Abstract
Sacbrood virus (SBV) is one of the most common viral infections of honeybees. The entire genome sequence for nine SBV infecting honeybees, Apis cerana and Apis mellifera, in Vietnam, namely AcSBV-Viet1, AcSBV-Viet2, AcSBV-Viet3, AmSBV-Viet4, AcSBV-Viet5, AmSBV-Viet6, AcSBV-Viet7, AcSBV-Viet8, and AcSBV-Viet9, was determined. These sequences were aligned with seven previously reported complete genome sequences of SBV from other countries, and various genomic regions were compared. The Vietnamese SBVs (VN-SBVs) shared 91–99% identity with each other, and shared 89–94% identity with strains from other countries. The open reading frames (ORFs) of the VN-SBV genomes differed greatly from those of SBVs from other countries, especially in their VP1 sequences. The AmSBV-Viet6 and AcSBV-Viet9 genome encodes 17 more amino acids within this region than the other VN-SBVs. In a phylogenetic analysis, the strains AmSBV-Viet4, AcSBV-Viet2, and AcSBV-Viet3 were clustered in group with AmSBV-UK, AmSBV-Kor21, and AmSBV-Kor19 strains. Whereas, the strains AmSBV-Viet6 and AcSBV-Viet7 clustered separately with the AcSBV strains from Korea and AcSBV-VietSBM2. And the strains AcSBV-Viet8, AcSBV-Viet1, AcSBV-Viet5, and AcSBV-Viet9 clustered with the AcSBV-India, AcSBV-Kor and AcSBV-VietSBM2. In a Simplot graph, the VN-SBVs diverged stronger in their ORF regions than in their 5′ or 3′ untranslated regions. The VN-SBVs possess genetic characteristics which are more similar to the Asian AcSBV strains than to AmSBV-UK strain. Taken together, our data indicate that host specificity, geographic distance, and viral cross-infections between different bee species may explain the genetic diversity among the VN-SBVs in A. cerana and A. mellifera and other SBV strains.
Keywords: Sacbrood virus, Apis cerana, Apis mellifera, entire genome sequence, phylogenetic analysis
In the last decade, the health of honeybees has been declining at an alarming rate, drawing into question the sustainability of our food production system (Van Engelsdorp et al. 2008, James et al. 2010, Evans and Schwars 2011). The health and vigor of honeybee colonies are affected by numerous parasites and pathogens, including viruses, bacteria, protozoa, fungi, and mites. A number of studies have suggested that, among these, RNA viral pathogens represent a serious threat to the health of managed honeybees. However, insufficient knowledge of honeybee pathogens compromises our ability to assess their importance and to develop control measures. This is especially true for honeybee viruses, although their importance in honeybee losses has become evident in recent years (Cox-Foster et al. 2007, Elke et al. 2010, Nazzi et al. 2012). Approximately 20 honeybee viruses have been described so far in each of Apis cerana and Apis mellifera (Chen and Siede 2007, Genersch and Aubert 2010, Cornman et al. 2012).
Sacbrood virus (SBV) is one of the most severe threats to the health of A. cerana and A. mellifera. SBV disease causes failure to pupate, and ecdysial fluid accumulates beneath the unshed skin. Infected larvae change color from white to pale yellow, and after death, they dry out quickly, forming a dark-brown gondola-shaped scale (Bailey 1975). SBV can also infect the adult bee, and infected workers have reduced life spans (Bailey 1976). However, when the adult bee is infected, it displays no clear physical signs (Bailey 1975, Berényi et al. 2006). According to previous studies, SBV has the greatest effect on A. cerana and A. mellifera in Asian countries and Vietnam (Ai et al. 2012, Choe et al. 2012a,b, Forsgren et al. 2015, Wu et al. 2015, Yanez et al. 2015, Ha et al. 2016, Sarwar 2016, Wang et al. 2016). SBV is a picorna-like virus of the genus Iflavirus, with a single positive-strand RNA genome of ~8.8 kb, with a single large open reading frame (ORF). The SBV genome is monopartite and monocistronic, with the structural genes at the 5′- end and the nonstructural gene at the 3′- end (Ghosh et al. 1999, Chen et al. 2006, Mingxiao et al. 2011, Choe et al. 2012c, Reddy et al. 2016).
In Vietnam, the beekeeping is also an important agricultural that brings in great profit. Vietnam stands in the sixth position in the list of the world’s top honey exporter countries, in 2014. The current, the A. cerana and A. mellifera species are widely breeding in all over of Vietnam, with about 180,000 colonies of A. cerana and 360,000 colonies of A. mellifera. The beekeeping in Vietnam is also facing many difficulties due to the diseases including the viruses. Among them, SBV that infect Vietnamese honeybees are referred to as Vietnamese SBVs (VN-SBVs), is the most common and dangerous virus in Vietnam. VN-SBV was first detected in the Asian honeybee A. cerana in 1974. Within a short period, A. cerana had been reduced by up to 90% of colonies by VN-SBV (Phung 1990). And VN-SBV was identified in the European honeybee A. mellifera in 2006 (Phung 2008). Therefore, it is important to determine and understand the disease distribution, the genetic variations of SBV strains on different hosts and geographic regions. These insights will be useful information in the prevention of diseases for honeybee.
