Abstract
Botanical monoterpenes are secondary metabolites present in essential oils produced by plants. Some of them are insect repellents. The bloodsucking bug Rhodnius prolixus Ståhl (Hemiptera: Reduviidae) is one of the main vectors of Chagas disease in the north of South America and some countries in Central America. In this study, we studied the repellence produced by two monoterpenes, menthyl acetate and geraniol, on fifth instar nymphs of R. prolixus . In the absence of other stimuli, both menthyl acetate and geraniol produced a repellent effect from 740 μg/cm 2 and 74 μg/cm 2 , respectively. Pre-exposure to each monoterpene reduced the repellent activity produced by the same substance. Additionally, pre-exposure to one monoterpene decreased the behavioral response of the nymphs to the other one. The repellent effect of both monoterpenes also decreased when nymphs’ antennae were previously treated with the nitric oxide donor S-nitroso-N-acetyl-cysteine.
Keywords: blood-sucking bug, geraniol, menthyl acetate
Essential oils are plant products obtained by hydrodistillation or other methods ( Isman and Machial 2006 ). This complex of compounds is produced by plants, giving them their characteristic smell and taste, and is usually composed of 20–80 or more substances ( Regnault-Roger et al. 2012 ). Their main components are monoterpenes (C 10 ) and sesquiterpenes (C 15 ) derived from isoprene.
Although there are exceptions, most monoterpenes present in essential oils have very low toxicity to mammals and are rapidly degraded in the environment ( Isman 2000 ). These characteristics and the discovery of the insecticide and repellent effects of some monoterpenes have awoken great interest in the possibility of using these compounds as alternative tools for pest control. Several monoterpenes have been reported as insect repellents ( Isman 2006 ).
An insect repellent is a substance that causes an organism to move away from the odor source ( Dethier et al. 1960 ). Insects perceive the volatile repellents by smell ( Bohbot and Dickens 2012 ). Many studies have explored the repellent properties of essential oils and their components. Most studies have been performed on mosquitoes with the purpose of identifying products to protect people from their bites ( Barnard 1999 , Ansari et al. 2000 , Choi et al. 2002 , Odalo et al. 2005 , Erler et al. 2006 , Maguranyi et al. 2009 , Sritabutra et al. 2011 ). To a lesser degree, repellent effects have also been studied on other hematophagous insects like lice and bloodsucking bugs ( Toloza et al. 2006 , Vilaseca et al. 2008 , Sfara et al. 2009 , Sainz et al. 2012 ) and on stored product pests ( García et al. 2005 , Wang et al. 2006 , Chaubey 2007 , Goel et al. 2007 ).
In insects exposed to repellents and other odors, the response of neurons subjected to stimulation depends on the chemical structure of the stimulus, its duration and intensity, previous neuronal experience, and the capacity to adapt to new situations produced by environmental changes or the individual’s development ( Colbert and Bargmann 1995 ). Sensory systems have the ability to adjust their sensitivity to the different intensities of a stimulus ( Zufall and Leinders-Zufall 2000 ). It implies a decrease in the capacity to perceive a stimulus, moving the perception threshold to higher concentrations, or producing a shift in the stimulus–response curve to higher concentrations.
A decrease in olfactory responses after exposure to specific odors has been described in moths, Trichoplusia ni ( Kuenen and Baker 1981 ) and Manduca sexta L. ( Dolzer et al. 2003 ), and silkworms, Antheraea polyphemus (Cramer) and Bombyx mori L. ( Ziegelberger et al. 1990 ), exposed to their respective sexual pheromones. It has also been reported for Drosophila melanogaster (Meigen) larvae exposed to alcohols, acids, and acetates ( Cobb and Domain 2000 ), benzaldehyde or isoamyl acetate ( Devaud et al. 2001 ).
In olfactory receptor neurons, the chemical interaction between the odor molecule and the receptor is dynamic and reversible. In a saturated system, molecules are continuously interacting with the receptor ( Zufall and Leinders-Zufall 2000 ). The result of this interaction is that a high amount of calcium enters the neuron, and this increase of intracellular calcium triggers an alternative signaling pathway that results in proportional responsiveness of the neuron to higher concentrations of the odor. In this way, the neuron (and the whole sensory system) adjusts its responsiveness to higher intensities of a stimulus that in normal conditions would be indistinguishable. This phenomenon is called sensory adaptation.
Nitric oxide (NO) is a colorless, slightly soluble water gas ( Liptrot 1980 ). In living beings, NO is produced during the conversion of l -arginine to l -citrulline, a reaction catalyzed by the NO synthase activity ( Bredt and Snyder 1992 ). NO acts as a molecular messenger in bacteria, protozoa, fungi, plants, and animals ( Torreilles 2001 ). Its main intracellular target is the heme group of soluble guanilate cyclase. NO activates this enzyme, which catalyzes cGMP synthesis. In turn, this nucleotide activates protein kinase G that regulates the conductivity of the ionic channels and the activity of phosphodiesterases ( Stamler et al. 1997 ). This series of reactions is known as the NO/cGMP system.
The NO/cGMP system participates in a variety of physiological processes related to the development and function of the insect’s nervous system, e.g., the formation of the visual system in fruit flies ( Gibbs and Truman 1998 ), the growth of axons forming the neuronal network in the antennae of the grasshopper Schistocerca gregaria (Forsskål) ( Seidel and Bicker 2000 ), the formation of long term memory in bees ( Müller 2000 ), and the pattern of the rhythmic movements of the ovipositor valves digging the ground where S. gregaria females deposit their eggs ( Newland and Yates 2007 , 2008 ). Regarding the sense of smell, evidences from experiments on the silkworm B. mori ( Redkozubov 2000 ) and meat fly Neobellieria bullata (Parker) ( Wasserman and Itagaki 2003 ) suggest that the NO/cGMP system is possibly responsible for the sensory adaptation phenomenon.
Most studies on NO and sensory perception in insects have approached the problem using immunocytochemical, electrophysiological, or pharmacological techniques. There are very few studies on the effect of NO on the behavior elicited by a sensory stimulus. In hematophagous insects, this phenomenon was studied in the bloodsucking bug Rhodnius prolixus (Ståhl) ( Sfara et al. 2011 ) and the mosquito Aedes aegypti L. ( Stanczyk et al. 2013 ). The treatment of R. prolixus antennae with an NO donor reduced the behavioral response to the insect repellent diethyltoluamide (N,N-diethyl-m-toluamide [DEET]) ( Sfara et al. 2008 ) and carbon dioxide ( Sfara et al. 2011 ).
