Abstract
Background
Effects of extreme sleep duration on risk of mortality and cardiovascular outcomes remain controversial. We aimed to quantify the dose‐response relationships of sleep duration with risk of all‐cause mortality, total cardiovascular disease, coronary heart disease, and stroke.
Methods and Results
PubMed and Embase were systematically searched for prospective cohort studies published before December 1, 2016, that examined the associations between sleep duration and at least 1 of the 4 outcomes in generally healthy populations. U‐shaped associations were indicated between sleep duration and risk of all outcomes, with the lowest risk observed for ≈7‐hour sleep duration per day, which was varied little by sex. For all‐cause mortality, when sleep duration was <7 hours per day, the pooled relative risk (RR) was 1.06 (95% CI, 1.04–1.07) per 1‐hour reduction; when sleep duration was >7 hours per day, the pooled RR was 1.13 (95% CI, 1.11–1.15) per 1‐hour increment. For total cardiovascular disease, the pooled RR was 1.06 (95% CI, 1.03–1.08) per 1‐hour reduction and 1.12 (95% CI, 1.08–1.16) per 1‐hour increment of sleep duration. For coronary heart disease, the pooled RR was 1.07 (95% CI, 1.03–1.12) per 1‐hour reduction and 1.05 (95% CI, 1.00–1.10) per 1‐hour increment of sleep duration. For stroke, the pooled RR was 1.05 (95% CI, 1.01–1.09) per 1‐hour reduction and 1.18 (95% CI, 1.14–1.21) per 1‐hour increment of sleep duration.
Conclusions
Our findings indicate that both short and long sleep duration is associated with an increased risk of all‐cause mortality and cardiovascular events.
Keywords: all‐cause death, cardiovascular disease, coronary heart disease, meta‐analysis, sleep, stroke
Subject Categories: Cardiovascular Disease, Lifestyle, Primary Prevention
Clinical Perspective
What Is New?
Uncertainty exists regarding the dose‐response relationship between sleep duration and the risk of all‐cause mortality and cardiovascular events.
In our systematic review and meta‐analysis, sleep duration that was either too short or too long was associated with higher risk of all‐cause mortality and cardiovascular events, with the lowest risk at sleep duration of ≈7 hours per day.
What Are the Clinical Implications?
The U‐shaped associations between sleep duration and adverse outcomes have clinical relevance with respect to recommendations for adequate sleep duration in routine clinical care as well as explicit suggestions for primary prevention in public health settings.
Introduction
According to the report of World Congress of Cardiology and Cardiovascular Health in 2016, cardiovascular diseases (CVDs) are the leading cause of death globally, with an estimate of >17 million deaths from total CVD. Of these deaths, >7 million were due to coronary heart disease (CHD) and >6 million were due to stroke. In <10 years, the premature deaths from CVDs could rise by a third.1 To reduce the risk of premature death from noncommunicable diseases by 25% by 2025, as a global target of the World Health Organization,2 it is imperative to identify modifiable lifestyle factors associated with lower occurrence of CVDs. Sleep is a complex set of brain processes that supports several physiological needs.3 Increased attention has been paid to understanding the extent of sleep duration problems at the population level and their associated negative effects on various health outcomes, such as metabolic syndrome, diabetes mellitus, and cancer.4, 5, 6 Previous publications suggest that the prevalence of short sleep duration (defined as <7 hours) may have gradually increased over past decades, whereas the prevalence of long sleep duration (defined as ≥9 hours) shows an opposite trend.7
In recent years, increasing evidence has suggested that extreme sleep duration is associated with the risk of mortality and cardiovascular outcomes; however, the results are not entirely consistent. Although several studies found that sleep duration that was either too short or too long was associated with increased risk of all‐cause mortality and cardiovascular events,8, 9, 10, 11, 12, 13 reverse associations were observed in other populations.14, 15 In addition, uncertainty exists about the dose‐response relationship between sleep duration and risk of the adverse outcomes because different quantitative categories of sleep duration were used in previous studies.8, 16, 17, 18 Two meta‐analyses reported the association between sleep duration and all‐cause mortality with dose‐response analysis, but the results were inconsistent.19, 20 A previous meta‐analysis published before 2011 reported the association between sleep duration and cardiovascular events21; however, without a dose‐response analysis, it remains unknown how many hours of habitual sleep are associated with the lowest risk of cardiovascular events. Since 2011, many more studies have been published and the number of prospective studies has nearly tripled, which allows quantitative analysis of the associations. Consequently, we conducted a comprehensive dose‐response meta‐analysis of prospective studies in generally healthy populations to determine the overall shape of the relationships and quantitative estimates between sleep duration and risk of all‐cause mortality, total CVD, CHD, and stroke.
Methods
Search Strategy
This study was conducted in accordance with the MOOSE (Meta‐Analysis of Observational Studies in Epidemiology) guidelines.22 We performed a literature search (up to December 1, 2016) of PubMed and Embase for prospective studies examining the association between sleep duration and risk of all‐cause mortality and selected cardiovascular outcomes (Data S1). In addition, we reviewed references from relevant original articles and review articles to identify further pertinent studies. Only articles published in the English language were considered.
Study Selection
Studies were included if they satisfied the following criteria: The study design was a prospective cohort study; the exposure of interest was sleep duration; the outcome was all‐cause mortality, CVD, CHD, or stroke; and the investigators reported relative risk (RR), hazard ratio, or odds ratio (OR) with 95% confidence intervals (CIs) for at least 3 quantitative categories of sleep duration. Given that primary prevention of CVD was the main focus of this work (rather than secondary prevention), we excluded studies if participants were not recruited from a generally healthy population (eg, those with diabetes mellitus or under regular dialysis therapy). In addition, we excluded reviews, editorials, nonhuman studies, and letters without sufficient data. Multiple reports from the same cohort study were reviewed, and only articles with the longest follow‐up for identical outcomes were included. If insufficient data were presented in the longer follow‐up study, we included the shorter follow‐up data. Study selection was conducted in 2 stages: an initial screening of titles and abstracts to identify potentially relevant articles, followed by screening of the full‐length articles. Two investigators (J.W.Y. and S.Z.L.) independently screened all studies by title or abstract and then by a full‐text evaluation. Any discrepancy between the 2 authors was solved by discussion with the senior investigator (X.L.J.).
Data Extraction and Quality Assessment
The extraction of data included authors, year of publication, study name, study location, years of follow‐up, sample size (number of participants and incident cases), participant characteristics (age and sex), measurement method of sleep duration (questionnaire and interview), types of sleep duration (24‐hour sleep, nighttime sleep), covariates adjusted in the multivariable analysis, and effect size (RR, hazard ratio, OR), with 95% CIs for all categories of sleep duration. When studies had several adjustment models, we extracted those that reflected the maximum extent of adjustment for potentially confounding variables.
Quality assessment was performed according to the Newcastle–Ottawa Quality Assessment Scale (NOS).23 Scores ranged from 0 to 9 points, with higher scores indicating higher study quality. We considered NOS scores of 0 to 3, 4 to 6, and 7 to 9 as low, medium, and high quality, respectively.
To evaluate potential dose‐response relationships, we further extracted numbers of cases, numbers of participants, and median sleep duration in each category. If the numbers of participants and cases were not provided, the corresponding authors were contacted for the data.
Data Synthesis and Analysis
In this meta‐analysis, the RR was used as the common measure of association across studies, and the hazard ratio was deemed equivalent to RR.24 If necessary, the OR was transformed into RR according to this formula: RR=OR/[(1−P0)+(P0×OR)], where P0 is the incidence of the outcome of interest in the nonexposed group.25 Any results stratified by sex were treated as 2 separate reports. Those articles reporting >1 outcome (eg, all‐cause mortality and total CVD) were also treated as separate reports and included in corresponding analyses. If the number of cases in each category was not available in 1 study and the authors did not give their reply, we used the method by Bekkering et al to provide approximate data.26
We recognized that sleeping 7 to 8 hours per night was treated as the reference category in the majority of studies. When the reference category was not 7 to 8 hours, we used the method proposed by Hamling and colleagues to convert risk estimates.27 We calculated pooled RRs and 95% CIs for the extreme categories of sleep duration versus the reference category of sleep duration. In addition, the reports with at least 3 quantitative categories of short or long sleep duration were included in dose‐response analyses. Potential nonlinear dose‐response relationships between sleep duration and all‐cause mortality and cardiovascular events were examined by using restricted cubic splines model with 4 knots at percentiles 5%, 35%, 65%, and 95% of the distribution.28, 29 We assigned the median or mean sleep duration in each category to the corresponding RR for each study. If the mean or median duration per category was not reported, the midpoint of the upper and lower boundaries in each category was assigned. When the shortest or the longest category was open‐ended, we assumed that the open‐ended interval length had the same length as the adjacent interval. The dose‐response curves are shown in the nonlinear figures. The RR estimates in the tables were based on the nonlinear figures but show RRs for selected sleep‐duration values. If a nonlinear shape association was observed, we treated the slope as 2 piecewise and conducted dose‐response analyses using the method by Greenland and Longnecker to calculate pooled RR and 95% CIs for 1‐hour increment or decrement compared with the reference category in sleep duration.30 We used a P value for curve linearity or nonlinearity to assess the difference between the linear and nonlinear models to test for nonlinearity.29 All pooled outcome measures were determined using random‐effects models, described by DerSimonian and Laird,31 to provide more conservative results than fixed‐effects models.