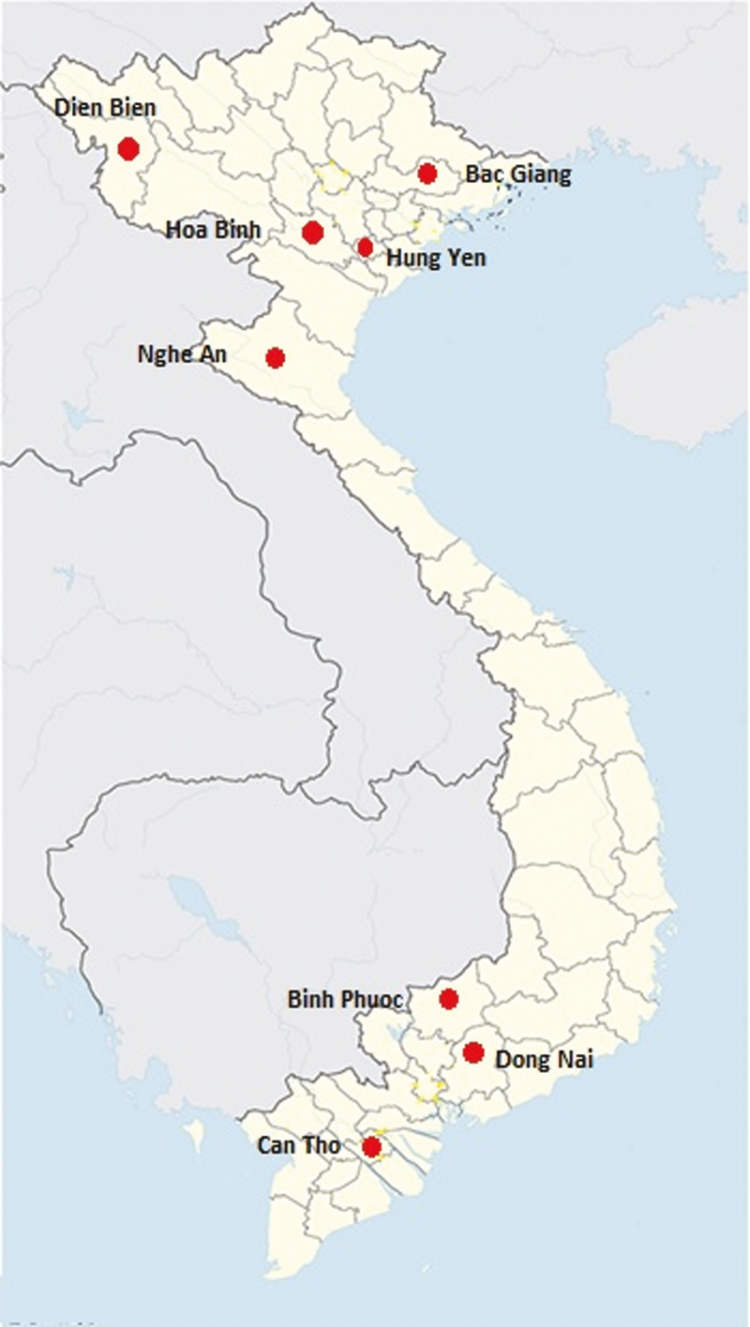
For this reason, we collected and analyzed SBV infected samples from both honeybee species in different provinces of Vietnam. We isolated nine complete genome sequences of VN-SBVs from honeybees in Vietnam. Seven samples were collected from A. cerana in Dien Bien, Bac Giang, Hoa Binh, Hung Yen, Nghe An provinces (in North of Vietnam) and Binh Phuoc, Can Tho province (in South of Vietnam). Two samples were collected from A. mellifera in Dien Bien province (in North of Vietnam) and Dong Nai province (in South of Vietnam). The genetic relationships and variations among these nine sequences were compared with those of SBV strains from other countries.
Materials and Methods
Sample Collection and RNA Extraction
Adult bees and larval samples of A. cerana and A. mellifera were collected from different provinces of Vietnam (Fig. 1) between August and December, 2014. In total, 216 samples were collected from the two bee species (180 A. cerana samples and 36 A. mellifera samples) and each sample contained of 10 honeybees from each colony in an apiary. All the samples were collected by the Institute of Biotechnology, Vietnam Academy of Science and Technology, Hanoi, Vietnam. The RNAs were extracted with an RNeasy Mini Kit (Qiagen, Hilden, Germany), according to the manufacturer’s instructions, and then sent to our Korean laboratory by express mail.
Fig. 1.
Sampling location in the different provinces of Vietnam.
Reverse Transcription PCR Amplification of Viral RNA
The RNAs were used to synthesize cDNAs in Korea using a reverse transcriptase method (Invitrogen, Massachusetts, USA) with an oligo(dT) primer, according to the manufacturer’s protocol. To screen the SBV-positive samples from various regions of Vietnam, a pair of oligo nucleotide primers was used: SBV-F (5′ - ACCAACCGATTCCTCAGTAG - 3′) and SBV-R (5′- CCTTGGAACTCTGCTGTGTA - 3′) (Grabensteiner et al. 2001). Fifteen sets of primers from our previous study (Choe et al. 2012c) were used to amplify overlapping PCR products that comprised the complete genome sequences of selected VN-SBV strains. The resulting cDNA (1 µl) was amplified in a 20 µl reaction mixture with TOPsimple DryMIX-HOT (Enzynomics, Deajon, South Korea). PCR was performed with the following thermal cycling parameters: one cycle of initial denaturation at 95°C for 10 min, followed by 35 cycles of denaturation at 95°C for 30 s, annealing at 55°C for 30 s, and extension at 72°C for 1 min. Finally, the samples were maintained at 72°C for 10 min. The reactions were performed in a C1000 Thermal Cycler (Bio-Rad, Canifornia, USA). Negative controls (H2O) were included with each PCR. The amplified products were separated electrophoretically in agarose gel containing ethidium bromide, visualized with UV transillumination, and photographed with the Digital Science Electrophoresis Documentation and Analysis System. The product sizes were determined with reference to a 100-bp molecular weight ladder (Enzynomics, Daejon, South Korea).