The bloodsucking bug R. prolixus Ståhl (Hemiptera: Reduviidae) is a hemimetabolous insect that during its entire life cycle (including five nymph stages) feeds exclusively on the blood of mammals and other vertebrates ( Lehane 1991 ). This insect transmits the protozoan Trypanosoma cruzi (Chagas), the causal agent of Chagas disease, through deposition of feces while feeding ( Rassi et al. 2010 ). R. prolixus is the main vector of this disease in the north of South America and some Central America countries ( World Health Organization [WHO] 2002 ).
The objects of this study were to 1) quantify the repellent effect of the monoterpenes menthyl acetate and geraniol on fifth instar nymphs of R. prolixus ; 2) determine if prior exposure to these substances reduces the behavioral response to the repellent activity produced by them, and 3) obtain evidence of the possible participation of NO in this phenomenon.
We chose to work with menthyl acetate and geraniol because both substances are known to have repellent activity in other insects such as the mosquitoes A. aegypti and Anopheles quadrimaculatus (Say) ( Barnard 1999 ), and the German cockroach, Blattella germanica L. ( Alzogaray et al. 2013 ). Furthermore, these monoterpenes showed both a very low fumigant activity and a high repellent effect on first instar nymphs of R. prolixus (fumigant activity is that shown by insecticides when applied in its gaseous state). The simultaneous presence of these two characteristics in both products is favorable for studying repellence because when the thresholds for repellent activity and toxicity are close, symptoms of intoxication may confuse the assessments.
Materials and Methods
Biological Material
Experiments were carried out using fifth instar nymphs of R. prolixus originated from a stable colony bred in the Centro de Investigaciones de Plagas e Insecticidas (CIPEIN-UNIDEF/CONICET), which were kept in chambers under controlled conditions of temperature and photoperiod (28°C and 12:12 [L:D] h, respectively). Insects were fed ad libitum on pigeon once a week. Nymphs used in bioassays were starved during a period of 15–20 d from molt.
Chemicals
Menthyl acetate and geraniol were purchased from Sigma-Aldrich (Buenos Aires, Argentina). Analytical grade acetone was purchased from Merck (Darmstadt, Germany). S-nitroso-N-acetyl-cisteine (SNAC, Sigma-Aldrich, Buenos Aires, Argentina) was synthesized by acid-catalyzed nitrosation of acetyl- l -cysteine as described by Mathews and Kerr (1993) . Briefly, 32.6 mg of N-acetyl- l -cysteine was dissolved in 250 μl of distilled water (Solution A). Similarly, 46 mg of NO 2 Na was dissolved in 500 μl of a 0.1% EDTA solution (Solution B). Then, 150 μl of Solution B was gently added to Solution A. These compounds react immediately, and the resulting solution becomes red. This red solution was acidified to pH = 2 by adding HCl 1N and left to stand at room temperature for 5 min. The solution was then neutralized with NaOH 0.5 N and made up to a final volume of 5 ml with cold acetone, obtaining a 40-mM solution of SNAC in acetone. This solution was finally diluted 1:10 with acetone to obtain a 4-mM solution of SNAC. Both solutions were deaerated with argon. SNAC was prepared daily, and the diluted stock solution was kept in the dark at −15°C until use. Synthesis by this method produces S-nitroso-N-acetyl-cysteine, i.e., >99% pure and stable at pH < 3.0 ( Byler et al. 1983 ). The final concentration of SNAC was determined using a spectrophotometer Shimadzu UV-160 (Shimadzu Corp., Kyoto, Japan) at 330 nm, based on the molar extinction coefficient of 727.
Quantification of Repellence
Figure 1 shows the device used for evaluating repellence. The test arena was a circle of filter paper 11 cm in diameter (Whatman No. 1, Whatman International Ltd., Maidstone, UK). The circle was cut into half. One half was impregnated with 0.35 ml of acetone and the other with the same quantity of a monoterpene solution in acetone (the tested concentrations were 0.74, 7.4, 74.0, and 740 µg/cm 2 ). After 10 min, once the solvent had evaporated, the halves were joined at the back with adhesive tape. A glass ring (10 cm in diameter, 5 cm in height) was placed on the paper to prevent the insects from leaving the test arena. After 10 min (for allowing acetone to evaporate), one-fifth instar nymph was carefully placed in the middle of the test arena.
Fig. 1.
Device used to determine the spatial distribution of nymphs in the test arena. (1) Video camera; (2) optic fiber of light source; (3) dark chamber; (4) filter paper; (5) insect; (6) glass ring; and (7) monitor.
Images of the test arena were captured with a digital camera (Sony, Tokio, Japan) located 30 cm above the test arena and connected to a color monitor (Sony, Tokio, Japan). The time the nymph remained on each filter paper half was recorded visually for 5 min. To quantify the distribution of the nymph in the test arena, we calculated a distribution coefficient (DC) = [(Tto – Ttr)/Tto], where Tto is the total experimental time and Ttr the time the nymph remains in the area treated with the monoterpene. The DC ranges between 0 (total attraction) and 1 (total repellent effect). A DC = 0.5 indicates that the nymph spent the same time on each half of the test arena (no preference for any area).
Pre-Exposure Bioassays
The pre-exposure was performed using circular pieces of filter paper 9 cm in diameter (Whatman No. 1) impregnated with 0.5 ml of a 250 mg/ml monoterpene solution (2 mg/cm 2 ). Once the solvent had evaporated, the treated filter paper was placed in a plastic circular box with a top (4 cm in height and 9 cm in diameter) that remained closed for 5 min. A nymph was then placed into it for 10 or 20 min.
Two experimental series were carried out with pre-exposed nymphs. In the first one, nymphs were pre-stimulated with a monoterpene, and then the repellence produced by the same substance was determined (applied at the minimum concentration producing repellence according to the previous experiments: 740 µg/cm 2 for menthyl acetate or 74 µg/cm 2 for geraniol). In the second experimental series, nymphs were exposed to each monoterpene as described above, but the repellence produced by the other compound was determined.
Each test included two controls. In one of them, nymphs were exposed to untreated filter paper before assessing repellence. In the other controls, nymphs were exposed to filter papers treated with acetone alone. Ten independent replicates were carried out in each test.