The heterogeneity among studies was estimated by the Cochran Q test (P≤0.1 to be indicative of statistically significant heterogeneity) and I2 statistic.32 We conducted subgroup and metaregression analyses stratified by sex, study location, number of participants, number of cases, duration of follow‐up, sleep assessment, sleep duration type, study quality, incidence or mortality (only in total CVD, stroke and CHD), and adjustment for confounders to investigate potential sources of heterogeneity between subgroups. Moreover, stratified analyses were performed to evaluate the influences of selected study and participant characteristics on the results. Publication bias was assessed by inspection of the funnel plots for asymmetry with the Egger test33 and Begg test.34 The Duval and Tweedie35 nonparametric trim‐and‐fill method was used to further assess the possible effect of publication bias. Additional sensitivity analyses were performed by omitting 1 study at each time to test the robustness of the results and the influence of an individual study on heterogeneity.36 All statistical analyses were performed with Stata version 12 (StataCorp LP), and all tests were 2‐sided with a significance level of 0.05 unless otherwise noted.
Results
Literature Search
Figure 1 shows the results of literature research and selection. We identified 836 articles from PubMed and 837 articles from Embase before December 1, 2016. After exclusion of duplicates and studies that did not fulfill the inclusion criteria, 101 remaining articles seemed to be relevant for this meta‐analysis. After evaluating the full texts of these 101 publications and counting 1 study obtained by hand searching, the final meta‐analysis included 67 articles with 141 independent reports. Among these 67 articles, 43 articles with 57 reports provided statistical effects relevant to the meta‐analyses on all‐cause mortality, 26 articles with 37 reports on total CVD, 22 articles with 27 reports on CHD, and 16 articles with 20 reports on stroke (Data S1).
Figure 1.
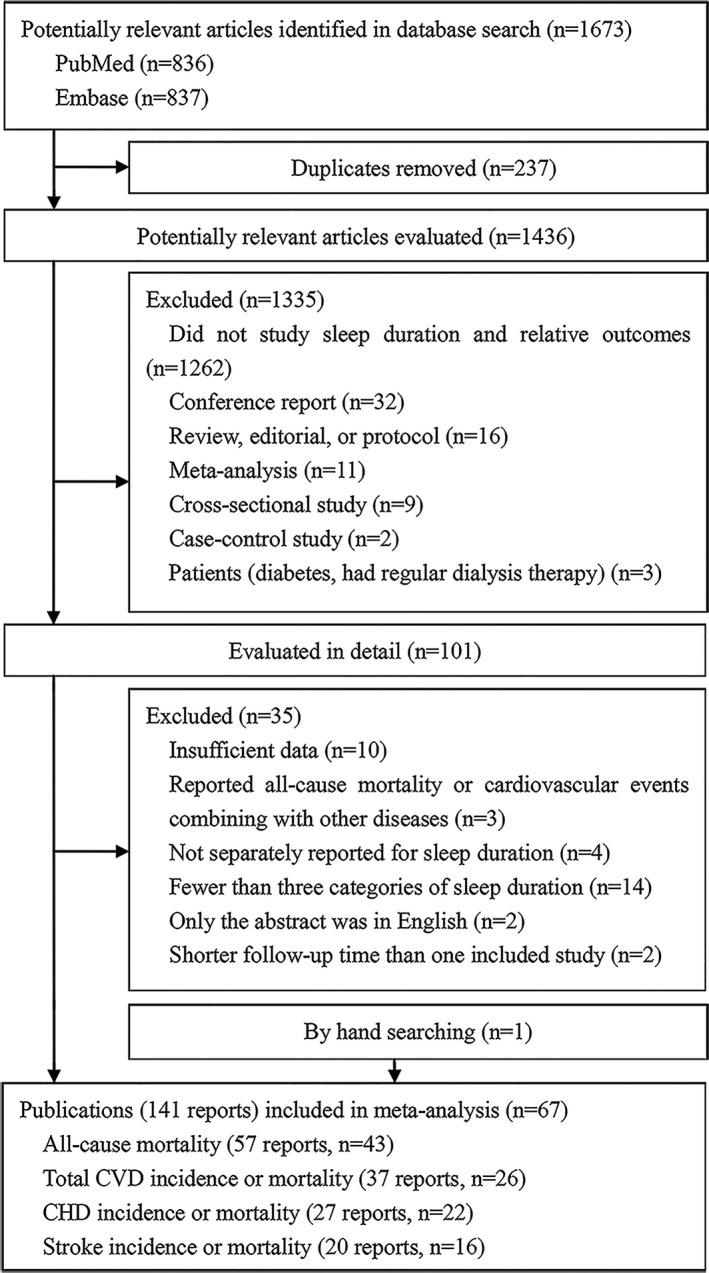
Flowchart of article selection. CHD indicates coronary heart disease; CVD, cardiovascular disease.
Study Characteristics
A summary of the study characteristics is shown in Tables S1 through S4. The sample sizes ranged from 724 to 1 116 936, with a total of 3 582 016 participants, including 241 107 cases of all‐cause mortality, 58 919 cases of total CVD, 22 511 cases of CHD, and 15 476 cases of stroke. The follow‐up periods ranged from 2.3 to 34 years. Among these 67 articles, most were conducted in Europe (n=22), the United States (n=16), and Asia (n=27); the others were done in Australia (n=2). Sleep duration was measured by self‐report questionnaires in 48 studies and by interview in 19 studies. The majority of the included studies had high quality, as indicated by the NOS score, and the mean study quality scores were 6.9 for all‐cause mortality, 7.0 for CVD, 7.0 for CHD, and 7.1 for stroke out of a maximum of 9 points (Tables S5 through S8).
Sleep Duration and Risk of All‐Cause Mortality
In total, 57 reports were included in the analysis of all‐cause mortality and extreme sleep duration. The pooled RR of the shortest and longest sleep duration versus reference sleep duration was 1.13 (95% CI, 1.09–1.17), with low to moderate heterogeneity (I2=37.5%, P<0.01), and 1.35 (95% CI, 1.29–1.41), with high heterogeneity (I2=76.2%, P<0.01), respectively (Table 1, Figure S1).
Table 1.
Associations of Sleep Duration With All‐Cause Mortality, Total CVD, CHD, and Stroke
| n | Shortest vs Reference | Longest vs Reference | |||||
|---|---|---|---|---|---|---|---|
| RRa (95% CI) | I2 | P Valueb | RRa (95% CI) | I2 | P Valueb | ||
| All‐cause mortality | 57 | 1.13 (1.10–1.17) | 37.5 | <0.01 | 1.35 (1.29–1.41) | 76.2 | <0.01 |
| Total CVD | 37 | 1.14 (1.09–1.20) | 31.1 | 0.04 | 1.36 (1.26–1.48) | 71.2 | <0.01 |
| CHD | 27 | 1.22 (1.13–1.31) | 39.6 | 0.02 | 1.21 (1.12–1.30) | 37.4 | 0.03 |
| Stroke | 20 | 1.09 (0.99–1.19) | 40.6 | 0.03 | 1.45 (1.30–1.62) | 63.5 | <0.01 |
CHD indicates coronary heart disease; CI, confidence interval; CVD, cardiovascular disease; RR, relative risk.
RR favors the analyses of shortest and longest vs reference sleep duration.
P for heterogeneity.
Reports with at least 3 quantitative categories of short or long sleep duration were included in dose‐response analysis. When using a restricted cubic splines model, we observed a U‐shape curvilinear association with the lowest risk of all‐cause mortality at a sleep duration of about 7 hours per day (P<0.01 for nonlinearity; Figure 2A). Both short and long sleep duration was associated with an increased risk of all‐cause mortality. Table 2 shows the RR estimates for selected sleep duration values, which were derived from the nonlinear figures. In the linear trend analyses for short sleep, no evidence of nonlinear association between short sleep duration and all‐cause mortality was found (P=0.12), and the pooled RR for all‐cause mortality was 1.06 (95% CI, 1.04–1.07) per 1‐hour reduction of sleep duration, with moderate to high heterogeneity (I2=55.5%, P<0.01; Figure 3A).8, 9, 10, 11, 12, 13, 18, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53 The heterogeneity was reduced when we excluded 2 reports9, 38 (I2=13.0%, P=0.26), but the association was not substantially altered (pooled RR: 1.06; 95% CI, 1.05–1.07). For long sleep, nonlinear association between long sleep duration and all‐cause mortality was found (P=0.02), and the pooled RR for all‐cause mortality was 1.13 (95% CI, 1.11–1.15) per 1‐hour increment of sleep duration, with high heterogeneity (I2=76.5%, P<0.01) (Figure 3B).1 The heterogeneity seemed to be mainly generated by 8 reports,8, 13, 40, 42, 44, 45, 53, 56 and when these were all excluded, the association still remained similar (RR: 1.12; 95% CI, 1.10–1.13) with low heterogeneity (I2=21.7%, P=0.15).
Figure 2.