Nucleotide Sequencing and Computational Analysis
Specific amplification products were excised from the agarose gel and extracted with a QIAquick Gel Extraction Kit (Qiagen, Hilden, Germany), according to the manufacturer’s instructions. The purified products were sequenced by Macrogen (Seoul, Korea). The consensus sequences were compared with sequences in GenBank using the Basic Local Alignment Search Tool (BLAST) on the National Center for Biotechnology Information (NCBI) website. The nucleotide sequences of all the fragments were compiled and aligned to build a continuous complete genome sequence using BioEdit version 7.0.9.0, based on the published Korean SBV sequence (JQ390592) as a reference.
Phylogenetic Tree Construction and Simplot Analysis of SBV
A phylogenetic tree was constructed for the complete genome sequences of seven previously reported SBVs from various countries and the nine VN-SBVs investigated: AcSBV-Viet1 (A. cerana SBV Vietnam1; Hoa Binh, North Vietnam), AcSBV-Viet2 (Dien Bien, North Vietnam), AcSBV-Viet3 (Hung Yen, North Vietnam), AmSBV-Viet4 (A. mellifera SBV Vietnam4; Dien Bien, North Vietnam), AcSBV-Viet5 (Can Tho, South Vietnam), AmSBV-Viet6 (Dong Nai, South Vietnam), AcSBV-Viet7 (Bac Giang, North Vietnam), AcSBV-Viet8 (Nghe An, North Vietnam), and AcSBV-Viet9 (Binh Phuoc, South Vietnam). A multiple sequence alignment was performed using ClustalW (Thompson et al. 1997) and a phylogenetic tree was constructed with MEGA 7 (Kumar et al. 2016), using the neighbor-joining method with Kimura’s two-parameter model (Kimura 1980). Bootstrap values were based on 1,000 replicates. The differences between the complete genome sequences of the VN-SBVs and those from other countries were plotted using Simplot (Ray 2003), with the two-parameter distance model (Kimura 1980).
Results
Detecting and Analyzing Genetic Variation of VN-SBV in A. cerana and A. mellifera
A specific primer pair was used to screen for the presence of SBV infection in 216 Vietnamese bee samples, 180 samples of A. cerana and 36 samples of A. mellifera. We identified 59 bee samples in A. cerana and two samples in A. mellifera that infected with SBV. To isolate the complete VN-SBV genomes, we randomly selected the samples that infected in both species from provinces in different regions of Vietnam.
Nine complete genome sequences of VN-SBV from A. cerana and A. mellifera in various regions of Vietnam were determined. They were aligned and compared with the genome sequences of SBV strains from different countries. The complete sequence of VN-SBV strains varied little in size: AcSBV-Viet1 (KM884990), AcSBV-Viet3 (KM884992), and AmSBV-Viet4 (KM884993) contained 8787 nucleotides (nt), AcSBV-Viet2 (KM884991) contained 8786 nt, AcSBV-Viet5 (KM884994) and AcSBV-Viet7 (KX668141) contained 8784 nt, AmSBV-Viet6 (KM884995) contained 8836 nt, AcSBV-Viet8 (KX668140) contained 8791 nt, and AcSBV-Viet9 (KX668139) contained 8831 nt. The complete sequences of VN-SBV contained a single large ORF flanked by 5′ and 3′ untranslated regions (UTRs). The VN-SBV has structural polyprotein regions, motifs, and other partial of gene as well as were described in previously studies (Ghosh et al. 1999, Mingxiao et al. 2011, Choe et al. 2012c). The similarities of the complete nucleotide sequences and the various genomic regions of the VN-SBVs and the SBVs from other countries are shown in Table 1.
Table 1.
Nucleotide sequence homology (%) of the complete genome sequences and different genomic regions of VN-SBVs and other reference SBVs
| Genomic region/complete genome | Vietnamese genome | AmSBV- Kor19 | AmSBV- Kor21 | AcSBV- Kor | AcSBV- China | AcSBV- India | AcSBV- VietSBM2 | AmSBV- UK |
|---|---|---|---|---|---|---|---|---|
| Complete nucleotide sequence | AcSBV-Viet1 | 93.7 | 90.0 | 94.4 | 92.8 | 92.1 | 93.1 | 89.9 |
| AcSBV-Viet2 | 93.6 | 90.0 | 94.4 | 92.8 | 92.0 | 93.1 | 89.9 | |
| AcSBV-Viet3 | 93.9 | 89.9 | 94.5 | 92.7 | 92.0 | 93.0 | 89.8 | |
| AmSBV-Viet4 | 93.8 | 90.2 | 94.4 | 93.0 | 92.3 | 93.3 | 90.0 | |
