Abstract
Background
Acute myeloid leukemia (AML) is a common hematologic malignancy of adults. The pathophysiological mechanism of AML is not well understood. The purpose of this study was to examine the crucial miRNAs and mRNAs associated with AML survival.
Material/Methods
The full clinical dataset of miRNA and mRNA expression profiling of AML patients was downloaded from The Cancer Genome Atlas database. Univariate Cox regression analysis was performed to obtain those miRNAs and mRNAs associated with AML survival. A miRNA-mRNA interaction network was constructed. The underlying functions of mRNAs were predicted through Kyoto Encyclopedia of Genes and Genomes (KEEG) pathway enrichment. The expression levels of miRNAs and mRNAs were detected by quantitative real-time polymerase chain reaction (qRT-PCR).
Results
Fourteen miRNAs and 830 mRNAs associated with AML survival were identified. Of the 14 miRNAs, hsa-mir-425, hsa-mir-1201, and hsa-mir-1978 were identified as risk factors and the other 11 miRNAs were identified as protective factors of AML survival. For target-genes of miRNAs, GTSF1, RTN4R, and CD44 were the top risk factor target-genes associated with AML survival. An interaction network was constructed that including 607 miRNA-target gene pairs associated with AML survival. Target-genes associated with AML survival were significantly enriched in several pathways including pancreatic secretion, calcium signaling pathway, natural killer cell mediated cytotoxicity, and Alzheimer’s disease. The qRT-PCR results were consistent with our bioinformatics analyses.
Conclusions
The miRNA hsa-mir-425 was identified as the top risk factor miRNA of AML survival and CD44 was identified as one of the top three risk factor target-genes associated with AML survival. Both hsa-mir-425 and CD44 may play key roles in progression and development of AML through calcium signaling pathway and natural killer cell mediated cytotoxicity.
MeSH Keywords: Database; Leukemia, Myeloid, Acute; Prognosis; Survival Analysis
Background
Acute myeloid leukemia (AML) is a common hematological malignancy with myeloblast proliferation in the bone marrow of adults and children. AML patients have highly heterogeneous disease course and outcomes. According to the French-American-British (FAB) classification, AML is defined as having eight subtypes: M0, M1, M2, M3, M4, M5, M6, and M7 [1,2].
It has been estimated that 50%–60% of AML patients display cytogenetic abnormalities and significantly heterogeneous outcomes [3,4]. Cytogenetic abnormalities include chromosomes translocation, inversion, and deletion; some chromosome abnormalities, such as t(8;21), t(16;16), inv(16), and t(15;17), predict better prognosis of AML patients. Chromosomes abnormalities, including −5,−7, abn(3q), del(5q) and other complex karyotypes, predict poor prognosis [5]. AML patients with normal karyotypes, FLT3 mutation, and negative mutation of NPM1 possess intermediate prognosis. A number of groups have endeavored to come to an agreement on prognosis risk evaluation of AML chromosomes abnormalities [6–9], but discrepancy exists among different group standards. For example, del(7q) is considered to be an intermediate-risk abnormality based on CALGB standards but it is considered to be a poor-risk based on SWOG standards [10].
Although there has been progress in understanding the mechanisms of pathogenesis of AML, it is still difficult to predict clinical outcomes for AML patients. Over the past decades, the prognostic prediction of AML patients has largely depended on cytogenetic abnormalities. More recently, molecular genetic changes have been identified to predict prognosis and guide clinical treatment. Molecular genetic changes consist of mutations of CEBPA, NPM1, FLT3, and c-KIT. CEBPA mutation and NPM1 mutation (absence of FLT3 mutation) suggest a favorable prognosis for AML patients with longer complete remission duration and overall survival. However, c-KIT mutation suggests an intermediate prognosis, and FLT3 mutation suggests an unfavorable prognosis [11].
In this study, we applied bioinformatics and univariate Cox regression analysis to identify the miRNAs and target-genes associated with overall survival of AML patients, and aimed to provide valuable information for further AML patient classification and therapy planning.
Material and Methods
Patients and samples
Two hundred study patients with AML were retrieved from The Cancer Genome Atlas (TCGA) data portal. The full clinical dataset was downloaded (up to March 16, 2015). The exclusion criteria for AML patients were as follows: 1) history of other malignancy; 2) history of AML treatment; and 3) samples with clinical data but without miRNA or mRNA sequence data. In the TCGA database, 49 AML patients had a history of treatment before collection of blood samples and all of them received treatment with hydroxyurea. Overall, 120 AML patients with available corresponding clinical data, including age, gender, race, vital status, and follow-up, were included in our study.
miRNA and mRNA expression data
The miRNA and mRNA expression data (level 3) of the AML patients were downloaded from the TCGA data portal (up to March 16, 2015). The miRNA and mRNA expression profiling was generated from the Illumina Genome Analyzer sequencing platforms (Illumina Inc., San Diego, CA, USA).
Survival analysis
The association between miRNA expression and overall survival was carried out using univariate Cox regression. A set of miRNAs that significantly correlated with survival was identified with the threshold of p-value less than 0.05. The association between mRNA expression and overall survival was sequentially performed using univariate Cox regression; the threshold was a p-value less than 0.05, which indicated that a set of mRNAs was significantly associated with survival. Hazard ratio (HR) >1 indicated a risk factor associated with AML survival and HR <1 indicated a protective factor associated with AML survival.
Identification of target-genes of miRNAs
To obtain the target-genes of miRNAs associated with AML survival, the miRNAs were integrated into the mirWalk database (http://www.umm.uni-heidelberg.de/apps/zmf/mirwalk/), in which the correlations between target-genes and miRNAs have been confirmed. Six algorithms including RNA22, miRanda, miRDB, miRWalk, PICTAR2, and Targetscan were conducted to predict target-genes of miRNAs. The genes, simultaneously predicted by more than four algorithms, were identified as the target-genes of the miRNAs.
miRNA-target gene network
miRNAs associated with AML survival and target-genes associated with AML survival were identified to construct the interaction network using Cytoscape software (http://cytoscape.org). In the miRNA-target gene network, a circular node represented mRNA and a rectangle node represented miRNA, and their association was represented by a solid line.
KEGG pathway enrichment
The underlying functions of target-genes associated with AML survival were predicted by Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis using the GeneCodis software. FDR <0.05 was the cut-off for selecting significant KEGG pathway.
Quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA of peripheral blood of AML patients was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. The SuperScript III Reverse Transcription Kit (Invitrogen, Carlsbad, CA, USA) was used to synthesize the cDNA according to the manufacturer’s instructions. Then qRT-PCR reactions were performed using Power SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA, USA) on the Applied Biosystems 7500 (Applied Biosystems, Foster City, CA, USA). For detection of miRNA expression level, we used miRcute miRNA First-Strand cDNA Kit (Tiangen, Bejing, China) and miRcute miRNA qPCR Detection Kit (Tiangen, Bejing, China). U6 and β-actin were used as internal controls for miRNA and mRNA expression, respectively. The relative expression of target-genes was calculated using the ΔCT equation. The PCR primers were used as follows:
IL15RA forward-5′CAACAGCCAAGAACTGGGAAC3′;
IL15RA reverse-5′AGTTTGCCTTGACTTGAGGTAGC3′;
CD44 forward-5′ ATCATCTTGGCATCCCTCTTG 3′;
CD44 reverse-5′TGTCCTCCACAGCTCCATTG 3′;
β-actin forward-5′ CTGAAGTACCCCATCGAGCAC 3′;
β-actin reverse- 5′ ATAGCACAGCCTGGATAGCAAC 3′;
hsa-miR-200c forward-5′ CGTCTTACCCAGCAGTGTTTG3′;
hsa-miR-425 forward-5′ AATGACACGATCACTCCCGTTGA 3′;
U6 forward-5′ CTCGCTTCGGCAGCACA 3′;
U6 reverse-5′ AACGCTTCACGAATTTGCGT 3′.