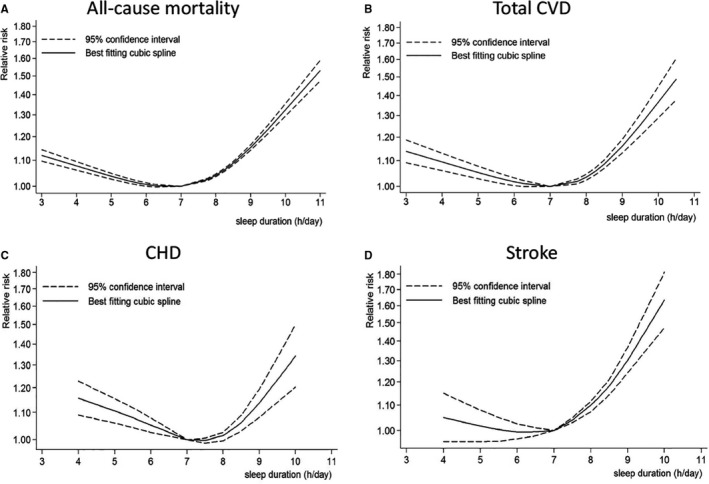
Nonlinear dose‐response analyses of sleep duration and risk of all‐cause mortality (A), total CVD (B), CHD (C), and stroke (D). CHD indicates coronary heart disease; CVD, cardiovascular disease.
Table 2.
Association Between Sleep Duration and All‐Cause Mortality, Total CVD, CHD and Stroke From Non‐Linear Dose‐Response Analysis
| Sleep Duration | All‐Cause Mortality (n=40a) | Total CVD (n=26a) | CHD (n=20a) | Stroke (n=17a) |
|---|---|---|---|---|
| 3 h | 1.12 (1.10–1.14) | 1.14 (1.09–1.19) | ··· | ··· |
| 4 h | 1.08 (1.06–1.09) | 1.09 (1.06–1.13) | 1.16 (1.09–1.23) | 1.05 (0.96–1.15) |
| 5 h | 1.04 (1.03–1.05) | 1.05 (1.03–1.08) | 1.11 (1.06–1.16) | 1.02 (0.96–1.08) |
| 6 h | 1.01 (1.00–1.01) | 1.02 (1.00–1.03) | 1.05 (1.03–1.08) | 0.99 (0.96–1.03) |
| 7 h | 1.00 | 1.00 | 1.00 | 1.00 |
| 8 h | 1.04 (1.04–1.05) | 1.03 (1.02–1.05) | 1.01 (0.99–1.03) | 1.08 (1.06–1.11) |
| 9 h | 1.15 (1.14–1.16) | 1.16 (1.13–1.19) | 1.14 (1.08–1.20) | 1.30 (1.24–1.37) |
| 10 h | 1.32 (1.29–1.35) | 1.37 (1.29–1.45) | 1.34 (1.20–1.50) | 1.64 (1.47–1.82) |
| 11 h | 1.53 (1.47–1.59) | ··· | ··· | ··· |
CHD indicates coronary heart disease; CVD, cardiovascular disease.
n denotes number of risk estimates.
Figure 3.
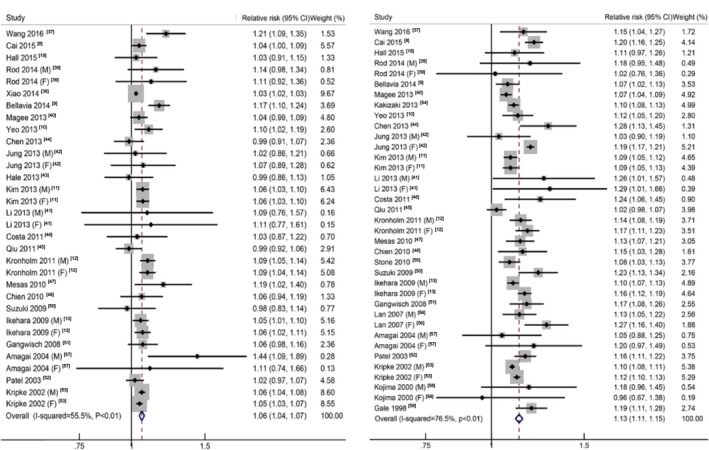
The forest plots between sleep duration (per hour) and risk of all‐cause mortality for short sleep (A) and long sleep (B). CI indicates confidence interval.
Sleep Duration and Risk of Total CVD
Overall, 37 reports were included in the analysis of total CVD and extreme sleep duration. A U‐shaped association was observed with the lowest risk of total CVD at a sleep duration of ≈7 hours per day (P<0.01 for nonlinearity; Figure 2B, Table 2). Both short and long sleep duration was associated with an increased risk of total CVD.
For short sleep, the pooled RR of the shortest sleep duration versus the reference sleep duration was 1.14 (95% CI, 1.09–1.20), with low to moderate heterogeneity (I2=31.1%, P=0.04; Table 1, Figure S2). We found no evidence of nonlinear association between short sleep duration and total CVD (P=0.19), and the pooled RR was 1.06 (95% CI, 1.03–1.08) per 1‐hour reduction of sleep duration, with moderate heterogeneity (I2=52.0%, P<0.01; Figure 4A).2 The heterogeneity was reduced when we excluded 1 report9 (I2=24.8%, P=0.63), and the association remained similar (pooled RR: 1.04; 95% CI, 1.02–1.06).
Figure 4.
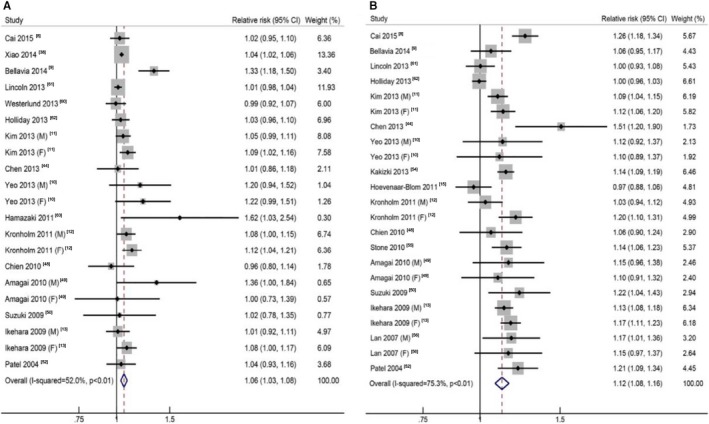
The forest plots between sleep duration (per hour) and risk of total cardiovascular disease for short sleep (A) and long sleep (B). CI indicates confidence interval.
For long sleep, the pooled RR of the longest sleep duration versus the reference sleep duration was 1.36 (95% CI, 1.26–1.48), with high heterogeneity (I2=71.2%, P<0.01; Table 1, Figure S2). A nonlinear association between long sleep duration and total CVD was found (P=0.02), and the pooled RR was 1.12 (95% CI, 1.08–1.16) per 1‐hour increment of sleep duration, with high heterogeneity (I2=75.3%, P<0.01; Figure 4B).3 The heterogeneity seemed to be generated mainly by 4 reports, and when those were all excluded, the association not substantially altered (RR: 1.13; 95% CI, 1.11–1.16) with low heterogeneity (I2=14.6%, P=0.28).
Sleep Duration and Risk of CHD
In total, 27 reports were included in the analysis of CHD and extreme sleep duration. A U‐shaped association was observed, with the lowest risk of CHD at a sleep duration of ≈7 hours per day (P<0.01 for nonlinearity; Figure 2C, Table 2). Both short and long sleep duration was associated with an increased risk of CHD.
For short sleep, the pooled RR of the shortest sleep duration versus the reference sleep duration was 1.22 (95% CI, 1.13–1.31), with low to moderate heterogeneity (I2=39.6%, P=0.02; Table 1, Figure S3). In the linear trend analyses for short sleep, a nonlinear association was noted between short sleep duration and CHD (P=0.02), and the pooled RR was 1.07 (95% CI, 1.03–1.12) per 1‐hour reduction of sleep duration, with moderate to high heterogeneity (I2=59.3%, P<0.01) (Figure 5A).4 The heterogeneity was reduced when we excluded 2 reports13, 66 (I2=23.2%, P=0.19), and the association remained similar (pooled RR: 1.04; 95% CI, 1.01–1.08).
Figure 5.
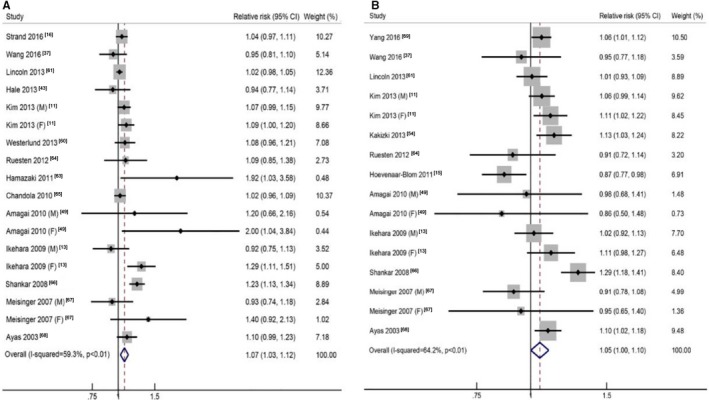
The forest plots between sleep duration (per hour) and risk of coronary heart disease for short sleep (A) and long sleep (B). CI indicates confidence interval.