| AcSBV-Viet5 | 93.4 | 89.6 | 94.1 | 92.6 | 91.9 | 92.9 | 89.8 | |
| AmSBV-Viet6 | 92.0 | 90.0 | 92.2 | 93.3 | 93.6 | 94.3 | 90.1 | |
| AcSBV-Viet7 | 92.6 | 90.1 | 94.2 | 93.4 | 93.4 | 94.2 | 90.2 | |
| AcSBV-Viet8 | 92.4 | 90.0 | 94.3 | 93.5 | 93.2 | 94.3 | 90.2 | |
| AcSBV-Viet9 | 92.3 | 90.2 | 93.3 | 93.4 | 93.0 | 93.9 | 90.1 | |
| 5′ UTR | AcSBV-Viet1 | 89.9 | 89.3 | 89.8 | 89.8 | 83.4 | 88.1 | 89.2 |
| AcSBV-Viet2 | 89.8 | 89.3 | 89.8 | 89.8 | 83.4 | 88.1 | 89.3 | |
| AcSBV-Viet3 | 91.5 | 91.0 | 91.5 | 91.5 | 85.5 | 89.8 | 91.0 | |
| AmSBV-Viet4 | 90.4 | 89.8 | 90.4 | 90.4 | 83.4 | 88.1 | 90.9 | |
| AcSBV-Viet5 | 89.7 | 89.2 | 89.2 | 89.7 | 84.8 | 89.2 | 90.3 | |
| AmSBV-Viet6 | 91.5 | 90.9 | 90.9 | 92.0 | 85.5 | 90.9 | 90.4 | |
| AcSBV-Viet7 | 91.4 | 90.3 | 90.6 | 92.1 | 85.3 | 89.1 | 91.2 | |
| AcSBV-Viet8 | 91.0 | 90.5 | 90.8 | 92.0 | 85.5 | 89.3 | 91.0 | |
| AcSBV-Viet9 | 91.4 | 90.2 | 90.1 | 92.3 | 85.3 | 90.9 | 90.2 | |
| ORF region | AcSBV-Viet1 | 93.7 | 89.9 | 94.5 | 92.8 | 92.0 | 93.0 | 89.9 |
| AcSBV-Viet2 | 93.7 | 89.9 | 94.5 | 92.8 | 92.0 | 93.1 | 89.9 | |
| AcSBV-Viet3 | 93.9 | 89.8 | 94.6 | 92.7 | 92.0 | 93.0 | 89.8 | |
| AmSBV-Viet4 | 93.9 | 90.2 | 94.5 | 93.0 | 92.3 | 93.3 | 90.0 | |
| AcSBV-Viet5 | 93.5 | 89.7 | 94.2 | 92.6 | 91.9 | 92.9 | 89.8 | |
| AmSBV-Viet6 | 91.9 | 89.9 | 92.3 | 93.3 | 93.6 | 94.3 | 90.7 | |
| AcSBV-Viet7 | 93.5 | 89.9 | 94.5 | 92.9 | 92.3 | 93.2 | 89.2 | |
| AcSBV-Viet8 | 93.2 | 90.2 | 94.6 | 92.5 | 92.4 | 92.4 | 89.3 | |
| AcSBV-Viet9 | 91.6 | 89.7 | 92.3 | 93.1 | 93.2 | 94.2 | 90.3 | |
| 3′ UTR | AcSBV-Viet1 | 94.7 | 94.7 | 94.8 | 94.6 | 86.6 | 93.5 | 96.1 |
| AcSBV-Viet2 | 94.8 | 94.8 | 94.8 | 94.8 | 86.6 | 93.5 | 96.1 | |
| AcSBV-Viet3 | 94.8 | 94.8 | 94.8 | 93.5 | 86.6 | 93.5 | 96.1 | |
| AmSBV-Viet4 | 94.8 | 94.8 | 94.8 | 93.5 | 86.6 | 93.5 | 96.1 | |
| AcSBV-Viet5 | 92.2 | 89.6 | 92.2 | 91.1 | 88.8 | 90.9 | 90.9 | |
| AmSBV-Viet6 | 97.4 | 92.2 | 97.4 | 94.8 | 91.1 | 96.1 | 90.9 | |
| AcSBV-Viet7 | 94.1 | 94.9 | 94.7 | 93.4 | 86.2 | 93.1 | 96.1 | |
| AcSBV-Viet8 | 94.5 | 94.3 | 94.2 | 93.6 | 86.1 | 92.7 | 95.7 | |
| AcSBV-Viet9 | 97.2 | 92.3 | 96.6 | 94.7 | 90.8 | 96.4 | 90.3 |
When we compared the different genomic regions of the VN-SBVs and other SBVs, we identified very interesting variations among them. The 5′ UTRs of VN-SBVs showed 82–99% identity with one another and 83–91% identity with the 5′ UTRs of the strains from other countries. The 5′ UTRs of VN-SBVs varied in size between strains: the AcSBV-Viet1 and AcSBV-Viet9 contained 179 nt, AcSBV-Viet2, AcSBV-Viet3, and AmSBV-Viet4 contained 178 nt, AcSBV-Viet5 contained 176 nt, AcSBV-Viet6 contained 177 nt, AcSBV-Viet7 contained 183 nt, and AcSBV-Viet8 contained 181 nt.
The 3′ UTR is very short and conserved in all strains. The 3′ UTRs of AcSBV-Viet1, AcSBV-Viet2, AcSBV-Viet5, and AmSBV-Viet6 are 79 nt, AcSBV-Viet3 and AcSBV-Viet4 are 80 nt, AcSBV-Viet7 and AcSBV-Viet9 are 72 nt, and AmSBV-Viet8 is 81 nt (excluding polyA tail). The 3′ UTR region of VN-SBVs are highly conserved, sharing 91–100% identity with one another. AcSBV-Viet1 and AcSBV-Viet2, AcSBV-Viet3 and AmSBV-Viet4 are 100% identical, respectively. These regions of VN-SBVs are 86–97% similar to those of strains from other countries.
The ORF regions displayed higher diversity rates (91–99%) between VN-SBV strains than the 5′ UTRs or 3′ UTRs. The sizes of the genomes varied in this region, and especially in the polyprotein region. Seven of VN-SBV genomes contained 8529 nt, whereas the AmSBV-Viet6 and AcSBV-Viet9 genomes contained 8580 nt, with an extra continuous 51-nt region in the polyprotein sequence (between nt positions 2134–2185, with reference to the complete Korean genome [HQ322114]). The AmSBV-Viet6 and AcSBV-Viet9 genomes are more similar to the AcSBV-VietSBM2 [KC007374], AcSBV-China [AF469603], and AcSBV-India [JX270800] genomes than to the other VN-SBV genomes. The VN-SBV ORF regions shared 89–94% similarity with those of the strains from other countries.