Application of SNAC
A solution of SNAC in acetone was topically applied onto the antennae of one nymph using a microsyringe provided with a dispenser (Hamilton, Reno, NV). A 1 µl of solution was applied onto each antennae. The nymph was then placed in the experimental arena and observed for 5 min as above. Two concentrations of SNAC were tested (0.78 and 7.8 µg/antenna), and the repellence produced by 740 µg/cm 2 of menthyl acetate or 74 µg/cm 2 of geraniol was assessed. Nymphs treated with 1 µl of acetone alone were used as control. Ten independent replications were performed for each assay.
Statistical Analysis
All results were analyzed using two approaches: 1) as data failed to meet the assumptions of analysis of variance, they were analyzed with nonparametric statistics using the Kruskal–Wallis one-way analysis by ranks, followed by Dunn’s test for post-hoc comparisons; Bonferroni correction was made to the test α level for controlling the family wise error rate (this analysis allowed us to establish the significance of differences between means from different treatments); and 2) separately, data were also analyzed using one-sample t -test (this analysis allowed us to establish the significance of each mean respect to 0.5 [random distribution of nymphs on the test arena]).
Results
Menthyl acetate and geraniol produced a repellent effect on fifth instar nymphs of R. prolixus . Figure 2 shows the mean DC values calculated for nymphs exposed to four concentrations of menthyl acetate or geraniol (0.74; 7.4; 74.0 and 740.0 μg/cm 2 ). Only the highest concentration of menthyl acetate produced a significant repellent effect compared with control (Dunn’s test; α = 0.005) ( Fig. 2 a). Geraniol showed a more potent repellent effect. It produced a significant repellence starting at 74 μg/cm 2 (Dunn’s test; α = 0.005) ( Fig. 2 b).
Fig. 2.
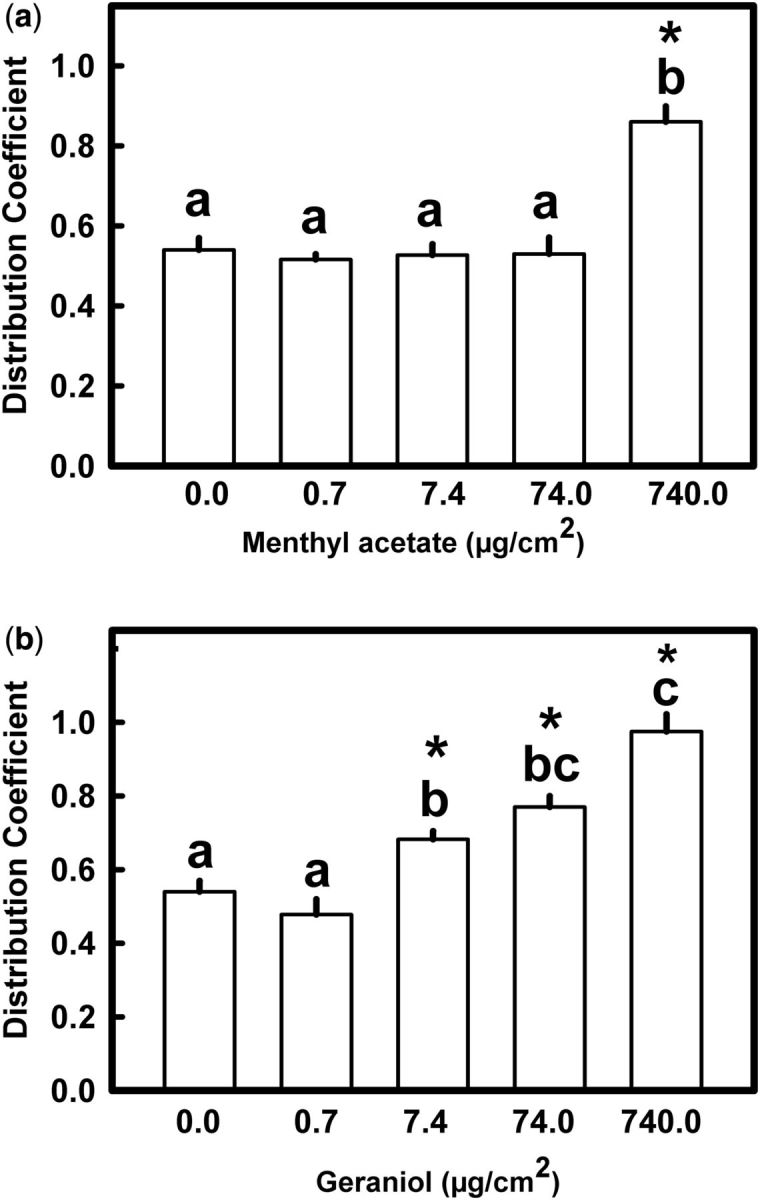
Repellence of (a) menthyl acetate or (b) geraniol to fifth instar nymphs of R. prolixus . Each bar is the mean of 10 replicates. Vertical lines are SE. Bars with the same letter are not significantly different (Dunn’s test; α = 0.005). Asterisks indicate significant differences from DC = 0.5 (one-sample t -test; P < 0.05).
Pre-exposure for 20 min to menthyl acetate abolished completely the repellent effect of this compound: the respective DC value was not significantly different from 0.5 (random distribution of nymphs on the test arena) (one-sample t -test; P = 0.058) ( Fig. 3 a). A similar pre-exposure to geraniol partially abolished the repellence produced by this substance: the respective DC value was significantly lower than control (Dunn’s test; α = 0.008), but significantly higher than 0.5 (one-sample t -test, P = 0.002) ( Fig. 3 b).
Fig. 3.