For long sleep, the pooled RR of the longest sleep duration versus the reference sleep duration was 1.21 (95% CI, 1.12–1.30), with low to moderate heterogeneity (I2=37.4%, P=0.03; Table 1, Figure S3). A nonlinear association was noted between long sleep duration and CHD (P<0.01), and the pooled RR was 1.05 (95% CI, 1.00–1.10) per 1‐hour increment of sleep duration, with moderate to high heterogeneity (I2=64.2%, P<0.01; Figure 5B).5 The heterogeneity was reduced when we excluded 2 reports15, 66 (I2=4.0%, P=0.41), and the association remained similar (pooled RR: 1.06; 95% CI, 1.03–1.09).
Sleep Duration and Risk of Stroke
Twenty reports were included in the analysis of stroke and extreme sleep duration. An approximate U‐shape curvilinear association was observed, with the lowest risk of stroke at a sleep duration of ≈6 to 7 hours per day (P<0.01 for nonlinearity; Figure 2D, Table 2). Both short and long sleep duration was associated with an increased risk of stroke.
For short sleep, the pooled RR of the shortest sleep duration versus the reference sleep duration was 1.09 (95% CI, 0.99–1.19), with low to moderate heterogeneity (I2=40.6%, P=0.03; Table 1, Figure S4). In the linear trend analyses for short sleep, we found no evidence of nonlinear association between short sleep duration and stroke (P=0.23), and the pooled RR for stroke was 1.05 (95% CI, 1.01–1.09) per 1‐hour reduction of sleep duration, with no significant heterogeneity (I2=0.0%, P=0.55) (Figure 6A).6
Figure 6.
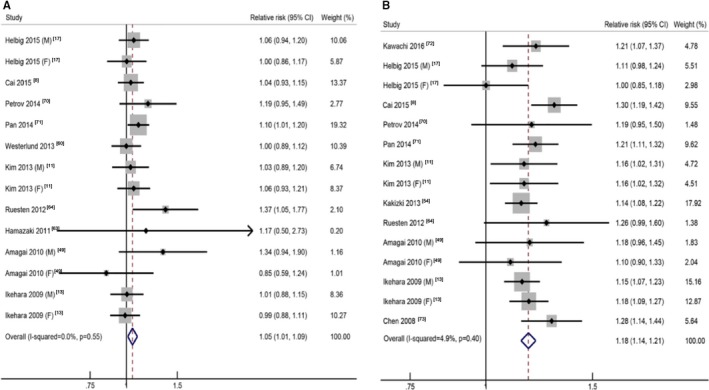
The forest plots between sleep duration (per hour) and risk of stroke for short sleep (A) and long sleep (B). CI indicates confidence interval.
For long sleep, the pooled RR of the longest sleep duration versus the reference sleep duration was 1.45 (95% CI, 1.30–1.62), with moderate to high heterogeneity (I2=63.5%, P<0.01; Table 1, Figure S4). No evidence of nonlinear dose‐response relationship was detected (P=0.13), and the pooled RR for stroke was 1.18 (95% CI, 1.14–1.21) per 1‐hour increment of sleep duration, with low heterogeneity (I2=4.9%, P=0.40; Figure 6B).7
Publication Bias
For the shortest or longest sleep duration versus the reference sleep duration, the publication bias was found between longest sleep duration and total CVD. The Begg rank correlation test indicated no publication bias (P=0.41), but the Egger linear regression test indicated possible publication bias for the association (P=0.01). We used the trim‐and‐fill method to recalculate our pooled risk estimate, and 13 missing studies were imputed to produce a symmetrical funnel plot (Figure S5). The analysis suggested that the imputed risk estimate was 1.22 (95% CI, 1.12–1.32), which is slightly decreased in risk but still identical to our original risk estimate. No significant publication bias was observed for other outcomes.
For the dose‐response analysis, we analyzed the publication bias of short sleep duration and all‐cause mortality and found that the Begg rank correlation test indicated no publication bias (P=0.59), but the Egger linear regression test indicated possible publication bias for the association (P=0.01). The trim‐and‐fill method was used to recalculate our pooled risk estimate, and 10 missing studies were imputed to produce a symmetrical funnel plot (Figure S6). The analysis suggested that the imputed risk estimate was 1.04 (95% CI, 1.03–1.06), which is identical to our original risk estimate. No significant publication bias was observed for other outcomes.
Subgroup, Metaregression, and Sensitivity Analyses
Tables S9 through S12 shows the different subgroup analyses of studies on all‐cause mortality, total CVD, CHD, and stroke. To explore potential sources of heterogeneity between subgroups, we carried out metaregression analyses of prespecified moderator variables. In the analyses of all‐cause mortality, the association between sleep duration and risk were not substantially changed in most subgroups. There was indication of heterogeneity (P=0.01) when we stratified studies by sleep duration type, and the pooled RRs for 1‐hour increment in long sleep duration were 1.16 (95% CI, 1.13–1.18; n=24) and 1.11 (95% CI, 1.10–1.13; n=13) for nighttime and 24‐hour sleep duration, respectively. In the nonlinear dose‐response analysis, slight variations in the risk estimates from the nonlinear dose‐response analyses were observed (Figure S7).
In the analyses of total CVD, the associations between sleep duration and risk were not substantially changed in most subgroups. Heterogeneity was indicated (P<0.01) when we stratified studies by incidence or mortality, and the pooled RRs for 1‐hour increment in long sleep duration were 1.00 (95% CI, 0.97–1.03; n=6) and 1.15 (95% CI, 1.12–1.16; n=16) for incidence and mortality, respectively. In the nonlinear analysis restricted to studies that reported the incidence of total CVD, there was no significantly increased risk of total CVD at the extreme sleep duration, whereas the U‐shaped association was more pronounced among the studies that reported mortality of total CVD (Figure S8). There was evidence of heterogeneity by study location in the linear dose‐response analysis of all participants (P=0.01), and the lowest RR was observed at 8‐hour sleep duration in Europe (Figure S9).
In the analyses of CHD, the pooled RRs for 1‐hour increment in long sleep duration were 0.89 (95% CI, 0.82–0.97; n=4) for Europe with indication of heterogeneity (P=0.02) by study location, which was inconsistent with other results. There was indication of heterogeneity (P=0.02) when we stratified studies by incidence or mortality, and the pooled RRs for 1‐hour increment in long sleep duration were 1.01 (95% CI, 0.97–1.07; n=12) and 1.13 (95% CI, 1.06–1.20; n=7) for incidence and mortality, respectively. There was no significantly increased risk of CHD at the extreme sleep duration; the U‐shaped association was more pronounced among the studies that reported mortality of CHD (Figure S10).
In the analyses of stroke, the association between sleep duration and risk was not substantially changed in most subgroups. There was indication of heterogeneity (P=0.01) when we stratified studies by duration of follow‐up, with a weaker association among studies with increasing durations of follow‐up (Figure S11).
To further confirm the robustness of the results, the dose‐response analyses were repeated using a fixed‐effects model; the pooled estimates were consistent for short and long sleep duration in relation to risk of all‐cause mortality and cardiovascular events. Sensitivity analyses omitting 1 study at a time did not substantially alter the pooled results for both short and long sleep duration and all‐cause mortality, total CVD, and CHD. For stroke, when we excluded 1 study,72 there was a statistically significant association in the analysis of the shortest versus reference sleep duration, and short sleep duration was associated with an increased risk of stroke (Figures S12 and S13).
Discussion
To our knowledge, the present work is the largest and most comprehensive study on the association of sleep duration with all‐cause mortality and cardiovascular events. Our study demonstrated U‐shaped associations between sleep duration and risk of all‐cause mortality, total CVD, CHD, and stroke, with the lowest risk observed with ≈7 hours of sleep duration. Sleep duration that was too short or too long was significantly associated with elevated risks of all‐cause mortality, total CVD, CHD, and stroke. Compared with 7 hours per day, a 1‐hour decrease was associated with 6%, 6%, 7%, and 5% increased risk of all‐cause mortality, total CVD, CHD, and stroke, respectively, and a 1‐hour increase in sleep duration was associated with 13%, 12%, 5%, and 18% increased risk, respectively.
To date, association between extreme sleep duration and increased risk of all‐cause mortality was reported previously in studies with large sample sizes and high quality,8, 9, 10, 11, 12, 13 which was consistent with our results. Heslop and colleagues,14 however, analyzed data from a workplace‐based study of Scottish men and women who were followed over a 25‐year period and found that long sleep was associated with decreased all‐cause mortality in men. But this study reported RRs with only 3 quantitative categories of sleep duration; meanwhile, long sleep duration was defined as >8 hours, which may result in inaccurate assessment of extreme long sleep. Recently, 2 systematic reviews,19, 20 both exploring the association between all‐cause mortality and sleep duration (separate analysis of 24‐hour sleep duration and nighttime sleep duration), observed markedly inconsistent results for short sleep duration. Results from Liu et al20 showed that short sleep duration was not associated with higher risk of all‐cause mortality in nighttime sleep duration. Nevertheless, results from Shen et al19 showed that for both 24‐hour and nighttime sleep duration, U‐shaped relationships were found, and the lowest risk of all‐cause mortality was observed with 7 hours per day of sleep duration, in line with our results; however, in the study by Shen et al, 1 cohort study74 was included twice in analysis. Moreover, the linear associations on the 2 sides of 7‐hour sleep duration were not detected.
