Abstract
Background
Sleep‐disordered breathing (SDB) after acute ischemic stroke is frequent and may be linked to stroke‐induced autonomic imbalance. In the present study, the interaction between SDB and peripheral endothelial dysfunction (ED) was investigated in patients with acute ischemic stroke and at 1‐year follow‐up.
Methods and Results
SDB was assessed by transthoracic impedance records in 101 patients with acute ischemic stroke (mean age, 69 years; 61% men; median National Institutes of Health Stroke Scale, 4) while being on the stroke unit. SDB was defined by apnea‐hypopnea index ≥5 episodes per hour. Peripheral endothelial function was assessed using peripheral arterial tonometry (EndoPAT‐2000). ED was defined by reactive hyperemia index ≤1.8. Forty‐one stroke patients underwent 1‐year follow‐up (390±24 days) after stroke. SDB was observed in 57% patients with acute ischemic stroke. Compared with patients without SDB, ED was more prevalent in patients with SDB (32% versus 64%; P<0.01). After adjustment for multiple confounders, presence of SDB remained independently associated with ED (odds ratio, 3.1; [95% confidence interval, 1.2–7.9]; P<0.05). After 1 year, the prevalence of SDB decreased from 59% to 15% (P<0.001). Interestingly, peripheral endothelial function improved in stroke patients with normalized SDB, compared with patients with persisting SDB (P<0.05).
Conclusions
SDB was present in more than half of all patients with acute ischemic stroke and was independently associated with peripheral ED. Normalized ED in patients with normalized breathing pattern 1 year after stroke suggests a mechanistic link between SDB and ED.
Clinical Trial Registration
URL: https://drks-neu.uniklinik-freiburg.de. Unique identifier: DRKS00000514.
Keywords: clinical trial, endothelial dysfunction, sleep disorders, sympathetic nervous system
Subject Categories: Autonomic Nervous System, Endothelium/Vascular Type/Nitric Oxide, Ischemic Stroke
Clinical Perspective
What Is New?
Our observational study demonstrates an association of peripheral endothelial dysfunction with sleep‐disordered breathing in patients with acute ischemic stroke.
Normalization of sleep‐disordered breathing pattern 1 year after stroke was associated with improved endothelial function.
What Are the Clinical Implications?
Whereas sleep‐disordered breathing is a frequent finding in the acute phase of stroke, our study suggests a transient character of SDB in the majority of stroke patients with mild‐to‐moderate neurological deficit on admission.
Large, prospective stroke studies including a systematic screening for sleep‐disordered breathing and endothelial dysfunction are needed to assess an impact on the frequency of cerebrovascular and cardiovascular complications.
Introduction
Sleep‐disordered breathing (SDB), including obstructive sleep apnea and central sleep apnea, is frequently observed in patients with cardiovascular diseases or stroke.1 SDB is characterized by periodic breathing frequency and depth. The prolonged episodes of breathing cessation lead to hypoxia, hypercapnia, and sympathetic activation, subsequently increasing the risk for cardiovascular events.2 Several clinical studies observed an association of obstructive sleep apnea—caused by the collapse of upper airways—with systemic inflammation, atrial fibrillation, (recurrent) stroke, and heart failure.3, 4
Compared with wakefulness, natural sleep is associated with increased carbon dioxide pressure in blood.5 After cerebral stroke, an impaired sensitivity of the medullary respiratory center to carbon dioxide leads to relative hyperventilation and subsequent to hypocapnia.6 Therefore, nocturnal as well as diurnal periodic breathing (eg, Cheyne–Stoke respiration) with episodes of hypoxia and hypercapnia might serve as a compensatory mechanism for normalization of carbon dioxide pressure.7 Indeed, nocturnal hypocapnia was independently associated with Cheyne–Stoke respiration after stroke.6 In addition, input from the cerebral cortex has been suggested as a suppresser of periodic pathological breathing patterns, generated in the brainstem. Therefore, the loss of the cortical control on the brainstem after brain injury might be causal for central sleep apnea.