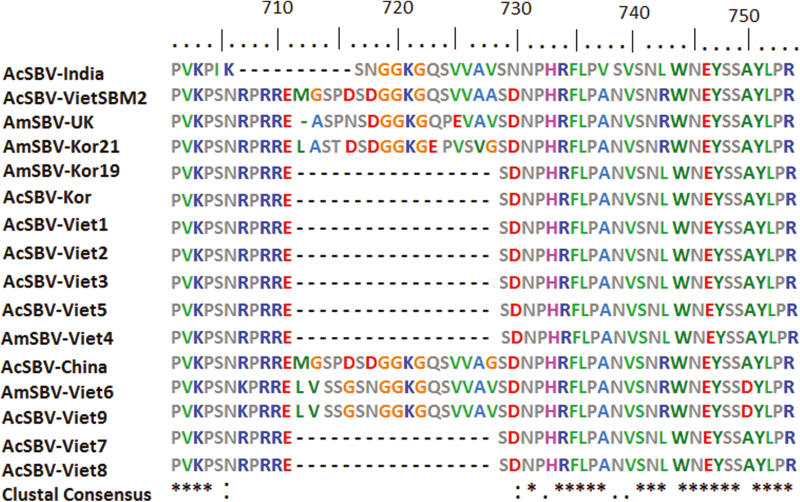
The amino acid sequences for Vietnamese SBV and the other virus strains were then aligned and compared (Table 2). The results showed that the genetic relationship of VN-SBV strains were 95–100% and with the strains from other countries were 93–97%. The deduced amino acid sequences for part of the VP1 protein in the nine VN-SBVs and seven SBVs from other countries were aligned (Fig. 2). Except for AmSBV-Viet6 and AcSBV-Viet9, all the VN-SBVs were very similar to one another in this region, and lacked the 17 continuous amino acids of these VN-SBVs (between amino acid positions 712–730 in the ORF region, with reference to the complete AmSBV-Kor21 genome [JQ390591]). However, the genome of VN-SBV strain which was previously published (AcSBV-VietSBM2 strain) also consist of 17 amino acids, is very similar to the genomes of AcSBV-China, AcSBV-India, AmSBV-Kor21, and AmSBV-UK.
Table 2.
Amino acid sequence homology (%) of VN-SBVs and other reference SBVs
| Vietnamese SBV | AmSBV-Kor19 | AmSBV-Kor21 | AcSBV-Kor | AcSBV-China | AcSBV-India | AcSBV-VietSBM2 | AmSBV-UK |
|---|---|---|---|---|---|---|---|
| AcSBV-Viet1 | 97.0 | 94.8 | 97.6 | 96.3 | 94.7 | 96.1 | 95.8 |
| AcSBV-Viet2 | 96.9 | 94.8 | 97.5 | 96.2 | 94.7 | 96.1 | 95.8 |
| AcSBV-Viet3 | 96.7 | 94.5 | 97.3 | 96.1 | 94.5 | 95.9 | 95.5 |
| AmSBV-Viet4 | 96.8 | 94.7 | 97.6 | 96.3 | 94.9 | 96.3 | 95.8 |
| AcSBV-Viet5 | 96.5 | 94.3 | 97.2 | 95.8 | 94.2 | 95.6 | 95.3 |
| AmSBV-Viet6 | 95.0 | 94.7 | 95.2 | 95.2 | 95.4 | 96.8 | 95.8 |
| AcSBV-Viet7 | 95.5 | 94.1 | 95.9 | 94.8 | 94.7 | 95.9 | 95.2 |
| AcSBV-Viet8 | 96.0 | 94.6 | 96.5 | 95.3 | 95.1 | 96.2 | 95.6 |
| AcSBV-Viet9 | 93.8 | 93.6 | 94.1 | 94.1 | 94.4 | 95.7 | 94.7 |
Fig. 2.
Multiple sequence alignment of the deduced amino acid sequences of the VP1 protein in the nine VN-SBV genomes in this study and seven SBV genomes from other countries. Bar above the aligned sequences indicates position of amino acids deduced from the ORFs. Majority of SBV genomes showed the lack of 17 continuous amino acids in their polyprotein sequence.
Phylogenetic Analysis of Complete VN-SBV Genomes and Their Molecular Evolutionary Relationships
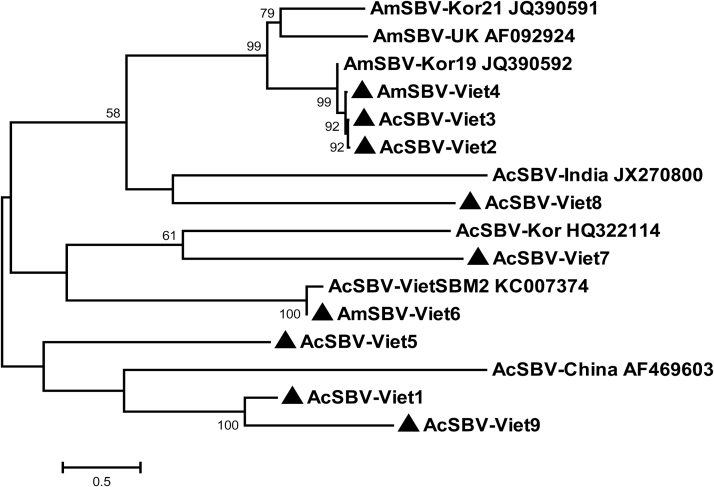
A phylogenetic tree was constructed using the complete genome sequences of AcSBV-Viet1, AcSBV-Viet2, AcSBV-Viet3, AmSBV-Viet4, AcSBV-Viet5, AmSBV-Viet6, AcSBV-Viet7, AcSBV-Viet8, AcSBV-Viet9 and the previously reported complete SBV genome sequences from other countries (Fig. 3). As shown in Fig. 3, the phylogenetic tree diverged to four main branches. In the first main branch, AcSBV-Viet2, AcSBV-Viet3, and AmSBV-Viet4 strains formed a subbranch with AmSBV-UK, AmSBV-Kor21, and AmSBV-Kor19 strains. The second branch included AcSBV-Viet8 and AcSBV-India. The SBV strains in the third main branch split into two subbranches. The first subbranch contained AcSBV-Kor and AcSBV-Viet7 sequences and the second subbranch contained AcSBV-VietSBM2 and AmSBV-Viet6 sequences. The other group contained AcSBV-China, AcSBV-Viet1, AcSBV-Viet5, and AcSBV-Viet9.
