Abstract
Background
Retrograde type A aortic dissection (RTAD) is a potentially lethal complication after thoracic endovascular aortic repair (TEVAR). However, data are limited regarding the development of RTAD post‐TEVAR. This systematic review aims to define the incidence, mortality, and potential risk factors of RTAD post‐TEVAR.
Methods and Results
Multiple electronic searches were performed. Fifty publications with a total of 8969 patients were analyzed. Pooled estimates for incidence and mortality of RTAD were 2.5% (95% confidence interval [CI], 2.0–3.1) and 37.1% (95% CI, 23.7–51.6), respectively. Metaregression analysis evidenced that RTAD rate was associated with hypertension (P=0.043), history of vascular surgery (P=0.042), and American Surgical Association (P=0.044). The relative risk of RTAD was 1.81 (95% CI, 1.04–3.14) for acute dissection (relative to chronic dissection) and 5.33 (95% CI, 2.70–10.51) for aortic dissection (relative to a degenerative aneurysm). Incidence of RTAD was significantly different in patients with proximal bare stent and nonbare stent endografts (relative risk [RR]=2.06; 95% CI, 1.22–3.50). RTAD occurrence rate in zone 0 was higher than other landing zones.
Conclusions
The pooled RTAD rate after TEVAR was calculated at 2.5% with a high mortality rate (37.1%). Incidence of RTAD is significantly more frequent in patients treated for dissection than those with an aneurysm (especially for acute dissection), and when the proximal bare stent was used. Rate of RTAD after TEVAR varied significantly according to the proximal Ishimaru landing zone. The more‐experienced centers tend to have lower RTAD incidences.
Keywords: complication, endograft, retrograde type A aortic dissection, TEVAR
Subject Categories: Aortic Dissection, Aneurysm
Clinical Perspective
What Is New?
This article first provides an overview of multicenter clinical data, which is robust to support the study in incidence, mortality, and potential risk factors of retrograde type A aortic dissection after thoracic endovascular aortic repair via meta‐analysis.
What Are the Clinical Implications?
The mechanism of retrograde type A aortic dissection after thoracic endovascular aortic repair has been controversial, while this article well indicates the etiological factors of retrograde type A aortic dissection through a large sample of clinical data, and provides a good guide for clinicians to avoid catastrophic complications such as retrograde type A aortic dissection.
Introduction
Thoracic endovascular aortic repair (TEVAR) has been increasingly used in the treatment of pathology affecting the descending thoracic aorta and distal aortic arch.1 However, TEVAR bears the risk of unusual, previously unanticipated, severe complications. One of the most feared complications of this procedure is retrograde type A aortic dissection (RTAD), which has a low incidence but high mortality rate.2, 3 The majority of published reports on RTAD post‐TEVAR are based on a small number of patients with ambiguous results. There have been many etiological factors that have been proposed as a cause of RTAD, but interpretation was difficult because of the heterogeneity of data quality and the reported parameters.
We undertook a systematic review and meta‐analysis to identify all published reports on RTAD post‐TEVAR with the intention of recording the incidence, mortality, and potential risk factors of RTAD. This information may be helpful in designing appropriate clinical strategies not only to minimize the occurrence, but also to diagnose and treat this complication early and effectively in the hope of improving future procedural safety and outcomes.
Methods
Several electronic health databases (including EMBASE and MEDLINE) were searched to identified all articles reporting RTAD post‐TEVAR between January 2000 and December 2014. The searching terms included “TEVAR,” “retrograde dissection,” “thoracic stent‐graft,” “endograft,” and “graft” with the Boolean operator “OR.” In addition, the references of all included articles were examined for additional relevant series. The literature search was limited to the English language. All studies were independently assessed by 2 reviewers (S.Z. and Y.C.), and the full text of the studies was retrieved.
Study Design and Definitions
Studies included in the meta‐analysis met all the following inclusion criteria:
All articles reporting complications, including RTAD post‐TEVAR, were identified by definition as those who underwent endovascular repair or hybrid repair of thoracic aortic pathology.
Diagnosis of aortic pathology had been made by computed tomography scan of the thorax, abdomen, or pelvis.
Series included more than 10 patients with TEVAR.
Demographic data and comorbidities of the patients were provided.
At least 1 of the basic outcome criteria (number of patients with TEVAR, number of patients with RTAD, or mortality of RTAD) was selected.
The article mentioned variables more than 25% of all reservations.
An extensive effort was made to minimize the impact of covert duplicate or metachronous republication from the same surgical groups on the patient sample size; for these cases, only the latest report was included.
Aortic dissection was considered an acute event if it occurred within the first 14 days from the onset of symptoms, whereas it was considered chronic beyond 14 days.4 Classification of a proximal stent graft landing zone in the aortic arch was performed using the order proposed by Ishimaru et al.5
Data Extraction
Two authors independently searched and assessed the full‐text articles, and the discrepancy was solved by discussion. The following data were extracted: the year of publication, number of patients with TEVAR, sex, and mean age; comorbidities; aortic pathology; procedural data (implanted stent grafts type, landing zone, proximal stent configuration, and proximal stent graft oversizing); mean follow‐up period; number of patients with RTAD; and the mortality of RTAD.
Statistical Analysis
Standard descriptive statistics (continuous variables were reported as mean with 95% confidence interval [CI] and categorical variables were reported as percentage with 95% CI) were used to summarize demographical and baseline data of the recruited patients from all eligible published studies. In Table 1, for the continuous variables such as age, the means and 95% CIs were calculated based on the means and the sample size reported by each study using SPSS software (SPSS, Inc., Chicago, IL). For categorical variables, such as sex, averaged proportions were estimated by using the “double‐Arcsine transformation” function in STATA (StatCorp LP, College Station, TX), based on the proportion in each study. Furthermore, a meta‐analysis was carried out on all included studies for incidence and mortality of RTAD post‐TEVAR. Clopper–Pearson interval (“exact” binomial interval) was used for individual study. “Meta” package was used with “metaprop” function in the package. “Arcsine transformation” was used in order to make the distribution close to normal distribution. “DerSimonian‐Laird” was used to estimate the random effects. In addition, 0.5 is added to all cell frequencies of studies with a zero cell count. Then, an examination of heterogeneity was performed using the I2 test. I2>50% is considered significant heterogeneity.6 Heterogeneity and robustness of pooled proportions were explored by subgroup analyses. In order to explore potential risk factors of RTAD post‐TEVAR, individual study risk ratio (RR) and 95% CI were calculated. A metaregression analysis was performed to explore the heterogeneity observed among studies. The meta‐analysis was conducted using R statistical software (R Foundation for Statistical Computing, Vienna, Austria), whereas the metaregression analysis was performed using STATA statistical software (StatCorp LP).
Table 1.