Statistical analysis
At least three independent experiments were performed for statistical evaluation. The SPSS19.0 program was used for general statistical analysis. The qRT-PCR experimental data were expressed as means ± standard deviation. The statistical significance was evaluated using the Student’s t-test; p<0.05 was considered a significant difference.
The peripheral blood samples for qRT-PCR examination were obtained from AML patients diagnosed at the Department of Hematology, Taian City Central Hospital. Our study was approved by the Ethics Committee of Taian City Central Hospital and complied with the Declaration of Helsinki. All participants provided written informed consent to participate in this study.
Results
miRNAs associated with survival time of AML patients
The miRNA expression and survival time of AML patients were obtained from the TCGA database and univariate Cox regression analysis was performed. We obtained 14 miRNAs associated with survival time; the threshold was p<0.05, as shown in Table 1. HR >1 indicated a risk factor and HR <1 indicated a protective factor. In our study, hsa-mir-1201, hsa-mir-1978, and hsa-mir-425 were identified as risk factors, and hsa-mir-10b, hsa-mir-193b, hsa-mir-194-1, hsa-mir-196a-1, hsa-mir-1976, hsa-mir-200c, hsa-mir-23b, hsa-mir-30a, hsa-mir-452, hsa-mir-509-1, and hsa-mir-589 were identified as protective factors for survival time of AML patients.
Table 1.
miRNAs associated with AML survival.
| miRNA | Beta | HR | 95 %CI (low) | 95%CI (high) | P-value |
|---|---|---|---|---|---|
| hsa-mir-425 | 0.78098 | 2.1836 | 1.1866 | 4.0184 | 0.012081 |
| hsa-mir-1978 | 0.77576 | 2.1722 | 1.1343 | 4.1601 | 0.019282 |
| hsa-mir-1201 | 0.65208 | 1.9195 | 1.0231 | 3.6012 | 0.042227 |
| hsa-mir-589 | −0.64776 | 0.52322 | 0.27722 | 0.98751 | 0.045633 |
| hsa-mir-23b | −0.65778 | 0.518 | 0.28334 | 0.94702 | 0.03261 |
| hsa-mir-196a-1 | −0.65789 | 0.51794 | 0.2715 | 0.98809 | 0.045895 |
| hsa-mir-200c | −0.66085 | 0.51641 | 0.27139 | 0.98263 | 0.044075 |
| hsa-mir-509-1 | −0.6679 | 0.51278 | 0.27829 | 0.94487 | 0.032206 |
| hsa-mir-1976 | −0.69919 | 0.49699 | 0.25874 | 0.95462 | 0.035777 |
| hsa-mir-194-1 | −0.72271 | 0.48543 | 0.24412 | 0.9653 | 0.039331 |
| hsa-mir-193b | −0.80362 | 0.4477 | 0.22526 | 0.88981 | 0.021841 |
| hsa-mir-452 | −0.82757 | 0.43711 | 0.2298 | 0.83143 | 0.011644 |
| hsa-mir-10b | −0.84543 | 0.42937 | 0.22207 | 0.8302 | 0.011965 |
| hsa-mir-30a | −0.85097 | 0.427 | 0.22278 | 0.81843 | 0.010358 |
HR – hazard ration; CI – confidence interval; Beta – regression coefficient; AML – acute myeloid leukemia.
mRNA associated with survival time of AML patients
The mRNA expression and survival time of AML patients were obtained from the TCGA database and univariate Cox regression analysis was performed. We obtained 830 mRNAs associated with survival time; the threshold was p<0.05, as shown in Table 2. We identified 830 mRNAs associated with AML survival including 482 mRNAs identified as risk factors and 348 mRNAs identified as protective factors (data not shown). The top 20 risk factor mRNAs and top 20 protective factor mRNAs associated with survival time (p<0.05) are presented in Table 2; POLR3C, TULP2, CIRBP, WNT7A, and FARS2 were the top 5 risk factor mRNAs. SPIC, ZBTB2, VCAM1, AMPD1, and SOX9 were the top 5 protective factor mRNAs associated with survival time of AML patients.
Table 2.
mRNAs associated with AML survival.
| Gene symbol | Beta | HR | 95%CI (low) | 95%CI (high) | P-value |
|---|---|---|---|---|---|
| Risky mRNAs (top 20) | |||||
| POLR3C | 1.299177 | 3.666278842 | 1.822439 | 7.375612 | 0.00027 |
| TULP2 | 1.271691 | 3.56687887 | 1.659339 | 7.667284 | 0.001126 |
| CIRBP | 1.212915 | 3.363273616 | 1.676527 | 6.747048 | 0.000638 |
| WNT7A | 1.13283 | 3.104429621 | 1.563505 | 6.164025 | 0.001207 |
| FARS2 | 1.128418 | 3.090761914 | 1.589044 | 6.011672 | 0.000886 |
| HSBP1L1 | 1.101514 | 3.008717605 | 1.49912 | 6.038464 | 0.001941 |
| SPATA24 | 1.093419 | 2.984460963 | 1.507142 | 5.909864 | 0.001708 |
| GTSF1 | 1.088786 | 2.97066512 | 1.516522 | 5.81914 | 0.001504 |
| LOC150197 | 1.087166 | 2.965857274 | 1.512986 | 5.813873 | 0.001547 |
| PRR4 | 1.085033 | 2.959538025 | 1.426825 | 6.138709 | 0.003558 |
| ZSCAN16 | 1.059951 | 2.886230787 | 1.496316 | 5.567227 | 0.001565 |
| tAKR | 1.057348 | 2.878727159 | 1.41916 | 5.83942 | 0.003389 |
