tau inversion recovery of magnetic resonance imaging late after surgery demonstrating high signal intensity on the right side of the tongue, suggesting hypoglossal nerve palsy
%PSTS before surgery and at 2 or 3, 7, 30, and 60–90 days after surgery was expressed as mean ± standard deviation at each spine level of the cervical spine. %PSTS was highest at 2 or 3 days after surgery and returned to preoperative levels within 1 month after surgery [Figure 3]. %PSTS early after surgery was significantly higher than %PSTS before surgery. Positive %PSTS with dysphagia was recognized in three participants at the C3 level, in two participants at the C4 level, and in one participant at the C5, C6, and C7 levels, respectively. Univariate analysis suggested that fusion level, positive %PSTS at C3 (C3-%PSTS) and C4 (C4-%PSTS) at 2 or 3 days after surgery were the significant factors affecting the incidence of postoperative dysphagia [Table 3]. Multivariate analysis revealed that the positive C3-%PSTS at 2 or 3 days after surgery was significantly associated with postoperative dysphagia within 30 days [Table 4]. Patients with positive C3-%PSTS at 2 or 3 days after surgery are 8.7 times more likely to have postoperative dysphagia, and patients with positive C4-%PSTS at 2 or 3 days after surgery are 4.7 times more likely to have postoperative dysphagia.
Figure 3.
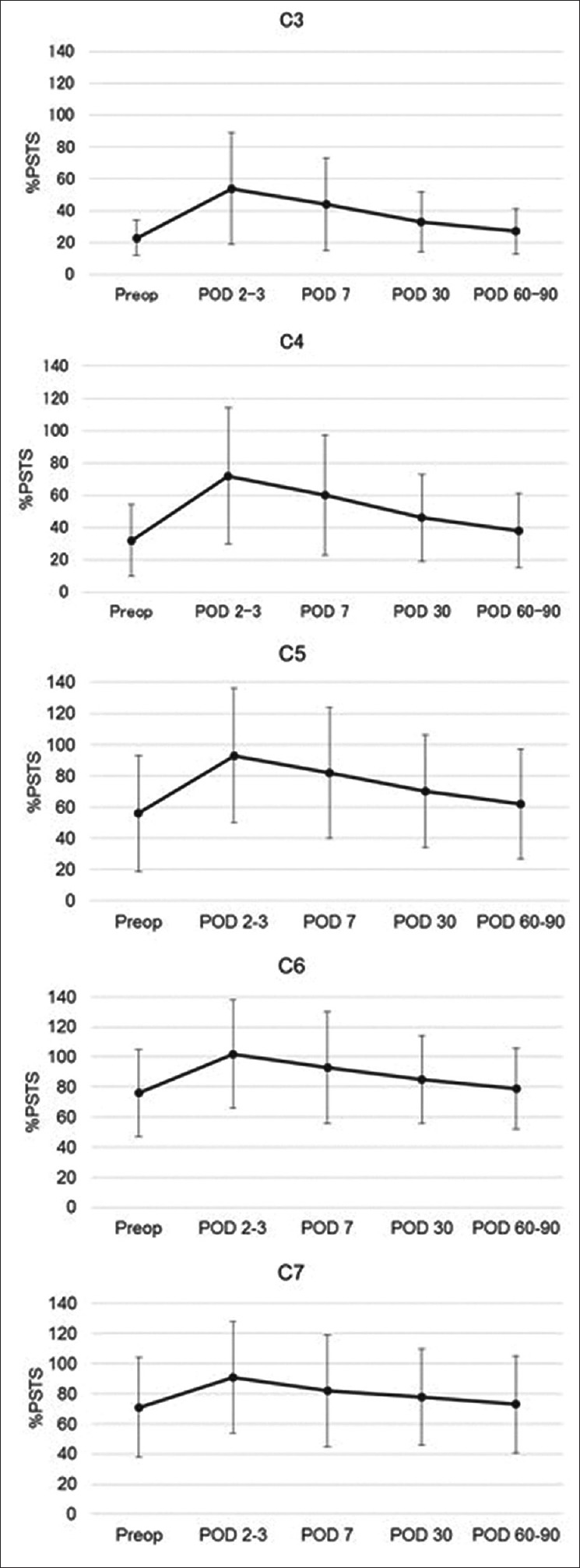
Graphs showing the sequential change in percentage of prevertebral soft tissue swelling before surgery and at 2 or 3, 7, 30, and 60–90 days after surgery, which is expressed as the mean ± standard deviation at each cervical spine level. Percent prevertebral soft tissue swelling was highest at 2 or 3 days after surgery and returned to preoperative levels within 30 days after surgery
Table 4.