Patient Demographics and Baseline Characteristics
| 95% CIs | ||
|---|---|---|
| Total patients, n | 8969 | … |
| Sex (% male) | 75.7 (3238/4398) | 72.7 to 78.6 |
| Age, y | 61.5 (4471) | 55.9 to 67.0 |
| Comorbidities, % | ||
| Hypertension | 85.6 (3635/4422) | 80.5 to 88.2 |
| DM | 12.5 (399/3373) | 9.8 to 15.5 |
| CAD | 18.4 (342/2038) | 13.3 to 24.0 |
| Renal impairment | 12.0 (440/3324) | 9.2 to 15.2 |
| COPD | 21.7 (761/3444) | 17.2 to 26.7 |
| ASA | 81.3 (1477/2274) | 67.8 to 91.8 |
| Nicotine consumption | 48.6 (1802/3523) | 40.6 to 56.6 |
| Connective tissue disorders/Marfan, % | 2.1 (51/3650) | 1.1 to 3.3 |
| History of vascular surgery (thorax or abdomen), % | 21.4 (359/2147) | 13.4 to 30.7 |
| Indication for TEVAR, % | ||
| Degenerative aneurysm, % | 15.5 (1704/5959) | 6.3 to 27.9 |
| AD, % | 79.4 (3604/5959) | 65.7 to 90.4 |
| Other aortic pathologies, % | 5.2 (651/5959) | 2.0 to 9.7 |
| Proximal landing zone, % | ||
| Zone 0 | 13.5 (201/2823) | 5.7 to 23.9 |
| Zone 1 | 11.3 (305/2823) | 5.9 to 18.1 |
| Zone 2 | 24.7 (972/2823) | 17.2 to 33.1 |
| Zone 3 | 20.8 (904/2823) | 10.0 to 34.5 |
| Zone 4 | 2.8 (357/2823) | 0.3 to 9.2 |
| Urgent procedure, % | 30.4 (794/2935) | 22.0 to 39.4 |
| General anesthesia, % | 84.2 (1138/1343) | 66.8 to 95.9 |
| Covered LSA, % | 49.1 (860/2314) | 37.2 to 61.1 |
| 30‐day mortality, % | 6.6 (272/4892) | 5.0 to 8.3 |
| Follow‐up, months | 26.6 | 15.5 to 37.7 |
AD indicates aortic dissection; ASA, American Surgical Association; CAD, coronary artery disease; CI, confidence interval; COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; LSA, left subclavian artery; TEVAR, thoracic endovascular aortic repair.
Results
Our initial search yielded 448 potential literature citations; 67 duplicates and 299 nonrelevant studies were excluded after scanning titles and abstracts, and 82 citations were left for further evaluation. Among the remaining citations, 32 were excluded for the following reasons: 6 were reviews or invited commentaries, 17 were single‐case reports, 3 were TEVAR or hybrid repair only for type A aortic dissection, and 6 studies were lack of outcomes of interest (Figure 1). Therefore, 50 studies were included in the meta‐analysis with a total of 8969 patients.1, 2, 3, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53
Figure 1.
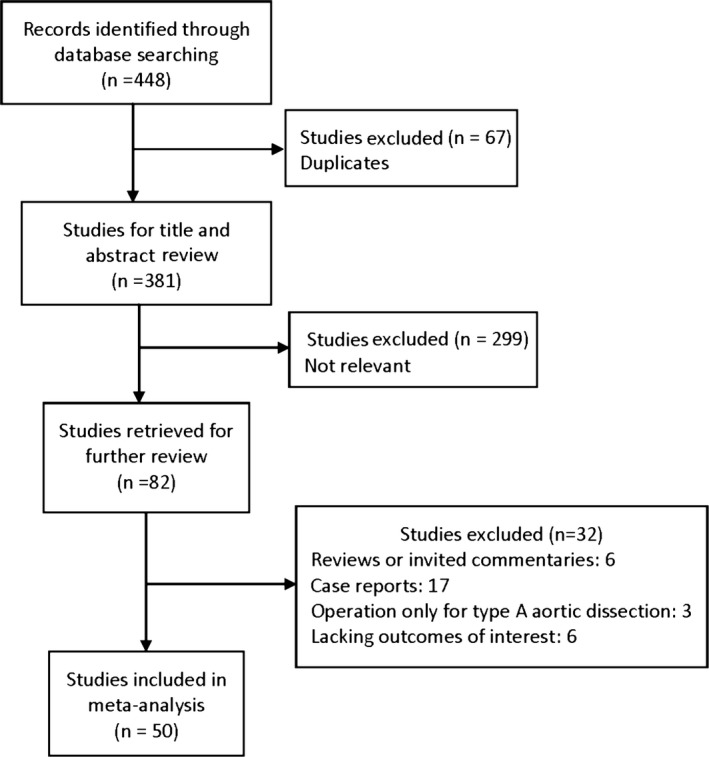
Flow diagram of study selection for meta‐analysis.
Information on sex was available in 4398 patients, of which 3238 (75.7%; 95% CI, 72.7–78.6) were men. Mean age was 61.5 years old (95% CI, 55.9–67.0), based on the available ages in 4471 patients. Patient demographics are detailed in Table 1. Indication for TEVAR included degenerative aneurysms (15.5%; 95% CI, 6.3–27.9), aortic dissections (79.4%; 95% CI, 65.7–90.4), and other aortic pathologies (penetrating ulcers, intramural hematomas, and mycotic aneurysms; 5.2%; 95% CI, 2.0–9.7).
In 794 patients (30.4%; 95% CI, 22.0–39.4), the procedure was urgent; 1138 patients (84.2%; 95% CI, 66.8–95.9) underwent general anesthesia; 860 patients (49.1%; 95% CI, 37.2–61.1) were covered with left subclavian artery; 30‐day mortality was (6.6%; 95% CI, 5.0–8.3), and the mean follow‐up period was 26.6 months (95% CI, 15.5–37.7).
Incidence of RTAD
A meta‐analysis of RTAD incidence showed a pooled rate of 2.5% (95% CI, 2.0–3.1; Figure 2) with acceptable heterogeneity among the 50 studies (I2=48.6%), indicating the presence of publication bias (P=0.0004).
Figure 2.