Fig. 3.
Phylogenetic tree construct based on the complete genome sequences of the nine VN-SBVs from A. melifera and A. cerana and seven SBV strains from other countries. The phylogenetic tree were constructed with the MEGA 7 package using the neighbor-joining method with Kimura’s two-parameter model and bootstrap values of 1,000 replicates.
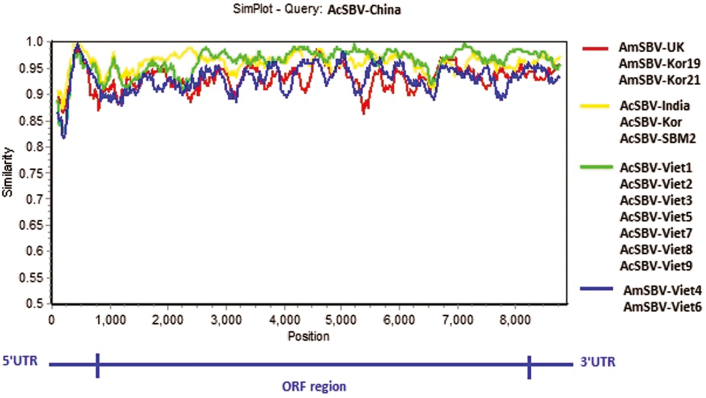
To identify differences in the full-length sequences and various genomic regions of the nine VN-SBVs and seven SBVs from other countries, they were plotted by using Simplot taking AcSBV-China as the reference sample (Fig. 4). As shown in Fig. 4, the AcSBV-VN and AmSBV-VN genomes were very similar and varied from the genomes of SBVs from other countries from the end of the 5′ UTR across the entire ORF region. The VP1 protein region, in particular, varied most extensively among the strains (between nt positions 2134–2185). The VN-SBV genomes were higher similar to those of AcSBV-VietSBM2, AcSBV-Kor, and AcSBV-India than those of AmSBV-UK, AmSBV-Kor19, and AmSBV-Kor21. Most of the A. mellifera—derived SBV genomes varied from the A. cerana—derived genomes in the polyprotein region.
Fig. 4.
Similarity plot of the nine complete VN-SBV genome sequences investigated and seven other complete SBV genome sequences (available in the GenBank database). The complete AcSBV-China strain genome sequence was used as reference.
Discussion
Molecular comparison of different genome sequences offered an accurate and reliable method of detecting variations within the same types of genomes, and the results can be evaluated statistically. In this study, we determined nine complete SBV genome sequences from A. cerana and A. mellifera species in various regions of Vietnam.
Almost of the VN-SBV genomes lack of continuous 51-nt region in the VP1 sequence compared with the SBV genomes from other countries. This region is not found in the genome sequence of the VN-SBV (AcSBV-VietSBM2 strain previously published). This data clearly show the presence of detectable variation in the genomes of the VN-SBV strains. Interestingly, the AmSBV-Viet4 genome also lack the continuous 51-nt sequence in the structural polyprotein region, and clustered with the A. cerana derived SBV strains that collected from different provinces in North of Vietnam. The strains AmSBV-Viet4 and AcSBV-Viet2 were collected from Dien Bien province, so there might be adaptations of the SBV strains in different hosts. This hypothesis have also been addressed in several previous studies such as Grabensteiner et al. (2001), Choe et al. (2012c). In addition, the strains AmSBV-Viet4 and AcSBV-Viet2, AcSBV-Viet3 (from North Vietnam) clustered into a group together with the AmSBV strains from UK and Korea. Whereas, the strains AmSBV-Viet6 and AcSBV-Viet7 clustered into a group with the AcSBV strains from Korea and AcSBV-VietSBM2. This data reinforce the finding that SBV can cross-infect between A. cerana and A. mellifera species.
Naturally, viruses infecting and circulating in the honeybee populations for a long time can lead to an exchange of viruses among the host populations, and as a consequence, the viruses have evolved more or less independently. It is believed that virus strains from the same continents or from the same countries have higher levels of similarity, and phylogenetic analyses can clearly point out the genetic clustering of the strains according to their geographic origins. Occasionally, distinct clustering patterns will occur due to genetic variations or recombinations at various positions in the genomes. Recombination has been identified in many insect- and vector-borne viruses (Holmes et al. 1999, Lukashev et al. 2003, Palacios et al. 2008, Noh et al. 2013). However, in this study the genetic similarity among the investigated strains does not appear to be influenced by geographical conditions. SBV strains usually show host specificity in the structural polyprotein regions of their genomes (Cheng et al. 2011, Choe et al. 2012b).
A multiple sequence alignment is built up using a series of VN-SBV genomes and genomes from other countries, and a Simplot graph was drawn to compare the different genomic regions (Fig. 4). This comparison of the SBV sequences identified interesting patterns in the diversity of the different genomic regions. The 5′ UTRs of the VN-SBV genomes were very similar to one another but diverged greatly from those of the SBVs from other countries (Fig. 4). This is a noncoding region located close to the start of the ORF region and plays a central role in transcription initiation and the regulation of gene expression (Nakashima and Uchiumi 2009). The VP1 regions of the VN-SBV genomes showed higher sequence divergence from the SBV genomes from other countries and higher diversity rates than the 5′ and 3′ UTRs. The SBV VP1 protein, in particular, showed great diversity and species specificity for A. cerana or A. mellifera (Choe et al. 2012b, Mingxiao et al. 2013). As shown in the Simplot graph (Fig. 4), the AmSBV-Viet4, AmSBV-Viet6 genomes and the genomes from other countries, including both of AmSBV and AcSBV strains, were more variable than the other VN-SBV genomes (especially in the VP1 region, at nt positions 2134–2185). The Simplot graph of these genomes showed the ORF region to be highly variable. Interestingly, there are big differences in the parts of the ORF region between VN-SBVs and AmSBVs from UK and Korea, whereas the VN-SBV genomes showed little variability between VN-SBVs and the AcSBVs from other countries.