Multivariate analysis using a logistic regression model of factors related to occurrence of dysphagia within 30 postoperative days
Receiver operating characteristic analysis
ROC curves of C3-%PSTS and C4-%PSTS at 2 or 3 days after surgery are demonstrated in Figure 4. Both of them had a predictable validity for postoperative dysphagia. The area under the curve in C3-%PSTS and C4-%PSTS was 0.773 and 0.762, respectively. From the results of ROC curve analysis, C3-%PSTS at 2 or 3 days after surgery of 71% was considered to be the good diagnostic cutoff value and had 58% sensitivity and 89% specificity, while C4-%PSTS at 2 or 3 days after surgery of 77% had 83% sensitivity and 66% specificity.
Figure 4.
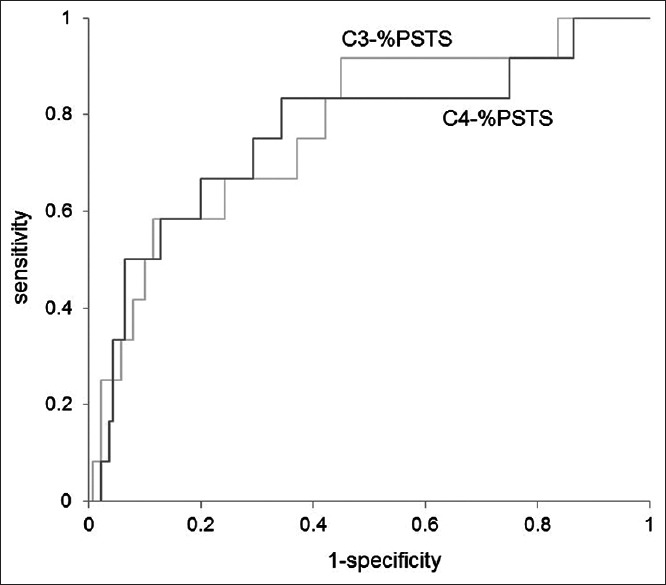
Receiver operating characteristic curves for C3-percentage of prevertebral soft tissue swelling C4-percentage of prevertebral soft tissue swelling at 2 or 3 days after surgery. An increase in percentage of prevertebral soft tissue swelling is a valid diagnostic indicator to predict the incidence of postoperative dysphagia. The area under the curve in C3-percentage of prevertebral soft tissue swelling and C4- percentage of prevertebral soft tissue swelling was 0.773 and 0.762, respectively. C3-percentage of prevertebral soft tissue swelling at 2 or 3 days after surgery of 71% was considered to be the good diagnostic cutoff value and had 58% sensitivity and 89% specificity, while C4-percentage of prevertebral soft tissue swelling at 2 or 3 days after surgery of 77% had 83% sensitivity and 66% specificity
Illustrative case 1
An 81-year-old man presented with a gradual onset of tetraparesis. Neurological examination revealed severe cervical myelopathy. Severe impairment of sensory function of the bilateral upper extremities was noted. Imaging studies suggested the origin of the myelopathy was at C4-5. The patient underwent 1-level ACDF using a rectangular titanium stand-alone cage with a left-sided approach. Although the surgery was well done without any troubles, plain lateral radiographs of the cervical spine clearly suggested the significant PSTS at 2 days after surgery that gradually recovered with 2 weeks [Figure 5a–c]. Dysphagia early after surgery was evident as Grade 2, but gradually improved to Grade 0 within 2 weeks after surgery. Although his early postoperative course was improved, aspiration pneumonia developed early after surgery [Figure 5d]. Possible reason for aspiration pneumonia may be decreased gag reflex early after surgery. A fasting treatment resulted in resolution of the aspiration pneumonia.