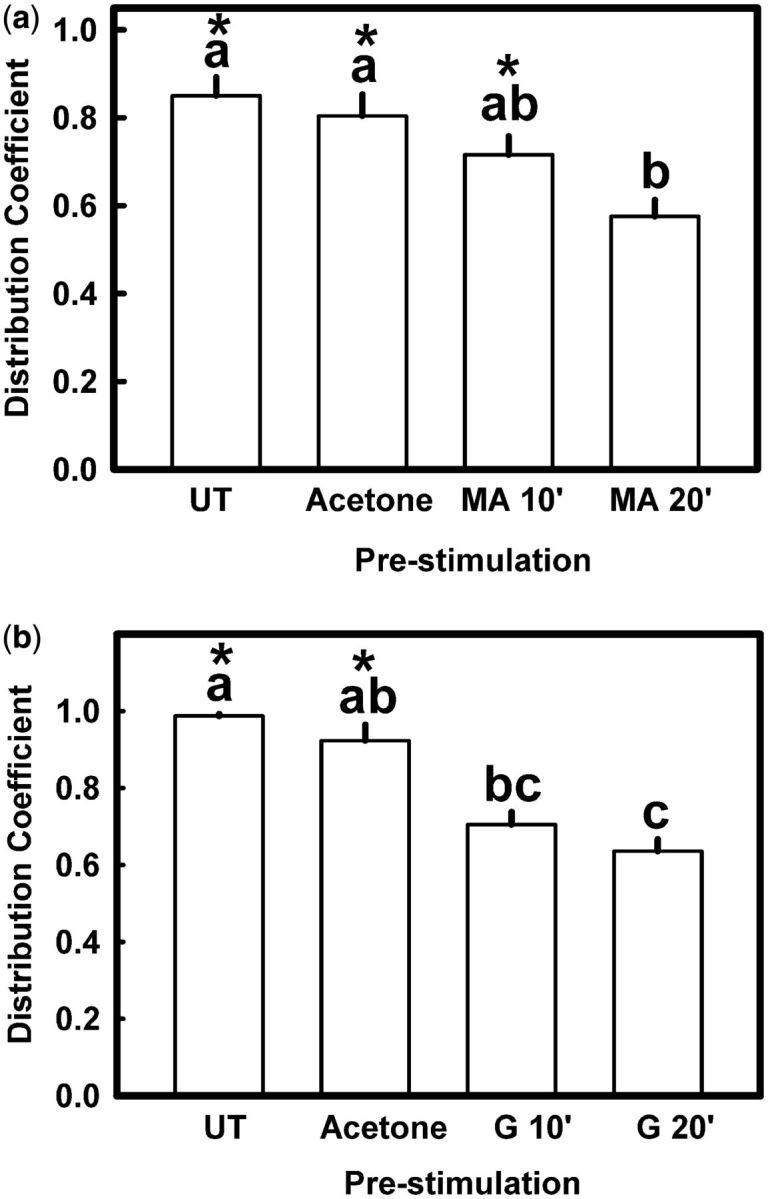
Effect of pre-exposure to (a) menthyl acetate or (b) geraniol (2 mg/cm 2 in both cases) on the repellent effect of menthyl acetate (740 µg/cm 2 ) and geraniol (74 µg/cm 2 ), respectively, on fifth instar R. prolixus nymphs. UT, nymphs pre-exposed to untreated filter paper; MA: menthyl acetate; G, geraniol; 10’ and 20’ indicate time of pre-exposure in min. Each bar is the mean of 10 replicates. Vertical lines are SE. Bars with the same letter are not significantly different (Dunn’s test; α = 0.008). Asterisks indicate significant differences from DC = 0.5 (one-sample t -test; P < 0.05).
Pre-exposure to either monoterpene reduced the behavioral response elicited by the other. Figure 4 a shows the repellent effect produced by 74 µg/cm 2 geraniol on nymphs pre-exposed to 2 mg/cm 2 menthyl acetate for 20 min. The mean DC value was significantly lower than in controls pre-exposed to filter papers treated with acetone alone (Kruskal–Wallis test; P = 0.027). In the same way, the mean DC value for 740 µg/cm 2 of menthyl acetate on nymphs prestimulated with 2 mg/cm 2 of geraniol for 20 min was significantly lower than in controls (Kruskal–Wallis test; P = 0.001) ( Fig. 4 b).
Fig. 4.
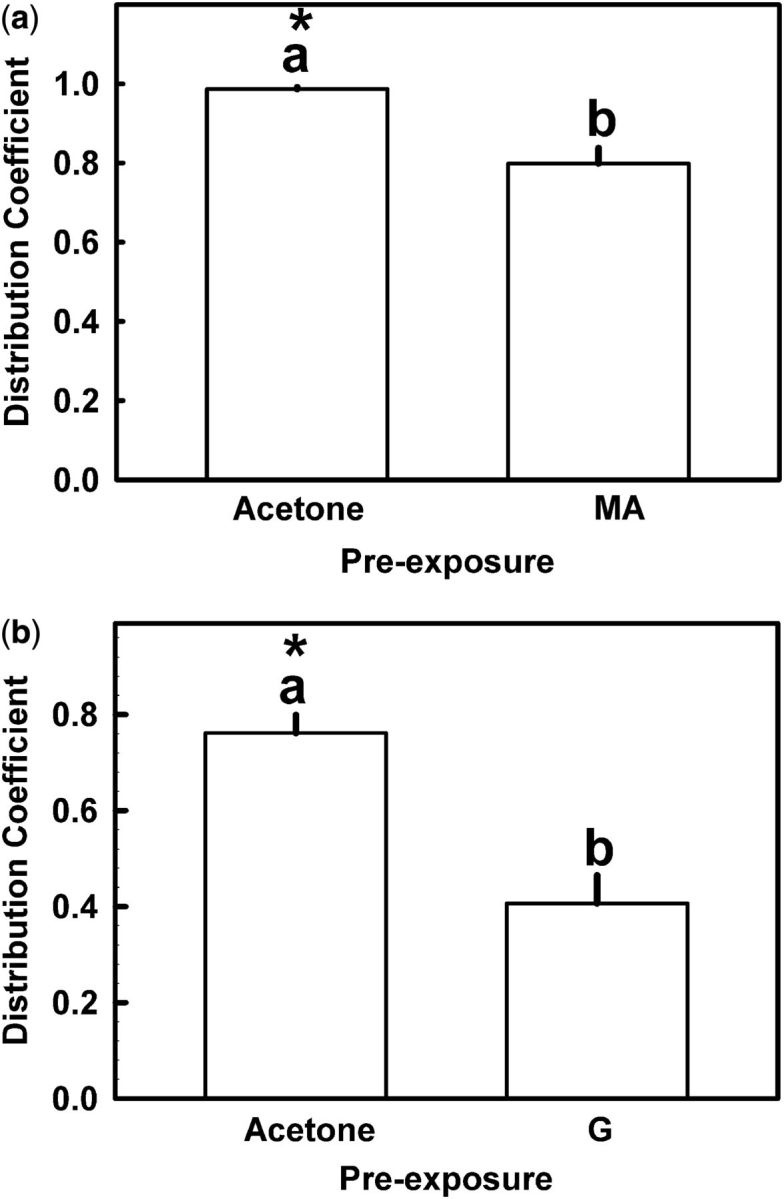
Effect of pre-exposure to (a) menthyl acetate or (b) geraniol (2 mg/cm 2 in both cases) on the repellent effect of geraniol and menthyl acetate, respectively, on fifth instar R. prolixus nymphs. MA, menthyl acetate; G: geraniol. Each bar is the mean of 10 replicates. Vertical lines are SE. Bars with the same letter are not significantly different (Dunn’s test; α = 0.05). Asterisks indicate significant differences from DC = 0.5 (one-sample t -test; P < 0.05).
