Abstract
Background
The effects of white matter hyperintensity volume and subclinical brain infarcts on the risk of incident stroke, its ischemic subtypes, and mortality require further study in diverse samples.
Methods and Results
Stroke‐free participants in the Northern Manhattan Study underwent magnetic resonance imaging (N=1287; mean age 71±9 years, 60% women, 15% non‐Hispanic white, 17% non‐Hispanic black, 68% Hispanic) and were followed for a median of 8 years (interquartile range: 6–9 years). Cox models estimated proportional hazards of incident stroke of all types, ischemic stroke (and its subtypes), and mortality and stratified by race/ethnicity. In total 72 participants (6%) had incident strokes and 244 died (19%). In fully adjusted models, those with larger white matter hyperintensity volume had greater risk of all stroke types (hazard ratio [HR]: 1.4; 95% CI, 1.1–1.9), ischemic stroke (HR: 1.3; 95% CI, 1.0–1.8), and cryptogenic stroke (HR: 2.2; 95% CI, 1.1–4.4). White and black but not Hispanic participants had increased stroke risk (P<0.05 for heterogeneity for all and ischemic stroke). Those with subclinical brain infarct had greater risk for all stroke types (HR: 1.9; 95% CI, 1.1–3.3), ischemic stroke (HR: 2.2; 95% CI, 1.3–3.8), lacunar (HR: 4.0; 95% CI, 1.3–12.3), and cryptogenic stroke (HR: 3.6; 95% CI, 1.0–12.7), without significant heterogeneity across race/ethnic groups. Greater white matter hyperintensity volume increased both vascular (HR: 1.3; 95% CI, 1.1–1.7) and nonvascular (HR: 1.2; 95% CI, 1.0–1.5) mortality among Hispanic and white but not black participants (P=0.040 for heterogeneity). Subclinical brain infarct was associated with increased vascular mortality among Hispanic participants only (HR: 2.9; 95% CI, 1.4–5.8).
Conclusions
In this urban US sample, subclinical cerebrovascular lesions increased the risk of clinical stroke and vascular mortality and varied by race/ethnicity and lesion type.
Keywords: cerebrovascular disease/stroke, epidemiology, mortality, stroke, white matter disease
Subject Categories: Cerebrovascular Disease/Stroke, Epidemiology, Race and Ethnicity, Biomarkers, Ischemia
Clinical Perspective
What Is New?
These observational, prospective data show that both white matter hyperintensities and subclinical infarcts were associated with elevated stroke risk in a population‐based cohort of Hispanic and non‐Hispanic white and black participants from the same community and that there were lesion‐specific differences in stroke risk across race/ethnic groups and by ischemic stroke subtypes.
What Are the Clinical Implications?
The study suggests subclinical lesion type‐specific heterogeneity in stroke risk that could help inform the design of future clinical trials targeting silent cerebrovascular damage to prevent stroke.
Introduction
The United Nations and the World Health Organization (WHO) have identified cardiovascular disease as a key noncommunicable disease and targeted its prevention and control as a priority area.1 To achieve WHO goals of reducing mortality due to noncommunicable diseases, there is a need for better markers of stroke risk. Numerous large cohort studies have documented a prevalence of ≈10% to 20% for subclinical brain infarcts (SBIs) in people with no clear history of stroke.2 Even more ubiquitous are hyperintensities on T2 MRI that occur in >90% of older adults and usually represent small vessel damage.3 Such lesions share many risk factors with cardiovascular disease and stroke, but their importance as predictors of vascular outcomes is not fully understood, especially in minorities. Although some incidence studies suggest these lesions confer a greater risk of both stroke and mortality, most studies have been limited to white participants, few have included black participants, and none have included Hispanic participants.4, 5, 6, 7, 8, 9 Moreover, even less is known about the association of subclinical cerebral small vessel disease (SVD) with increased risk of specific ischemic stroke subtypes.
White matter hyperintensities (WMHs) often represent underlying SVD, but many SBIs are superficial, suggestive of branch artery occlusions due to thromboembolism. Some population‐based data have associated SBI with both atrial fibrillation and cardioembolic stroke; however, community‐based studies examining SBI subtype and incident stroke risk are few in number.8, 9, 10 Known racial and ethnic differences in the prevalence of vascular risk factors and stroke subtypes suggest these subclinical vascular lesions could also have different effects in minority populations, but studies in diverse samples are limited.9, 11 We hypothesized that SBI and WMH would be predictors of incident stroke and mortality and would differ across racial and ethnic groups by ischemic stroke subtype in an urban US cohort study of Hispanic, black, and non‐Hispanic white people living in the same community.
Methods
Study Population
NOMAS (Northern Manhattan Study) is a population‐based cohort, and sampling details have been published.11 Briefly, eligible participants were enrolled between 1993 and 2001 and were stroke‐free, aged >40 years (≥55 years beginning in 1998), and residents of northern Manhattan, New York, for at least 3 months in a household with a telephone. Audits and Surveys, Inc, performed random digit dialing using dual‐frame sampling (telephone response rate was 91%), and participants were invited to enroll with an in‐person interview and neurological assessment (enrollment response rate was 75%). The overall participation rate was 69%, with a total of 3298. All participants signed written informed consent, and the institutional review boards of Columbia University Medical Center and the University of Miami approved the study.
Baseline Evaluation
Trained research assistants collected data through interviews in English or Spanish, depending on the language spoken at home, and study physicians did the neurological examinations. Race and ethnicity were determined based on self‐identification using questions modeled after the US census.11 Standardized questions were adapted from the Behavioral Risk Factor Surveillance System by the Centers for Disease Control and Prevention regarding hypertension, diabetes mellitus (DM), smoking, and cardiac conditions.12 Hypertension was defined as systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg based on the average of the 2 measurements taken in a seated position 5 minutes apart with a manual sphygmomanometer, a patient's self‐report of a history of hypertension, or antihypertensive medication use. DM was defined as fasting blood glucose ≥126 mg/dL, the participant's self‐reported history of DM, or diabetes medication use. Hypercholesterolemia was defined as total serum cholesterol >240 mg/dL, a patient's self‐report of hypercholesterolemia, or use of lipid‐lowering treatment. Current and past tobacco use was recorded. Coronary artery disease was defined as a history of myocardial infarction, coronary artery bypass grafting, percutaneous coronary intervention, typical angina, or use of anti‐ischemic medication. Atrial fibrillation was defined using ECG at the time of echocardiography or self‐reported history. Fasting glucose and lipids were measured with automated spectrometers.13
Brain MRI Cohort
Surviving stroke‐free participants were invited to participate in the MRI substudy during annual telephone follow‐up beginning in 2003 and were screened for head MRI safety. Those with pacemakers or other implanted devices or metal objects were excluded. A total of 1091 participants were enrolled from 2003 to 2008. To supplement the sample, original cohort participants were asked if there were other persons aged ≥50 years and stroke free who were living in their household that might wish to participate. An additional 199 stroke‐free people were thus added to the prospective cohort from 2006 to 2008 (N=1290). Participants were imaged on a 1.5‐T Philips Intera scanner at Columbia University Medical Center. Images were transferred electronically to University of California Davis for morphometric analysis of subcortical WMH volume (WMHV), as described previously.14, 15, 16 Intrarater reliability for WMH was highly significant.15 Briefly, nonbrain elements were manually removed from the image by operator‐guided tracing of the dura mater within the cranial vault, including the middle cranial fossa but excluding posterior fossa structures. The resulting cranial vault measure was defined as total intracranial volume. Analyses were performed using semiautomated measurements of pixel distributions using mathematical modeling of pixel‐intensity histograms for cerebrospinal fluid and brain to identify the optimal pixel‐intensity threshold to distinguish cerebrospinal fluid from brain matter. WMHV was calculated as the sum of voxels ≥3.5 SD above the mean image intensity multiplied by voxel dimensions and section thickness and is expressed as a percentage of total intracranial volume to correct for individual differences in head size. WMHV was natural log transformed to normalize the distribution. Presence and absence of SBI was based on a previously established protocol.10 Subcortical SBIs were cavitated lesions >3 mm in axial diameter on the fluid‐attenuated inversion recovery sequence (or similar characteristics on proton density‐, T2‐, and T1‐weighted sequences). Subcortical infarcts were distinct from a vessel (due to the lack of signal void on T2 sequence) and of equal intensity to cerebrospinal fluid. SBIs were categorized as superficial if they affected the cerebral cortex or cerebellum, suggestive of branch occlusion due to thromboembolism. Interobserver agreement for SBI detection was 93%.17 Raters were blinded to participant‐identifying information.