Figure 5.
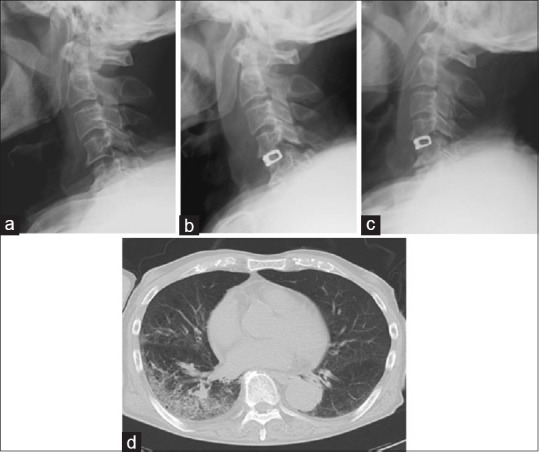
Illustrative sequential images of lateral cervical radiographs before surgery (a) and at 2 (b), and 2 weeks (c) after surgery. C3-percentage of prevertebral soft tissue swelling and C4-percentage of prevertebral soft tissue swelling at 2 days after surgery was 91% and 123%, respectively. (d) Lung computed tomography scans demonstrating the typical images of postoperative aspiration pneumonia
Illustrative case 2
A 72-year-old man presented with gradual onset of moderate disability of the bilateral upper extremities. Neurological examination revealed moderate cervical myelopathy. Moderate impairment of sensory function of the bilateral upper extremities was noted. Imaging studies suggested the origin of the myelopathy was at C3-4-5. The patient underwent 2-level ACDF using a rectangular titanium stand-alone cage with a left-sided approach. Although the surgery was well done without any troubles, the patient complained of moderate dysphagia. Plain lateral radiographs of the cervical spine clearly suggested the significant PSTS at 2 days after surgery that gradually recovered with 4 weeks [Figure 6a–c]. Dysphagia early after surgery was evident as Grade 2, but gradually improved to Grade 0 within 2 weeks after surgery. Laryngoscopy 3 days after surgery revealed slow clearance at the level of the epiglottis, suggesting dysphagia. A fasting treatment resulted in improvement of the dysphagia [Figure 6d and e].
Figure 6.