SDB is accompanied by increased sympathetic activation. The most common manifestations of autonomic dysfunction observed in association with acute stroke are cardiac arrhythmias, changes of heart rate variability, fluctuations of blood pressure, endothelial dysfunction (ED), and myocardial injury.8, 9, 10, 11, 12 Previously, we reported the presence of peripheral ED in patients with acute ischemic stroke.13 However, only a few studies investigated an association between SDB and ED in stroke.14, 15
We hypothesized that nocturnal SDB after acute ischemic stroke may contribute to development of peripheral ED. Subsequently, we prospectively examined patients in the acute phase after ischemic stroke and 1 year afterward.
Patients and Methods
The Study Protocol
The present analysis is a part of the prospective, single‐center observational BoSSS (Body Size in Stroke Study; German registry for clinical trials number DRKS00000514), performed at the stroke unit (Charité–Medical School, Berlin, Germany).
One hundred one consecutive patients (aged 35–89 years) with acute ischemic stroke of the middle cerebral artery territory were recruited to this study within 48 hours after symptom onset. In the acute phase after stroke, patients were treated according to the current guidelines recommendations individually adjusted (including antiplatelet drugs, statins, angiotensin‐converting enzyme inhibitors, and β‐blocker). Thirty‐one patients (31%) received thrombolytic therapy (Actilyse; Boehringer Ingelheim, Ingelheim am Rhein, Germany) according to the appropriate therapeutic window.
The study protocol has been published previously.16 Briefly, inclusion criteria were: age ≥21 years; presence of acute ischemic stroke within the middle cerebral artery territory; and neurological deficit according to the National Institutes of Health Stroke Scale (NIHSS) ≤12. Exclusion criteria were acute and chronic inflammatory diseases, immune suppressive therapy, history of cancer shorter than 5 years, and women with known pregnancy.
The protocol was approved by the Charité Ethics Committee, and written informed consent was obtained from all subjects.
Baseline Examinations
Baseline examinations were completed in‐hospital in the acute phase after stroke. Stroke‐related neurological deficit was evaluated by the NIHSS and estimation of ischemic brain injury volume was assessed using the Alberta Stroke Program Early CT score.17 Functional impairment and disability were evaluated by the modified Rankin Scale (mRS) and the Barthel Index (BI). Venous blood samples were obtained under standardized conditions after overnight fasting. Standard biochemical parameters were assessed immediately in the routine clinical laboratory. Body mass index was calculated as a ratio of body weight and squared height (kg/m²).
Assessment of SDB
Screening for SDB was performed 4±2 days after symptom onset using transthoracic impedance recording integrated into a Holter system (CardioDay; Getemed, Teltow, Germany). Analyses of SDB were performed visually as previously described.18 Presence of SDB was defined by apnea‐hypopnea index (AHI) ≥5 episodes per hour.19
Peripheral endothelial function
Quantitative determination of peripheral endothelial function was assessed by application of finger plethysmograph (EndoPAT2000; Itamar Medical, Caesarea, Israel) as described previously.13 Assessments were performed 4±2 days after symptom onset under standardized conditions after at least 15 minutes of supine rest in a quiet, air‐conditioned room. An estimation of endothelial function was based on peripheral arterial tonometry of the index finger of the nonparetic arm. A reactive hyperemia index (RHI) was defined as a ratio between the post‐ and preoccluded measurement of the peripheral arterial tonometry signal corrected for signal of the nonoccluded contralateral to the brain lesion arm. ED was considered with RHI ≤1.8.
Cardiovascular assessment
Echocardiographic evaluation of myocardial morphology and global left ventricular function was performed (Vivid S5 with 3S‐RS 1.5–3.6 MHz transducer; GE Medical Systems, Marlborough, MA). Left ventricular ejection fraction (LVEF) was calculated according to the Simpson biplane method. Left ventricular diastolic dysfunction was determined according to the diagnostic criteria of the European Society of Cardiology: septal (<7 cm/s) or lateral (<10 cm/s) mitral annular early‐diastolic (e′) peak velocity by pulsed‐waved spectral tissue Doppler imaging and LVEF 50% to 55%.20 Left ventricular systolic dysfunction was considered in patients with clinical sings of heart failure (HF) and LVEF ≤50%. Patients with normal LVEF (≥55%) and without clinical signs of HF were considered to have no HF.