| TMCO6 | 1.048799 | 2.854219977 | 1.445137 | 5.637231 | 0.002525 |
| GLRA1 | 1.046813 | 2.848559639 | 1.431752 | 5.667387 | 0.002858 |
| KCNQ5 | 1.040786 | 2.83144076 | 1.412616 | 5.675325 | 0.003349 |
| CCDC61 | 1.030272 | 2.801828015 | 1.446379 | 5.427514 | 0.002258 |
| METRN | 1.014433 | 2.757800065 | 1.305514 | 5.825647 | 0.007844 |
| HIST2H2BF | 1.012048 | 2.751231083 | 1.452881 | 5.209838 | 0.001892 |
| RTN4R | 1.011228 | 2.748975915 | 1.384951 | 5.456416 | 0.003839 |
| PKP3 | 1.008338 | 2.741042689 | 1.428346 | 5.26015 | 0.002429 |
| Protective mRNAs (top 20) | |||||
| SPIC | −1.31893 | 0.2674205 | 0.129581 | 0.551884 | 0.00036 |
| ZBTB2 | −1.27059 | 0.2806665 | 0.142188 | 0.554012 | 0.00025 |
| VCAM1 | −1.26516 | 0.2821953 | 0.142927 | 0.557167 | 0.000267 |
| AMPD1 | −1.21566 | 0.2965143 | 0.147273 | 0.59699 | 0.000662 |
| SOX9 | −1.19687 | 0.3021391 | 0.15331 | 0.595449 | 0.000545 |
| CXCL10 | −1.18323 | 0.306289 | 0.146201 | 0.641673 | 0.001714 |
| MOCS1 | −1.17538 | 0.3087017 | 0.155751 | 0.611851 | 0.000759 |
| CXCL12 | −1.16587 | 0.3116504 | 0.159361 | 0.60947 | 0.000657 |
| SDC3 | −1.14539 | 0.3181006 | 0.16393 | 0.617262 | 0.000708 |
| SDC1 | −1.14055 | 0.3196427 | 0.161413 | 0.632981 | 0.001068 |
| FER1L4 | −1.12792 | 0.3237057 | 0.160942 | 0.651075 | 0.001558 |
| TRIM25 | −1.12791 | 0.32371 | 0.161198 | 0.650058 | 0.00152 |
| EBF3 | −1.1233 | 0.3252052 | 0.169878 | 0.622555 | 0.000698 |
| CDH15 | −1.11564 | 0.3277049 | 0.15995 | 0.671401 | 0.002299 |
| CD163L1 | −1.10857 | 0.3300322 | 0.16721 | 0.651404 | 0.001396 |
| IGF1 | −1.10036 | 0.3327518 | 0.171385 | 0.646052 | 0.001152 |
| ADAMTS9 | −1.07894 | 0.3399546 | 0.174127 | 0.663706 | 0.001573 |
| C10orf81 | −1.0656 | 0.34452 | 0.172913 | 0.686437 | 0.002448 |
| ZCCHC24 | −1.06124 | 0.3460263 | 0.172931 | 0.692381 | 0.00271 |
| CCL18 | −1.04882 | 0.3503516 | 0.180175 | 0.681262 | 0.001993 |
HR – hazard ration; CI – confidence interval; Beta – regression coefficient; AML – acute myeloid leukemia.
The target-gene prediction of miRNA associated with survival time
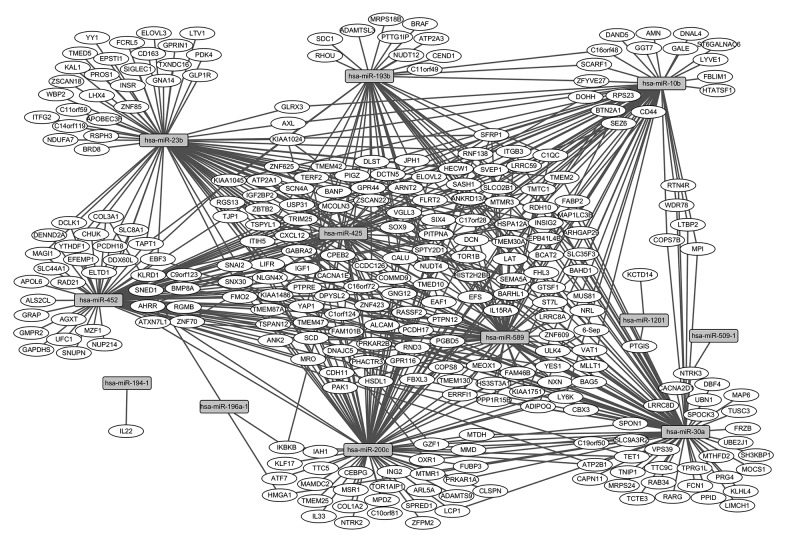
The target genes of 14 miRNAs associated with survival time were predicted and target-genes of 12 miRNAs were available in the mirWalk database. A total of 607 miRNA-target gene pairs were obtained, as shown in the Supplementary Table 1. The predictive target-genes of miRNAs (Figure 1) and mRNAs associated with survival time overlapped; 109 predictive target-genes associated with survival time were obtained (underlined genes in Supplementary Table 1). GTSF1, RTN4R, and CD44 were the top risk factor target-genes associated with AML survival.
Figure 1.
Interaction network of miRNAs-target-genes associated with AML. Circular nodes represent target-genes and rectangle nodes represented miRNAs. The solid line represents association between miRNA and target-gene.
Regulatory network construction of miRNA and mRNA associated with survival time
To obtain insights into the regulatory relationship between the 12 miRNAs and 109 target-genes associated with survival time, a regulatory network was constructed. As shown in Figure 1, rectangle nodes represent miRNAs associated with survival time and circular nodes represent mRNAs associated with survival time. The miRNAs hsa-mir-30a, hsa-mir-589, hsa-mir-10b, hsa-mir-452, and hsa-mir-200c had high connectivity with the mRNAs associated with AML patient survival time, and regulated 27, 24, 22, 22, 21 mRNAs, respectively.
KEGG pathway enrichment
KEGG pathway analysis was used to identify biological functions of miRNA target-genes. In all, 105 of the 109 target-genes associated with survival time were enriched in the KEGG database. Four signaling pathways were significantly enriched, including pancreatic secretion, calcium signaling pathway, natural killer cell mediated cytotoxicity, and Alzheimer’s disease, as shown in Table 3.
Table 3.
KEGG signaling pathway enrichment.
| KEGG ID | KEGG term | No. | FDR | Genes |
|---|---|---|---|---|
| hsa 04972 | Pancreatic secretion | 3 | 0.000131 | ATP2B1, ATP2A1, ATP2A3 |
| hsa04020 | Calcium signaling pathway | 3 | 0.000131 | ATP2B1, ATP2A1, ATP2A3 |
| hsa04650 | Natural killer cell mediated cytotoxicity | 3 | 0.006809 | BRAF, KLRD1, LAT |
| hsa05010 | Alzheimer’s disease | 3 | 0.013968 | ATP2A1, ATP2A3, NDUFA7 |
FDR – false discovery rate; No. – number of genes.