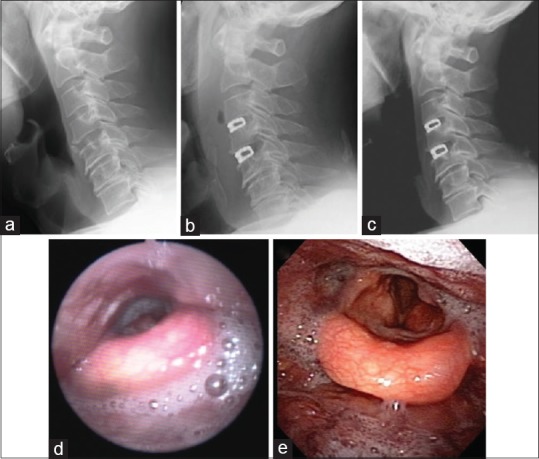
Illustrative sequential images of lateral cervical radiographs before surgery (a) and at 2 (b), and 4 weeks (c) after surgery. C3-percentage of prevertebral soft tissue swelling and C4-percentage of prevertebral soft tissue swelling at 2 days after surgery was 88% and 92%, respectively. Laryngoscopy at 3 days after surgery revealing slow clearance at the level of the epiglottis, suggesting dysphagia (d), which was improved by a fasting treatment (e)
DISCUSSION
The true incidence of oropharyngeal complications after anterior cervical spine surgery is still unknown and varies widely in the literature. The incidence of dysphagia within 1 week after anterior cervical spine surgery varies from 1% to 79%.[9,13,14,15,16,17] This wide variation may be attributed to differences in patient age and sex, surgical technique, extent of surgery, spine level, operative time, or use of spinal instrumentation. Bazaz et al. reported that postoperative dysphagia was found in 50.2%, 32.2%, 17.8%, and 12.5% at 1, 2, 6, and 12 months, respectively.[9] However, only 4.8% of those patients were still experiencing moderate or severe dysphagia at 6 months after surgery. Surgery at multiple disc levels increased the risk of postoperative dysphagia at 1 and 2 months. Although its etiology was poorly understood in majority of their cases, motor paralysis of the larynx was found in 1.3% of patients at 12 months after surgery. Singh et al. performed a retrospective database analysis of a total of 159, 590 cases of anterior cervical fusion (ACF).[16] The incidence of dysphagia in three or more heat ACF was double that of 1- or 2-level fusions. Dysphagia was more common in men who underwent 1- or 2-level surgery. The use of bone morphogenetic proteins was linked to an increased incidence of postoperative dysphagia in 1- or 2-level surgery. Wu et al. retrospectively reviewed the medical charts of 358 patients who underwent anterior cervical decompression and fusion.[21] All patients were followed for 6 months postoperatively. The incidence of dysphagia was 10.9% at 1–5 days, 6.4% at 3 months, and 2.7% at 6 months after surgery. Although there were no patients with severe dysphagia at 3 months after surgery, mild or moderate dysphagia slightly affected quality of life. They suggested that multilevel cervical spine and high-level cervical spine surgeries are strong risk factors for postoperative dysphagia. Olsson et al. performed a cross-sectional cohort study of 100 patients who had undergone ACDF.[18] The rate of postoperative dysphagia at an average of 2.75 years was 26%. Rare and mild dysphagia was reported by 2% and 7% of patients, respectively. Moderate and severe dysphagia was reported by 12% and 5%, respectively. Smokers were more likely to report dysphagia symptoms. They suggested that dysphagia symptoms persist in a significant proportion of patients >1 year after ACDF. Carucci et al. analyzed a radiologic evaluation of modified barium swallow studies and esophagrams in 74 patients who demonstrated dysphagia secondary to ACF.[17] Serious complications of ACF that resulted in dysphagia included surgical hardware displacement or bone graft displacement, esophageal perforation, and retropharyngeal abscess. Pharyngeal functional abnormalities were detected in 50 patients (67.6%), with penetration, aspiration, or both, seen in 32 patients (43.2%). The possible reasons for postoperative dysphagia may be multifactorial and the relation between dysphagia and PSTS is still under debate. Song et al. found a relation between marked PSTS and dysphagia at 3 months after anterior cervical spine surgery.[14] On the other hand, Stachniak et al. found no correlation between PSTS and dysphagia at 2 weeks after the procedure.[24] Khaki et al. failed to show a relation between PSTS and the development of chronic dysphagia in a cohort of 67 patients undergoing multilevel surgery.[25] Kang et al. evaluated factors related to swallowing dysfunction after ACDF using videofluoroscopic swallowing studies (VFSS).[26] They found that patients after ACDF with their highest surgery level at C3 and C4 showed more abnormal VFSS findings, significantly increased soft tissue thickness, and decreased maximal distance of upper esophageal sphincter opening. Hyoid bone movement did not change significantly after surgery. In the present study, positive C3-%PSTS or positive C4-%PSTS at 2 or 3 days after surgery was the significant factor affecting the incidence of postoperative dysphagia within 30 days. Possible anatomical reasons for the occurrence of dysphagia after ACDF may include decreased gag reflex, decreased pharyngeal clearance, or decreased maximal distance of upper esophageal sphincter opening. Anatomical complex between hyoid bone and pharynx at the spine level of C3 or C4 may affect the incidence of postoperative dysphagia.