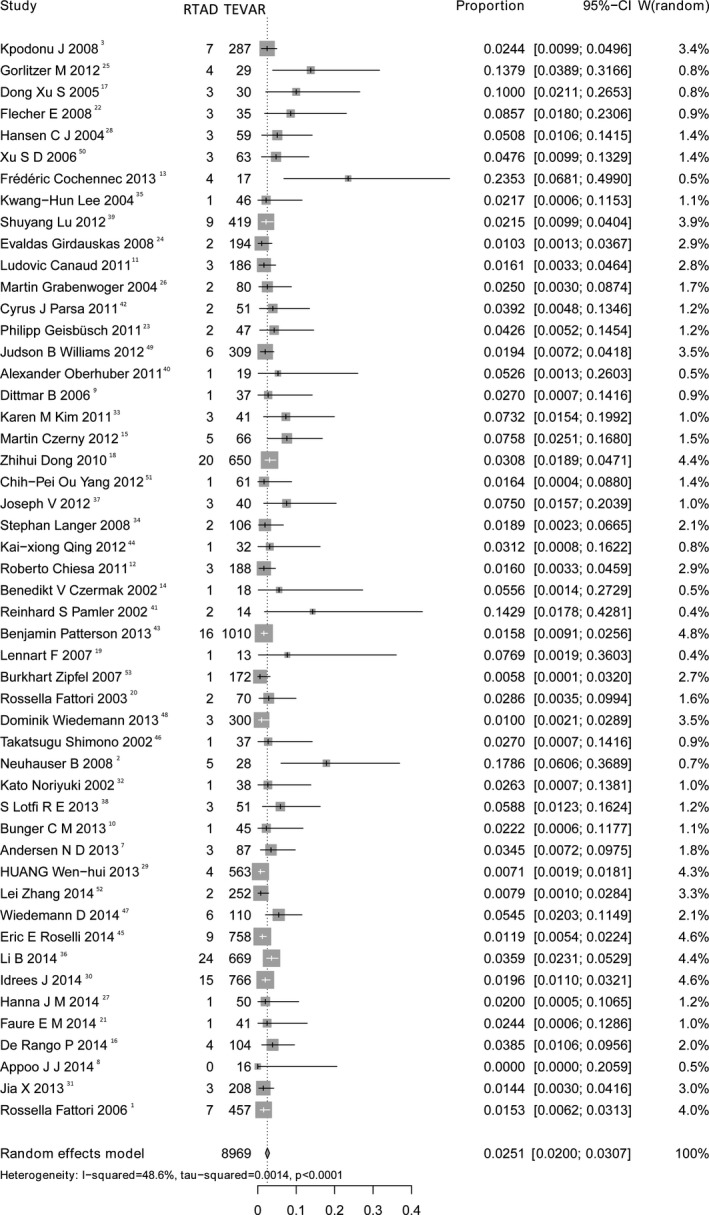
Forest plot shows the random‐effects proportion meta‐analysis for RTAD rates post‐TEVAR. CI indicates confidence interval; RTAD indicates retrograde type A aortic dissection; TEVAR, thoracic endovascular aortic repair.
Mortality of RTAD
Data concerning mortality of RTAD were available in 33 studies. The proportional meta‐analysis of mortality showed a pooled rate of 37.1% (95% CI, 23.7–51.6; Figure 3) with great heterogeneity (I2=63.4%) and no publication bias (P=0.11).
Figure 3.
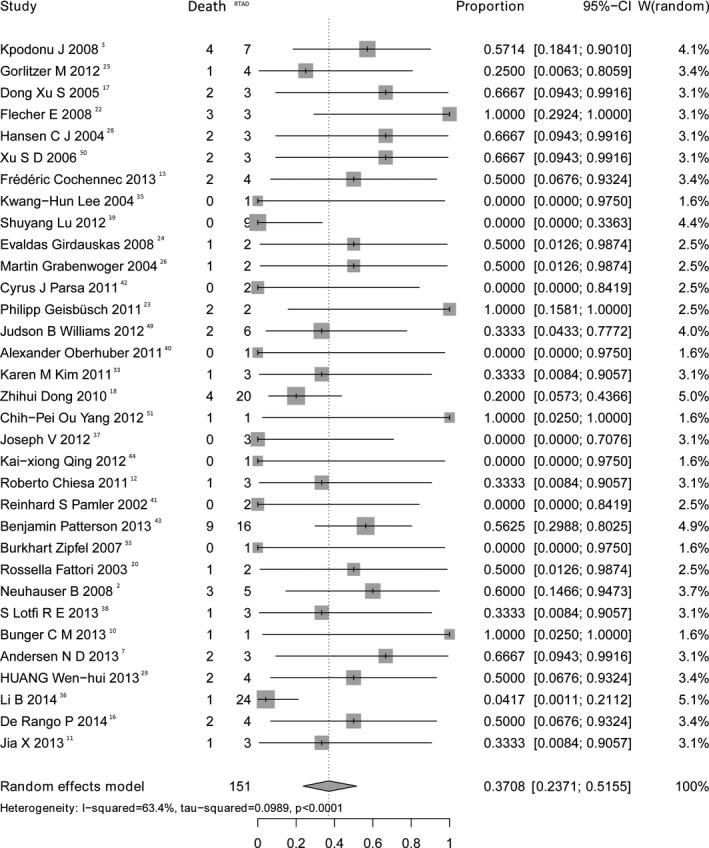
Forest plot shows the random‐effects proportion meta‐analysis for mortality of RTAD. CI indicates confidence interval; RTAD, retrograde type A aortic dissection.
Subgroup Analysis
To explain the observed within‐study heterogeneity, a subgroup analysis was performed according the following standards: (1) whether the study was published before 2010 and (2) the center experience.
Nineteen studies published before 2010 have the pooled RTAD rate of 3.8% (95% CI, 2.4–5.9) and the remaining 31 studies have the rate of 2.3% (95% CI, 1.7–2.9). In both subgroups, there was significant within‐study heterogeneity (I2=57.5% and I2=53.6%, respectively). There was no publication bias in those studies before 2010 (P=0.48), but there was evidence of it in studies after 2010 (P=0.0005).
In terms of center experience, the pooled RTAD rate from the 30 experienced centers (reporting ≥50 cases each) was 2.1% (95% CI, 1.7–2.7) without significant between‐study heterogeneity (I2=42.5%). In contrast, the RTAD rate from less experienced teams was 5.7% (95% CI, 3.7–8.0) with no significant heterogeneity (I2=25.1%). There was no publication bias in either subgroup (P=0.70 for more‐experienced teams; and P=0.17 for less‐experienced teams).
Indication for TEVAR
The results of the 13 series that simultaneously reported the incidence of RTAD for acute and chronic dissection revealed that incidence of RTAD was 3.65% (22 of 603) in patients treated for acute dissection and 2.17% (16 of 738) in patients treated for chronic dissection (RR=1.81; 95% CI, 1.04–3.14). There was no heterogeneity among the studies (I2=6.3%; P=0.383; Figure 4A). Incidence of RTAD was 5.10% (28 of 549) in patients treated for dissection and 0.66% (7 of 1055) in patients treated for degenerative aneurysm (RR=5.33; 95% CI, 2.70–10.51). There was no heterogeneity among the studies (I2=0%; P=0.971; Figure 4B).