Some studies have found an adverse association between extreme sleep duration and cardiovascular events. In our study, both short and long sleep duration was indicated to be associated with an increased risk of total CVD, which was inconsistent with a previous systematic review21 in 2011. In that study, short duration of sleep was not significantly associated with a greater risk of total CVD, possibly because of limited included studies. Nineteen prospective cohort studies (26 reports) have been published since 2011 and were included in our study to describe the dose‐response relationship between sleep duration and risk of total CVD. To our surprise, the findings from our subgroup analyses showed a decreased risk of CHD with long sleep duration in Europe, which should be interpreted carefully, given limited included studies. The association disappeared when we omitted the MOGEN study.15 This research showed that long sleep duration tended to be protective for CHD; however, U‐shaped associations were observed in the subgroup analysis of sleep quality in participants with available data. Notably, the proportion of women among long sleepers was significantly higher than that of men in the baseline population, whereas higher mortality rates and risks of CHD were observed in men than in women in published studies.75 This may lead to the different result. Moreover, our subgroup analyses for total CVD and CHD showed indications of heterogeneity when we stratified studies by incidence and mortality. The U‐shaped association was more pronounced among the studies that reported the mortality of total CVD or CHD compared with those that reported the incidence of total CVD or CHD. The association between cardiovascular events and sleep duration might be enhanced in the process through which patients tended to go from the occurrence of disease to death. It may also indicate that appropriate sleep duration is particularly important for delaying death among those people with chronic CVDs, and this needs to be identified further in additional studies. In our study, the adverse effect of short sleep for stroke was not observed in the shortest sleep duration versus reference analysis, whereas short sleep duration was associated with a higher risk of stroke in the dose‐response analysis. By sensitivity analysis, we found that 1 study72 had an obvious influence on the result of the shortest sleep duration versus reference analysis. The research indicated that a decreased risk of mortality from stroke was associated with short duration of sleep. Nonetheless, the small number of participants with short sleep duration limited the ability to separately analyze the effect of ≤5 and 6 hours of sleep, and the study was not included in the dose‐response analysis because it had too few categories of short sleep. After omitting the studies with <3 categories of short sleep, the pooled RR of the shortest versus reference sleep duration was 1.16 (95% CI, 1.03–1.31), which was in line with the dose‐response analysis.
Sex and age are important variables in risk of death and CVDs; this was generally accepted. In light of previous studies, the association between sleep duration and mortality8, 57, 58 and cardiovascular events16, 67 varies by sex; however, in our subgroup analyses, extreme sleep durations were significantly associated with elevated risks of all‐cause mortality, total CVD, CHD, and stroke in both men and women. Our metaregression analyses further demonstrated that there was no potential source of heterogeneity from the sex variable; therefore, a sex difference in the association of sleep duration with death and CVDs must be interpreted with caution. In addition, several studies found a stronger U‐shaped association between sleep duration and CVDs in older adults compared with younger adults (cutoff at age 65 years).10, 16 Nevertheless, the result in a study including 60 000 Chinese participants (cutoff at age 60 years) was not entirely consistent.66 Considering that the age range of the study population varied widely and the length of follow‐up was different among the included studies, we did not conduct subgroup analyses stratified by age. Further studies concentrated on sleep duration and adverse outcomes among different age groups are warranted in the future.
Short and long sleep duration may share some relevant mechanisms in relation to all‐cause mortality and cardiovascular events. As elucidated in published articles, extreme sleep duration on both sides was associated with elevated C‐reactive protein.76 As widely accepted, however, distinctive mechanisms with their own characteristics may operate at either end of the distribution of sleep duration.77
Several potential mechanisms may contribute to the relationship between short sleep duration and adverse outcomes. First, sleep restriction during the night has multiple effects on endocrine and metabolic function such as decreases of testosterone78 and melatonin secretion,79 which also may be implicated with mortality or cardiovascular events.80, 81 Second, observational studies also found that short duration of sleep was associated with vascular damage, such as coronary artery calcification.82 Third, short duration of sleep was associated with reduced levels of leptin and elevated levels of ghrelin.83, 84 The serum leptin and ghrelin levels are independent predictors of cardiovascular morbidity and mortality.85, 86 Finally, individuals with sleep deprivation, especially shift workers, have irregular sleep schedules, resulting in circadian misalignment, which may aggravate CVD in humans.87
The potential mechanisms underlying the association between long sleep duration and adverse outcomes are considered more speculative. Some insisted that the elevated risk of long sleep duration most likely represented the confounding effects of subhealthy status or uncontrolled chronic illness, such as obstructive sleep apnea, a known cause of increased need for sleep and an identified risk factor for mortality and cardiovascular events.88 As mentioned, changes in inflammatory markers and vascular health come with long sleep duration, as shown by new evidence in recent years. First, long sleep duration may be associated with an increased risk of atherosclerosis.82 Second, excessive time in bed has been linked to increased sleep fragmentation,89 which was considered to be associated with more severe arteriolosclerosis and subcortical macroscopic infarcts. These were independent risk factors of CVD and several medical comorbidities.90 Third, long sleep duration has been linked with feelings of fatigue and lethargy, which in turn would cause sleep extension. These states may fail to provide sufficient restoration against stress and disease and then lead to increased mortality.91 Finally, long duration of sleep was associated with depressive symptoms, low socioeconomic status, unemployment, low household income, low level of education, and other risk factors for mortality and cardiovascular events.92 Further experimental studies are warranted to explore the potential effects of sleep extension on health outcomes.
This meta‐analysis has several strengths. All studies included in our meta‐analysis used a prospective design, thus the differential misclassification of sleep duration attributable to recall bias was minimized. The majority of the included studies had relatively high quality. Moreover, we investigated a dose‐response relationship between sleep duration and the outcomes, allowing us to examine the shape of this possible association. Linear and nonlinear relationships were also tested to assess the dose‐response relationship.
Several limitations of our study should also be acknowledged. First, nearly all studies relied on sleep duration that was self‐reported by questionnaire or interview; 1 study93 provided the RRs between all‐cause mortality and both subjective and objective sleep duration, but no substantial difference was observed. Meanwhile, in the big data era, the widespread availability and acceptance of electronic wearable devices, such as consumer‐level activity monitors, may allow accurate, reliable, and scalable objective sleep‐duration assessment in large epidemiological studies.94 Second, sleep duration is a dynamic biological process. A single measure of exposure may not fully capture the sustained effects of sleep duration over time when related to long‐term disease incidence. One included study95 addressed this issue by measuring changes in sleep duration twice, several years apart, and found that stable short and stable long sleep was associated with a significantly increased risk of mortality; moreover, moving to either shorter of longer sleep from average sleep was also associated with increased mortality. This finding was in line with our result that appropriate sleep duration was important for the delay or prevention of premature mortality. Third, we cannot rule out the possibility of residual or unmeasured confounding, even though we have taken into consideration major confounding factors by using adjusted risk estimates from multivariate models from each contributing study. Finally, sleep quality affected by factors like sleep apnea is an independent predictor of risk of adverse outcomes96 but was not assessed in our study. Despite the limitations, at this stage, results from prospective cohort studies are still the best evidence available to assess the longitudinal effect of sleep duration on all‐cause mortality and cardiovascular events.
Conclusions
In summary, our dose‐response meta‐analysis of prospective studies provides further evidence that sleep duration that is either too short or too long is associated with higher risk of all‐cause mortality and cardiovascular events, with the lowest risk with ≈7 hours per day of sleep duration. Longer term randomized controlled trials are needed to establish causality and to elucidate the underlying mechanisms.
Author Contributions
Yin, Shan, Chen, and Liu conceived the study. Yin searched the databases, checked them according to the eligible criteria and exclusion criteria, extracted and analyzed the data, and wrote the draft of the article. S.Z. Li and Jin helped extract quantitative data from some articles and contributed to writing, reviewing, or revising the article. Huang, P.Y. Li, Shan, Bao, Yang, X.B. Peng, Z. Peng and Yu critically reviewed and revised for important intellectual content. Shan and Bao provided advice on meta‐analysis methodology and contributed to reviewing, or revising the article. Liu is the guarantor and had full access to all the data and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Sources of Funding
This work was funded by the National Natural Science Foundation of China (NSFC 81472978), the National Science and Technology Support Program (2012BAI02B02) and China Postdoctoral Science Foundation (2016M602314). Integrated Innovative Team for Major Human Diseases Program of Tongji Medical College, HUST. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the article.
Disclosures
None.
Supporting information
Data S1.