One Year Follow‐up
Repeated measurements of the baseline study examinations were conducted in 41 patients at 1‐year follow‐up (FU). Sixty patients were lost to FU.
Statistical Analysis
All data were presented as means± SD, median (interquartile range; IQR) or percentage, as appropriate. Data were tested for normal distribution using the Kolmogorov–Smirnov test. Statistical comparisons were made using paired or unpaired Student t tests or Mann–Whitney U test or Kruskal–Wallis test. The chi‐squared test was used to assess categorical distribution between groups. Simple linear regression and Pearson correlation and uni‐ and multivariate logistic regression analyses were used, as appropriate. A P<0.05 was considered statistically significant. Statistical analyses were performed with the StatView software package (version 5.0; SAS Institute Inc, Cary, NC).
Results
One‐hundred one patients with acute ischemic stroke (69±12 years; body mass index, 28.2±4.6 kg/m2) were studied within 4±2 days after symptom onset. The study cohort consisted of 62 (61%) male and 39 female patients. Patients were mild to moderate disabled (median NIHSS, 4.0 [IQR, 2–7]; mean BI, 71±33; mean mRS, 2.3±1.5; Table 1). Median AHI in the entire study cohort was 5.7 [IQR, 3–13] episodes per hour. Baseline characteristics of all patients are given in Table 1.
Table 1.
Baseline Characteristics of Study Population
| Clinical Parameter | All Patients (N=101) | Patients Without SDB (N=43) | Patients With SDB (N=58) | P Values |
|---|---|---|---|---|
| Age, y | 69±12 | 68±13 | 69±11 | 0.7 |
| Male sex, % | 61 | 47 | 72 | 0.008 |
| BMI, kg/m2 | 28.2±4.6 | 28.7±5.7 | 27.8±3.5 | 0.3 |
| Diastolic BP, mm Hg | 80±14 | 81±15 | 79±13 | 0.5 |
| Systolic BP, mm Hg | 141±21 | 142±24 | 140±18 | 0.7 |
| Thrombolytic therapy, % | 31 | 23 | 21 (36) | 0.2 |
| AHI, episodes/h | 5.7 [3–13] | 2.5 [1–3] | 11.6 [7–19] | <0.0001 |
| Stroke severity | ||||
| ASPECT | 8.4±1.4 | 8.9±1.1 | 8.1±1.6 | 0.007 |
| NIHSS | 4.0 [2–7] | 3 [2–4] | 5 [2.75–8] | 0.01 |
| NIHSS ≥5, % | 41 | 23 | 53 | 0.002 |
| Barthel Index | 71±33 | 80±25 | 64±37 | 0.014 |
| Modified Rankin | 2.3±1.5 | 1.8±1.1 | 2.8±1.6 | <0.001 |
| Right hemispheric stroke, % | 55 | 49 | 61 | 0.3 |
| Medical history | ||||
| Atrial fibrillation, % | 18 | 14 | 21 | 0.5 |
| History of sleep apnea, % | 2 | ··· | 3 | ··· |
| Hypertension, % | 69 | 61 | 76 | 0.1 |
| Diabetes mellitus, % | 26 | 21 | 29 | 0.4 |
| Dyslipidemia, % | 33 | 33 | 33 | 0.9 |
| Biochemistry | ||||
| Hemoglobin, g/L | 14.5±1.6 | 14.2±2.0 | 14.8±1.3 | 0.2 |
| Glucose, mg/dL | 112±42 | 115±47 | 109±38 | 0.5 |
| HbA1C, mg/dL | 6.1±1.1 | 6.2±1.3 | 5.9±0.9 | 0.2 |
| Sodium, mmol/L | 140.6±3.5 | 140.1±3.9 | 141.1±3.2 | 0.2 |
| Potassium, mmol/L | 4.0±0.4 | 4.1±0.4 | 4.0±0.5 | 0.3 |
| Triglyceride, mg/dL | 139±61 | 138±62 | 139±61 | 0.9 |
| Cholesterol, mg/dL | 185±44 | 191±46 | 178±43 | 0.1 |
| Low‐density lipoprotein, mg/dL | 108±39 | 113±37 | 104±41 | 0.2 |
| High‐density lipoprotein, mg/dL | 48.0±15.0 | 51.1±1 6.5 | 46.0±13.7 | 0.1 |
| Creatinine, mg/dL | 0.9±0.2 | 0.9±0.2 | 1.0±0.2 | 0.2 |
| C‐reactive protein, mg/dL | 4.8 [2–8] | 4.8 [2–8] | 5.0 [2–9] | 0.3 |
| Uric acid, mg/dL | 5.3±1.4 | 5.1±1.4 | 5.5±1.4 | 0.3 |
Values are mean±SD, median [interquartile range], or percentage. AHI indicates Apnea‐hypopnea index; ASPECT, Alberta Stroke Program Early CT; BMI, body mass index; BP, blood pressure; HbA1C, hemoglobin A1C; NIHSS, National Institutes of Health Stroke Scale; SDB, sleep‐disordered breathing.