Figure 4.
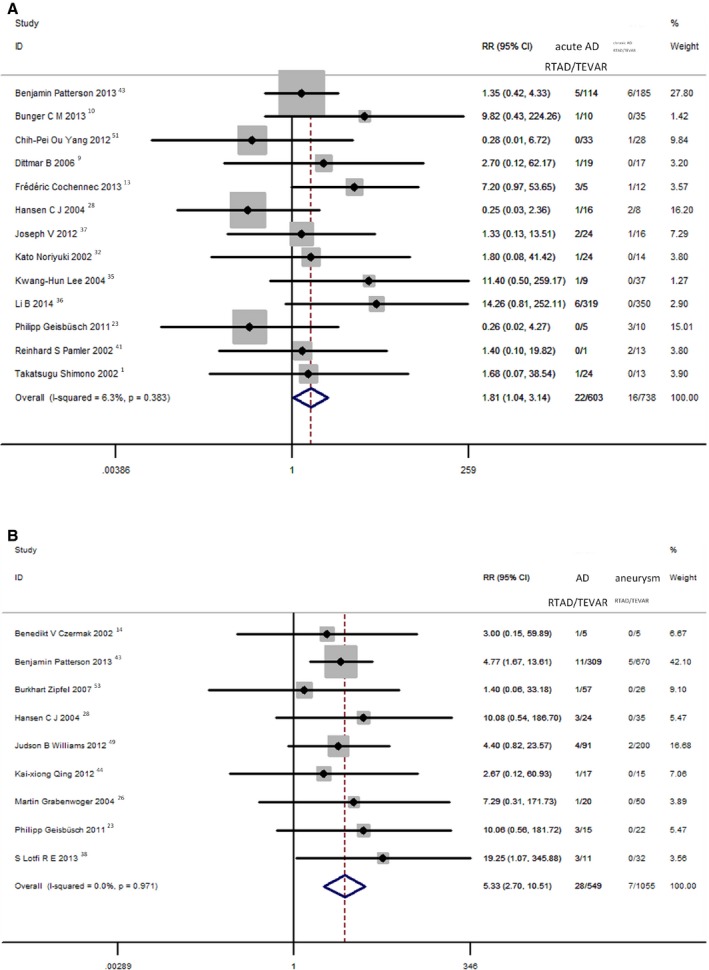
A, Comparison of RTAD rates post‐TEVAR between acute and chronic dissection. B, Comparison of RTAD rates post‐TEVAR between dissection and aneurysm. AD indicates aortic dissection; CI, confidence interval; RR, risk ratio; RTAD, retrograde type A aortic dissection; TEVAR, thoracic endovascular aortic repair.
Proximal Stent‐Graft Configuration
Data concerning proximal stent‐graft configuration were available in 14 studies. The concluded incidence of RTAD was 2.31% (40 of 1728) in patients treated with proximal bare stent and 1.24% (14 of 1126) in patients treated with proximal nonbare stent grafts (RR=2.06; 95% CI, 1.22–3.50). There was no heterogeneity among the studies (I2=31.8%; P=0.121; Figure 5).
Figure 5.
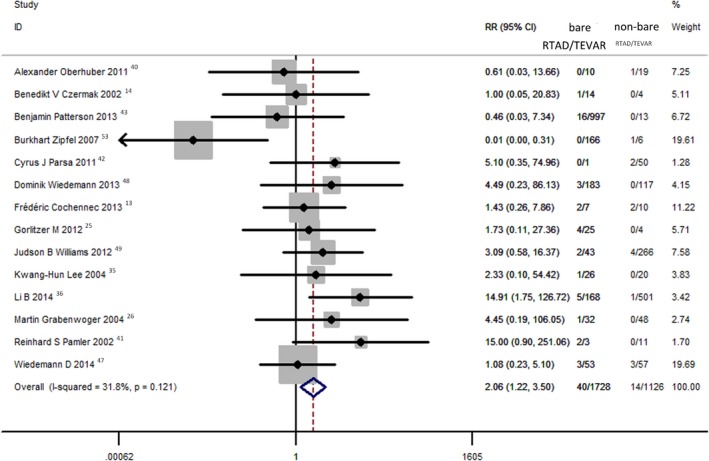
Comparison of RTAD rates post‐TEVAR between proximal bare stent and nonbare stent. RTAD indicates retrograde type A aortic dissection; TEVAR, thoracic endovascular aortic repair.
Proximal Landing Zone
Fifteen series reporting both the incidence of RTAD and proximal landing zone showed that the rate of RTAD post‐TEVAR varied significantly according to the proximal Ishimaru landing zone: 8.12% (16 of 197) in zone 0; 2.57% (7 of 272) in zone 1; 2.66% (24 of 903) in zone 2; and 0.67% (8 of 1195) in zones 3 and 4 (P<0.0001; Table 2).
Table 2.
Incidence, Proximal Landing Zone
| Incidence, % | P Value | |
|---|---|---|
| Proximal landing zone | ||
| Zone 0 | 8.12 (16/197) | <0.0001 |
| Zone 1 | 2.57 (7/272) | |
| Zone 2 | 2.66 (24/903) | |
| Zones 3 and 4 | 0.67 (8/1195) | |
Stent Graft Oversizing
Although there are 25 studies describing oversizing in TEVAR, most of them provided interval ranges without a detailed numerical description. Only 3 studies including 27 patients provided specific values, with the mean value 14.3±5.2%. There was no statistical significance by regression analysis (P=0.389).
Results of the Metaregression Analysis
The metaregression analysis evidenced that the pooled estimate for RTAD rate was associated at a statistically significant level with hypertension (P=0.043), history of vascular surgery (P=0.042), and American Surgical Association (P=0.044). No other investigated moderator variables were found to be statistically significantly related to RTAD (Table 3).
Table 3.
Individual Effect of Covariates on the Rate of RTAD; Results of the Random‐Effects Meta‐Regression Analyses
| Covariates | Studies N | t | P Value | Coefficient |
|---|---|---|---|---|
| Age, y | 34 | 0.98 | 0.335 | 0.0048371 |
| Male | 33 | 0.01 | 0.989 | 0.00452 |
| Hypertension | 30 | 2.12 | 0.043 | 0.7417532 |
| DM | 20 | 1.31 | 0.206 | 0.7039571 |
| CAD | 20 | 0.66 | 0.515 | 0.2248837 |
| Renal impairment | 22 | 0.09 | 0.929 | 0.0389217 |
| COPD | 24 | 0.49 | 0.629 | 0.1377187 |
| ASA | 11 | 2.35 | 0.044 | 0.309096 |
| Nicotine consumption | 20 | −0.68 | 0.506 | −0.165958 |
| History of vascular surgery | 19 | 2.19 | 0.042 | 0.5205373 |
| Follow‐up | 40 | −1.56 | 0.128 | −0.0032653 |
ASA indicates American Surgical Association; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; RTAD, retrograde type A aortic dissection.