Table S1. Sleep Duration and All‐Cause Mortality
Table S2. Sleep Duration and Total Cardiovascular Disease
Table S3. Sleep Duration and Coronary Heart Disease
Table S4. Sleep Duration and Stroke
Table S5. Study Quality of Studies Included in the Analysis of Sleep Duration and All‐Cause Mortality
Table S6. Study Quality of Studies Included in the Analysis of Sleep Duration and Total Cardiovascular Disease
Table S7. Study Quality of Studies Included in the Analysis of Sleep Duration and Coronary Heart Disease
Table S8. Study Quality of Studies Included in the Analysis of Sleep Duration and Stroke
Table S9. Subgroup Analyses of Sleep Duration and All‐Cause Mortality Per Hour, Per Day
Table S10. Subgroup Analyses of Sleep Duration and Total Cardiovascular Disease Per Hour, Per Day
Table S11. Subgroup Analyses of Sleep Duration and Coronary Heart Disease Per Hour, Per Day
Table S12. Subgroup Analyses of Sleep Duration and Stroke Per Hour, Per Day
Figure S1. Sleep duration and all‐cause mortality, shortest and longest vs reference analysis.
Figure S2. Sleep duration and total cardiovascular disease, shortest and longest vs reference analysis.
Figure S3. Sleep duration and coronary heart disease, shortest and longest vs reference analysis.
Figure S4. Sleep duration and stroke, shortest and longest vs reference analysis.
Figure S5. Trim‐and‐fill correction for publication bias for total cardiovascular disease, longest vs reference analysis.
Figure S6. Trim‐and‐fill correction for publication bias for all‐cause mortality, dose‐response analysis for short sleep.
Figure S7. Nonlinear dose‐response analysis of sleep duration and all‐cause mortality by nighttime sleep duration (A) and 24‐hour sleep duration (B).
Figure S8. Nonlinear dose‐response analysis of sleep duration and total cardiovascular disease by incidence (A) and mortality (B).
Figure S9. Nonlinear dose‐response analysis of sleep duration and total cardiovascular disease by Asia (A), Europe (B), and the United States (C).
Figure S10. Nonlinear dose‐response analysis of sleep duration and coronary heart disease by incidence (A) and mortality (B).
Figure S11. Nonlinear dose‐response analysis of sleep duration and stroke by follow‐up duration <10 years (A) and follow‐up duration ≥10 years (B).
Figure S12. Sensitive analysis of stroke and sleep duration, shortest vs reference analysis.
Figure S13. Sensitive analysis of stroke and sleep duration after excluding the study of Kawachi (2016), shortest vs reference analysis.
(J Am Heart Assoc. 2017;6:e005947 DOI: 10.1161/JAHA.117.005947.)28889101
Notes
Contributor Information
Xiaoyi Chen, Email: wwchenxy1@163.com.
Liegang Liu, Email: lgliu@mails.tjmu.edu.cn.
References
- 1. World Heart Federation . World congress of cardiology & cardiovascular health 2016. Available at: www.world-heart-federation.org/resources/world-congress-cardiology-cardiovascular-health-2016/. Accessed December 1, 2016.
- 2. World Health Organization . Global action plan for the prevention and control of NCDS 2013–2020. Available at: www.Who.Int/nmh/publications/ncd-action-plan/en/. Accessed December 1, 2016.
- 3. Kirszenblat L, van Swinderen B. The yin and yang of sleep and attention. Trends Neurosci. 2015;38:776–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. St‐Onge MP, Grandner MA, Brown D, Conroy MB, Jean‐Louis G, Coons M, Bhatt DL. Sleep duration and quality: impact on lifestyle behaviors and cardiometabolic health: a scientific statement from the American Heart Association. Circulation. 2016;134:e367–e386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shan Z, Ma H, Xie M, Yan P, Guo Y, Bao W, Rong Y, Jackson CL, Hu FB, Liu L. Sleep duration and risk of type 2 diabetes: a meta‐analysis of prospective studies. Diabetes Care. 2015;38:529–537. [DOI] [PubMed] [Google Scholar]
- 6. Qin Y, Zhou Y, Zhang X, Wei X, He J. Sleep duration and breast cancer risk: a meta‐analysis of observational studies. Int J Cancer. 2014;134:1166–1173. [DOI] [PubMed] [Google Scholar]
- 7. Jean‐Louis G, Williams NJ, Sarpong D, Pandey A, Youngstedt S, Zizi F, Ogedegbe G. Associations between inadequate sleep and obesity in the US adult population: analysis of the national health interview survey (1977–2009). BMC Public Health. 2014;14:290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cai H, Shu XO, Xiang YB, Yang G, Li H, Ji BT, Gao J, Gao YT, Zheng W. Sleep duration and mortality: a prospective study of 113 138 middle‐aged and elderly Chinese men and women. Sleep. 2015;38:529–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bellavia A, Akerstedt T, Bottai M, Wolk A, Orsini N. Sleep duration and survival percentiles across categories of physical activity. Am J Epidemiol. 2014;179:484–491. [DOI] [PubMed] [Google Scholar]
- 10. Yeo Y, Ma SH, Park SK, Chang SH, Shin HR, Kang D, Yoo KY. A prospective cohort study on the relationship of sleep duration with all‐cause and disease‐specific mortality in the Korean Multi‐Center Cancer Cohort Study. J Prev Med Public Health. 2013;46:271–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kim Y, Wilkens LR, Schembre SM, Henderson BE, Kolonel LN, Goodman MT. Insufficient and excessive amounts of sleep increase the risk of premature death from cardiovascular and other diseases: the Multiethnic Cohort Study. Prev Med. 2013;57:377–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kronholm E, Laatikainen T, Peltonen M, Sippola R, Partonen T. Self‐reported sleep duration, all‐cause mortality, cardiovascular mortality and morbidity in Finland. Sleep Med. 2011;12:215–221. [DOI] [PubMed] [Google Scholar]
- 13. Ikehara S, Iso H, Date C, Kikuchi S, Watanabe Y, Wada Y, Inaba Y, Tamakoshi A. Association of sleep duration with mortality from cardiovascular disease and other causes for Japanese men and women: the JACC study. Sleep. 2009;32:295–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Heslop P, Smith GD, Metcalfe C, Macleod J, Hart C. Sleep duration and mortality: the effect of short or long sleep duration on cardiovascular and all‐cause mortality in working men and women. Sleep Med. 2002;3:305–314. [DOI] [PubMed] [Google Scholar]
- 15. Hoevenaar‐Blom MP, Spijkerman AM, Kromhout D, van den Berg JF, Verschuren WM. Sleep duration and sleep quality in relation to 12‐year cardiovascular disease incidence: the MORGEN study. Sleep. 2011;34:1487–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Strand LB, Tsai MK, Gunnell D, Janszky I, Wen CP, Chang SS. Self‐reported sleep duration and coronary heart disease mortality: a large cohort study of 400,000 Taiwanese adults. Int J Cardiol. 2016;207:246–251. [DOI] [PubMed] [Google Scholar]
- 17. Helbig AK, Stockl D, Heier M, Ladwig KH, Meisinger C. Symptoms of insomnia and sleep duration and their association with incident strokes: findings from the population‐based MONICA/KORA Augsburg cohort study. PLoS One. 2015;10:e0134480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hall MH, Smagula SF, Boudreau RM, Ayonayon HN, Goldman SE, Harris TB, Naydeck BL, Rubin SM, Samuelsson L, Satterfield S, Stone KL, Visser M, Newman AB. Association between sleep duration and mortality is mediated by markers of inflammation and health in older adults: the Health, Aging and Body Composition Study. Sleep. 2015;38:189–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shen X, Wu Y, Zhang D. Nighttime sleep duration, 24‐hour sleep duration and risk of all‐cause mortality among adults: a meta‐analysis of prospective cohort studies. Sci Rep. 2016;6:21480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liu TZ, Xu C, Rota M, Cai H, Zhang C, Shi MJ, Yuan RX, Weng H, Meng XY, Kwong JS, Sun X. Sleep duration and risk of all‐cause mortality: a flexible, non‐linear, meta‐regression of 40 prospective cohort studies. Sleep Med Rev. 2017;32:28–36. [DOI] [PubMed] [Google Scholar]
- 21. Cappuccio FP, Cooper D, D'Elia L, Strazzullo P, Miller MA. Sleep duration predicts cardiovascular outcomes: a systematic review and meta‐analysis of prospective studies. Eur Heart J. 2011;32:1484–1492. [DOI] [PubMed] [Google Scholar]
- 22. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta‐analysis of observational studies in epidemiology: a proposal for reporting. Meta‐analysis of Observational Studies in Epidemiology (MOOSE) Group. JAMA. 2000;283:2008–2012. [DOI] [PubMed] [Google Scholar]
- 23. Wells GS, Shea B, O'Connell D, Robertson J, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta‐analyses. Available at: www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed December 1, 2016.