Patients were divided according to the presence or absence of SDB. In 58 patients (57%), the presence of SDB was identified (median AHI, 11.6 [IQR, 7–18.25] episodes per hour). This subgroup consisted mainly of male patients (72%). Compared with patients without SDB, patients with SDB had more‐severe neurological deficit according to the NIHSS (Figure 1A), larger infarct volume in approximation by Alberta Stroke Program Early CT score (Figure 1B), as well as higher functional impairment according to the mRS and BI (Figure 1C and 1D; Table 1). No further differences were observed regarding comorbidities or clinical and biochemical characteristics between both of the subgroups (Table 1).
Figure 1.
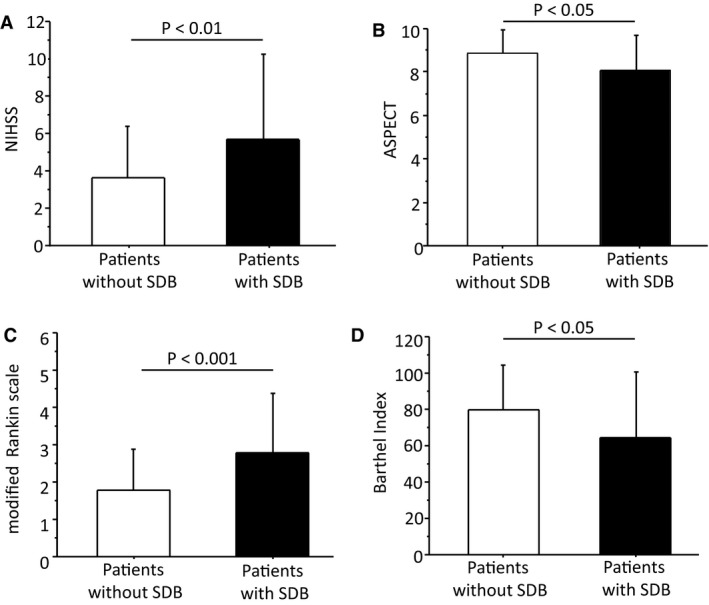
Neurological deficit at baseline according to the National Institutes of Health Stroke Scale (NIHSS); (A); estimation of stroke‐related brain lesion according to the Alberta Stroke Program Early CT (ASPECT) score (B); functional impairment at baseline according to the modified Rankin scale (mRS) (C); functional disability at baseline according to the Barthel index (D) in patients without sleep‐disordered breathing (SDB) compared with the patients with SDB.
Peripheral Endothelial Function in Relation to SDB After Acute Ischemic Stroke
Patients with SDB showed peripheral ED—as indicated by RHI—compared with patients without SDB (RHI 1.7±0.5 versus 2.0±0.4; P=0.001; Figure 2A). Peripheral ED was present in 64% of patients with SDB and in 32% of patients without SDB (P=0.003). ED was strongly associated with higher AHI in simple regression analysis (r=−0.38; P<0.001; Figure 2B). In univariate logistic regression, presence of SDB was associated with presence of peripheral ED (odds ratio [OR], 3.9 [95% confidential interval {CI}, 1.6–9.4]; P=0.003; Table 2). After adjustment for sex, age, and body mass index, the presence of SDB remained independently associated with the presence of ED (OR, 3.1 [CI, 1.2–7.9]; P<0.05).