Discussion
TEVAR has become a first‐choice therapy for many diseases of aortic pathologies.54 RTAD as a catastrophic complication of TEVAR has been extensively described, but the precise mechanism has not been elucidated. Etiological factors that have been suggested include device and procedure related, unfavorable aortic‐dissection anatomy, and natural progression of initial aortic dissection.
A multicenter study that analyzed the European Registry on Endovascular Aortic Repair Complications data involving 4750 procedures estimated the incidence of RTAD of 1.33%.55 The most promising outcome was reported by Appoo et al with a 0.0% incidence in a series of 16 patients.8 The most discouraging results were reported by Neuhauser et al2 with incidence at 17.9%. In our meta‐analysis, we reviewed 8969 patients and found that incidence of RTAD was 2.5% (95% CI, 2.0–3.1), with acceptable heterogeneity. Therefore, we can suggest that incidence of RTAD is roughly 2.5%.
In the subgroup analysis, we found that the RTAD of centers experienced in endovascular surgery was significantly lower than the less‐experienced centers. Occurrence of RTAD is associated with guide wire, catheter, delivery system, balloon dilation, and a series of operations during the TEVAR procedure. For example, when the guide wire is passing through the tortuous aortic arch, especially the areas where they are anatomically abnormal and where the aorta is extremely narrow or distorted, the guide wire or the catheter could contact the aortic wall, the friction between them can cause potential damage to the wall, and once the damage has occurred, the risk of the occurrence of RTAD will increase. The more‐experienced centers are well aware of the aforementioned risks, and the accumulated surgical experience allows them to effectively minimize or even avoid some of the potential risks. As a result, the more‐experienced centers tend to have lower RTAD incidences.
Interestingly, RTAD represents the most common complication among Marfan patients who were treated with the TEVAR procedure. The European Registry on Endovascular Aortic Repair Complications, by Eggebrecht et al, captured 48 TEVAR cases in patients with Marfan syndrome (RTAD incidence, 8.3%)56; Dong et al revealed that among 4 patients with Marfan syndrome, 3 developed retrograde dissection post‐TEVAR.57 Marfan patients are often affected by aortic middle‐level cystic necrosis, its pathological basis of the elastin and collagen peptide chain between the transverse joint.
In addition, RTAD seems to be much higher after surgical treatment of dissection compared with atherosclerotic or post‐traumatic aneurysm. Incidence of RTAD post‐TEVAR for acute type B aortic dissection was 8.4% (26 of 309) and 3% (10 of 325) for chronic dissection. The odds ratio of RTAD (relative to a degenerative aneurysm) was 10.0 (CI, 4.7–21.9) for an acute aortic dissection and 3.4 (CI, 1.3–8.8) for a chronic aortic dissection.55 In this study, the cumulative data revealed that the RR for RTAD in acute dissection was 1.81 compared with chronic dissection, and RR for patients with dissection was 5.33 when compared with that for patients with degenerative thoracic aneurysms. A possible explanation for RTAD, particularly in spontaneous thoracic aortic dissection, can be the progression of the initial aortic disease.58 Moreover, the natural progression of the diseased aortic wall as a cause of RTAD is suggested by public data, proving the extension of dissection in patients with medical treatment or after surgical repair of a thoracic dissection.59, 60 The influence of acute dissection and aortic fragility on RTAD has been illustrated in other reports. Hata et al60 reported a 2.2% incidence of RTAD in a group of 180 patients treated with the best medical therapy for acute type B dissection, which was similar to the rate reported post‐TEVAR.
In terms of proximal stent‐graft configuration design, reports have suggested that proximal bare stent configuration is associated with an increased risk of RTAD.2, 17, 49, 57 A recent system review of data from 4750 patients treated with TEVAR observed that there was no difference in the incidence of RTAD in patients treated with endografts with bare stent or nonbare stent configuration.55 However, our meta‐analysis demonstrated that proximal bare stent configuration is associated with an increased risk of RTAD (RR=2.06; 95% CI, 1.22–3.50).
This study also observed that deployment of the stent graft in the aortic arch (zones 0–2) is another significant risk factor of RTAD, with a high odds ratio in zone 0. The association of RTAD in proximal aortic arch procedures has been previously observed. In a transcontinental registry, the rate of 58 RTAD incidences has been reported up to 7.5% during zone 0 total debranching.14 A recent systematic review reported 73 RTAD cases following 3211 TEVAR cases with an incidence of 2.27%, whereas incidence increased up to 6.8% post‐TEVAR with a proximal landing in zone 0.55 Several attempts have been made to clarify this association. The increased risk may result from:
Spring‐back force when stent across‐arch placement and the common feature of the “spring” to spontaneously turn back to straight if passively curved. Moreover, the more the endograft is bent, the higher the stress might be. Dong et al57 reported 91.7% (22 of 24) retear in the great curvature, which is consistent with the route of the spring‐back movement of the endograft.
The Windkessel effect and the dynamic movement of the ascending aorta during the cardiac cycle may lead to an aggravated interaction with the rigid stent edge, which may lead to intimal injury with subsequent dissection.
Extensive radial force attributed to oversizing of the endografts more than 20% in relation to the diameter of the aorta has also been proposed as a potential cause of post‐TEVAR RTAD.3, 61 The larger the diameter of the stent graft, the greater the radial force, which provides better opposition to the aortic wall. On the other hand, in the weak aortic wall, oversizing of stent grafts might cause intimal injury. Canaud et al55 indicated that graft oversizing >9% translated to an increased relative risk of RTAD, by a factor of 1.4 for each additional percentage point of oversizing. Although in this research there was no correlation between RTAD and oversizing, the reasons can be explained as follows:
Only a few (3 of 50) studies clearly indicated oversizing specific values, and there is no uniform data format; therefore, selection bias or confounding may be present.
Oversizing choice at most current centers depends on the type of aortic disease or dissection phase (acute and chronic). In our meta‐analysis, oversizing was less than 10% in 4 studies, with incidence of RTAD at 5.9% (12 of 205) in dissection and 0.5% (1 of 200) in aneurysm; this has confirmed that the incidence of dissection was significantly higher than of aneurysm.
At present, the measurement of aortic diameter is not uniform; it defines either “aortic intima—intima” or “aortic adventitia—adventitia” as the diameter of the aorta. The magnification of the intravascular grafts varies widely, and the arterial diameter varies with the cardiac cycle as well. If dynamic measurements are used, the magnification bias will be greater. Therefore, how to choose a more‐appropriate measurement method is subject to further study.