- 24. Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The cochrane collaboration; 2011. Available at: www.Cochrane-handbook.Org. Accessed December 1, 2016. [Google Scholar]
- 25. Zhang J, Yu KF. What's the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA. 1998;280:1690–1691. [DOI] [PubMed] [Google Scholar]
- 26. Bekkering GE, Harris RJ, Thomas S, Mayer AM, Beynon R, Ness AR, Harbord RM, Bain C, Smith GD, Sterne JA. How much of the data published in observational studies of the association between diet and prostate or bladder cancer is usable for meta‐analysis? Am J Epidemiol. 2008;167:1017–1026. [DOI] [PubMed] [Google Scholar]
- 27. Hamling J, Lee P, Weitkunat R, Ambuhl M. Facilitating meta‐analyses by deriving relative effect and precision estimates for alternative comparisons from a set of estimates presented by exposure level or disease category. Stat Med. 2008;27:954–970. [DOI] [PubMed] [Google Scholar]
- 28. Jackson D, White IR, Thompson SG. Extending DerSimonian and Laird's methodology to perform multivariate random effects meta‐analyses. Stat Med. 2010;29:1282–1297. [DOI] [PubMed] [Google Scholar]
- 29. Orsini N, Li R, Wolk A, Khudyakov P, Spiegelman D. Meta‐analysis for linear and nonlinear dose‐response relations: examples, an evaluation of approximations, and software. Am J Epidemiol. 2012;175:66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Greenland S, Longnecker MP. Methods for trend estimation from summarized dose‐response data, with applications to meta‐analysis. Am J Epidemiol. 1992;135:1301–1309. [DOI] [PubMed] [Google Scholar]
- 31. DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials. 1986;7:177–188. [DOI] [PubMed] [Google Scholar]
- 32. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ. 2003;327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 35. Duval S, Tweedie R. Trim and fill: a simple funnel‐plot‐based method of testing and adjusting for publication bias in meta‐analysis. Biometrics. 2000;56:455–463. [DOI] [PubMed] [Google Scholar]
- 36. Wallace BC, Schmid CH, Lau J, Trikalinos TA. Meta‐analyst: software for meta‐analysis of binary, continuous and diagnostic data. BMC Med Res Methodol. 2009;9:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang X, Liu X, Song Q, Wu S. Sleep duration and risk of myocardial infarction and all‐cause death in a Chinese population: the Kailuan study. Sleep Med. 2016;19:13–16. [DOI] [PubMed] [Google Scholar]
- 38. Xiao Q, Keadle SK, Hollenbeck AR, Matthews CE. Sleep duration and total and cause‐specific mortality in a large US cohort: interrelationships with physical activity, sedentary behavior, and body mass index. Am J Epidemiol. 2014;180:997–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rod NH, Kumari M, Lange T, Kivimaki M, Shipley M, Ferrie J. The joint effect of sleep duration and disturbed sleep on cause‐specific mortality: results from the Whitehall II cohort study. PLoS One. 2014;9:e91965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Magee CA, Holliday EG, Attia J, Kritharides L, Banks E. Investigation of the relationship between sleep duration, all‐cause mortality, and preexisting disease. Sleep Med. 2013;14:591–596. [DOI] [PubMed] [Google Scholar]
- 41. Li Y, Sato Y, Yamaguchi N. Potential biochemical pathways for the relationship between sleep duration and mortality. Sleep Med. 2013;14:98–104. [DOI] [PubMed] [Google Scholar]
- 42. Jung KI, Song CH, Ancoli‐Israel S, Barrett‐Connor E. Gender differences in nighttime sleep and daytime napping as predictors of mortality in older adults: the Rancho Bernardo study. Sleep Med. 2013;14:12–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hale L, Parente V, Dowd JB, Sands M, Berger JS, Song Y, Martin LW, Allison MA. Fibrinogen may mediate the association between long sleep duration and coronary heart disease. J Sleep Res. 2013;22:305–314. [DOI] [PubMed] [Google Scholar]
- 44. Chen HC, Su TP, Chou P. A nine‐year follow‐up study of sleep patterns and mortality in community‐dwelling older adults in Taiwan. Sleep. 2013;36:1187–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Qiu L, Sautter J, Liu Y, Gu D. Age and gender differences in linkages of sleep with subsequent mortality and health among very old Chinese. Sleep Med. 2011;12:1008–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Castro‐Costa E, Dewey ME, Ferri CP, Uchoa E, Firmo JO, Rocha FL, Prince M, Lima‐Costa MF, Stewart R. Association between sleep duration and all‐cause mortality in old age: 9‐year follow‐up of the Bambui Cohort Study, Brazil. J Sleep Res. 2011;20:303–310. [DOI] [PubMed] [Google Scholar]
- 47. Mesas AE, Lopez‐Garcia E, Leon‐Munoz LM, Guallar‐Castillon P, Rodriguez‐Artalejo F. Sleep duration and mortality according to health status in older adults. J Am Geriatr Soc. 2010;58:1870–1877. [DOI] [PubMed] [Google Scholar]
- 48. Chien KL, Chen PC, Hsu HC, Su TC, Sung FC, Chen MF, Lee YT. Habitual sleep duration and insomnia and the risk of cardiovascular events and all‐cause death: report from a community‐based cohort. Sleep. 2010;33:177–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Amagai Y, Ishikawa S, Gotoh T, Kayaba K, Nakamura Y, Kajii E. Sleep duration and incidence of cardiovascular events in a Japanese population: the Jichi Medical School cohort study. J Epidemiol. 2010;20:106–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Suzuki E, Yorifuji T, Ueshima K, Takao S, Sugiyama M, Ohta T, Ishikawa‐Takata K, Doi H. Sleep duration, sleep quality and cardiovascular disease mortality among the elderly: a population‐based cohort study. Prev Med. 2009;49:135–141. [DOI] [PubMed] [Google Scholar]
- 51. Gangwisch JE, Heymsfield SB, Boden‐Albala B, Buijs RM, Kreier F, Opler MG, Pickering TG, Rundle AG, Zammit GK, Malaspina D. Sleep duration associated with mortality in elderly, but not middle‐aged, adults in a large US sample. Sleep. 2008;31:1087–1096. [PMC free article] [PubMed] [Google Scholar]
- 52. Patel SR, Ayas NT, Malhotra MR, White DP, Schernhammer ES, Speizer FE, Stampfer MJ, Hu FB. A prospective study of sleep duration and mortality risk in women. Sleep. 2004;27:440–444. [DOI] [PubMed] [Google Scholar]
- 53. Kripke DF, Garfinkel L, Wingard DL, Klauber MR, Marler MR. Mortality associated with sleep duration and insomnia. Arch Gen Psychiatry. 2002;59:131–136. [DOI] [PubMed] [Google Scholar]
- 54. Kakizaki M, Kuriyama S, Nakaya N, Sone T, Nagai M, Sugawara Y, Hozawa A, Fukudo S, Tsuji I. Long sleep duration and cause‐specific mortality according to physical function and self‐rated health: the Ohsaki Cohort Study. J Sleep Res. 2013;22:209–216. [DOI] [PubMed] [Google Scholar]
- 55. Stone KL, Ewing SK, Ancoli‐Israel S, Ensrud KE, Redline S, Bauer DC, Cauley JA, Hillier TA, Cummings SR. Self‐reported sleep and nap habits and risk of mortality in a large cohort of older women. J Am Geriatr Soc. 2009;57:604–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lan TY, Lan TH, Wen CP, Lin YH, Chuang YL. Nighttime sleep, Chinese afternoon nap, and mortality in the elderly. Sleep. 2007;30:1105–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Amagai Y, Ishikawa S, Gotoh T, Doi Y, Kayaba K, Nakamura Y, Kajii E. Sleep duration and mortality in Japan: the Jichi Medical School cohort study. J Epidemiol. 2004;14:124–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kojima M, Wakai K, Kawamura T, Tamakoshi A, Aoki R, Lin Y, Nakayama T, Horibe H, Aoki N, Ohno Y. Sleep patterns and total mortality: a 12‐year follow‐up study in Japan. J Epidemiol. 2000;10:87–93. [DOI] [PubMed] [Google Scholar]
- 59. Gale C, Martyn C. Larks and owls and health, wealth, and wisdom. BMJ. 1998;317:1675–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Westerlund A, Bellocco R, Sundstrom J, Adami HO, Akerstedt T, Trolle Lagerros Y. Sleep characteristics and cardiovascular events in a large Swedish cohort. Eur J Epidemiol. 2013;28:463–473. [DOI] [PubMed] [Google Scholar]
- 61. Sands‐Lincoln M, Loucks EB, Lu B, Carskadon MA, Sharkey K, Stefanick ML, Ockene J, Shah N, Hairston KG, Robinson JG, Limacher M, Hale L, Eaton CB. Sleep duration, insomnia, and coronary heart disease among postmenopausal women in the Women's Health Initiative. J Womens Health (Larchmt). 2013;22:477–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Holliday EG, Magee CA, Kritharides L, Banks E, Attia J. Short sleep duration is associated with risk of future diabetes but not cardiovascular disease: a prospective study and meta‐analysis. PLoS One. 2013;8:e82305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hamazaki Y, Morikawa Y, Nakamura K, Sakurai M, Miura K, Ishizaki M, Kido T, Naruse Y, Suwazono Y, Nakagawa H. The effects of sleep duration on the incidence of cardiovascular events among middle‐aged male workers in Japan. Scand J Work Environ Health. 2011;37:411–417. [DOI] [PubMed] [Google Scholar]