Outcomes
The primary outcomes were adjudicated incident stroke and mortality. Follow‐up procedures and outcome classifications have been published.18 Briefly, participants and/or family members are interviewed annually by telephone to determine changes in vital status, to detect neurologic events, and to document interval hospitalizations. The outcome surveillance network includes daily screening of admissions, review of neurology consult lists with covering house staff, hospital admission and discharge data (including screening of International Classification of Diseases, Ninth Revision codes), emergency room visits, and visits to the ambulatory care network. The average annual contact rate has been 99% with only 1% lost to follow‐up. A specially trained research assistant reviews all strokes and deaths, and medical records of all hospitalizations are reviewed to verify details of suspected events. Persons who screen positive for stroke undergo in‐person assessment, chart review, and examination by a study neurologist. Stroke events were classified as the first occurrence of ischemic stroke, intracerebral hemorrhage, subarachnoid hemorrhage, and unknown stroke type.
Ischemic stroke subtypes were classified according to a modified National Institute of Neurological Disorders and Stroke (NINDS) scheme, based on the medical history, neurological examination, and diagnostic evaluation (brain and vessel imaging, echocardiography, ECG or Holter monitoring, and conventional catheter angiography).19 A panel of NOMAS neurologists blinded to patient identifiers (except history of transient ischemic attack, atrial fibrillation, and any other heart condition) classified each case using modified NINDS methods.20 Two NOMAS vascular neurologists adjudicated each stroke case independently, and a third resolved disagreements.
Statistical Analysis
Two clinical end point outcomes analyzed were incident stroke and mortality. Incident stroke was defined as “all stroke” (hemorrhagic and ischemic), ischemic stroke alone, and ischemic stroke subtypes (cardioembolic, lacunar, cryptogenic, and large vessel). Mortality was defined as all deaths, vascular deaths, and nonvascular deaths. We also stratified by race/ethnicity for all stroke, ischemic stroke, and mortality outcomes. Person‐time of follow‐up accrued from MRI to the last follow‐up, the time of incident stroke, death, or loss to follow‐up, whichever came first. For each defined outcome, we used Cox models to estimate hazard ratios (HRs) for WMHV and SBI as separate predictors after adjusting for age, sex, race/ethnicity (except in analyses stratified by this factor), years of education, medical insurance status, body mass index, smoking status (current or past versus none), physical activity (moderate to heavy versus none), reported alcohol intake (moderate versus other), hypertension, DM, hypercholesterolemia, history of atrial fibrillation, coronary artery disease, and myocardial infarction.
For WMHV, we evaluated the associations using the natural log‐transformed WMHV as a continuous variable, expressed as percent total intracranial volume and also compared each of the top 3 quartiles with the bottom quartile. For SBI, we evaluated the association of SBI as a categorical variable, by presence (yes no), number (single or multiple), and location (subcortical cavitated, superficial). Because subcortical cavitated SBIs 3 to 15 mm in axial diameter are considered to result from SVD, but lesions >15 mm could be caused by other mechanisms, we excluded the latter from the subcortical SBI analysis (n=10). Given that a primary focus of NOMAS is to understand racial and ethnic differences in stroke risk, we tested for the heterogeneities and interactions between race/ethnicity and WMHV and SBI for stroke risk and stratified the analysis by race/ethnicity. Analyses were done using SAS version 9.3, and the level of statistical significance was set at P<0.05.
Results
Characteristics of the stroke‐free NOMAS MRI substudy sample (n=1287) are shown in Table 1. There were 72 incident strokes (63 ischemic), with a median time to stroke of 7.8 years (interquartile range: 6.3–9.1 years) and a median time to death of 7.9 years (interquartile range: 6.4–9.1 years). In total 192 participants had SBI (15% overall; 17% white, 22% black, 13% Hispanic). Those with SBI or greater WMHV (total intracranial volume, median: 0.36%; interquartile range: 0.21–0.77%) were more likely to be older than the median baseline age of 70 years, to have Medicaid or no insurance, to be current smokers, and to have a greater burden of vascular risk factors, although histories of cardiac disease and dyslipidemia were exceptions.
Table 1.