Figure 2.
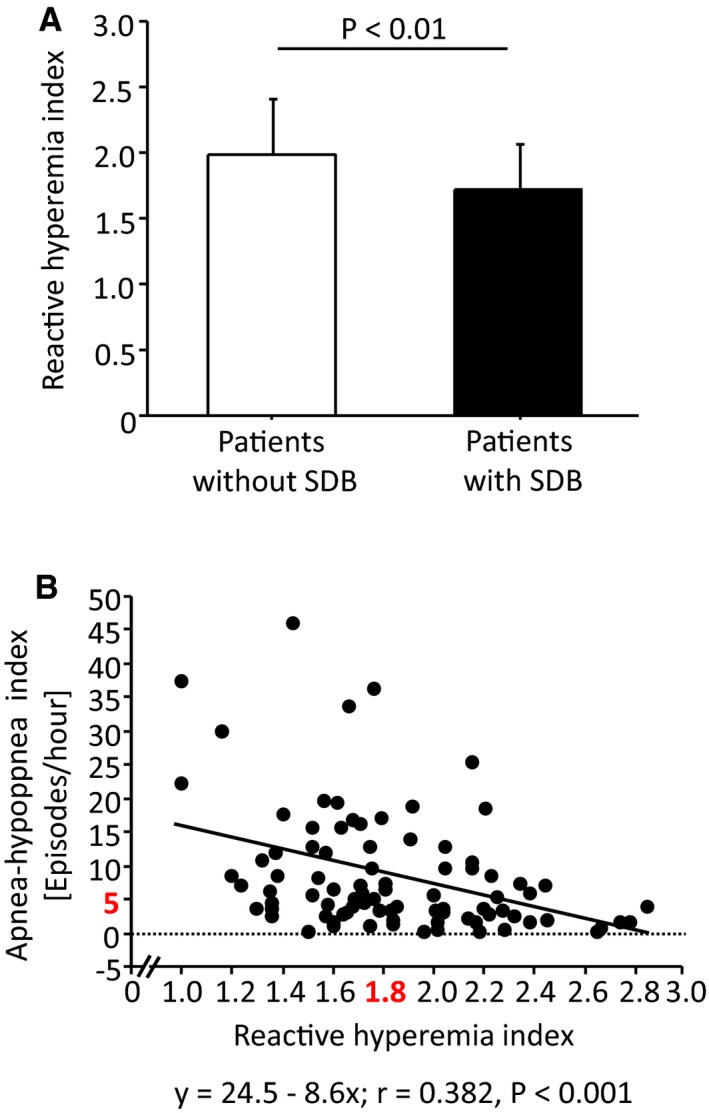
Peripheral endothelial function according to reactive hyperemia index (RHI) in patients without sleep‐disordered breathing (SDB) and in those with SDB after acute ischemic stroke (A). Association between the peripheral endothelial function according to RHI and the severity of SDB according to apnea‐hypopnea index (AHI) (B).
Table 2.