In summary, the nine Vietnamese SBV genomes (AcSBV-Viet1 to AcSBV-Viet9) are very similar to one another (91–99%). The AmSBV-Viet4 is >97% similar with AcSBV-Viet1, AcSBV-Viet2, and AcSBV-Viet3. And the AmSBV-Viet6 is 99% similar with AcSBV-Viet9. The high similarity between the VN-AcSBV and the VN-AmSBV strains may be due to that these strains are cross-infections in geographically close provinces. Overall, the VN-SBV genomes are more closely genetically related to the Asian AcSBV strains than to the AmSBV strains. The VN-SBVs are geographically separated from one another based on spatial distances, environmental conditions, and their host specificity, and all these conditions may have contributed to their separation from the SBVs of other countries. However, further studies are required to identify the exact processes underlying the variations in the VP1 protein sequences of SBVs infecting A. cerana and A. mellifera. These results extend our understanding of the viral pathogens of Vietnamese honeybee species, A. cerana and A. mellifera, their complete genome sequences, and how they vary in different genomic regions. These data will also be useful in the identification of geographic variants of SBVs across different countries in future studies.
Acknowledgments
This study was supported by Vietnam National Foundation for Science and Technology Development (NAFOSTED, grant 106-NN.02-2014.24) and the Animal and Plant Quarantine Agency, Republic of Korea.
References Cited
- Ai H., Yan X., and Han R.. 2012. Occurrence and prevalence of seven bee viruses in Apis mellifera and Apis cerana apiaries in China. J. Invertebr. Pathol. 109: 160–164. [DOI] [PubMed] [Google Scholar]
- Bailey L. 1975. Recent research on honeybee viruses. Bee World. 56: 55–64. [Google Scholar]
- Bailey L. 1976. Viruses attacking the honey bee. Adv. Virus Res. 20: 271–304. [DOI] [PubMed] [Google Scholar]
- Berényi O., Bakonyi T., Derakhshifar I., Köglberger H., and Nowotny N.. 2006. Occurrence of six honeybee viruses in diseased Austrian apiaries. Appl. Environ. Microbiol. 72: 2414–2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Evans J., and Feldlaufer M.. 2006. Horizontal and vertical transmission of viruses in the honey bee, Apis mellifera. J. Invertebr. Pathol. 92: 152–159. [DOI] [PubMed] [Google Scholar]
- Chen Y. P., and Siede R.. 2007. Honey bee viruses. Adv. Virus Res. 70: 33–80. [DOI] [PubMed] [Google Scholar]
- Cheng J., Zhang P., Ma M. X., Li M., and Yang S.. 2011. Predication of spatial structure and B cell epitope of VP1 protein of Chinese sacrbrood virus LN-QY strain. Chinese J. Biologicals 42: 280–284. [Google Scholar]
- Choe S. E., Nguyen L. T., Noh J. H., Koh H. B., Jean Y. H., Kweon C. H., and Kang S. W.. 2012a. Prevalence and distribution of six bee viruses in Korean Apis cerana populations. J. Invertebr. Pathol. 109: 330–333. [DOI] [PubMed] [Google Scholar]
- Choe S. E., Nguyen T. T., Hyun H. B., Noh J. H., Lee H. S., Lee C. H., and Kang S. W.. 2012b. Genetic and phylogenetic analysis of South Korean sacbrood virus isolates from infected honey bees (Apis cerana). Vet. Microbiol. 157: 32–40. [DOI] [PubMed] [Google Scholar]
- Choe S. E., Nguyen L. T., Noh J. H., Kweon C. H., Reddy K. E., Koh H. B., Chang K. Y., and Kang S. W.. 2012c. Analysis of the complete genome sequence of two Korean sacbrood viruses in the Honey bee, Apis mellifera. Virology. 432: 155–161. [DOI] [PubMed] [Google Scholar]
- Cornman R. S., Tarpy D. R., Chen Y., Jeffreys L., Lopez D., Pettis J. S., vanEngelsdorp D., and Evans J. D.. 2012. Pathogen webs in collapsing honey bee colonies. Plos One. 7: e43562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox-Foster D. L., Conlan S., Holmes E. C., Palacios G., Evans J. D., Moran N. A., Quan P. L., Briese T., Hornig M., Geiser D. M., . et al. 2007. A metagenomic survey of microbes in honey bee colony collapse disorder. Science. 318: 283–287. [DOI] [PubMed] [Google Scholar]
- Elke G., von Werner D. O., Hannes K., Annette S., Christoph O., Ralph B., Stefan B., Wolfgang R., Werner M., Sebastian G., . et al. 2010. The German bee monitoring project: a long term study to understand periodically high winter losses of honey bee colonies. Apidologie 41: 332–335. [Google Scholar]
- Evans J. D., and Schwarz R. S.. 2011. Bees brought to their knees: microbes affecting honey bee health. Trends Microbiol. 19: 614–620. [DOI] [PubMed] [Google Scholar]
- Forsgren E., Wei S., Guiling D., Zhiguang L., Tran T. V., Tang P. T., Truong T. A., Dinh Q. T., and Fries I.. 2015. Preliminary observation on possible pathogen spill-over from Apis mellifera to Apis cerana. Apidologie 46: 265–275. [Google Scholar]
- Genersch E., and Aubert M.. 2010. Emerging and re-emerging viruses of the honey bee (Apis mellifera L.). Vet. Res. 41: 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh R. C., Ball B. V., Willcocks M. M., and Carter M. J.. 1999. The nucleotide sequence of sacbrood virus of the honey bee: an insect picorna-like virus. J. Gen. Virol. 80: 1541–1549. [DOI] [PubMed] [Google Scholar]