qRT-PCR validation of miRNA and mRNA associated with survival
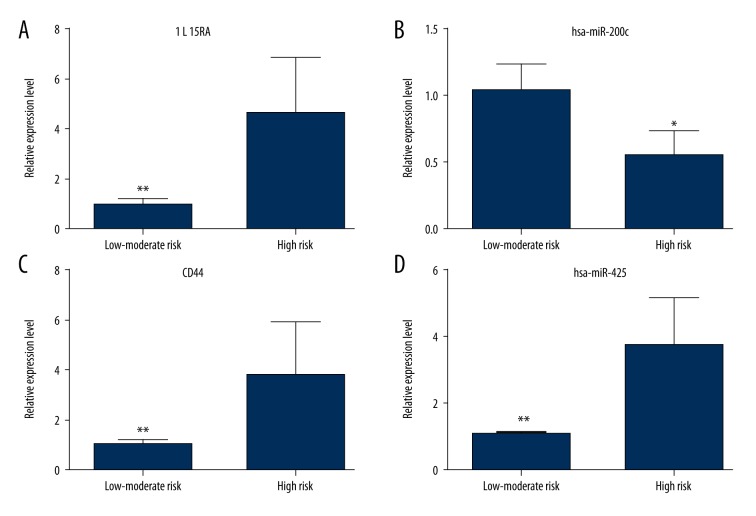
To validate the expression status of miRNAs and mRNAs associated with survival, miRNAs (hsa-miR-200c and hsa-miR-425) and mRNA (IL15RA and CD44) were preliminarily quantified by qRT-PCR. Sixteen AML patients were divided into two groups, a low-moderate risk group and a high-risk group, based on karyotype and gene mutation detection according to the standards of the Medical Research Council. As Figure 2A, 2C, and 2D show, the expression levels of IL15RA (p=0.0003), CD44 (p=0.028) and hsa-miR-425 (p=0.053) were significant upregulated in the high-risk group compared with the low-moderate risk group. As Figure 2B shows, the expression level hsa-miR-200c (p=0.033) was significant downregulated in the high-risk group compared with the low-moderate risk group.
Figure 2.
Verification of expression levels of miRNAs and mRNAs in the low-moderate risk group and the high risk group of AML patients by qRT-PCR. (A) IL15RA; (B) hsa-miR-200c; (C) CD44; (D) hsa-miR-425. * is p<0.05 and ** is p<0.01.
Discussion
In our study, hsa-mir-425 was the top risk factor associated with AML survival, as shown in Table 1. It has been reported that mir-425-5p is upregulated in human gastric cancer and contributes to gastric cancer cell proliferation, invasion and metastasis in vitro and in vivo [12–14]. Over-expression of miR-425 enhances cell proliferation, colony formation, and cell metastasis in esophageal squamous cell carcinoma by targeting SMAD2 [15]. Ge et al. reported that mir-425 was significantly associated with recurrence-free survival (RFS) and overall survival of chromophobe renal cell carcinoma, and miRNA expression signatures including mir-191, mir-19a, mir-210, and mir-425, were identified as predictors of clinical prognosis [16].
In our study, hsa-mir-30a was the top protective factor associated with AML survival (Table 1). Over-expression of BCR-ABL1 is associated with chronic myeloid leukemia; and downregulation of mir-30a enhances BCR-ABL expression and promotes chronic myeloid leukemia tumorigenesis [17]. The MYBL2 gene encodes a transcription factor; and over-expression of MYBL2 could be implicated in tumorigenesis of colorectal cancer and AML [18,19]. In AML, MYBL2 over-expression is associated with the miRNA-30 family (including mir-30a, mir-30b, mir-30c) and predicts unfavorable prognosis of AML patients [19,20].
CD44 was one of the top three risk factor target-genes associated with AML survival. A number of studies have reported that CD44 is associated with AML survival. In elderly patients with refractory AML, the overall survival of patients with PTEN-positive and CD44-negative expression is longer than patients with PTEN-negative and CD44-positive expression [21]. Knockdown of CD44 enhances chemo-sensitivity of AML cells to adriamycin (ADM) and cytosine arabinoside (Ara-C) [22]. CD44 activation enhances primary acute monoblastic leukemia blast survival and increases apoptosis resistance of THP-1 monoblastic leukemia cells. Moreover, CD44 activation upregulates the expression of anti-apoptotic Mcl-1 protein, which is essential for apoptosis resistance of THP-1 cells [23]. Calcium signaling pathway was one of the significantly enriched pathways in our study (Table 3). Calcium can act in signal transduction resulting from activation of ion channels or as a second messenger. Calcium signaling through ion channels is important to maintain depolarization of the heart and neuronal synaptic transmission. Calcium ions play a vital role in muscle contraction, neuronal transmission, cell motility, cell growth or proliferation [24–27]. It has been reported that calcium signaling pathway is related to progression and development of various cancers including lung adenocarcinoma [28], colorectal cancer [29], glioblastoma [30], breast cancer [31], and Burkitt lymphoma [32]. Calcium signaling pathway may play a key role in the progression and development of AML.
Natural killer cell mediated cytotoxicity was one of the significant enrichment KEGG pathways in our study (Table 3). Natural killer cell mediated cytotoxicity contributes to the innate immune response against numerous malignancies. Progression and development of malignancy is promoted by tumor cells escaping from immune surveillance of immune effector cells, including natural killer cells [33]. The phenomenon of tumor cells escaping from immune surveillance is observed in numerous malignancies including breast cancer [34], head-and-neck squamous carcinoma [35], and leukemia. Leukemia stem cells play a central role in the relapse and refractory of AML. Leukemic stem-like cells from AML cell line KG1a cells are resistant to chemotherapy and natural killer cell-mediated cytotoxicity [36]. Acute lymphoblastic leukemia could resist natural killer cell-mediated cytotoxicity [37].
Calcium signaling pathway and natural killer cell mediated cytotoxicity correlate with the progression and development of numerous tumors. It has been suggested that these two pathways may play key roles in AML progression and contribute to AML survival.
We identified the miRNAs and target-genes associated with AML survival. In our study, IL15RA, TNIP1, CD44, and hsa-mir-425 were risk-factor miRNA/target-genes associated with AML survival. In addition, hsa-mir-10b and hsa-mir-30a were protective-factor miRNAs associated with AML survival. As Figure 2 shows, IL15RA, CD44, and hsa-miR-425 were obviously upregulated in the high-risk group, and hsa-miR-200c was significantly downregulated in the high-risk group compared with the low-moderate risk group of AML patients. The qRT-PCR results were in accordance with other study results.
There were limitations to our study. First, although the miRNAs and mRNAs associated with survival time of AML patients identified in our study may have implications in the understanding of AML tumorigenesis and development of targeted therapy of AML, the roles of identified miRNAs and mRNAs in AML need to be further explored in laboratory work. Second, although we obtained miRNAs and target-genes associated with AML survival, we did not construct a prognostic model of AML survival. In future work, we will use miRNAs and target-genes to construct a prognostic signature to predict survival time of AML patients, and then verify the prognostic signature through clinical observations.
Conclusions
A number of miRNAs and mRNAs that correlated with survival of AML patients were identified in our study, which might provide valuable information for identification of potentially prognostic biomarker for AML survival.
Supplementary Table
Supplementary Table 1.