Logistic Regression Analyses Between Presence of SDB and Clinical Variables
| Parameter | OR | 95% CI | P Value |
|---|---|---|---|
| Univariate analyses | |||
| Reactive hyperemia index, per 0.1 point | 0.15 | 0.05 to 0.51 | 0.002 |
| Presence of endothelial dysfunction | 3.57 | 1.49 to 8.58 | 0.004 |
| NIHSS, per point | 1.19 | 1.05 to 1.36 | 0.009 |
| NIHSS ≥5 | 3.79 | 1.58 to 9.10 | 0.003 |
| Barthel index, per 10 points | 0.88 | 0.78 to 0.99 | 0.046 |
| Modified Rankin Scale, per point | 1.66 | 1.22 to 2.26 | 0.001 |
| ASPECT, per point | 0.62 | 0.43 to 0.89 | 0.01 |
| Male sex | 3.02 | 1.32 to 6.93 | 0.009 |
| Lesion of right hemisphere | 1.48 | 0.67 to 3.28 | 0.33 |
| Atrial fibrillation | 1.61 | 0.55 to 4.70 | 0.38 |
| LVEF, per 5% | 0.89 | 0.69 to 1.13 | 0.33 |
| E/e′ ratio | 1.00 | 0.92 to 1.09 | 0.92 |
| Multivariate analyses (adjusted for age, sex, BMI) | |||
| 1. Reactive hyperemia index | 0.17 | 0.05 to 0.60 | 0.006 |
| 2. Presence of endothelial dysfunction | 3.09 | 1.21 to 7.87 | 0.018 |
| 3. Presence of endothelial dysfunction and NIHSS ≥5 | 17.6 | 2.18 to 142.3 | 0.007 |
ASPECT indicates Alberta Stroke Program Early CT; BMI, body mass index; CI, confidence interval; LVEF, left ventricular ejection fraction; NIHSS, National Institutes of Health Stroke Scale; OR, odds ratio; SDB, sleep‐disordered breathing.
Logistic Regression Analyses
In univariate logistic regression an association between the presence of SDB and neurological deficit according to the NIHSS (OR, 1.2 [95% CI, 1.1–1.4]; P<0.01), functional impairment according to the mRS (OR, 1.7 [95% CI, 1.2–2.3]; P<0.001) or to the BI (OR, 0.9 [95% CI 0.8–1.0]; P<0.05), and male sex (OR, 3.0 [95% CI, 1.3–6.9]; P=0.01) was found at baseline (Table 2). The stroke affected hemisphere, atrial fibrillation, and cardiac function (E/e′ ratio or LVEF) were not correlated with presence of SDB in the acute phase after ischemic stroke (Table 2).
Cardiac Function and SDB in Acute Stroke
Basic echocardiographic parameters were similar in patients with or without SDB (Table 3). Comparing stroke patients without HF (n=21) with patients with Left ventricular diastolic dysfunction (n=62) or those with left ventricular systolic dysfunction (n=18) revealed higher AHI in patients with left ventricular systolic dysfunction (P=0.04; Figure 3). No significant difference in SDB prevalence was observed between these groups (no HF, 43%; left ventricular diastolic dysfunction, 58%; left ventricular systolic dysfunction, 72%).
Table 3.
Basic Echocardiographic Characteristics of the Study Groups
| Parameter | Patients Without SDB (N=43) | Patients With SDB (N=58) | P Values |
|---|---|---|---|
| HR, bpm | 71±11 | 72±12 | 0.9 |
| LVEF, % | 57±7 | 56±10 | 0.3 |
| LA diameter, mm | 41.3±5.3 | 42.0±6.0 | 0.5 |
| LV wall diastolic diameter, mm | 11.1±2.3 | 11.5±2.2 | 0.5 |
| LV diastolic diameter, mm | 47.3±8.3 | 48.3±6.9 | 0.5 |
| IVS diastolic diameter, mm | 12.4±2.4 | 13.1±2.4 | 0.1 |
| Septal e′ mitral annular velocity by TDI, cm/s | 7.0±2.7 | 6.5±2.6 | 0.5 |
| Lateral e′ mitral annular velocity by TDI, cm/s | 8.9±3.2 | 7.4±2.6 | 0.1 |
| E/e′ ratio | 11±5 | 12±5 | 0.9 |
| RA diameter, mm | 38.0±7.5 | 35.4±8.4 | 0.3 |
| RV diastolic diameter, mm | 2.9±0.7 | 3.5±1.7 | 0.3 |
| TAPSE, mm | 23±6 | 22±5 | 0.3 |
Values are mean±standard deviation. bpm indicates beats per minute; HR, heart rate; IVS, intraventricular septum; LA, left atrial; LV, left ventricular; LVEF, left ventricular ejection fraction; RA, right atrial; RV, right ventricular; SDB, sleep‐disordered breathing; TAPSE, tricuspid annular plane systolic excursion; TDI, tissue Doppler imaging.