Conclusion
Although RTAD post‐TEVAR is an uncommon complication with incidence around 2.5%, it has a high mortality rate (37.1%). The importance of considering the fragility of the aortic wall is emphasized by the fact that RTAD is significantly more frequent in patients treated for acute and chronic type B dissection compared with aneurysm, and when the proximal bare stent was used. The rate of RTAD post‐TEVAR varied significantly according to the proximal Ishimaru landing zone. The more‐experienced centers tend to have lower RTAD incidences. Further validation of the data in large‐scale clinical studies is needed to substantiate these results.
Disclosures
None.
Sources of Funding
This work was supported by National Natural Science Foundation of China (81170291).
Acknowledgment
We thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this article.
(J Am Heart Assoc. 2017;6:e004649 DOI: 10.1161/JAHA.116.004649.)28939705
Contributor Information
Qingsheng Lu, Email: luqs@xueguan.net.
Zaiping Jing, Email: 43670064@qq.com.
References
- 1. Fattori R, Nienaber CA, Rousseau H, Beregi JP, Heijmen R, Grabenwoger M, Piquet P, Lovato L, Dabbech C, Kische S, Gaxotte V, Schepens M, Ehrlich M, Bartoli JM. Results of endovascular repair of the thoracic aorta with the talent thoracic stent graft: the talent thoracic retrospective registry. J Thorac Cardiovasc Surg. 2006;132:332–339. [DOI] [PubMed] [Google Scholar]
- 2. Neuhauser B, Greiner A, Jaschke W, Chemelli A, Fraedrich G. Serious complications following endovascular thoracic aortic stent‐graft repair for type B dissection. Eur J Cardiothorac Surg. 2008;33:58–63. [DOI] [PubMed] [Google Scholar]
- 3. Kpodonu J, Preventza O, Ramaiah VG, Shennib H, Wheatley GH III, Rodriquez‐Lopez J, Williams J, Diethrich EB. Retrograde type A dissection after endovascular stenting of the descending thoracic aorta. Is the risk real?. Eur J Cardiothorac Surg. 2008;33:1014–1018. [DOI] [PubMed] [Google Scholar]
- 4. Crawford ES, Svensson LG, Coselli JS, Safi HJ, Hess KR. Surgical treatment of aneurysm and/or dissection of the ascending aorta, transverse aortic arch, and ascending aorta and transverse aortic arch. Factors influencing survival in 717 patients. J Thorac Cardiovasc Surg. 1989;98:659–673; discussion 673–654. [PubMed] [Google Scholar]
- 5. Ishimaru S. Endografting of the aortic arch. J Endovasc Ther. 2004;11(suppl 2):II62–II71. [DOI] [PubMed] [Google Scholar]
- 6. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ. 2003;327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Andersen ND, Williams JB, Hanna JM, Shah AA, McCann RL, Hughes GC. Results with an algorithmic approach to hybrid repair of the aortic arch. J Vasc Surg. 2013;57:655–667; discussion 666–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Appoo JJ, Herget EJ, Pozeg ZI, Ferris MC, Wong JK, Gregory AJ, Gupta AK, Merchant N, Kent WD. Midterm results of endovascular stent grafts in the proximal aortic arch (zone 0): an imaging perspective. Canadian J Cardiol. 2015;31:731–737. [DOI] [PubMed] [Google Scholar]
- 9. Bockler D, Schumacher H, Ganten M, von Tengg‐Kobligk H, Schwarzbach M, Fink C, Kauczor HU, Bardenheuer H, Allenberg JR. Complications after endovascular repair of acute symptomatic and chronic expanding stanford type B aortic dissections. J Thorac Cardiovasc Surg. 2006;132:361–368. [DOI] [PubMed] [Google Scholar]
- 10. Bunger CM, Kische S, Liebold A, Leissner M, Glass A, Schareck W, Ince H, Nienaber CA. Hybrid aortic arch repair for complicated type B aortic dissection. J Vasc Surg. 2013;58:1490–1496. [DOI] [PubMed] [Google Scholar]
- 11. Canaud L, Alric P, Gandet T, Albat B, Marty‐Ane C, Berthet JP. Surgical conversion after thoracic endovascular aortic repair. J Thorac Cardiovasc Surg. 2011;142:1027–1031. [DOI] [PubMed] [Google Scholar]
- 12. Chiesa R, Tshomba Y, Logaldo D, Civilini E, Bertoglio L, Melissano G. Hybrid repair of aortic aneurysms and dissections: the European perspective. Tex Heart Inst J. 2011;38:687–690. [PMC free article] [PubMed] [Google Scholar]
- 13. Cochennec F, Tresson P, Cross J, Desgranges P, Allaire E, Becquemin JP. Hybrid repair of aortic arch dissections. J Vasc Surg. 2013;57:1560–1567. [DOI] [PubMed] [Google Scholar]
- 14. Czermak BV, Waldenberger P, Perkmann R, Rieger M, Steingruber IE, Mallouhi A, Fraedrich G, Jaschke WR. Placement of endovascular stent‐grafts for emergency treatment of acute disease of the descending thoracic aorta. AJR Am J Roentgenol. 2002;179:337–345. [DOI] [PubMed] [Google Scholar]
- 15. Czerny M, Weigang E, Sodeck G, Schmidli J, Antona C, Gelpi G, Friess T, Klocker J, Szeto WY, Moeller P, Pochettino A, Bavaria JE. Targeting landing zone 0 by total arch rerouting and TEVAR: midterm results of a transcontinental registry. Ann Thorac Surg. 2012;94:84–89. [DOI] [PubMed] [Google Scholar]
- 16. De Rango P, Cao P, Ferrer C, Simonte G, Coscarella C, Cieri E, Pogany G, Verzini F. Aortic arch debranching and thoracic endovascular repair. J Vasc Surg. 2014;59:107–114. [DOI] [PubMed] [Google Scholar]
- 17. Dong Xu S, Zhong Li Z, Huang FJ, Yang JF, Wang XY, Zhang ZG, Du JH, Sun YQ. Treating aortic dissection and penetrating aortic ulcer with stent graft: thirty cases. Ann Thorac Surg. 2005;80:864–868. [DOI] [PubMed] [Google Scholar]
- 18. Dong Z, Fu W, Wang Y, Wang C, Yan Z, Guo D, Xu X, Chen B. Stent graft‐induced new entry after endovascular repair for stanford type B aortic dissection. J Vasc Surg. 2010;52:1450–1457. [DOI] [PubMed] [Google Scholar]