The target-genes of miRNAs associated with AML survival.
| miRNA | Count of targets | Count of target related to survival | Target mRNAs |
|---|---|---|---|
| hsa-mir-10b | 66 | 22 | JPH1, TRIM25, AXL, HTATSF1, VGLL3, SLCO2B1, RTN4R, C17orf28, CD44, IGF2BP2, ITGB3, NRL, SCARF1, HSPA12A, RDH10, SASH1, ANKRD13A, KIAA1024, MAP1LC3B, SFRP1, COPS7B, BTN2A1, NUDT4, FLRT2, SPTY2D1, ERRFI1, LTBP2, LRRC59, PPP1R15B, DNAJC5, C16orf48, GGT7, BAG5, WDR78, GPR44, FBLIM1, SEZ6, TMTC1, RPS23, CALU, DNAL4, GLRX3, DOHH, ZSCAN22, ST7L, ELOVL2, BAHD1, MPI, AMN, GALE, SIX4, KIAA1751, KIAA1045, INSIG2, TMEM2, ZFYVE27, MTMR3, ARNT2, FHL3, DAND5, FABP2, LYVE1, PITPNA, LY6K, ADIPOQ, ST6GALNAC6 |
| hsa-mir-1201 | 2 | 0 | KCTD14, PTGIS |
| hsa-mir-193b | 44 | 15 | SOX9, SDC1, TRIM25, C1QC, HECW1, EPB41L4B, ADAMTSL3, RHOU, ITGB3, SCARF1, SVEP1, TMEM30A, PTPRE, ANKRD13A, SLC35F3, CEND1, MRPS18B, NUDT4, TMED10, ZNF423, DNAJC5, C16orf48, ATP2A3, EAF1, LRRC8A, RNF138, CACNA1E, CALU, NUDT12, C11orf49, ST7L, BRAF, PTTG1IP, SNX30, ARHGAP29, COMMD9, SIX4, 6-Sep, KIAA1045, ZFYVE27, DLST, YAP1, DCTN5, TAPT1 |
| hsa-mir-194-1 | 1 | 0 | IL22 |
| hsa-mir-196a-1 | 3 | 2 | IKBKB, COL3A1, HMGA1 |
| hsa-mir-200c | 95 | 21 | IGF1, ADAMTS9, PTPN12, C10orf81, VGLL3, COL1A2, OXR1, MRO, C1orf124, CEBPG, EPB41L4B, GZF1, CD44, NTRK2, SLC9A3R2, KLF17, ITGB3, TTC5, HSPA12A, CDH11, RDH10, C16orf72, SASH1, TMEM47, KIAA1486, KIAA1024, MAP1LC3B, SFRP1, YES1, BTN2A1, PHACTR3, RND3, RND3, NUDT4, TOR1AIP1, TJP1, SPTY2D1, GNG12, ERRFI1, NLGN4X, TMED10, ZNF423, DNAJC5, ATP2B1, BAG5, ATF7, SNAI2, SNAI2, LRRC8A, IKBKB, TMTC1, RNF138, RPS23, CALU, ATXN7L1, FUBP3, ALCAM, AHRR, ZFPM2, ZFPM2, ING2, TSPAN12, CLSPN, MPDZ, SCD, TET1, FBXL3, MAMDC2, GPR116, ELOVL2, MTDH, TMEM25, SNX30, ARL5A, MSR1, SPRED1, PRKAR2B, TERF2, MMD, 6-Sep, IAH1, MTMR1, SPON1, PAK1, HSDL1, LCP1, IL33, YAP1, SEMA5A, PRKAR1A, PITPNA, LY6K, ADIPOQ, HS3ST3A1, COPS8 |
| hsa-mir-23b | 86 | 16 | ZBTB2, CXCL12, CXCL12, EBF3, IGF1, RASSF2, AXL, VGLL3, ITFG2, HECW1, GNA14, OXR1, PCDH17, MAGI1, EPB41L4B, GZF1, C17orf28, PROS1, ITIH5, EPSTI1, ITGB3, HSPA12A, NDUFA7, RDH10, RSPH3, C16orf72, LHX4, KIAA1024, SFRP1, NUDT4, FLRT2, KAL1, ZSCAN18, TJP1, SPTY2D1, NLGN4X, LIFR, TMED10, ZNF423, SIGLEC1, EAF1, RGS13, DCLK1, CHUK, GPRIN1, CACNA1E, CALU, PCDH18, ALCAM, GLRX3, CPEB2, TSPAN12, TXNDC16, SCN4A, CD163, DDX60L, INSR, ZSCAN22, C11orf59, ST7L, PDK4, PDK4, ELOVL2, LTV1, WBP2, USP31, YTHDF1, SNX30, BRD8, YY1, SLC8A1, TERF2, SIX4, ELOVL3, TMED5, INSIG2, TMEM2, ZNF85, ARNT2, SEMA5A, FCRL5, APOBEC3F, PITPNA, GLP1R, C14orf119, TAPT1 |
| hsa-mir-30a | 102 | 27 | SOX9, CXCL12, EBF3, MOCS1, SPOCK3, UBN1, VGLL3, NXN, RTN4R, FRZB, HECW1, OXR1, TUSC3, PCDH17, EPB41L4B, TTC9C, GZF1, TNIP1, MTHFD2, CACNA2D1, RARG, LRRC8D, SLC9A3R2, ITGB3, ITGB3, RAB34, TCTE3, RDH10, TMEM30A, NTRK3, TMEM47, ANKRD13A, VAT1, SLC35F3, KIAA1024, MAP1LC3B, YES1, COPS7B, DBF4, RND3, KLHL4, SPTY2D1, ERRFI1, LTBP2, LIMCH1, LIFR, TMED10, TMED10, PPP1R15B, FCN1, ATP2B1, WDR78, EAF1, PRG4, CBX3, LRRC8A, UBE2J1, UBE2J1, TMTC1, RNF138, CALU, MRPS24, MLLT1, MAP6, FUBP3, CPEB2, C19orf50, DPYSL2, VPS39, ZSCAN22, TET1, FBXL3, ELOVL2, MTDH, RGMB, PTGIS, BAHD1, MPI, SNX30, ARHGAP29, C9orf123, COMMD9, MMD, CAPN11, SIX4, PPID, 6-Sep, KIAA1751, INSIG2, TMEM2, TMEM87A, TMEM87A, MTMR1, SH3KBP1, SPON1, TPRG1L, HSDL1, LCP1, LCP1, PRKAR1A, HMGA1, LY6K |
| hsa-mir-425 | 57 | 20 | SOX9, JPH1, IGF1, TRIM25, HIST2H2BE, VGLL3, DCN, C17orf28, ITIH5, CCDC126, ITGB3, TMEM42, TMEM30A, C16orf72, GABRA2, ANKRD13A, ZNF625, KIAA1486, SFRP1, PHACTR3, SNED1, RND3, NUDT4, FLRT2, SPTY2D1, GNG12, NLGN4X, LRRC59, TMED10, ZNF423, ZNF70, ATP2A1, EAF1, RGS13, LRRC8A, IKBKB, SEZ6, BMP8A, CALU, AHRR, CPEB2, SCN4A, DPYSL2, DOHH, PIGZ, BANP, ZSCAN22, MUS81, GPR116, USP31, C9orf123, TSPYL1, KLRD1, MTMR3, HSDL1, DLST, MCOLN3 |
| hsa-mir-452 | 81 | 22 | EBF3, IGF1, TRIM25, RASSF2, PTPN12, VGLL3, PGBD5, FMO2, MRO, PCDH17, C1orf124, MAGI1, IGF2BP2, ALS2CL, RAD21, HSPA12A, ANK2, APOL6, SASH1, TMEM47, PTPRE, KIAA1486, SNUPN, MAP1LC3B, SFRP1, YES1, SNED1, FLRT2, GNG12, NLGN4X, LIFR, TMED10, GRAP, ATP2B1, ZNF70, GPR44, FAM101B, SNAI2, DCLK1, CHUK, DENND2A, COL3A1, TMTC1, BMP8A, RNF138, CACNA1E, PCDH18, UFC1, ATXN7L1, EFEMP1, AHRR, CPEB2, DPYSL2, MZF1, DDX60L, GAPDHS, SCD, TET1, SLC44A1, FBXL3, ELOVL2, ELTD1, RGMB, YTHDF1, SNX30, SLC8A1, PRKAR2B, C9orf123, COMMD9, TSPYL1, KLRD1, KIAA1045, TMEM87A, AGXT, ARNT2, GMPR2, YAP1, NUP214, DCTN5, TAPT1, COPS8 |
| hsa-mir-509-1 | 1 | 0 | NTRK3 |
| hsa-mir-589 | 69 | 24 | ZBTB2, GTSF1, C1QC, RASSF2, HIST2H2BE, VGLL3, NXN, SLCO2B1, PGBD5, FMO2, MRO, PCDH17, DCN, EPB41L4B, CCDC126, ITGB3, NRL, CDH11, SVEP1, ANK2, TMEM130, EFS, C16orf72, SASH1, PTPRE, ULK4, IL15RA, VAT1, KIAA1486, SFRP1, YES1, ZNF609, GNG12, ERRFI1, LIFR, PPP1R15B, DNAJC5, BAG5, FAM101B, CBX3, TMTC1, BMP8A, RPS23, ATXN7L1, MLLT1, LAT, DPYSL2, SCD, ZSCAN22, TOR1B, FBXL3, ST7L, BAHD1, FAM46B, COMMD9, SIX4, KIAA1751, BARHL1, PAK1, FHL3, FABP2, YAP1, SEMA5A, PITPNA, MEOX1, DCTN5, HS3ST3A1, BCAT2, COPS8 |
Footnotes
Conflict interest
All of the authors declare that they have no conflict interest.