Sample Characteristics, WMHV and SBI
| Characteristics | n (%) | WMHV (1/TIV%) | SBI | ||
|---|---|---|---|---|---|
| Median (IQR) | P Valuea | % | P Valueb | ||
| All | 1287 (100) | 0.36 (0.21–0.77) | 16 | ||
| Age (mean 70.6±9 y) | |||||
| <70 | 619 (48) | 0.25 (0.17–0.43) | Ref. | 10 | Ref. |
| ≥70 | 668 (52) | 0.56 (0.30–1.15) | <0.001 | 21 | <0.001 |
| Sex | |||||
| Female | 779 (60) | 0.38 (0.22–0.78) | Ref. | 13 | Ref. |
| Male | 508 (40) | 0.34 (0.19–0.72) | 0.363 | 19 | 0.002 |
| Race/ethnicity | |||||
| NH‐white | 191 (15) | 0.38 (0.20–0.64) | Ref. | 17 | Ref. |
| NH‐black | 222 (17) | 0.54 (0.26–1.19) | <0.001 | 22 | 0.214 |
| Hispanic | 845 (66) | 0.33 (0.20–0.69) | <0.001 | 13 | 0.971 |
| NH‐other | 29 (2) | 0.41 (0.22–0.79) | 0.038 | 26 | 0.078 |
| High school completion | |||||
| No | 697 (54) | 0.37 (0.22–0.75) | Ref. | 14 | Ref. |
| Yes | 590 (46) | 0.36 (0.20–0.80) | 0.976 | 17 | 0.230 |
| Medicaid/uninsured | |||||
| No | 675 (52) | 0.36 (0.21–0.76) | Ref. | 16 | Ref. |
| Yes | 612 (48) | 0.37 (0.21–0.77) | <0.001 | 15 | 0.566 |
| Body mass index | |||||
| <25 | 315 (25) | 0.44 (0.22–0.95) | Ref. | 20 | Ref. |
| 25 to <30 | 534 (41) | 0.35 (0.21–0.77) | 0.617 | 14 | 0.487 |
| ≥30 | 438 (34) | 0.34 (0.19–0.66) | 0.342 | 15 | 0.177 |
| Smoking | |||||
| Never | 609 (47) | 0.36 (0.22–0.74) | Ref. | 14 | Ref. |
| Former | 559 (43) | 0.34 (0.20–0.73) | 0.311 | 16 | 0.319 |
| Current | 119 (9) | 0.54 (0.24–1.04) | <0.001 | 20 | 0.045 |
| Physical activity | |||||
| No | 565 (44) | 0.35 (0.20–0.70) | Ref. | 16 | Ref. |
| Yes | 722 (56) | 0.38 (0.22–0.80) | 0.972 | 15 | 0.401 |
| Moderate alcohol drinking | |||||
| No | 856 (67) | 0.39 (0.23–0.82) | Ref. | 16 | Ref. |
| Yes | 431 (33) | 0.31 (0.18–0.60) | 0.004 | 16 | 0.277 |
| Hypertension | |||||
| No | 353 (27) | 0.28 (0.17–0.50) | Ref. | 9 | Ref. |
| Yes | 934 (73) | 0.41 (0.23–0.83) | <0.001 | 18 | 0.002 |
| Diabetes mellitus | |||||
| No | 996 (77) | 0.34 (0.20–0.72) | Ref. | 15 | Ref. |
| Yes | 291 (23) | 0.42 (0.24–0.86) | 0.019 | 19 | 0.061 |
| Hypercholesterolemia | |||||
| No | 783 (61) | 0.35 (0.21–0.82) | Ref. | 15 | Ref. |
| Yes | 504 (39) | 0.38 (0.22–0.69) | 0.184 | 17 | 0.254 |
| History of MI/AF/CAD | |||||
| No | 1084 (84) | 0.35 (0.20–0.74) | Ref. | 15 | Ref. |
| Yes | 203 (16) | 0.43 (0.25–0.93) | 0.316 | 18 | 0.706 |
AF indicates atrial fibrillation; CAD, coronary artery disease; IQR, interquartile range; MI, myocardial infarction; NH, non‐Hispanic; Ref. reference; SBI indicates subclinical brain infarction; TIV, total intracranial volume; WMHV, white matter hyperintensity volume.
WMHV was natural log transformed and comparisons were based on general linear models adjusted for age.
% SBI was compared using logistic regression adjusted for age.
WMHs, Subclinical Infarcts, and Stroke Risk
Participants with greater WMHV were ≈50% more likely to have incident strokes of all types (WMHV, adjusted HR per SD: 1.4; 95% confidence interval [CI], 1.1–1.9; Figure 2, Table S1) as well as ischemic stroke (adjusted HR per SD: 1.3; 95% CI, 1.0–1.8; Figure 2, Table S1). Exploring dose effects by dividing WMHV into quartiles, HRs for those in WMHV quartiles 2 to 4 were incrementally greater than those of the bottom quartile (P=0.014 for trend), but only the top quartile showed a significant association with all stroke, with a doubling of risk (adjusted HR: 2.6; 95% CI, 1.1–6.1). The risk was also doubled for those in the top quartile of WMHV compared with the bottom quartile in relation to ischemic stroke, but this did not reach significance (P=0.085; Figure 2, Table S1). Participants with ≥1 SBI were at significantly greater risk of all stroke (adjusted HR: 1.9; 95% CI, 1.1–3.3) and ischemic stroke (adjusted HR: 2.2; 95% CI, 1.3–3.8). A sensitivity analysis adjusting only for covariates significantly associated with WMHV or SBI as predictors of incident all or ischemic stroke did not alter our findings (data not shown).
We then examined subcortical cavitated and superficial SBIs as predictors of incident stroke (Figure 1 for examples). Compared with those without SBI, participants with subcortical cavitated SBI 3 to 15 mm in axial diameter were at twice the risk of incident all stroke (HR: 1.9; 95% CI, 1.1–3.4) and ischemic stroke (HR: 2.2; 95% CI, 0.8–5.6), whereas those with superficial SBI had twice the risk of ischemic stroke alone compared with those without superficial SBI (HR: 2.1; 95% CI, 1.1–3.9; Figure 2, Table S1). There were no outcome events among participants with subcortical cavitated SBI >15 mm in axial diameter. When examined separately, those with single and multiple SBIs were each at about twice the risk of incident ischemic stroke (Figure 2, Table S1). In separate analyses replacing categorical variables for hypertension, DM, and hypercholesterolemia with terms for systolic and diastolic blood pressure, fasting blood sugar, total cholesterol, and medications to control hypertension, DM, and lipids, HRs predicting stroke and mortality were very similar and remained significant (data not shown).
Figure 1.
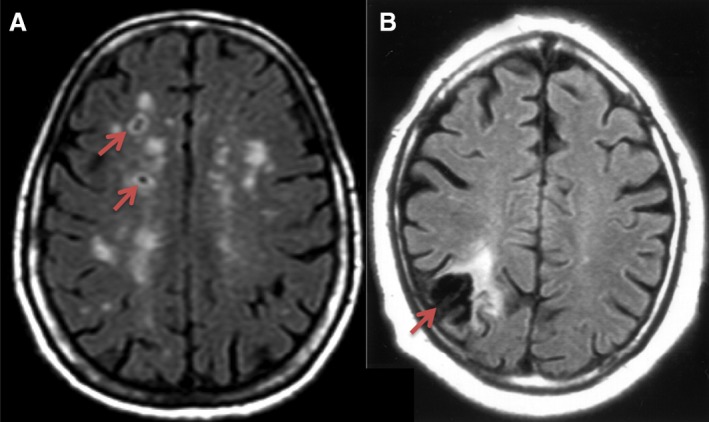
Examples of subclinical brain infarcts. A, Subcortical cavitated brain infarcts 3 to 15 mm in axial diameter (arrows) with scattered white matter hyperintensities. B, Wedge shaped superficial infarct of presumed embolic origin.
Figure 2.