Pretreatment of nymphs’ antennae with SNAC reduced the behavioral response elicited by the monoterpenes. Figure 5 a indicates that the mean DC for nymphs pretreated with 0.78 µg SNAC/antenna and exposed to menthyl acetate was not significantly different than the mean DC for the control group pretreated with acetone (Dunn’s test; α = 0.016). However, application of 7.8 µg SNAC/antenna reduced significantly the repellent effect (Dunn’s test; α = 0.03). Similar results were observed in nymphs treated with the same concentrations of SNAC and subsequently exposed to geraniol ( Fig. 5 b).
Fig. 5.
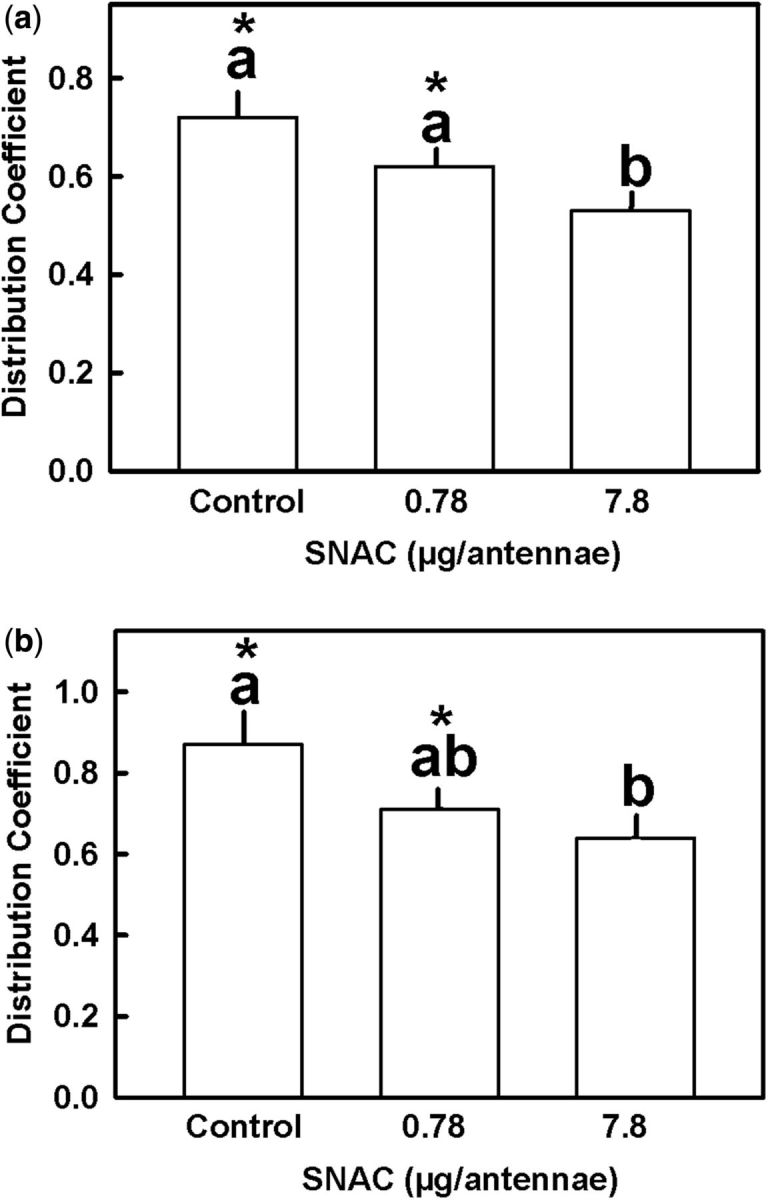
Effect of pretreatment with SNAC onto the antennae of fifth instar R. prolixus nymphs exposed to (a) menthyl acetate or (b) geraniol. Each bar is the mean of 10 replicates. Vertical lines are SE. Bars with the same letter are not significantly different (Dunn’s test; α = 0.02). Asterisks indicate significant differences from DC = 0.5 (one-sample t -test; P < 0.05).
Discussion
Various monoterpenes have been reported to have a repellent effect on insects ( Isman 2006 , Moore and Debboun 2007 ). In this study, menthyl acetate and geraniol had a repellent effect on R. prolixus fifth instar nymphs in absence of other stimuli. The minimum effective concentration was 740 µg/cm 2 for menthyl acetate and 74 µg/cm 2 for geraniol. Although it was not the aim of this study to compare the repellent effect of these substances with DEET, their repellent concentrations are in the range of the repellent effect of DEET on Triatoma infestans (Klug) ( Alzogaray et al. 2000 ) and on R. prolixus ( Sfara et al. 2008 ) nymphs.
There are many reports of reduced responsiveness in insects after being exposed to stimuli ( Kuenen and Baker 1981 , Cobb and Domain 2000 , Devaud et al. 2001 , Boyle and Cobb 2005 ). Several mechanisms have been proposed to explain this lack of responsiveness, either at the central (habituation) or at the receptor level (sensory adaptation). As both mechanisms are expressed at the behavioral level as a decrease in responsiveness either with constant or pulsed stimulation, the observation of the behavioral response makes it difficult to determine whether it is an adaptation or a habituation phenomenon. On the other hand, central phenomena such as habituation are stimulus specific. We found that exposure to one monoterpene produces a loss of responsiveness to the other one, suggesting an effect at the sensory level, rather than central. However, electrophysiological recordings of the antennae would confirm whether the loss of repellency observed occurs at the sensory level or not.