In the present study, unfortunately, we encountered 1 exceptional patient who demonstrated prolonged symptoms of hypoglossal nerve palsy. Although there was not any clear mechanism for the hypoglossal nerve palsy, its anatomical exception was suggested based on the operative findings. The incidence of hypoglossal nerve palsy after ACDF is not well known. The hypoglossal nerve consistently travels toward the midline at the C2-3 level and is considered to be safely caudal to C3-4.[27] There is no appreciable side-to-side variation. Its anatomical course is reliably consistent. Sengupta et al. reported hypoglossal nerve injury as a complication of anterior C2-5 corpectomy and fusion.[8] They reported that the hypoglossal nerve palsy persisted, although minimal functional disability remained at 18 months after surgery. The only other case report of a patient with hypoglossal nerve palsy after anterior cervical spine surgery also failed to recover. The authors concluded that hypoglossal nerve palsy after anterior cervical spine surgery is unlikely to recover spontaneously and suggested that the nerve should be carefully identified. Although an anterior cervical approach below the C3-4 level may be safe for the avoidance of hypoglossal nerve injury, surgeons should be aware of the regional anatomy as well as possible anatomical exceptions.
CONCLUSIONS
Twelve of 152 patients (7.9%) demonstrated prolonged symptoms of mild or moderate dysphagia within 30 PODs. %PSTS was most severe at 2 or 3 days after surgery and returned to preoperative levels within 30 days after surgery. All patients eventually demonstrated satisfactory or acceptable recovery late after surgery, except one case of prolonged symptoms of hypoglossal nerve palsy. Statistical analysis suggested that the positive C3-%PSTS or C4-%PSTS at 2 or 3 days after surgery was significantly associated with postoperative dysphagia. Patients with positive C3-%PSTS at 2 or 3 days after surgery are 8.7 times more likely to have postoperative dysphagia, and patients with positive C4-%PSTS at 2 or 3 days after surgery are 4.7 times more likely to have postoperative dysphagia. Although the possible reasons for postoperative dysphagia may not only be multifactorial but also be highly surgeon-dependent, such a complication is still underestimated and needs to be carefully resolved. %PSTS appeared to be easy and reliable index to judge the degree of postoperative dysphagia.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Schmieder K, Wolzik-Grossmann M, Pechlivanis I, Engelhardt M, Scholz M, Harders A, et al. Subsidence of the wing titanium cage after anterior cervical interbody fusion: 2-year follow-up study. J Neurosurg Spine. 2006;4:447–53. doi: 10.3171/spi.2006.4.6.447. [DOI] [PubMed] [Google Scholar]
- 2.Kolstad F, Nygaard ØP, Andresen H, Leivseth G. Anterior cervical arthrodesis using a “stand alone” cylindrical titanium cage: Prospective analysis of radiographic parameters. Spine (Phila Pa 1976) 2010;35:1545–50. doi: 10.1097/BRS.0b013e3181d259c1. [DOI] [PubMed] [Google Scholar]
- 3.Tani S, Nagashima H, Isoshima A, Akiyama M, Ohashi H, Tochigi S, et al. A unique device, the disc space-fitted distraction device, for anterior cervical discectomy and fusion: Early clinical and radiological evaluation. J Neurosurg Spine. 2010;12:342–6. doi: 10.3171/2009.10.SPINE09283. [DOI] [PubMed] [Google Scholar]