Source of support: Departmental sources
References
- 1.Walter RB, Othus M, Burnett AK, et al. Significance of FAB subclassification of “acute myeloid leukemia, NOS” in the 2008 WHO classification: Analysis of 5848 newly diagnosed patients. Blood. 2013;121:2424–31. doi: 10.1182/blood-2012-10-462440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Butrym A, Rybka J, Baczyńska D, et al. Low expression of microRNA-204 (miR-204) is associated with poor clinical outcome of acute myeloid leukemia (AML) patients. J Exp ClinCancer Res. 2015;34:68. doi: 10.1186/s13046-015-0184-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hackl H, Astanina K, Wieser R. Molecular and genetic alterations associated with therapy resistance and relapse of acute myeloid leukemia. 2017;10:51. doi: 10.1186/s13045-017-0416-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kamal AM, El-Hefny NH, Hegab HM, El-Mesallamy HO. Expression of thioredoxin-1 (TXN) and its relation with oxidative DNA damage and treatment outcome in adult AML and ALL: A comparative study. Hematology (Amsterdam, Netherlands) 2016;21:567–75. doi: 10.1080/10245332.2016.1173341. [DOI] [PubMed] [Google Scholar]
- 5.Jabbour E, Takahashi K, Wang X, et al. Acquisition of cytogenetic abnormalities in patients with IPSS defined lower-risk myelodysplastic syndrome is associated with poor prognosis and transformation to acute myelogenous leukemia. Am J Hematol. 2013;88:831–37. doi: 10.1002/ajh.23513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herold T, Metzeler KH, Vosberg S, et al. Acute myeloid leukemia with del(9q) is characterized by frequent mutations of NPM1, DNMT3A, WT1 and low expression of TLE4. Genes Chromosomes Cancer. 2017;56:75–86. doi: 10.1002/gcc.22418. [DOI] [PubMed] [Google Scholar]
- 7.Lemonnier F, Inoue S, Mak TW. Genomic classification in acute myeloid leukemia. N Engl J Med. 2016;375:900. doi: 10.1056/NEJMc1608739. [DOI] [PubMed] [Google Scholar]
- 8.Bochtler T, Granzow M, Stolzel F, et al. Marker chromosomes can arise from chromothripsis and predict adverse prognosis in acute myeloid leukemia. Blood. 2017;129:1333–42. doi: 10.1182/blood-2016-09-738161. [DOI] [PubMed] [Google Scholar]
- 9.Niederwieser C, Nicolet D, Carroll AJ, et al. Chromosome abnormalities at onset of complete remission are associated with worse outcome in patients with acute myeloid leukemia and an abnormal karyotype at diagnosis: CALGB 8461 (Alliance) Haematologica. 2016;101:1516–23. doi: 10.3324/haematol.2016.149542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walter RB, Othus M, Burnett AK, et al. Resistance prediction in AML: Analysis of 4601 patients from MRC/NCRI, HOVON/SAKK, SWOG and MD Anderson Cancer Center. Leukemia. 2015;29:312–20. doi: 10.1038/leu.2014.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gaballa S, Saliba R, Oran B, et al. Relapse risk and survival in patients with FLT3 mutated acute myeloid leukemia undergoing stem cell transplantation. Am J Hematol. 2017;92:331–37. doi: 10.1002/ajh.24632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Z, Li Y, Fan L, et al. microRNA-425-5p is upregulated in human gastric cancer and contributes to invasion and metastasis in vitro and in vivo. Exp Ther Med. 2015;9:1617–22. doi: 10.3892/etm.2015.2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peng W-Z, Ma R, Wang F, et al. Role of miR-191/425 cluster in tumorigenesis and diagnosis of gastric cancer. Int J Mol Sci. 2014;15:4031–48. doi: 10.3390/ijms15034031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma J, Liu J, Wang Z, et al. NF-kappaB-dependent microRNA-425 upregulation promotes gastric cancer cell growth by targeting PTEN upon IL-1β induction. Mol Cancer. 2014;13:40. doi: 10.1186/1476-4598-13-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu L, Zhao Z, Zhou W, et al. Enhanced expression of miR-425 promotes esophageal squamous cell carcinoma tumorigenesis by targeting SMAD2. J Genet Genomics. 2015;42:601–11. doi: 10.1016/j.jgg.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 16.Ge Y-Z, Xin H, Lu T-Z, et al. MicroRNA expression profiles predict clinical phenotypes and prognosis in chromophobe renal cell carcinoma. Sci Rep. 2015;5:10328. doi: 10.1038/srep10328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu Y, Song Y, Ma W, et al. Decreased microRNA-30a levels are associated with enhanced ABL1 and BCR-ABL1 expression in chronic myeloid leukemia. Leuk Res. 2013;37:349–56. doi: 10.1016/j.leukres.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 18.Ren F, Wang L, Shen X, et al. MYBL2 is an independent prognostic marker that has tumor-promoting functions in colorectal cancer. Am J Cancer Res. 2015;5:1542–52. [PMC free article] [PubMed] [Google Scholar]