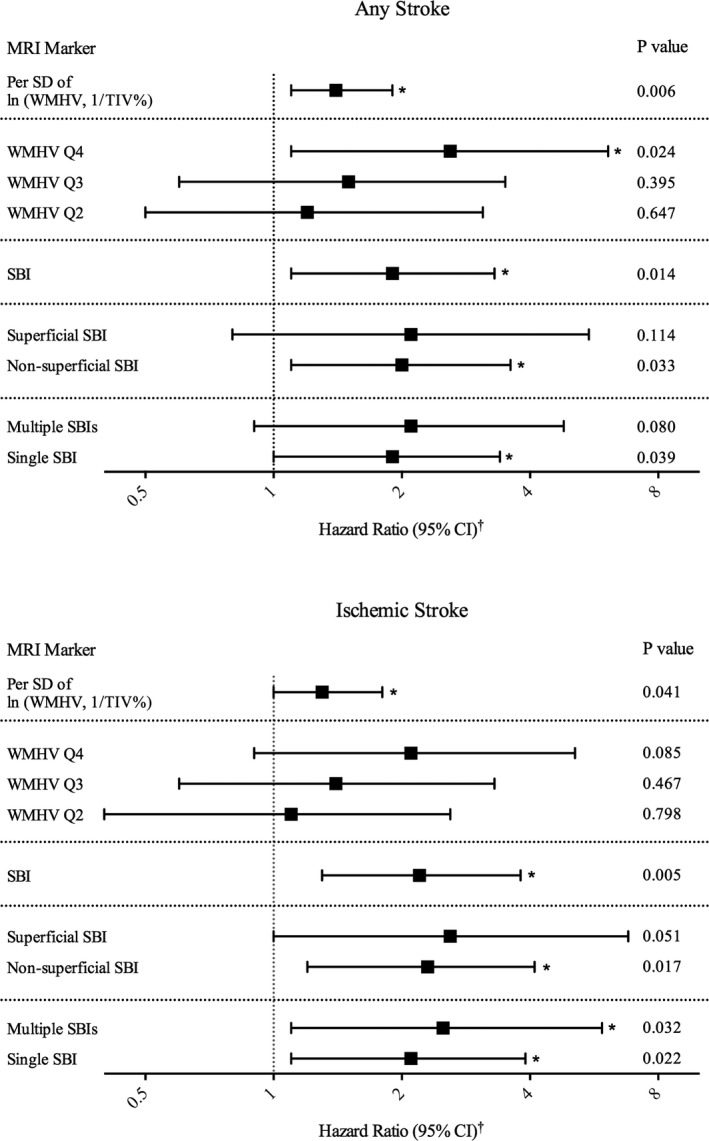
Subclinical brain lesions and risk of stroke. WMHV divided into quartiles based on WMHV, 1/TIV%, with Q1 as reference group. Absence of SBI used as reference group against presence, location, and quantity of SBI. *P<0.05. †Hazard ratios and 95% CIs were estimated using Cox proportional hazards models, adjusted for age, sex, race/ethnicity, education, medical insurance status, body mass index, smoking, physical activity, moderate alcohol drinking, hypertension, diabetes mellitus, hypercholesterolemia, history of atrial fibrillation, coronary artery disease, and myocardial infarction. Additional information can be found in Table S1. CI indicates confidence interval; Q, quartile; SBI, subclinical brain infarction; TIV, total intracranial volume; WMHV, white matter hyperintensity volume.
Examining incident ischemic stroke by subtype (24 cardioembolic, 15 lacunar, 12 cryptogenic, 8 large vessel), the adjusted HR per SD for WMHV showed a significantly increased risk of cryptogenic stroke (Figure 3, Table S2). When we also included SBI in the model, the effect for WMHV attenuated and was no longer statistically significant (P=0.054). Those with SBI were at elevated risk of both incident lacunar and cryptogenic strokes (Figure 3, Table S2). When we also included WMHV in these models, SBI still increased the risk of lacunar stroke (P=0.018), but the effect on cryptogenic stroke risk was attenuated and no longer significant.
Figure 3.
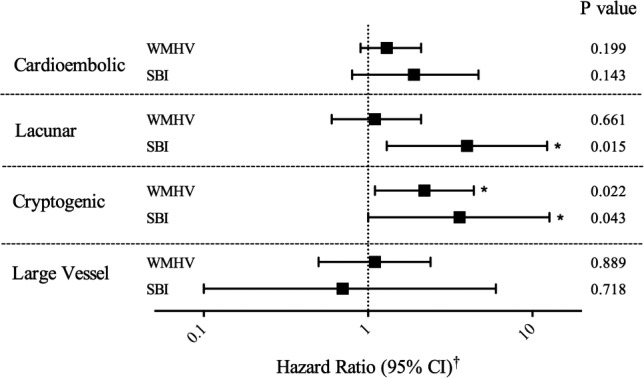
Subclinical brain lesions and risk of ischemic stroke subtypes. WMHV represents associations using the natural log‐transformed continuous variable (lnWMHV, 1/TIV%) per standard deviation. SBI represents association for SBI presence as a binary variable. *P<0.05. †HRs and 95% CIs were estimated using Cox proportional hazards models, adjusted for age, sex, race/ethnicity, education, medical insurance status, body mass index, smoking, physical activity, moderate alcohol drinking, hypertension, diabetes mellitus, hypercholesterolemia, history of atrial fibrillation, coronary artery disease, and myocardial infarction. Additional information can be found in Table S2. CI indicates confidence interval; HR, hazard ratio; NH, non‐Hispanic; SBI, subclinical brain infarction; TIV, total intracranial volume; WMHV, white matter hyperintensity volume.
There were interactions (P<0.2) between Hispanic ethnicity and WMHV and SBI for risk of all stroke (WMHV: Hispanic versus white, P=0.034; SBI: Hispanic versus white, P=0.170) and ischemic stroke (WMHV: Hispanic versus white, P=0.071). Stratifying by race/ethnicity, both non‐Hispanic white and Hispanic participants with SBI were at elevated risk of all stroke in unadjusted analysis, but the multivariable‐adjusted association remained significant only for non‐Hispanic white participants (HR: 3.6; 95% CI, 1.0–13.1; Figure 4, Table S3). We also found that greater WMHV was associated with greater risk of all and ischemic stroke across racial and ethnic groups in the unadjusted analysis. The adjusted HRs remained significant for non‐Hispanic white participants (all stroke: HR: 3.7; 95% CI, 1.5–9.0; ischemic stroke: HR: 2.9; 95% CI, 1.1–7.4) and black participants (all stroke, HR: 2.8 [95% CI, 1.3–6.2]; ischemic stroke, HR: 2.5 [95% CI, 1.1–5.4]) but not Hispanic participants (all stroke, HR: 1.1 [95% CI, 0.8–1.5]; ischemic stroke, HR: 1.0 [95% CI, 0.7–1.5]; Figure 4, Table S3).
Figure 4.