- 19. Duebener L, Hartmann F, Kurowski V, Richardt G, Geist V, Erasmi A, Sievers HH, Misfeld M. Surgical interventions after emergency endovascular stent‐grafting for acute type B aortic dissections. Interact Cardiovasc Thorac Surg. 2007;6:288–292. [DOI] [PubMed] [Google Scholar]
- 20. Fattori R, Napoli G, Lovato L, Grazia C, Piva T, Rocchi G, Angeli E, Di Bartolomeo R, Gavelli G. Descending thoracic aortic diseases: stent‐graft repair. Radiology. 2003;229:176–183. [DOI] [PubMed] [Google Scholar]
- 21. Faure EM, Canaud L, Agostini C, Shaub R, Boge G, Marty‐ane C, Alric P. Reintervention after thoracic endovascular aortic repair of complicated aortic dissection. J Vasc Surg. 2014;59:327–333. [DOI] [PubMed] [Google Scholar]
- 22. Flecher E, Cluzel P, Bonnet N, Aubert S, Gaubert A, Pavie A, Jault F, Leprince P. Endovascular treatment of descending aortic dissection (type B): short‐ and medium‐term results. Arch Cardiovasc Dis. 2008;101:94–99. [DOI] [PubMed] [Google Scholar]
- 23. Geisbusch P, Kotelis D, Muller‐Eschner M, Hyhlik‐Durr A, Bockler D. Complications after aortic arch hybrid repair. J Vasc Surg. 2011;53:935–941. [DOI] [PubMed] [Google Scholar]
- 24. Girdauskas E, Falk V, Kuntze T, Borger MA, Schmidt A, Scheinert D, Mohr FW. Secondary surgical procedures after endovascular stent grafting of the thoracic aorta: successful approaches to a challenging clinical problem. J Thorac Cardiovasc Surg. 2008;136:1289–1294. [DOI] [PubMed] [Google Scholar]
- 25. Gorlitzer M, Weiss G, Moidl R, Folkmann S, Waldenberger F, Czerny M, Grabenwoger M. Repair of stent graft‐induced retrograde type A aortic dissection using the E‐vita open prosthesis. Eur J Cardiothorac Surg. 2012;42:566–570. [DOI] [PubMed] [Google Scholar]
- 26. Grabenwoger M, Fleck T, Ehrlich M, Czerny M, Hutschala D, Schoder M, Lammer J, Wolner E. Secondary surgical interventions after endovascular stent‐grafting of the thoracic aorta. Eur J Cardiothorac Surg. 2004;26:608–613. [DOI] [PubMed] [Google Scholar]
- 27. Hanna JM, Andersen ND, Ganapathi AM, McCann RL, Hughes GC. Five‐year results for endovascular repair of acute complicated type B aortic dissection. J Vasc Surg. 2014;59:96–106. [DOI] [PubMed] [Google Scholar]
- 28. Hansen CJ, Bui H, Donayre CE, Aziz I, Kim B, Kopchok G, Walot I, Lee J, Lippmann M, White RA. Complications of endovascular repair of high‐risk and emergent descending thoracic aortic aneurysms and dissections. J Vasc Surg. 2004;40:228–234. [DOI] [PubMed] [Google Scholar]
- 29. Huang WH, Luo SY, Luo JF, Liu Y, Fan RX, Xue L, Yang F, Kang HY, Gu MN, Liu Z, Xie NJ, Dong HJ, Ni ZH, Huang MP, Chen JY. Perioperative aortic dissection rupture after endovascular stent graft placement for treatment of type B dissection. Chin Med J. 2013;126:1636–1641. [PubMed] [Google Scholar]
- 30. Idrees J, Arafat A, Johnston DR, Svensson LG, Roselli EE. Repair of retrograde ascending dissection after descending stent grafting. J Thorac Cardiovasc Surg. 2014;147:151–154. [DOI] [PubMed] [Google Scholar]
- 31. Jia X, Guo W, Li TX, Guan S, Yang RM, Liu XP, Zhang MH, Xiong J. The results of stent graft versus medication therapy for chronic type B dissection. J Vasc Surg. 2013;57:406–414. [DOI] [PubMed] [Google Scholar]
- 32. Kato N, Shimono T, Hirano T, Suzuki T, Ishida M, Sakuma H, Yada I, Takeda K. Midterm results of stent‐graft repair of acute and chronic aortic dissection with descending tear: the complication‐specific approach. J Thorac Cardiovasc Surg. 2002;124:306–312. [DOI] [PubMed] [Google Scholar]
- 33. Kim KM, Donayre CE, Reynolds TS, Kopchok GE, Walot I, Chauvapun JP, White RA. Aortic remodeling, volumetric analysis, and clinical outcomes of endoluminal exclusion of acute complicated type B thoracic aortic dissections. J Vasc Surg. 2011;54:316–324; discussion 324–315. [DOI] [PubMed] [Google Scholar]
- 34. Langer S, Mommertz G, Koeppel TA, Schurink GW, Autschbach R, Jacobs MJ. Surgical correction of failed thoracic endovascular aortic repair. J Vasc Surg. 2008;47:1195–1202. [DOI] [PubMed] [Google Scholar]
- 35. Lee KH, Won JY, Lee DY, Choi D, Shim WH, Chang BC. Elective stent‐graft treatment of aortic dissections. J Endovasc Ther. 2004;11:667–675. [DOI] [PubMed] [Google Scholar]
- 36. Li B, Pan XD, Ma WG, Zheng J, Liu YL, Zhu JM, Liu YM, Sun LZ. Stented elephant trunk technique for retrograde type A aortic dissection after endovascular stent graft repair. Ann Thorac Surg. 2014;97:596–602. [DOI] [PubMed] [Google Scholar]
- 37. Lombardi JV, Cambria RP, Nienaber CA, Chiesa R, Teebken O, Lee A, Mossop P, Bharadwaj P. Prospective multicenter clinical trial (stable) on the endovascular treatment of complicated type B aortic dissection using a composite device design. J Vasc Surg. 2012;55:629–640.e622. [DOI] [PubMed] [Google Scholar]
- 38. Lotfi S, Clough RE, Ali T, Salter R, Young CP, Bell R, Modarai B, Taylor P. Hybrid repair of complex thoracic aortic arch pathology: long‐term outcomes of extra‐anatomic bypass grafting of the supra‐aortic trunk. Cardiovasc Intervent Radiol. 2013;36:46–55. [DOI] [PubMed] [Google Scholar]
- 39. Lu S, Lai H, Wang C, Sun X, Hong T, Song K, Yuan Z, Liu X. Surgical treatment for retrograde type a aortic dissection after endovascular stent graft placement for type B dissection. Interact Cardiovasc Thorac Surg. 2012;14:538–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Oberhuber A, Winkle P, Schelzig H, Orend KH, Muehling BM. Technical and clinical success after endovascular therapy for chronic type B aortic dissections. J Vasc Surg. 2011;54:1303–1309. [DOI] [PubMed] [Google Scholar]