- 19.Dolz S, García P, Llop M, et al. Study of the S427G polymorphism and of MYBL2 variants in patients with acute myeloid leukemia. Leuk Lymphoma. 2015 doi: 10.3109/10428194.2015.1049167. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 20.Fuster Ó, Llop M, Dolz S, et al. Adverse prognostic value of MYBL2 overexpression and association with microRNA-30 family in acute myeloid leukemia patients. Leuk Res. 2013;37:1690–96. doi: 10.1016/j.leukres.2013.09.015. [DOI] [PubMed] [Google Scholar]
- 21.Huang X, Li D, Li T, et al. Prognostic value of the expression of phosphatase and tensin homolog and CD44 in elderly patients with refractory acute myeloid leukemia. Oncol Lett. 2015;10:103–10. doi: 10.3892/ol.2015.3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang N-s, Wei M, Ma W-l, et al. Knockdown of CD44 enhances chemosensitivity of acute myeloid leukemia cells to ADM and Ara-C. Tumor Biol. 2014;35:3933–40. doi: 10.1007/s13277-013-1523-3. [DOI] [PubMed] [Google Scholar]
- 23.Sansonetti A, Bourcier S, Durand L, et al. CD44 activation enhances acute monoblastic leukemia cell survival via Mcl-1 upregulation. Leuk Res. 2012;36:358–62. doi: 10.1016/j.leukres.2011.09.022. [DOI] [PubMed] [Google Scholar]
- 24.Isik A, Gursul C, Peker K, et al. Metalloproteinases and their inhibitors in patients with inguinal hernia. World J Surg. 2017 doi: 10.1007/s00268-016-3858-6. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 25.Saghiri MA, Asatourian A, Orangi J, et al. Functional role of inorganic trace elements in angiogenesis – Part I: N, Fe, Se, P, Au, and Ca. Crit Rev Oncol Hematol. 2015;96(1):129–42. doi: 10.1016/j.critrevonc.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 26.Capiod T. Extracellular calcium has multiple targets to control cell proliferation. Adv Exp Med Biol. 2016;898:133–56. doi: 10.1007/978-3-319-26974-0_7. [DOI] [PubMed] [Google Scholar]
- 27.Isik A, Idiz O, Firat D. Novel approaches in pilonidal sinus treatment. Prague Med Rep. 2016;117:145–52. doi: 10.14712/23362936.2016.15. [DOI] [PubMed] [Google Scholar]
- 28.Wu X, Zhang W, Hu Y, Yi X. Bioinformatics approach reveals systematic mechanism underlying lung adenocarcinoma. Tumori. 2015;101:281–86. doi: 10.5301/tj.5000278. [DOI] [PubMed] [Google Scholar]
- 29.Kou Y, Zhang S, Chen X, Hu S. Gene expression profile analysis of colorectal cancer to investigate potential mechanisms using bioinformatics. Onco Targets Ther. 2015;8:745–52. doi: 10.2147/OTT.S78974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wei B, Wang L, Du C, et al. Identification of differentially expressed genes regulated by transcription factors in glioblastomas by bioinformatics analysis. Mol Med Rep. 2015;11:2548–54. doi: 10.3892/mmr.2014.3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen WY, Wu F, You ZY, et al. Analyzing the differentially expressed genes and pathway cross-talk in aggressive breast cancer. J Obstet Gynaecol Res. 2015;41:132–40. doi: 10.1111/jog.12495. [DOI] [PubMed] [Google Scholar]
- 32.Gu L, Xie L, Zuo C, et al. Targeting mTOR/p70S6K/glycolysis signaling pathway restores glucocorticoid sensitivity to 4E-BP1 null Burkitt Lymphoma. BMC Cancer. 2015;15:529. doi: 10.1186/s12885-015-1535-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pahl J, Cerwenka A. Tricking the balance: NK cells in anti-cancer immunity. Immunobiology. 2017;222:11–20. doi: 10.1016/j.imbio.2015.07.012. [DOI] [PubMed] [Google Scholar]
- 34.Nieto-Velazquez NG, Torres-Ramos YD, Munoz-Sanchez JL, et al. Altered expression of natural cytotoxicity receptors and NKG2D on peripheral blood NK cell subsets in breast cancer patients. Transl Oncol. 2016;9:384–91. doi: 10.1016/j.tranon.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim MJ, Lee JC, Lee JJ, et al. Association of CD47 with natural killer cell-mediated cytotoxicity of head-and-neck squamous cell carcinoma lines. Tumor Biol. 2008;29:28–34. doi: 10.1159/000132568. [DOI] [PubMed] [Google Scholar]
- 36.She M, Niu X, Chen X, et al. Resistance of leukemic stem-like cells in AML cell line KG1a to natural killer cell-mediated cytotoxicity. Cancer Lett. 2012;318:173–79. doi: 10.1016/j.canlet.2011.12.017. [DOI] [PubMed] [Google Scholar]
- 37.Dolstra H, Roeven MW, Spanholtz J, et al. Successful transfer of umbilical cord blood CD34+ hematopoietic stem and progenitor-derived NK cells in older acute myeloid leukemia patients. Clin Cancer Res. 2017 doi: 10.1158/1078-0432.CCR-16-2981. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1.
The target-genes of miRNAs associated with AML survival.
| miRNA | Count of targets | Count of target related to survival | Target mRNAs |
|---|---|---|---|