- Grabensteiner E., Ritter W., Carter M. J., Davison S., Pechhacker H., Kolodziejek J., Boecking O., Derakhshifar I., Moosbeckhofer R., Licek E., . et al. 2001. Sacbrood virus of the honeybee (Apis mellifera): rapid identification and phylogenetic analysis using reverse transcription-PCR. Clin. Diagn. Lab. Immunol. 8: 93–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha T. T., Nguyen T. K. L., Mai T. L., Le T. H., Nguyen T. H., Pham H. T., Reddy K. E., Yoo M. S., Kim J. H., Cho Y. S., . et al. 2016. Prevalence of bee viruses among Apis cerana population in Vietnam. J. Apic. Res. 55: 379–385. [Google Scholar]
- Holmes E. C., Worobey M., and Rambaut A.. 1999. Phylogenetic evidence for recombination in dengue virus. Mol. Biol. Evol. 16: 405–409. [DOI] [PubMed] [Google Scholar]
- James D. E., Jay D. E., Jeff P.. 2010. Colony losses, managed colony population decline, and Colony Collapse Disorder in the United States. J. Apic. Res. 49: 134–136. [Google Scholar]
- Kimura M. 1980. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 16: 111–120. [DOI] [PubMed] [Google Scholar]
- Kumar S., Stecher G., and Tamura K.. 2016. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 33: 1870–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukashev A. N., Lashkevich V. A., Ivanova O. E., Koroleva G. A., Hinkkanen A. E., and Ilonen J.. 2003. Recombination in circulating enteroviruses. J. Virol. 77: 10423–10431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mingxiao M., Ming L., Jian C., Song Y., Shude W., and Pengfei L.. 2011. Molecular and Biological Characterization of Chinese Sacbrood Virus LN Isolate. Comp. Funct. Genomics. 2011: 409386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mingxiao M., Yanna Y., Xiaoli X., Lin Z., Yongfei L., and Zhidong L.. 2013. Genetic characterization of VP1 gene of seven Sacbrood virus isolated from three provinces in northern China during the years 2008-2012. Virus Res. 176: 78–82. [DOI] [PubMed] [Google Scholar]
- Nakashima N., and Uchiumi T.. 2009. Functional analysis of structural motifs in dicistroviruses. Virus Res. 139: 137–147. [DOI] [PubMed] [Google Scholar]
- Nazzi F., Brown S. P., Annoscia D., Del Piccolo F., Di Prisco G., Varricchio P., Della Vedova G., Cattonaro F., Caprio E., and Pennacchio F.. 2012. Synergistic parasite-pathogen interactions mediated by host immunity can drive the collapse of honeybee colonies. Plos Pathog. 8: e1002735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noh J. H., Reddy K. E., Choe S. E., Yoo M. S., Doan H. T., Kweon C. H., Ramya M., Yoon B. S., Nguyen L. T., Nguyen T. T., . et al. 2013. Phylogenetic analysis of black queen cell virus genotypes in South Korea. Virus Genes. 46: 362–368. [DOI] [PubMed] [Google Scholar]
- Palacios G., Hui J., Quan P. L., Kalkstein A., Honkavuori K. S., Bussetti A. V., Conlan S., Evans J., Chen Y. P., vanEngelsdorp D., . et al. 2008. Genetic analysis of Israel acute paralysis virus: distinct clusters are circulating in the United States. J. Virol. 82: 6209–6217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phung H. C. 1990. Some diseases of larvae A. cerana A. cerana. Agriculture Publisher, Hanoi, Vietnam. [Google Scholar]
- Phung H. C. 2008. Sacbrood virus disease on foreign bee in Vietnam. J. Sci. Tech. 1: 2–5. [Google Scholar]
- Ray S. C. 2003. Simplot for Windows 98/NT/2000/XP Version 3.5.1. [Google Scholar]
- Reddy K. E., Yoo M. S., Kim Y. H., Kim N. H., Ramya M., Jung H. N., Thao L. E. T. B., Lee H. S., and Kang S. W.. 2016. Homology differences between complete Sacbrood virus genomes from infected Apis mellifera and Apis cerana honeybees in Korea. Virus Genes. 52: 281–289. [DOI] [PubMed] [Google Scholar]
- Sarwar M. 2016. Prevalence of multiple viral diseases associated with honey bees colony collapse and control of disorders. Int. J. Zool. Stud. 1: 29–34. [Google Scholar]
- Thompson J. D., Gibson T. J., Plewniak F., Jeanmougin F., and Higgins D. G.. 1997. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25: 4876–4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Engelsdorp D., Hayes J. Jr., Underwood R. M., and Pettis J.. 2008. A survey of honey bee colony losses in the U.S., fall 2007 to spring 2008. Plos One. 3: e4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Bi J., Wang L., Zhou D., Ma X., Li W., Zhao W., Yin G., Liu J., and He S.. 2016. Prevalence of four common bee RNA viruses in Eastern bee populations in Yunnan province, China. Vet. Sci. Technol. 7: 1. [Google Scholar]
- Wu Y. Y., Jia H. R., Wang Q., Dai P. L., Diao Q. Y., Xu S. F., Wang X., and Zhou T.. 2015. Multiple virus infections and the characteristics of chronic bee paralysis virus in diseased honey bees (Apis mellifera L.) in China. J. Apic. Sci. 59: 95–106. [Google Scholar]
- Yanez O., Zheng H. Q., Su X. L., Hu F. L., Neumann P., and Dietemann V.. 2015. Potential for virus transfer betwween the honey bees Apis mellifera and A. cerana. J. Apic. Res. 54: 179–191. [Google Scholar]