- 41. Pamler RS, Kotsis T, Gorich J, Kapfer X, Orend KH, Sunder‐Plassmann L. Complications after endovascular repair of type B aortic dissection. J Endovasc Ther. 2002;9:822–828. [DOI] [PubMed] [Google Scholar]
- 42. Parsa CJ, Williams JB, Bhattacharya SD, Wolfe WG, Daneshmand MA, McCann RL, Hughes GC. Midterm results with thoracic endovascular aortic repair for chronic type B aortic dissection with associated aneurysm. J Thorac Cardiovasc Surg. 2011;141:322–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Patterson B, Holt P, Nienaber C, Cambria R, Fairman R, Thompson M. Aortic pathology determines midterm outcome after endovascular repair of the thoracic aorta: report from the Medtronic Thoracic Endovascular Registry (MOTHER) Database. Circulation. 2013;127:24–32. [DOI] [PubMed] [Google Scholar]
- 44. Qing KX, Yiu WK, Cheng SW. A morphologic study of chronic type B aortic dissections and aneurysms after thoracic endovascular stent grafting. J Vasc Surg. 2012;55:1268–1275; discussion 1275–1266. [DOI] [PubMed] [Google Scholar]
- 45. Roselli EE, Abdel‐Halim M, Johnston DR, Soltesz EG, Greenberg RK, Svensson LG, Sabik JF III. Open aortic repair after prior thoracic endovascular aortic repair. Ann Thorac Surg. 2014;97:750–756. [DOI] [PubMed] [Google Scholar]
- 46. Shimono T, Kato N, Yasuda F, Suzuki T, Yuasa U, Onoda K, Hirano T, Takeda K, Yada I. Transluminal stent‐graft placements for the treatments of acute onset and chronic aortic dissections. Circulation. 2002;106:I241–I247. [PubMed] [Google Scholar]
- 47. Wiedemann D, Ehrlich M, Amabile P, Lovato L, Rousseau H, Evangelista‐Masip A, Moeller P, Bavaria J. Emergency endovascular stent grafting in acute complicated type B dissection. J Vasc Surg. 2014;60:1204–1208. [DOI] [PubMed] [Google Scholar]
- 48. Wiedemann D, Mahr S, Vadehra A, Schoder M, Funovics M, Lowe C, Plank C, Lammer J, Laufer G, Stelzmuller ME, Kocher A, Ehrlich MP. Thoracic endovascular aortic repair in 300 patients: long‐term results. Ann Thorac Surg. 2013;95:1577–1583. [DOI] [PubMed] [Google Scholar]
- 49. Williams JB, Andersen ND, Bhattacharya SD, Scheer E, Piccini JP, McCann RL, Hughes GC. Retrograde ascending aortic dissection as an early complication of thoracic endovascular aortic repair. J Vasc Surg. 2012;55:1255–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Xu SD, Huang FJ, Yang JF, Li ZZ, Wang XY, Zhang ZG, Du JH. Endovascular repair of acute type B aortic dissection: early and mid‐term results. J Vasc Surg. 2006;43:1090–1095. [DOI] [PubMed] [Google Scholar]
- 51. Yang CP, Hsu CP, Chen WY, Chen IM, Weng CF, Chen CK, Shih CC. Aortic remodeling after endovascular repair with stainless steel‐based stent graft in acute and chronic type B aortic dissection. J Vasc Surg. 2012;55:1600–1610. [DOI] [PubMed] [Google Scholar]
- 52. Zhang L, Zhou J, Lu Q, Zhao Z, Bao J, Jing Z. Potential risk factors of re‐intervention after endovascular repair for type B aortic dissections. Catheter Cardiovasc Interv. 2015;86:E1–E10. [DOI] [PubMed] [Google Scholar]
- 53. Zipfel B, Hammerschmidt R, Krabatsch T, Buz S, Weng Y, Hetzer R. Stent‐grafting of the thoracic aorta by the cardiothoracic surgeon. Ann Thorac Surg. 2007;83:441–448; discussion 448–449. [DOI] [PubMed] [Google Scholar]
- 54. Svensson LG, Kouchoukos NT, Miller DC, Bavaria JE, Coselli JS, Curi MA, Eggebrecht H, Elefteriades JA, Erbel R, Gleason TG, Lytle BW, Mitchell RS, Nienaber CA, Roselli EE, Safi HJ, Shemin RJ, Sicard GA, Sundt TM III, Szeto WY, Wheatley GH III. Expert consensus document on the treatment of descending thoracic aortic disease using endovascular stent‐grafts. Ann Thorac Surg. 2008;85:S1–S41. [DOI] [PubMed] [Google Scholar]
- 55. Canaud L, Ozdemir BA, Patterson BO, Holt PJ, Loftus IM, Thompson MM. Retrograde aortic dissection after thoracic endovascular aortic repair. Ann Surg. 2014;260:389–395. [DOI] [PubMed] [Google Scholar]
- 56. Eggebrecht H, Thompson M, Rousseau H, Czerny M, Lonn L, Mehta RH, Erbel R. Retrograde ascending aortic dissection during or after thoracic aortic stent graft placement: insight from the European registry on endovascular aortic repair complications. Circulation. 2009;120:S276–S281. [DOI] [PubMed] [Google Scholar]
- 57. Dong ZH, Fu WG, Wang YQ, da Guo Q, Xu X, Ji Y, Chen B, Jiang JH, Yang J, Shi ZY, Zhu T, Shi Y. Retrograde type A aortic dissection after endovascular stent graft placement for treatment of type B dissection. Circulation. 2009;119:735–741. [DOI] [PubMed] [Google Scholar]
- 58. Neuhauser B, Czermak BV, Fish J, Perkmann R, Jaschke W, Chemelli A, Fraedrich G. Type A dissection following endovascular thoracic aortic stent‐graft repair. J Endovasc Ther. 2005;12:74–81. [DOI] [PubMed] [Google Scholar]
- 59. Di Cesare E, Costanzi A, Fedele F, Di Renzi P, D'Eusanio G, Lupattelli L, Passariello R. MRI postoperative monitoring in patients surgically treated for aortic dissection. Magn Reson Imaging. 1996;14:1149–1156. [DOI] [PubMed] [Google Scholar]
- 60. Hata M, Shiono M, Inoue T, Sezai A, Niino T, Negishi N, Sezai Y. Optimal treatment of type B acute aortic dissection: long‐term medical follow‐up results. Ann Thorac Surg. 2003;75:1781–1784. [DOI] [PubMed] [Google Scholar]
- 61. Rubin S, Bayle A, Poncet A, Baehrel B. Retrograde aortic dissection after a stent graft repair of a type B dissection: how to improve the endovascular technique. Interact Cardiovasc Thorac Surg. 2006;5:746–748. [DOI] [PubMed] [Google Scholar]