| hsa-mir-10b | 66 | 22 | JPH1, TRIM25, AXL, HTATSF1, VGLL3, SLCO2B1, RTN4R, C17orf28, CD44, IGF2BP2, ITGB3, NRL, SCARF1, HSPA12A, RDH10, SASH1, ANKRD13A, KIAA1024, MAP1LC3B, SFRP1, COPS7B, BTN2A1, NUDT4, FLRT2, SPTY2D1, ERRFI1, LTBP2, LRRC59, PPP1R15B, DNAJC5, C16orf48, GGT7, BAG5, WDR78, GPR44, FBLIM1, SEZ6, TMTC1, RPS23, CALU, DNAL4, GLRX3, DOHH, ZSCAN22, ST7L, ELOVL2, BAHD1, MPI, AMN, GALE, SIX4, KIAA1751, KIAA1045, INSIG2, TMEM2, ZFYVE27, MTMR3, ARNT2, FHL3, DAND5, FABP2, LYVE1, PITPNA, LY6K, ADIPOQ, ST6GALNAC6 |
| hsa-mir-1201 | 2 | 0 | KCTD14, PTGIS |
| hsa-mir-193b | 44 | 15 | SOX9, SDC1, TRIM25, C1QC, HECW1, EPB41L4B, ADAMTSL3, RHOU, ITGB3, SCARF1, SVEP1, TMEM30A, PTPRE, ANKRD13A, SLC35F3, CEND1, MRPS18B, NUDT4, TMED10, ZNF423, DNAJC5, C16orf48, ATP2A3, EAF1, LRRC8A, RNF138, CACNA1E, CALU, NUDT12, C11orf49, ST7L, BRAF, PTTG1IP, SNX30, ARHGAP29, COMMD9, SIX4, 6-Sep, KIAA1045, ZFYVE27, DLST, YAP1, DCTN5, TAPT1 |
| hsa-mir-194-1 | 1 | 0 | IL22 |
| hsa-mir-196a-1 | 3 | 2 | IKBKB, COL3A1, HMGA1 |
| hsa-mir-200c | 95 | 21 | IGF1, ADAMTS9, PTPN12, C10orf81, VGLL3, COL1A2, OXR1, MRO, C1orf124, CEBPG, EPB41L4B, GZF1, CD44, NTRK2, SLC9A3R2, KLF17, ITGB3, TTC5, HSPA12A, CDH11, RDH10, C16orf72, SASH1, TMEM47, KIAA1486, KIAA1024, MAP1LC3B, SFRP1, YES1, BTN2A1, PHACTR3, RND3, RND3, NUDT4, TOR1AIP1, TJP1, SPTY2D1, GNG12, ERRFI1, NLGN4X, TMED10, ZNF423, DNAJC5, ATP2B1, BAG5, ATF7, SNAI2, SNAI2, LRRC8A, IKBKB, TMTC1, RNF138, RPS23, CALU, ATXN7L1, FUBP3, ALCAM, AHRR, ZFPM2, ZFPM2, ING2, TSPAN12, CLSPN, MPDZ, SCD, TET1, FBXL3, MAMDC2, GPR116, ELOVL2, MTDH, TMEM25, SNX30, ARL5A, MSR1, SPRED1, PRKAR2B, TERF2, MMD, 6-Sep, IAH1, MTMR1, SPON1, PAK1, HSDL1, LCP1, IL33, YAP1, SEMA5A, PRKAR1A, PITPNA, LY6K, ADIPOQ, HS3ST3A1, COPS8 |
| hsa-mir-23b | 86 | 16 | ZBTB2, CXCL12, CXCL12, EBF3, IGF1, RASSF2, AXL, VGLL3, ITFG2, HECW1, GNA14, OXR1, PCDH17, MAGI1, EPB41L4B, GZF1, C17orf28, PROS1, ITIH5, EPSTI1, ITGB3, HSPA12A, NDUFA7, RDH10, RSPH3, C16orf72, LHX4, KIAA1024, SFRP1, NUDT4, FLRT2, KAL1, ZSCAN18, TJP1, SPTY2D1, NLGN4X, LIFR, TMED10, ZNF423, SIGLEC1, EAF1, RGS13, DCLK1, CHUK, GPRIN1, CACNA1E, CALU, PCDH18, ALCAM, GLRX3, CPEB2, TSPAN12, TXNDC16, SCN4A, CD163, DDX60L, INSR, ZSCAN22, C11orf59, ST7L, PDK4, PDK4, ELOVL2, LTV1, WBP2, USP31, YTHDF1, SNX30, BRD8, YY1, SLC8A1, TERF2, SIX4, ELOVL3, TMED5, INSIG2, TMEM2, ZNF85, ARNT2, SEMA5A, FCRL5, APOBEC3F, PITPNA, GLP1R, C14orf119, TAPT1 |
| hsa-mir-30a | 102 | 27 | SOX9, CXCL12, EBF3, MOCS1, SPOCK3, UBN1, VGLL3, NXN, RTN4R, FRZB, HECW1, OXR1, TUSC3, PCDH17, EPB41L4B, TTC9C, GZF1, TNIP1, MTHFD2, CACNA2D1, RARG, LRRC8D, SLC9A3R2, ITGB3, ITGB3, RAB34, TCTE3, RDH10, TMEM30A, NTRK3, TMEM47, ANKRD13A, VAT1, SLC35F3, KIAA1024, MAP1LC3B, YES1, COPS7B, DBF4, RND3, KLHL4, SPTY2D1, ERRFI1, LTBP2, LIMCH1, LIFR, TMED10, TMED10, PPP1R15B, FCN1, ATP2B1, WDR78, EAF1, PRG4, CBX3, LRRC8A, UBE2J1, UBE2J1, TMTC1, RNF138, CALU, MRPS24, MLLT1, MAP6, FUBP3, CPEB2, C19orf50, DPYSL2, VPS39, ZSCAN22, TET1, FBXL3, ELOVL2, MTDH, RGMB, PTGIS, BAHD1, MPI, SNX30, ARHGAP29, C9orf123, COMMD9, MMD, CAPN11, SIX4, PPID, 6-Sep, KIAA1751, INSIG2, TMEM2, TMEM87A, TMEM87A, MTMR1, SH3KBP1, SPON1, TPRG1L, HSDL1, LCP1, LCP1, PRKAR1A, HMGA1, LY6K |
| hsa-mir-425 | 57 | 20 | SOX9, JPH1, IGF1, TRIM25, HIST2H2BE, VGLL3, DCN, C17orf28, ITIH5, CCDC126, ITGB3, TMEM42, TMEM30A, C16orf72, GABRA2, ANKRD13A, ZNF625, KIAA1486, SFRP1, PHACTR3, SNED1, RND3, NUDT4, FLRT2, SPTY2D1, GNG12, NLGN4X, LRRC59, TMED10, ZNF423, ZNF70, ATP2A1, EAF1, RGS13, LRRC8A, IKBKB, SEZ6, BMP8A, CALU, AHRR, CPEB2, SCN4A, DPYSL2, DOHH, PIGZ, BANP, ZSCAN22, MUS81, GPR116, USP31, C9orf123, TSPYL1, KLRD1, MTMR3, HSDL1, DLST, MCOLN3 |
| hsa-mir-452 | 81 | 22 | EBF3, IGF1, TRIM25, RASSF2, PTPN12, VGLL3, PGBD5, FMO2, MRO, PCDH17, C1orf124, MAGI1, IGF2BP2, ALS2CL, RAD21, HSPA12A, ANK2, APOL6, SASH1, TMEM47, PTPRE, KIAA1486, SNUPN, MAP1LC3B, SFRP1, YES1, SNED1, FLRT2, GNG12, NLGN4X, LIFR, TMED10, GRAP, ATP2B1, ZNF70, GPR44, FAM101B, SNAI2, DCLK1, CHUK, DENND2A, COL3A1, TMTC1, BMP8A, RNF138, CACNA1E, PCDH18, UFC1, ATXN7L1, EFEMP1, AHRR, CPEB2, DPYSL2, MZF1, DDX60L, GAPDHS, SCD, TET1, SLC44A1, FBXL3, ELOVL2, ELTD1, RGMB, YTHDF1, SNX30, SLC8A1, PRKAR2B, C9orf123, COMMD9, TSPYL1, KLRD1, KIAA1045, TMEM87A, AGXT, ARNT2, GMPR2, YAP1, NUP214, DCTN5, TAPT1, COPS8 |
| hsa-mir-509-1 | 1 | 0 | NTRK3 |
| hsa-mir-589 | 69 | 24 | ZBTB2, GTSF1, C1QC, RASSF2, HIST2H2BE, VGLL3, NXN, SLCO2B1, PGBD5, FMO2, MRO, PCDH17, DCN, EPB41L4B, CCDC126, ITGB3, NRL, CDH11, SVEP1, ANK2, TMEM130, EFS, C16orf72, SASH1, PTPRE, ULK4, IL15RA, VAT1, KIAA1486, SFRP1, YES1, ZNF609, GNG12, ERRFI1, LIFR, PPP1R15B, DNAJC5, BAG5, FAM101B, CBX3, TMTC1, BMP8A, RPS23, ATXN7L1, MLLT1, LAT, DPYSL2, SCD, ZSCAN22, TOR1B, FBXL3, ST7L, BAHD1, FAM46B, COMMD9, SIX4, KIAA1751, BARHL1, PAK1, FHL3, FABP2, YAP1, SEMA5A, PITPNA, MEOX1, DCTN5, HS3ST3A1, BCAT2, COPS8 |