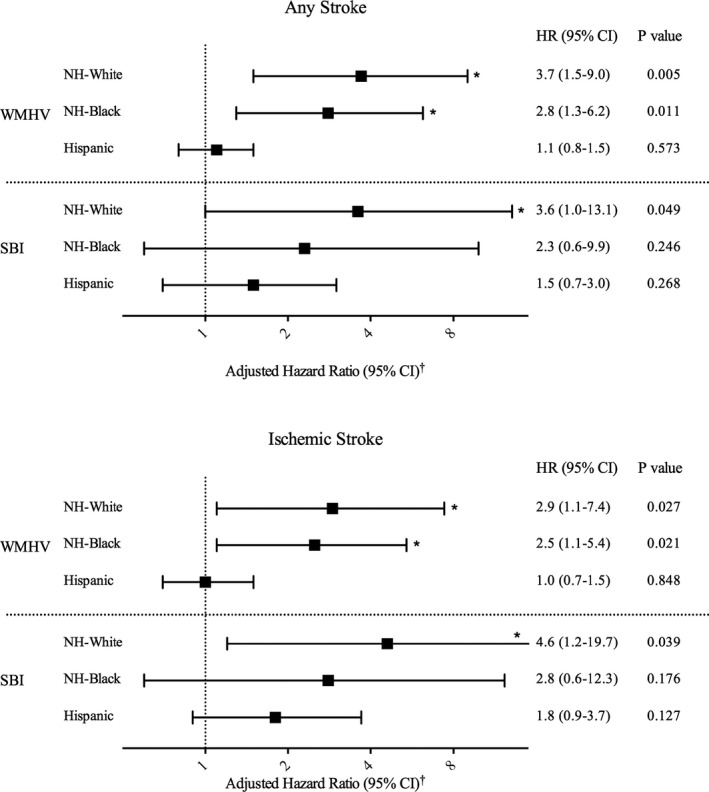
Subclinical brain lesions and stroke risk by race/ethnicity. WMHV represents associations using the natural log‐transformed continuous variable (lnWMHV, 1/TIV%, 1/TIV%) per standard deviation. SBI represents association for SBI presence vs absence. *P<0.05. †HRs and 95% CIs were estimated using Cox proportional hazards models, adjusted for age, sex, race‐ethnicity, education, medical insurance status, body mass index, smoking, physical activity, moderate alcohol drinking, hypertension, diabetes mellitus, hypercholesterolemia, history of atrial fibrillation, coronary artery disease, and myocardial infarction. Additional information can be found in Table S3. CI indicates confidence interval; HR, hazard ratio; NH, non‐Hispanic; SBI, subclinical brain infarction; TIV, total intracranial volume; WMHV, white matter hyperintensity volume.
WMHs, Subclinical Infarcts, and Mortality
Participants were at significantly greater risk per SD of natural log‐transformed WMHV of both vascular mortality (adjusted HR: 1.3; 95% CI, 1.1–1.7) and nonvascular mortality (adjusted HR: 1.2; 95% CI, 1.0–1.5; Table 2). When divided into quartiles of WMHV, those in the upper 3 had greater risk of all mortality, and this reached significance for the top quartile (HR: 2.0; 95% CI, 1.3–3.1), which was also associated with vascular mortality (HR: 2.0; 95% CI, 1.3–3.1), with a trend for an association with nonvascular mortality that did not reach statistical significance (Table 2). Those with SBI showed a nonsignificant trend toward greater risk of vascular mortality (HR: 1.6; 95% CI, 1.0–2.6) but not nonvascular, mortality (HR: 1.0; 95% CI, 0.6–1.5). A sensitivity analysis adjusting only for covariates significantly associated with WMHV or SBI as predictors of mortality did not alter our findings (data not shown).
Table 2.
Subclinical Brain Lesions and Mortality
| MRI Marker | All | Vascular | Nonvascular | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Events | Rate (/1000 PYS) | HR (95% CI)a | P Value | No. of Event | Rate (/1000 PYS) | HR (95% CI)a | P Value | No. of Event | Rate (/1000 PYS) | HR (95% CI)a | P Value | |
| Per SD of ln(WMHV, 1/TIV%) | 244 | 24.7 | 1.3 (1.1–1.4) | 0.001 | 86 | 8.7 | 1.3 (1.1–1.7) | 0.017 | 134 | 13.6 | 1.2 (1.0–1.5) | 0.027 |
| WMHV, 1/TIV% | ||||||||||||
| Q1 (n=322) | 28 | 10.9 | Ref. | 8 | 3.1 | Ref. | 19 | 7.4 | Ref. | |||
| Q2 (n=321) | 44 | 18.0 | 1.6 (1.0–2.5) | 0.071 | 11 | 4.5 | 1.2 (0.5–3.1) | 0.661 | 24 | 9.8 | 1.3 (0.7–2.5) | 0.344 |
| Q3 (n=322) | 66 | 26.3 | 1.5 (0.9–2.3) | 0.087 | 22 | 8.8 | 1.6 (0.7–3.7) | 0.255 | 40 | 16.0 | 1.5 (0.8–2.6) | 0.176 |
| Q4 (n=322) | 106 | 45.0 | 2.0 (1.3–3.1) | 0.004 | 45 | 19.1 | 2.6 (1.2–5.9) | 0.018 | 51 | 21.7 | 1.6 (0.9–2.9) | 0.107 |
| SBI | ||||||||||||
| No (n=1043) | 173 | 21.3 | Ref. | 55 | 6.8 | Ref. | 102 | 12.6 | Ref. | |||
| Yes (n=192) | 62 | 42.7 | 1.2 (0.9–1.6) | 0.343 | 28 | 19.3 | 1.6 (1.0–2.6) | 0.059 | 28 | 19.3 | 1.0 (0.6–1.5) | 0.826 |
CI indicates confidence interval; HR, hazard ratio; ln, natural log transformed; PYS, person‐years; Q, quartile; Ref., reference; SBI, subclinical brain infarction; TIV, total intracranial volume; WMHV, white matter hyperintensity volume.
HRs and 95% CIs were estimated using Cox proportional hazards models adjusted for age, sex, race/ethnicity, education, medical insurance status, body mass index, smoking, physical activity, moderate alcohol drinking, hypertension, diabetes mellitus, hypercholesterolemia, history of atrial fibrillation, coronary artery disease, and myocardial infarction.
We found racial and ethnic differences in the effects of WMHV and SBI on mortality (Table 3). For Hispanic and non‐Hispanic white participants, the adjusted HR was greater for both all mortality (non‐Hispanic white, HR: 1.5 [95% CI, 1.1–2.0]; Hispanic white, HR: 1.4 [95% CI, 1.2–1.8]) and vascular mortality (non‐Hispanic white, HR: 2.0 [95% CI, 1.2–3.3]; Hispanic, HR: 1.6 [95% CI, 1.1–2.2]), and Hispanic participants were also at greater risk of nonvascular mortality (HR: 1.4; 95% CI, 1.1–1.8). Having SBI increased the risk of all mortality (HR: 1.7; 95% CI, 1.1–2.7) and vascular mortality (HR: 2.9; 95% CI, 1.4–5.8) for Hispanic participants only, and in this group, a significant effect on nonvascular mortality lost statistical significance after adjusting for other covariates. Neither greater WMHV nor SBI carried an increased risk of mortality for black participants.
Table 3.