In this study, pre-exposure to menthyl acetate or geraniol reduced the repellent effect of both substances in fifth instar nymphs of R. prolixus . Adaptation depended on the time of pretest exposure. Furthermore, continuous exposure of nymphs to one of the monoterpenes reduced the repellent effect of the other. A similar phenomenon was previously reported in D. melanogaster . A decrease in the behavioral and electrophysiological responses to ethyl acetate, butanol, and benzaldehyde was observed in adults adapted to isoamyl acetate ( Störtkuhl et al. 1999 ). In experiments using D. melanogaster larvae, exposure to hexanol completely abolished the behavioral response elicited by six alcohols ( Cobb and Domain 2000 ). These results suggest that substances involved share at least some part of the olfactory way from the chemoreceptor to the central nervous system. This could be the case for menthyl acetate and geraniol in R. prolixus .
The activity of NO in the nervous system of insects has mainly been studied by biochemical ( Stengl and Zintl 1996 , Redkozubov 2000 , Krannich and Stengl 2008 , Newland and Yates 2008 ) or electrophysiological ( Stengl et al. 2001 , Dolzer et al. 2003 , Wasserman and Itagaki 2003 ) experiments, but there have been very few behavioral approaches.
The frequency of abdominal movements that the locust S. gregaria performs to dig the substrate to oviposit increased in insects treated with S -nitroso- N -acetyl-penicillamine (an NO donor) or with 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (a soluble guanilate cyclase inhibitor) ( Newland and Yates 2007 ). On the contrary, application of 2-phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl 3-oxide (an NO scavenger) or cGMP analog decreased the frequency.
Treatment of male Chorthippus biguttulus L. grasshoppers with sodium nitroprusside (an NO donor), different drugs activating the NO/GMPc system, or a cGMP analog suppressed the courtship noises it makes for finding and courting partners ( Wenzel et al. 2005 ).
In R. prolixus , treatment of antennae with SNAC or db-cGMP reduced the behavioral response elicited by DEET ( Sfara et al. 2008 ). In both cases, the effect was dose dependent.
In this study, topical application of SNAC onto the antennae of R. prolixus nymphs reduced the repellent effect produced by menthyl acetate and geraniol. This result can be considered as evidence of the action of these compounds via olfactory pathway. Because NO is a very reactive molecule, it elicits its action near the site of application of the donor. It is quite possible that NO modulates the response of neurons present in the insect’s antennae when exposed to monoterpenes.
In our experiments, nymphs walked on a monoterpene-treated surface. Chemoreceptors present in insects tarsi may be involved, in addition to the antennae in the detection of these compounds as repellents. However, treatment of the antennae with the NO donor SNAC produced a total loss of responsiveness to monoterpenes. If chemoreceptors of the tarsi are involved in the detection of these chemicals, at least a minor response of treated insects should have been observed. NO donors have to be applied as near of their target site as possible, because NO is a very reactive molecule, which interacts rapidly with its target. For this reason, our results suggest that monoterpenes are detected only by chemoreceptors located on antennae.
Why does R. prolixus , a highly specialized blood-sucking insect, detects synthetic ( Sfara et al. 2008 ) and botanical ( Sfara et al. 2009 , this work) repellents? Electrophysiological and pharmacological studies suggest the existence of “generalist” insect olfactory receptors that recognize compounds with very different chemical structures, including repellents and other synthetic molecules ( Carey et al. 2010 , Jones et al. 2011 , Pellegrino et al. 2011 ).
In conclusion, this study demonstrated that menthyl acetate and geraniol have a repellent effect on R. prolixus nymphs in the absence of other stimuli. Pre-exposure to these monoterpenes produced a decrease in the behavioral response to them. Treatment with NO donor SNAC elicits the release of NO in the internal compartment of the insect. We presume that previous exposure to monoterpenes produces an increase in the intracellular amount of NO in the neurons of insects’ antennae. We tested this hypothesis by measuring the response to the compounds after treating the insects antennae with NO donor. In this case, no previous exposure to monoterpenes was needed. We observed a similar decrease in the response to monoterpenes either when insects were pre-exposed to monoterpenes or were treated with SNAC. These results are consistent with the hypothesis that pre-exposure to monoterpenes elicits an increase in intracellular NO concentration.
Acknowledgments
We thank Javier Calcagno (Área de Investigaciones Biomédicas y Biotecnológicas, Universidad Maimónides) for assistance with statistical analysis. Comments from three anonymous reviewers were helpful in improving the manuscript. V.S. and R.A.A. are members of the Carrera del Investigador Científico del Consejo Nacional de Investigaciones Científicas y Técnicas from Argentina (CONICET). This work was supported by CONICET (PIP 2010-0887) and from the Agencia Nacional de Promoción Científica y Tecnológica from Argentina (PICT 2008-1331).