- 4.Sugawara T, Itoh Y, Hirano Y, Higashiyama N, Mizoi K. β-tricalcium phosphate promotes bony fusion after anterior cervical discectomy and fusion using titanium cages. Spine (Phila Pa 1976) 2011;36:E1509–14. doi: 10.1097/BRS.0b013e31820e60d9. [DOI] [PubMed] [Google Scholar]
- 5.Yamagata T, Takami T, Uda T, Ikeda H, Nagata T, Sakamoto S, et al. Outcomes of contemporary use of rectangular titanium stand-alone cages in anterior cervical discectomy and fusion: Cage subsidence and cervical alignment. J Clin Neurosci. 2012;19:1673–8. doi: 10.1016/j.jocn.2011.11.043. [DOI] [PubMed] [Google Scholar]
- 6.Iwasaki K, Ikedo T, Hashikata H, Toda H. Autologous clavicle bone graft for anterior cervical discectomy and fusion with titanium interbody cage. J Neurosurg Spine. 2014;21:761–8. doi: 10.3171/2014.7.SPINE131000. [DOI] [PubMed] [Google Scholar]
- 7.Yamagata T, Naito K, Arima H, Yoshimura M, Ohata K, Takami T, et al. A minimum 2-year comparative study of autologous cancellous bone grafting versus beta-tricalcium phosphate in anterior cervical discectomy and fusion using a rectangular titanium stand-alone cage. Neurosurg Rev. 2016;39:475–82. doi: 10.1007/s10143-016-0714-y. [DOI] [PubMed] [Google Scholar]
- 8.Sengupta DK, Grevitt MP, Mehdian SM. Hypoglossal nerve injury as a complication of anterior surgery to the upper cervical spine. Eur Spine J. 1999;8:78–80. doi: 10.1007/s005860050131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bazaz R, Lee MJ, Yoo JU. Incidence of dysphagia after anterior cervical spine surgery: A prospective study. Spine (Phila Pa 1976) 2002;27:2453–8. doi: 10.1097/00007632-200211150-00007. [DOI] [PubMed] [Google Scholar]
- 10.Pompili A, Canitano S, Caroli F, Caterino M, Crecco M, Raus L, et al. Asymptomatic esophageal perforation caused by late screw migration after anterior cervical plating. Spine (Phila Pa 1976) 2002;27:499–502. doi: 10.1097/00007632-200212010-00016. [DOI] [PubMed] [Google Scholar]
- 11.Patel NP, Wolcott WP, Johnson JP, Cambron H, Lewin M, McBride D, et al. Esophageal injury associated with anterior cervical spine surgery. Surg Neurol. 2008;69:20–4. doi: 10.1016/j.surneu.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 12.Lee SH, Kim KT, Suk KS, Park KJ, Oh KI. Effect of retropharyngeal steroid on prevertebral soft tissue swelling following anterior cervical discectomy and fusion: A prospective, randomized study. Spine (Phila Pa 1976) 2011;36:2286–92. doi: 10.1097/BRS.0b013e318237e5d0. [DOI] [PubMed] [Google Scholar]
- 13.Skeppholm M, Ingebro C, Engström T, Olerud C. The dysphagia short questionnaire: An instrument for evaluation of dysphagia: A validation study with 12 months' follow-up after anterior cervical spine surgery. Spine (Phila Pa 1976) 2012;37:996–1002. doi: 10.1097/BRS.0b013e31823a7a5b. [DOI] [PubMed] [Google Scholar]
- 14.Song KJ, Choi BW, Kim HY, Jeon TS, Chang H. Efficacy of postoperative radiograph for evaluating the prevertebral soft tissue swelling after anterior cervical discectomy and fusion. Clin Orthop Surg. 2012;4:77–82. doi: 10.4055/cios.2012.4.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cho SK, Lu Y, Lee DH. Dysphagia following anterior cervical spinal surgery: A systematic review. Bone Joint J. 2013;95-B:868–73. doi: 10.1302/0301-620X.95B7.31029. [DOI] [PubMed] [Google Scholar]