Subclinical Brain Lesions and Mortality by Race/Ethnicity
| Mortality | Sample | No. of Event | Rate (1/1000 PYS) | Model | Per SD of ln(WMHV, 1/TIV%) | SBI (Yes vs No) | ||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI)a | P Value | HR (95% CI)a | P Value | |||||
| All | NH‐white (n=191) | 59 | 39.9 | Unadjusted | 1.7 (1.3–2.2) | <0.001 | 1.3 (0.7–2.5) | 0.402 |
| Adjusteda | 1.5 (1.1–2.0) | 0.056 | 0.8 (0.4–1.5) | 0.472 | ||||
| NH‐black (n=222) | 60 | 34.9 | Unadjusted | 1.4 (1.1–1.8) | 0.004 | 1.4 (0.8–2.4) | 0.284 | |
| Adjusteda | 1.0 (0.8–1.4) | 0.788 | 0.7 (0.4–1.5) | 0.397 | ||||
| Hispanic (n=845) | 116 | 18.0 | Unadjusted | 1.9 (1.6–2.3) | <0.001 | 2.7 (1.8–4.1) | <0.001 | |
| Adjusteda | 1.4 (1.2–1.8) | <0.001 | 1.7 (1.1–2.7) | 0.017 | ||||
| P for heterogeneityb | Unadjusted | 0.166 | 0.073 | |||||
| Adjusteda | 0.167 | 0.059 | ||||||
| Vascular | NH‐white (n=191) | 21 | 14.2 | Unadjusted | 2.3 (1.5–3.6) | <0.001 | 1.6 (0.6–4.3) | 0.382 |
| Adjusteda | 2.0 (1.2–3.3) | 0.008 | 1.0 (0.3–2.9) | 0.954 | ||||
| NH‐black (n=222) | 21 | 12.2 | Unadjusted | 1.2 (0.8–1.7) | 0.465 | 1.6 (0.6–4.0) | 0.369 | |
| Adjusteda | 0.9 (0.6–1.4) | 0.665 | 1.1 (0.4–3.3) | 0.849 | ||||
| Hispanic (n=845) | 38 | 5.9 | Unadjusted | 2.3 (1.7–3.1) | <0.001 | 4.2 (2.2–8.1) | <0.001 | |
| Adjusteda | 1.6 (1.1–2.2) | 0.015 | 2.9 (1.4–5.8) | 0.004 | ||||
| P for heterogeneityb | Unadjusted | 0.012 | 0.132 | |||||
| Adjusteda | 0.040 | 0.153 | ||||||
| Nonvascular | NH‐white (n=191) | 31 | 21.5 | Unadjusted | 1.4 (1.0–2.1) | 0.061 | 1.2 (0.5–3.0) | 0.625 |
| Adjusteda | 1.3 (0.8–2.0) | 0.311 | 0.7 (0.3–2.1) | 0.629 | ||||
| NH‐black (n=222) | 28 | 16.2 | Unadjusted | 1.4 (1.0–2.0) | 0.064 | 0.8 (0.3–2.1) | 0.635 | |
| Adjusteda | 1.0 (0.7–1.5) | 0.999 | 0.4 (0.1–1.2) | 0.089 | ||||
| Hispanic (n=845) | 74 | 11.5 | Unadjusted | 1.8 (1.4–2.3) | <0.001 | 2.3 (1.3–3.9) | 0.003 | |
| Adjusteda | 1.4 (1.1–1.8) | 0.007 | 1.3 (0.8–2.5) | 0.287 | ||||
| P for heterogeneityb | Unadjusted | 0.398 | 0.126 | |||||
| Adjusteda | 0.664 | 0.173 | ||||||
CI indicates confidence interval; HR, hazard ratio; ln, natural log transformed; PYS, person‐years; Q, quartile; SBI, subclinical brain infarction; TIV, total intracranial volume; WMHV, white matter hyperintensity volume.
HRs and 95% CIs were estimated using Cox proportional hazards models adjusted for age, sex, race/ethnicity, education, medical insurance status, body mass index, smoking, physical activity, moderate alcohol drinking, hypertension, diabetes mellitus, hypercholesterolemia, history of atrial fibrillation, coronary artery disease, and myocardial infarction.
Weighted sums‐of‐squares Q statistics were computed to test for heterogeneity in effect sizes of WMHV and SBI across racial and ethnic groups.
Discussion
In this prospective cohort study of 3 racial and ethnic groups living in the same urban US community, participants with greater WMHV or subclinical infarcts were at greater risk of incident stroke and mortality independent of traditional vascular risk factors. We found racial and ethnic variations in the effects of these subclinical brain findings that suggest differential risk of both stroke and mortality in these groups. In addition, we found evidence that subclinical lesion types affect subsequent stroke risk and incident stroke subtype.
Our findings suggest that SBI and WMH are both risk factors for incident ischemic stroke. Of 7 population‐based studies examining SVD and incident stroke,4, 6, 7, 21, 22, 23, 24 we found only 4 that examined ischemic stroke as a separate outcome, and all found similar associations.4, 9, 24 We found only 1 prior study examining ischemic stroke subtypes, for which presence of high WMH grade was associated with cardioembolic and unknown ischemic stroke subtypes, and we found no other reports that SBIs increase both lacunar and cryptogenic stroke risk.24 However, when we adjusted for both WMHV and SBI, the effect of SBI and WMHV on cryptogenic stroke risk was no longer significant, although the HR for WMHV suggests increased risk that may be clinically important. Because those with SBI are also likely to have high white matter grade, these effects are difficult to disentangle. Interestingly, WMHV load was not associated with incident lacunar stroke, whereas SBIs were. Even though WMHV often represents cerebral SVD, it is likely that subcortical cavitated SBI 3 to 15 mm in axial diameter is a more specific marker of small vessel ischemia. Finally, data on SBI subtype in relation to stroke risk are limited. We found that those with superficial and subcortical cavitated SBI 3 to 15 mm in axial diameter each had an increased risk of ischemic stroke, similar to findings from the Cardiovascular Health Study, as well as from the ARIC (Atherosclerosis Risk in Communities) study for lesions ≥3 mm (including nonlacunes).8, 9
We were unable to find other studies that stratified by race/ethnicity and examined the risk of incident stroke associated with SVD, and our study provided a valuable opportunity to do so. Participants from the same neighborhood with a shared environment, such as in NOMAS, may improve comparability over studies in which racial and ethnic groups live in different communities. In the ARIC study, black participants were more likely to have SBI >3 mm in size, but there was no significant interaction by race, and a stratified analysis was not performed.9 We found that SVD was associated with increased risk of all stroke and ischemic stroke among white participants. In addition, WMHV burden increased stroke risk for black participants. We found one other cohort study that included a large number of black participants, and WMHV load was associated with incident stroke, but no stratified analysis comparing racial groups was done.23 For Hispanic participants, the associations between SVD markers and stroke risk were more complex. Both WMHV and SBI increased risk for Hispanic participants in unadjusted analyses, but these associations lost significance when we adjusted for sociodemographic and vascular risk factors. Because this group had a greater number of incident strokes than either of the other racial and ethnic groups, these findings are probably not due to limited statistical power. We previously reported that Hispanic participants were at elevated risk of both large and small vessel strokes compared with white participants in this cohort, suggesting that the Hispanic participants have a number of vascular comorbidities.19 In addition, we have found that the impact of stroke risk factors differs across racial and ethnic groups in NOMAS, and this may partly account for differences between the adjusted and unadjusted HRs for stroke risk that we observed in the Hispanic group.11 Understanding contributors to stroke and mortality risk is becoming increasingly important as low‐ and middle‐income countries face aging populations at risk for noncommunicable diseases, and international organizations such as the United Nations and the WHO identify them as targets.25 To optimize healthcare spending, specific markers of risk are essential; however, strong treatment recommendations for patients with evidence of subclinical SVD have not been possible, given the lack of randomized clinical trials. Further study of subclinical cerebrovascular lesions such as WMH and SBI may provide the next wave of targets for prevention of stroke.