References Cited
- Alzogaray R. A., Fontán A., Zerba E. N. . 2000. . Repellency of DEET to nymphs of Triatoma infestans . Med. Vet. Entomol. 14 : 6 – 10 . [DOI] [PubMed] [Google Scholar]
- Alzogaray R. A., Sfara V., Moretti A. N., Zerba E. N. . 2013. . Behavioural and toxicological responses of Blattella germanica (Dictyoptera: Blattellidae) to monoterpenes . Eur. J. Entomol. 110 : 247 – 252 . [Google Scholar]
- Ansari M. A., Vasudevan P., Tandon M., Razdan R. K. . 2000. . Larvicidal and mosquito repellent action of pepermint ( Mentha piperita ) oil . Bioresour. Technol. 71 : 267 – 271 . [Google Scholar]
- Barnard D. R. 1999. . Repellency of essential oils to mosquitoes (Diptera: Culicidae) . J. Med. Entomol. 36 : 625 – 629 . [DOI] [PubMed] [Google Scholar]
- Bohbot J. D., Dickens J. C. . 2012. . Odorant receptor modulation: Ternary paradigm for mode of action of insect repellents . Neuropharmacology 62 : 2086 – 2095 . [DOI] [PubMed] [Google Scholar]
- Boyle J., Cobb M. . 2005. . Olfactory coding in Drosophila larvae by cross-adaptation . J. Exp. Biol. 208 : 3483 – 3491 . [DOI] [PubMed] [Google Scholar]
- Bredt D. S., Snyder S. H. . 1992. . Nitric oxide, a novel neuronal messenger . Neuron 8 : 3 – 11 . [DOI] [PubMed] [Google Scholar]
- Byler D., Gosser D., Susi H. . 1983. . Spectroscopic estimation of the extent of S-nitrosothiol formation by nitric action on sulfhydryl groups . J. Agric. Food Chem. 31 : 523 – 527 . [Google Scholar]
- Carey A. F., Wang G., Su C., Zwiebel L. J., Carlson J. R. . 2010. . Odorant reception in the malaria mosquito Anopheles gambiae . Nature 464 : 66 – 71 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaubey M. K. 2007. . Insecticidal activity of Trachyspermum ammi (Umbelliferae), Anethum graveolens (Umbelliferae) and Nigella sativa (Ranunculaceae) essential oils against stored-product beetle Tribolium castaneum Herbst (Coleoptera: Tenebrionidae) . Afr. J. Agric. Res. 2 : 596 – 600 . [Google Scholar]
- Choi W., Park B., Ku S., Lee S. . 2002. . Repellent activities of essential oils and monoterpenes against Culex pipiens pallens . J. Am. Mosq. Control Assoc. 18 : 348 – 351 . [PubMed] [Google Scholar]
- Cobb M., Domain I. . 2000. . Olfactory coding in a simple system: adaptation in Drosophila larvae . Proc. R. Soc. London B 267 : 2119 – 2125 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbert H. A., Bargmann C. I. . 1995. . Odorant-specific adaptation pathways generate olfactory plasticity in C . elegans . Neuron 14 : 803 – 812 . [DOI] [PubMed] [Google Scholar]
- Dethier V. G., Browne L. B., Smith C. N. . 1960. . The designation of chemicals in terms of the response they elicit from insects . J. Econ. Entomol. 53 : 134 – 136 . [DOI] [PubMed] [Google Scholar]
- Devaud J. M., Acebes A., Ferrús A. . 2001. . Odour exposure causes central adaptation and morphological changes in selected olfactory glomeruli in Drosophila . J. Neurosci. 21 : 6274 – 6282 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolzer J., Fischer K., Stengl M. . 2003. . Adaptation in pheromone-sensitive trichoid sensilla of the hawkmoth Manduca sexta . J. Exp. Biol. 206 : 1575 – 1588 . [DOI] [PubMed] [Google Scholar]
- Erler F., Ulug I., Yalcinkaya B. . 2006. . Repellent activity of five essential oils against Culex pipiens . Fitoterapia 77 : 491 – 494 . [DOI] [PubMed] [Google Scholar]
- García M., Donadel O. J., Ardanaz C. E., Tonn C. E., Sosa M. E. . 2005. . Toxic and repellent effects of Baccharis salicifolia essential oil on Tribolium castaneum . Pest Manage. Sci. 61 : 612 – 618 . [DOI] [PubMed] [Google Scholar]
- Gibbs S. M., Truman J. W. . 1998. . Nitric oxide and cyclic GMP regulate retinal patterning in the optic lobe in Drosophila . Neuron 20 : 83 – 93 . [DOI] [PubMed] [Google Scholar]
- Goel D., Goel R., Singh V., Ali M., Mallavarapu G., Kumar S. . 2007. . Composition of the essential oil from the root of Artemisia annua . J. Nat. Med. 61 : 458 – 461 . [Google Scholar]
- Isman M. B. 2000. . Plant essential oils for pest and disease management . Crop Prot. 19 : 603 – 608 . [Google Scholar]
- Isman M. B. 2006. . Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world . Annu. Rev. Entomol. 51 : 45 – 66 . [DOI] [PubMed] [Google Scholar]
- Isman M. B., Machial C. M. . 2006. . Pesticides based on plant essential oils: from traditional practice to commercialization , pp. 29 – 44 . InRai M., Carpinella M. C. (eds.), Naturally occurring bioactive compounds . Elsevier; , Amsterdam: . [Google Scholar]
- Jones P. L., Pask G. M., Rinker D. C., Zwiebel L. J. . 2011. . Functional agonism of insect odorant receptor ion channels . Proc. Natl Acad. Sci. USA. 108 : 8821 – 8825 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krannich S., Stengl M. . 2008. . Cyclic nucleotide-activated currents in cultured olfactory receptor neurons of the hawkmoth Manduca sexta . J. Neurophysiol. 100 : 2866 – 2877 . [DOI] [PubMed] [Google Scholar]
- Kuenen L. S., Baker T. C. . 1981. . Habituation versus sensory adaptation as the cause of reduced attraction following pulsed and constant sex pheromone pre-exposure in Trichoplusia ni . J. Insect Physiol. 27 : 721 – 726 . [Google Scholar]
- Lehane M. J. 1991. . Biology of blood-sucking insects , 288 pp. Chapman and Hall, London, United Kingdom . [Google Scholar]
- Liptrot G. F. 1980. . Química inorgánica moderna . Compañía Editorial Continental, Mexico . [Google Scholar]
- Maguranyi S. K., Webb C. E., Mansfield S., Russell R. C. . 2009. . Are commercially available essential oils from Australian native plants repellents to mosquitoes? J. Am. Mosq. Control Assoc. 25 : 292 – 300 . [DOI] [PubMed] [Google Scholar]
- Mathews W. R., Kerr S. W. . 1993. . Biological activity of S-nitrosothiols: the role of nitric oxide . J. Pharmacol. Exp. Ther. 267 : 1529 – 1537 . [PubMed] [Google Scholar]
- Moore S., Debboun M. . 2007. . History of insect repellent , pp. 3 – 29 . InDebboun M., Frances S. P., Strickman D. (eds.), Insect repellents . Principles, methods and uses. CRC Press, Boca Raton . [Google Scholar]