- 16.Singh K, Marquez-Lara A, Nandyala SV, Patel AA, Fineberg SJ. Incidence and risk factors for dysphagia after anterior cervical fusion. Spine (Phila Pa 1976) 2013;38:1820–5. doi: 10.1097/BRS.0b013e3182a3dbda. [DOI] [PubMed] [Google Scholar]
- 17.Carucci LR, Turner MA, Yeatman CF. Dysphagia secondary to anterior cervical fusion: Radiologic evaluation and findings in 74 patients. AJR Am J Roentgenol. 2015;204:768–75. doi: 10.2214/AJR.14.13148. [DOI] [PubMed] [Google Scholar]
- 18.Olsson EC, Jobson M, Lim MR. Risk factors for persistent dysphagia after anterior cervical spine surgery. Orthopedics. 2015;38:e319–23. doi: 10.3928/01477447-20150402-61. [DOI] [PubMed] [Google Scholar]
- 19.Erwood MS, Hadley MN, Gordon AS, Carroll WR, Agee BS, Walters BC, et al. Recurrent laryngeal nerve injury following reoperative anterior cervical discectomy and fusion: A meta-analysis. J Neurosurg Spine. 2016;25:198–204. doi: 10.3171/2015.9.SPINE15187. [DOI] [PubMed] [Google Scholar]
- 20.Halani SH, Baum GR, Riley JP, Pradilla G, Refai D, Rodts GE, Jr, et al. Esophageal perforation after anterior cervical spine surgery: A systematic review of the literature. J Neurosurg Spine. 2016;25:285–91. doi: 10.3171/2016.1.SPINE15898. [DOI] [PubMed] [Google Scholar]
- 21.Wu B, Song F, Zhu S. Reasons of dysphagia after operation of anterior cervical decompression and fusion. Clin Spine Surg. 2016 doi: 10.1097/BSD.0000000000000180. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 22.Hakuba A. Trans-unco-discal approach. A combined anterior and lateral approach to cervical discs. J Neurosurg. 1976;45:284–91. doi: 10.3171/jns.1976.45.3.0284. [DOI] [PubMed] [Google Scholar]
- 23.Hanley JA, McNeil BJ. A representation and interpretation of the area under a receiver operating characteristic (ROC) Radiology. 1982;143:29–36. doi: 10.1148/radiology.143.1.7063747. [DOI] [PubMed] [Google Scholar]
- 24.Stachniak JB, Diebner JD, Brunk ES, Speed SM. Analysis of prevertebral soft-tissue swelling and dysphagia in multilevel anterior cervical discectomy and fusion with recombinant human bone morphogenetic protein-2 in patients at risk for pseudarthrosis. J Neurosurg Spine. 2011;14:244–9. doi: 10.3171/2010.9.SPINE09828. [DOI] [PubMed] [Google Scholar]
- 25.Khaki F, Zusman NL, Nemecek AN, Ching AC, Hart RA, Yoo JU. Postoperative prevertebral soft tissue swelling does not affect the development of chronic dysphagia following anterior cervical spine surgery. Spine (Phila Pa 1976) 2013;38:E528–32. doi: 10.1097/BRS.0b013e31828a2992. [DOI] [PubMed] [Google Scholar]
- 26.Kang SH, Kim DK, Seo KM, Lee SY, Park SW, Kim YB, et al. Swallowing function defined by videofluoroscopic swallowing studies after anterior cervical discectomy and fusion: A prospective study. J Korean Med Sci. 2016;31:2020–5. doi: 10.3346/jkms.2016.31.12.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haller JM, Iwanik M, Shen FH. Clinically relevant anatomy of high anterior cervical approach. Spine (Phila Pa 1976) 2012;15:97–100. doi: 10.1097/BRS.0b013e31820408af. [DOI] [PubMed] [Google Scholar]