Like previous studies, we found WMHV burden increased mortality risk.4, 26, 27 Both WMH and SBI increased the risk of vascular mortality. Although the latter did not reach statistical significance (P=0.059), the HR suggests that SBI confers some risk, and larger studies are needed. Our finding that greater WMHV burden was associated with nonvascular mortality as well as vascular mortality may be due to the heterogeneity of white matter lesions. Certainly, those who die of nonvascular causes often have concomitant vascular disease, but it is possible that other mechanisms such as blood–brain barrier breakdown caused by systemic inflammation from nonvascular diseases explain their presence.28 The regional distribution of WMHV may have implications for the underlying cause of death, with posterior WMHV being more representative of Alzheimer disease (and possibly related mortality), but we lacked regional data for this analysis.29 Most prior studies on SVD and risk of mortality either have not stratified by race or did not include Hispanic participants and have been mainly in white populations (we found one that included Asian participants).4, 5, 6, 22 In NOMAS, however, WMHV and SBI both increased all mortality and vascular mortality among Hispanic participants. Our finding that Hispanic participants with more WMHs were at elevated risk of nonvascular mortality may again be attributable to the heterogeneous nature of some of these lesions and highlights the importance of studies examining WMHV in relation to diverse outcomes.
Several limitations are noteworthy. The number of outcome events in our study limited statistical power to detect significant associations, especially for the analyses of incident stroke subtype. As events accumulate, our findings will require confirmation. Another limitation to our study is the observational design and the inability to avoid unmeasured confounding; however, our study was designed to detect stroke and vascular outcomes and has 99% follow‐up over the life of the study, increasing the validity of our findings. It is also important to note that this sample included people who had survived from baseline enrollment and were well enough to participate in the MRI study, resulting in survivor healthy sample bias compared with the original random sample of northern Manhattan. We did not evaluate lesions <3 mm in size as part of the original MRI study, but we plan to collect these data to allow comparisons with other population‐based studies such as ARIC.9 Strengths of our study include the racially and ethnically diverse urban population‐based prospective design and the confirmed stroke‐free status of participants at the time of MRI. We also assessed the impact of different SVD measures (ie, WMHV and SBI) on similar outcomes, allowing comparison across markers.
Conclusions
After adjusting for possible potential confounders, people with greater WMH load or evidence of subclinical infarcts were at elevated risk of subsequent stroke and mortality in an urban and racially and ethnically diverse population‐based sample. Both WMH and SBI increased the risk of ischemic stroke, especially cryptogenic stroke, and the latter also raised the risk of lacunar stroke. Both superficial and subcortical cavitated SBIs raised the risk of incident stroke, whereas the presence of multiple SBI did not result in incremental risk. Racial and ethnic differences were present in the risk of stroke and mortality attributable to SVD. Greater WMHs increased the risk of both vascular and nonvascular mortality among white and Hispanic participants, whereas subclinical infarcts increased the risk of vascular mortality in Hispanic participants alone. Better understanding of how different racial and ethnic groups may be differentially affected by subclinical evidence of vascular damage are warranted to design appropriate primary prevention trials.
Sources of Funding
The Northern Manhattan Study is funded by the National Institute of Neurological Disorders and Strokea (NINDS). The NINDS was not involved in the design of this study or in the writing of this report.
Disclosures
All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: Dr Elkind receives unrelated compensation from Biogen IDEC, Biotelemetry/Cardionet, BMS‐Pfizer Partnership, Boehringer‐Ingelheim, Daiichi‐Sankyo, and Janssen Pharmaceuticals, and from the American Academy of Neurology for service as an associate editor of the journal Neurology. He serves on the National, Founders Affiliate, and New York City chapter boards of the American Heart Association/American Stroke Association, and receives royalties from UpToDate for a chapter related to cryptogenic stroke. Dr Wright receives royalties from UpToDate for 2 chapters on vascular dementia. Dr Sacco served as a consultant for Boehringer Ingelheim for the design and conduct of a secondary stroke prevention trial using dabigatran.
Supporting information
Table S1. Subclinical Brain Lesions and Risk of Stroke
Table S2. Subclinical Brain Lesions and Risk of Ischemic Stroke Subtypes
Table S3. Subclinical Brain Lesions and Stroke Risk by Race/Ethnicity
Acknowledgments
All authors had full access to all of the data in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis.
(J Am Heart Assoc. 2017;6:e004069 DOI: 10.1161/JAHA.116.004069.)28847914
Some data from this study were presented as an abstract at the American Academy of Neurology Annual Meeting, April 21, 2015, in Washington, DC.