- Müller U. 2000. . Prolonged activation of cAMP-dependent protein kinase during conditioning induces long-term memory in honeybees . Neuron 27 : 159 – 168 . [DOI] [PubMed] [Google Scholar]
- Newland P. L., Yates P. . 2007. . Nitrergic modulation of an oviposition digging rhythm in locusts . J. Exp. Biol. 210 : 4448 – 4456 . [DOI] [PubMed] [Google Scholar]
- Newland P. L., Yates P. . 2008. . Nitric oxide modulates salt and sugar responses via different signaling pathways . Chem. Senses 33 : 347 – 356 . [DOI] [PubMed] [Google Scholar]
- Odalo J. O., Omolo M. O., Malebo H., Angira J., Njeru P. M., Ndiege I. O., Hassanali A. . 2005. . Repellency of essential oils of some plants from the Kenyan coast against Anopheles gambiae . Acta Tropica 95 : 210 – 218 . [DOI] [PubMed] [Google Scholar]
- Pellegrino M., Steinbach N., Stensmyr M. C., Hansson B. S., Vosshall L. . 2011. . A natural polymorphism alters odour and DEET sensitivity in an insect odorant receptor . Nature 478 : 511 – 514 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassi A., Jr., Rassi A., Marin-Neto J. A. . 2010. . Chagas disease . Lancet 375 : 1388 – 1402 . [DOI] [PubMed] [Google Scholar]
- Redkozubov A. 2000. . Guanosine 3´,5´-cyclic monophosphate reduces the response of the moth’s olfactory receptor neuron to pheromone . Chem. Senses 25 : 381 – 385 . [DOI] [PubMed] [Google Scholar]
- Regnault-Roger C., Vincent C., Arnason L. . 2012. . Essential oils in insect control: low-risk products in a high-stakes world . Annu. Rev. Entomol. 57 : 405 – 424 . [DOI] [PubMed] [Google Scholar]
- Sainz P., Sanz J., Burillo J., González-Coloma A., Bailén M., Martínez-Díaz R. A. . 2012. . Essential oils for the control of reduviid insects . Phytochem. Rev. 11 : 361 – 369 . [Google Scholar]
- Seidel C., Bicker G. . 2000. . Nitric oxide and cGMP influence axonogenesis pioneer neurons . Development 127 : 4541 – 4549 . [DOI] [PubMed] [Google Scholar]
- Sfara V., Zerba E. N., Alzogaray R. A. . 2008. . Decrease in DEET repellency caused by nitric oxide in Rhodnius prolixus . Arch. Insect Biochem. Physiol. 67 : 1 – 8 . [DOI] [PubMed] [Google Scholar]
- Sfara V., Zerba E. N., Alzogaray R. A. . 2009. . Fumigant insecticidal activity and repellent effect of five essential oils and seven monoterpenes on first-instar nymphs of Rhodnius prolixus . J. Med. Entomol. 46 : 511 – 515 . [DOI] [PubMed] [Google Scholar]
- Sfara V., Zerba E. N., Alzogaray R. A. . 2011. . Deterrence of feeding in Rhodnius prolixus (Hemiptera: Reduviidae) after treatment of antennae with a nitric oxide donor . Eur. J. Entomol. 108 : 701 – 704 . [Google Scholar]
- Sritabutra D., Soonwera M., Waltanachanobon S., Poungjai S. . 2011. . Evaluation of herbal essential oil as repellent against Aedes aegypti (L.) and Anopheles dirus . Asian Pac. J. Trop. Biomed. 1 : S214 – S218 . [Google Scholar]
- Stamler J. S., Jia L., Eu J. P., McMahon Demchenko I. T., Bonaventura J., Gernert K., Piantadosi C. A. . 1997. . Blood flow regulation by S-nitrosohemoglobin in the physiological oxygen gradient . Science 276 : 2034 – 2037 . [DOI] [PubMed] [Google Scholar]
- Stanczyk N. M., Brookfield J.F.Y., Field L. M., Logan J. G. . 2013. . Aedes aegypti mosquitoes exhibit decreased repellency by DEET following previous exposure . PLoS One 8 : e54438 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stengl M., Zintl R. . 1996. . NADPH-diaphorase staining in the antenna of the moth Manduca sexta . J. Exp. Biol. 199 : 1063 – 1072 . [DOI] [PubMed] [Google Scholar]
- Stengl M., Zintl R., De Vente J., Nighorn A. . 2001. . Localization of cGMP immunoreactivity and soluble guanylyl cyclase in antennal sensilla of the hawkmoth Manduca sexta . Cell. Tissue Res. 304 : 409 – 421 . [DOI] [PubMed] [Google Scholar]
- Störtkuhl K. F., Hovemann B. T., Carlson J. R. . 1999. . Olfactory adaptation depends on the Trp Ca21 channel in Drosophila . J. Neurosci. 19 : 4839 – 4846 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toloza A. C., Zygadlo J., Mougabure Cueto G., Biurrin F., Zerba E., Picollo M. I. . 2006. . Fumigant and repellent properties of essential oils and component compounds against permethrin-resistant Pediculus humanus capitis (Anoplura: Pediculidae) from Argentina . J. Med. Entomol. 43 : 889 – 895 . [DOI] [PubMed] [Google Scholar]
- Torreilles J. 2001. . Nitric oxide: one of the more conserved and widespread signalling molecules . Front. Biosci. 6 : 1161 – 1172 . [DOI] [PubMed] [Google Scholar]
- Vilaseca L. A., Laurent D., Ballivian C., Chantraine J. M., Ibañez R. . 2008. . Repellent activity of the essential oils in Triatoma infestans . Planta Medica 74 : PI4 . [Google Scholar]
- Wang J., Zhua F., Zhoua X. M., Niua C. Y., Lei C. L. . 2006. . Repellent and fumigant activity of essential oil from Artemisia vulgaris to Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae) . J. Stored Prod. Res. 42 : 339 – 347 . [Google Scholar]
- Wasserman S. L., Itagaki H. . 2003. . The olfactory responses of the antenna and maxillary palp of the fleshfly, Neobellieria bullata (Diptera: Sarcophagidae), and their sensitivity to blockage of nitric oxide synthase . J. Insect Physiol. 49 : 271 – 280 . [DOI] [PubMed] [Google Scholar]
- Wenzel B., Kunst M., Günther C., Ganter G. K., Lakes-Harlan R., Elsner N., Heinrich R. . 2005. . Nitric oxide/cyclic guanosine monophosphate signalling in the central complex of the grasshopper brain inhibits singing behaviour . J. Comp. Neurol. 488 : 129 – 139 . [DOI] [PubMed] [Google Scholar]
- (WHO) World Health Organization . 2002. . Control of Chagas disease . WHO, Geneva . [PubMed] [Google Scholar]
- Ziegelberger G., van den Berg M. J., Kaissling K.-E., Klumpp S., Schultz J. E. . 1990. . Cyclic GMP levels and guanylate cyclase activity in pheromone-sensitive antennae of the silkmoths Antheraea polyphemus and Bombyx mori . J. Neurosci. 10 : 1217 – 1225 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zufall F., Leinders-Zufall T. . 2000. . The cellular and molecular basis of odor adaptation . Chem. Senses 25 : 473 – 481 . [DOI] [PubMed] [Google Scholar]