- 64. von Ruesten A, Weikert C, Fietze I, Boeing H. Association of sleep duration with chronic diseases in the European Prospective Investigation into Cancer and Nutrition (EPIC)‐Potsdam study. PLoS One. 2012;7:e30972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Chandola T, Ferrie JE, Perski A, Akbaraly T, Marmot MG. The effect of short sleep duration on coronary heart disease risk is greatest among those with sleep disturbance: a prospective study from the Whitehall II cohort. Sleep. 2010;33:739–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Shankar A, Koh WP, Yuan JM, Lee HP, Yu MC. Sleep duration and coronary heart disease mortality among Chinese adults in Singapore: a population‐based cohort study. Am J Epidemiol. 2008;168:1367–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Meisinger C, Heier M, Lowel H, Schneider A, Doring A. Sleep duration and sleep complaints and risk of myocardial infarction in middle‐aged men and women from the general population: the MONICA/KORA Augsburg cohort study. Sleep. 2007;30:1121–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ayas NT, White DP, Manson JE, Stampfer MJ, Speizer FE, Malhotra A, Hu FB. A prospective study of sleep duration and coronary heart disease in women. Arch Intern Med. 2003;163:205–209. [DOI] [PubMed] [Google Scholar]
- 69. Yang L, Yang H, He M, Pan A, Li X, Min X, Zhang C, Xu C, Zhu X, Yuan J, Wei S, Miao X, Hu FB, Wu T, Zhang X. Longer sleep duration and midday napping are associated with a higher risk of CHD incidence in middle‐aged and older Chinese: the Dongfeng‐Tongji Cohort Study. Sleep. 2016;39:645–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ruiter Petrov ME, Letter AJ, Howard VJ, Kleindorfer D. Self‐reported sleep duration in relation to incident stroke symptoms: nuances by body mass and race from the REGARDS study. J Stroke Cerebrovasc Dis. 2014;23:e123–e132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Pan A, De Silva DA, Yuan JM, Koh WP. Sleep duration and risk of stroke mortality among Chinese adults: Singapore Chinese Health Study. Stroke. 2014;45:1620–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kawachi T, Wada K, Nakamura K, Tsuji M, Tamura T, Konishi K, Nagata C. Sleep duration and the risk of mortality from stroke in Japan: the Takayama Cohort Study. J Epidemiol. 2016;26:123–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Chen JC, Brunner RL, Ren H, Wassertheil‐Smoller S, Larson JC, Levine DW, Allison M, Naughton MJ, Stefanick ML. Sleep duration and risk of ischemic stroke in postmenopausal women. Stroke. 2008;39:3185–3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Ferrie JE, Shipley MJ, Cappuccio FP, Brunner E, Miller MA, Kumari M, Marmot MG. A prospective study of change in sleep duration: associations with mortality in the Whitehall II cohort. Sleep. 2007;30:1659–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Albrektsen G, Heuch I, Lochen ML, Thelle DS, Wilsgaard T, Njolstad I, Bonaa KH. Lifelong gender gap in risk of incident myocardial infarction: the Tromso Study. JAMA Intern Med. 2016;176:1673–1679. [DOI] [PubMed] [Google Scholar]
- 76. Grandner MA, Buxton OM, Jackson N, Sands‐Lincoln M, Pandey A, Jean‐Louis G. Extreme sleep durations and increased C‐reactive protein: effects of sex and ethnoracial group. Sleep. 2013;36:769–779e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Knutson KL, Turek FW. The U‐shaped association between sleep and health: the 2 peaks do not mean the same thing. Sleep. 2006;29:878–879. [DOI] [PubMed] [Google Scholar]
- 78. Leproult R, Van Cauter E. Effect of 1 week of sleep restriction on testosterone levels in young healthy men. JAMA. 2011;305:2173–2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Blask DE. Melatonin, sleep disturbance and cancer risk. Sleep Med Rev. 2009;13:257–264. [DOI] [PubMed] [Google Scholar]
- 80. Brugger P, Marktl W, Herold M. Impaired nocturnal secretion of melatonin in coronary heart disease. Lancet. 1995;345:1408. [DOI] [PubMed] [Google Scholar]
- 81. Kloner RA, Carson C III, Dobs A, Kopecky S, Mohler ER III. Testosterone and cardiovascular disease. J Am Coll Cardiol. 2016;67:545–557. [DOI] [PubMed] [Google Scholar]
- 82. King CR, Knutson KL, Rathouz PJ, Sidney S, Liu K, Lauderdale DS. Short sleep duration and incident coronary artery calcification. JAMA. 2008;300:2859–2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Taheri S, Lin L, Austin D, Young T, Mignot E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004;1:e62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Spiegel K, Tasali E, Penev P, Van Cauter E. Brief communication: sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med. 2004;141:846–850. [DOI] [PubMed] [Google Scholar]
- 85. Sader S, Nian M, Liu P. Leptin: a novel link between obesity, diabetes, cardiovascular risk, and ventricular hypertrophy. Circulation. 2003;108:644–646. [DOI] [PubMed] [Google Scholar]
- 86. Rodriguez A. Novel molecular aspects of ghrelin and leptin in the control of adipobiology and the cardiovascular system. Obes Facts. 2014;7:82–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Morris CJ, Purvis TE, Hu K, Scheer FA. Circadian misalignment increases cardiovascular disease risk factors in humans. Proc Natl Acad Sci USA. 2016;113:E1402–E1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Basner RC. Cardiovascular morbidity and obstructive sleep apnea. N Engl J Med. 2014;370:2339–2341. [DOI] [PubMed] [Google Scholar]
- 89. Youngstedt SD, Kripke DF. Long sleep and mortality: rationale for sleep restriction. Sleep Med Rev. 2004;8:159–174. [DOI] [PubMed] [Google Scholar]
- 90. Lim AS, Yu L, Schneider JA, Bennett DA, Buchman AS. Sleep fragmentation, cerebral arteriolosclerosis, and brain infarct pathology in community‐dwelling older people. Stroke. 2016;47:516–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Grandner MA, Kripke DF. Self‐reported sleep complaints with long and short sleep: a nationally representative sample. Psychosom Med. 2004;66:239–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Patel SR, Sotres‐Alvarez D, Castaneda SF, Dudley KA, Gallo LC, Hernandez R, Medeiros EA, Penedo FJ, Mossavar‐Rahmani Y, Ramos AR, Redline S, Reid KJ, Zee PC. Social and health correlates of sleep duration in a US Hispanic population: results from the Hispanic Community Health Study/Study of Latinos. Sleep. 2015;38:1515–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Zuurbier LA, Luik AI, Hofman A, Franco OH, Van Someren EJ, Tiemeier H. Fragmentation and stability of circadian activity rhythms predict mortality: the Rotterdam study. Am J Epidemiol. 2015;181:54–63. [DOI] [PubMed] [Google Scholar]
- 94. Ferguson T, Rowlands AV, Olds T, Maher C. The validity of consumer‐level, activity monitors in healthy adults worn in free‐living conditions: a cross‐sectional study. Int J Behav Nutr Phys Act. 2015;12:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Hublin C, Partinen M, Koskenvuo M, Kaprio J. Sleep and mortality: a population‐based 22‐year follow‐up study. Sleep. 2007;30:1245–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353:2034–2041. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1.
Table S1. Sleep Duration and All‐Cause Mortality
Table S2. Sleep Duration and Total Cardiovascular Disease
Table S3. Sleep Duration and Coronary Heart Disease
Table S4. Sleep Duration and Stroke
Table S5. Study Quality of Studies Included in the Analysis of Sleep Duration and All‐Cause Mortality
Table S6. Study Quality of Studies Included in the Analysis of Sleep Duration and Total Cardiovascular Disease
Table S7. Study Quality of Studies Included in the Analysis of Sleep Duration and Coronary Heart Disease
Table S8. Study Quality of Studies Included in the Analysis of Sleep Duration and Stroke
Table S9. Subgroup Analyses of Sleep Duration and All‐Cause Mortality Per Hour, Per Day
Table S10. Subgroup Analyses of Sleep Duration and Total Cardiovascular Disease Per Hour, Per Day
Table S11. Subgroup Analyses of Sleep Duration and Coronary Heart Disease Per Hour, Per Day
Table S12. Subgroup Analyses of Sleep Duration and Stroke Per Hour, Per Day
Figure S1. Sleep duration and all‐cause mortality, shortest and longest vs reference analysis.
Figure S2. Sleep duration and total cardiovascular disease, shortest and longest vs reference analysis.
Figure S3. Sleep duration and coronary heart disease, shortest and longest vs reference analysis.
Figure S4. Sleep duration and stroke, shortest and longest vs reference analysis.
Figure S5. Trim‐and‐fill correction for publication bias for total cardiovascular disease, longest vs reference analysis.
Figure S6. Trim‐and‐fill correction for publication bias for all‐cause mortality, dose‐response analysis for short sleep.
Figure S7. Nonlinear dose‐response analysis of sleep duration and all‐cause mortality by nighttime sleep duration (A) and 24‐hour sleep duration (B).
Figure S8. Nonlinear dose‐response analysis of sleep duration and total cardiovascular disease by incidence (A) and mortality (B).
Figure S9. Nonlinear dose‐response analysis of sleep duration and total cardiovascular disease by Asia (A), Europe (B), and the United States (C).
Figure S10. Nonlinear dose‐response analysis of sleep duration and coronary heart disease by incidence (A) and mortality (B).
Figure S11. Nonlinear dose‐response analysis of sleep duration and stroke by follow‐up duration <10 years (A) and follow‐up duration ≥10 years (B).
Figure S12. Sensitive analysis of stroke and sleep duration, shortest vs reference analysis.
Figure S13. Sensitive analysis of stroke and sleep duration after excluding the study of Kawachi (2016), shortest vs reference analysis.