References
- 1. Smith SC Jr, Collins A, Ferrari R, Holmes DR Jr, Logstrup S, McGhie DV, Ralston J, Sacco RL, Stam H, Taubert K, Wood DA, Zoghbi WA. Our time: a call to save preventable death from cardiovascular disease (heart disease and stroke). Circulation. 2012;126:2769–2775. [DOI] [PubMed] [Google Scholar]
- 2. Fanning JP, Wong AA, Fraser JF. The epidemiology of silent brain infarction: a systematic review of population‐based cohorts. BMC Med. 2014;12:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fazekas F, Kleinert R, Offenbacher H, Schmidt R, Kleinert G, Payer F, Radner H, Lechner H. Pathologic correlates of incidental MRI white matter signal hyperintensities. Neurology. 1993;43:1683–1689. [DOI] [PubMed] [Google Scholar]
- 4. Debette S, Beiser A, DeCarli C, Au R, Himali JJ, Kelly‐Hayes M, Romero JR, Kase CS, Wolf PA, Seshadri S. Association of MRI markers of vascular brain injury with incident stroke, mild cognitive impairment, dementia, and mortality: the Framingham Offspring Study. Stroke. 2010;41:600–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liebetrau M, Steen B, Hamann GF, Skoog I. Silent and symptomatic infarcts on cranial computerized tomography in relation to dementia and mortality: a population‐based study in 85‐year‐old subjects. Stroke. 2004;35:1816–1820. [DOI] [PubMed] [Google Scholar]
- 6. Bokura H, Kobayashi S, Yamaguchi S, Iijima K, Nagai A, Toyoda G, Oguro H, Takahashi K. Silent brain infarction and subcortical white matter lesions increase the risk of stroke and mortality: a prospective cohort study. J Stroke Cerebrovasc Dis. 2006;15:57–63. [DOI] [PubMed] [Google Scholar]
- 7. Vermeer SE, Hollander M, van Dijk EJ, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and white matter lesions increase stroke risk in the general population: the Rotterdam Scan Study. Stroke. 2003;34:1126–1129. [DOI] [PubMed] [Google Scholar]
- 8. Bernick C, Kuller L, Dulberg C, Longstreth WT Jr, Manolio T, Beauchamp N, Price T; Group* ftCHSCR . Silent MRI infarcts and the risk of future stroke. Neurology. 2001;57:1222–1229. [DOI] [PubMed] [Google Scholar]
- 9. Windham BG, Deere B, Griswold ME, Wang W, Bezerra DC, Shibata D, Butler K, Knopman D, Gottesman RF, Heiss G, Mosley TH Jr. Small brain lesions and incident stroke and mortality: a cohort study. Ann Intern Med. 2015;163:22–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Prabhakaran S, Wright CB, Yoshita M, Delapaz R, Brown T, DeCarli C, Sacco RL. Prevalence and determinants of subclinical brain infarction: the Northern Manhattan Study. Neurology. 2008;70:425–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sacco RL, Boden‐Albala B, Abel G, Lin IF, Elkind M, Hauser WA, Paik MC, Shea S. Race‐ethnic disparities in the impact of stroke risk factors: the Northern Manhattan Stroke Study. Stroke. 2001;32:1725–1731. [DOI] [PubMed] [Google Scholar]
- 12. Boden‐Albala B, Cammack S, Chong J, Wang C, Wright C, Rundek T, Elkind MS, Paik MC, Sacco RL. Diabetes, fasting glucose levels, and risk of ischemic stroke and vascular events: findings from the Northern Manhattan Study (NOMAS). Diabetes Care. 2008;31:1132–1137. [DOI] [PubMed] [Google Scholar]
- 13. Sacco RL, Gan R, Boden‐Albala B, Lin IF, Kargman DE, Hauser WA, Shea S, Paik MC. Leisure‐time physical activity and ischemic stroke risk: the Northern Manhattan Stroke Study. Stroke. 1998;29:380–387. [DOI] [PubMed] [Google Scholar]
- 14. Wright CB, Paik MC, Brown TR, Stabler SP, Allen RH, Sacco RL, DeCarli C. Total homocysteine is associated with white matter hyperintensity volume: the Northern Manhattan Study. Stroke. 2005;36:1207–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Decarli C, Maisog J, Declan MMG, Teichberg D, Rapoport SI, Horwitz B. Method for quantification of brain, ventricular, and subarachnoid CSF volumes from MR images. J Comput Assist Tomogr. 1992;16:274–284. [DOI] [PubMed] [Google Scholar]
- 16. Decarli C, Miller BL, Swan GE, Reed T, Wolf PA, Garner J, Jack L, Carmelli D. Predictors of brain morphology for the men of the NHLBI twin study. Stroke. 1999;30:529–536. [DOI] [PubMed] [Google Scholar]
- 17. Willey JZ, Moon YP, Paik MC, Yoshita M, Decarli C, Sacco RL, Elkind MS, Wright CB. Lower prevalence of silent brain infarcts in the physically active: the Northern Manhattan Study. Neurology. 2011;76:2112–2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Munoz Mendoza J, Isakova T, Ricardo AC, Xie H, Navaneethan SD, Anderson AH, Bazzano LA, Xie D, Kretzler M, Nessel L, Hamm LL, Negrea L, Leonard MB, Raj D, Wolf M; Chronic Renal Insufficiency C . Fibroblast growth factor 23 and inflammation in CKD. Clin J Am Soc Nephrol. 2012;7:1155–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. White H, Boden‐Albala B, Wang C, Elkind MS, Rundek T, Wright CB, Sacco RL. Ischemic stroke subtype incidence among whites, blacks, and Hispanics: the Northern Manhattan Study. Circulation. 2005;111:1327–1331. [DOI] [PubMed] [Google Scholar]
- 20. Foulkes MA, Wolf PA, Price TR, Mohr JP, Hier DB. The Stroke Data Bank: design, methods, and baseline characteristics. Stroke. 1988;19:547–554. [DOI] [PubMed] [Google Scholar]
- 21. Kobayashi S, Okada K, Koide H, Bokura H, Yamaguchi S. Subcortical silent brain infarction as a risk factor for clinical stroke. Stroke. 1997;28:1932–1939. [DOI] [PubMed] [Google Scholar]
- 22. Buyck JF, Dufouil C, Mazoyer B, Maillard P, Ducimetiere P, Alperovitch A, Bousser MG, Kurth T, Tzourio C. Cerebral white matter lesions are associated with the risk of stroke but not with other vascular events: the 3‐City Dijon Study. Stroke. 2009;40:2327–2331. [DOI] [PubMed] [Google Scholar]
- 23. Wong TY, Klein R, Sharrett AR, Couper DJ, Klein BE, Liao DP, Hubbard LD, Mosley TH. Cerebral white matter lesions, retinopathy, and incident clinical stroke. JAMA. 2002;288:67–74. [DOI] [PubMed] [Google Scholar]
- 24. Kuller LH, Longstreth WT Jr, Arnold AM, Bernick C, Bryan RN, Beauchamp NJ Jr. White matter hyperintensity on cranial magnetic resonance imaging: a predictor of stroke. Stroke. 2004;35:1821–1825. [DOI] [PubMed] [Google Scholar]
- 25. Lim SS, Gaziano TA, Gakidou E, Reddy KS, Farzadfar F, Lozano R, Rodgers A. Prevention of cardiovascular disease in high‐risk individuals in low‐income and middle‐income countries: health effects and costs. Lancet. 2007;370:2054–2062. [DOI] [PubMed] [Google Scholar]
- 26. Kuller LH, Arnold AM, Longstreth WT Jr, Manolio TA, O'Leary DH, Burke GL, Fried LP, Newman AB. White matter grade and ventricular volume on brain MRI as markers of longevity in the Cardiovascular Health Study. Neurobiol Aging. 2007;28:1307–1315. [DOI] [PubMed] [Google Scholar]
- 27. Ikram MA, Vernooij MW, Vrooman HA, Hofman A, Breteler MM. Brain tissue volumes and small vessel disease in relation to the risk of mortality. Neurobiol Aging. 2009;30:450–456. [DOI] [PubMed] [Google Scholar]
- 28. Wright CB, Moon Y, Paik MC, Brown TR, Rabbani L, Yoshita M, DeCarli C, Sacco R, Elkind MS. Inflammatory biomarkers of vascular risk as correlates of leukoariosis. Stroke. 2009;40:3466–3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wiegman AF, Meier IB, Provenzano FA, Schupf N, Manly JJ, Stern Y, Luchsinger JA, Brickman AM. Regional white matter hyperintensity volume and cognition predict death in a multiethnic, community cohort of older adults. J Am Geriatr Soc. 2013;61:2246–2248. DOI: 10.1111/jgs.12568. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Subclinical Brain Lesions and Risk of Stroke
Table S2. Subclinical Brain Lesions and Risk of Ischemic Stroke Subtypes
Table S3. Subclinical Brain Lesions and Stroke Risk by Race/Ethnicity