Figure 3.
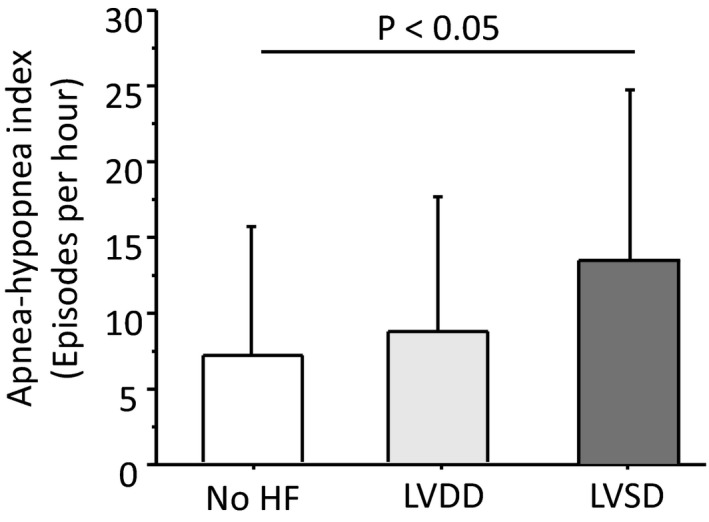
Severity of sleep‐disordered breathing (SDB) according to apnea‐hypopnea index (AHI) in patients without heart failure (HF), in those with left ventricular diastolic dysfunction (LVDD) and in those with left ventricular systolic dysfunction (LVSD).
SDB and Endothelial Function 1 Year After Ischemic Stroke
Clinical characteristics of 41 patients who were available at 1‐year FU are given in Table 4. One year after stroke, 19 patients (46%) improved significantly their functional capacity according to the BI (delta BI, 27±21; P<0.001) compared to baseline, 18 (44%) remained unchanged, whereas 4 worsened their functional capacity (delta BI, −19±13; P=0.058). Furthermore, 63% of the patients revealed a body weight increase compared to baseline (delta body weight 6.2±5.6 kg; P=0.002), whereas 37% showed a body weight lost (delta body weight, −3.7±3.8 kg; P<0.0001). Prevalence of SDB decreased from 59% at baseline to 15% at 1 year follow‐up (P<0.001). Median AHI decreased from 7.1 [IQR, 3–13.75] episodes per hour at baseline to 2.15 [2–3.75] episodes per hour at 1‐year FU (P<0.001). All 6 stroke patients with sustained SDB had right hemispheric middle cerebral artery stroke. Notably, only stroke patients with normalized nocturnal breathing pattern compared with baseline showed improved peripheral endothelial function after 1 year (RHI, 2.0±0.6 versus 1.7±0.3 at baseline, P=0.03, respectively; Figure 4). By contrast, patients with sustained SDB after 1 year still showed ED (RHI, 1.7±0.5 at FU versus 1.7±0.3 at baseline, P=0.9; Figure 4).
Table 4.
Clinical Characteristics of Patients Completed 1 Year FU Examinations
| Baseline (N=41) | 1 Year FU (N=41) | P Values | |
|---|---|---|---|
| Age, y | 68±12 | 69±11 | <0.001 |
| BMI, kg/m² | 27.6±4.0 | 28.5±4.5 | 0.03 |
| Male sex, n (%) | 28 (70) | 28 (70) | ··· |
| Days after stroke | 3±2 | 390±24 | <0.001 |
| Thrombolytic therapy, n (%) | 11 (27) | ··· | ··· |
| Presence of SDB, n (%) | 24 (58.5) | 6 (14.6) | <0.001 |
| AHI, episodes/h | 7.0 [3–13] | 2.15 [2–3.75] | <0.001 |
| RHI | 1.8±0.4 | 1.9±0.5 | 0.2 |
| Barthel Index | 80±27 | 93±17 | 0.001 |
| Modified Rankin Scale | 2.0±1.2 | 1.3±1.1 | <0.001 |
AHI indicates apnea‐hypopnea index; BMI, body mass index; FU, follow‐up; RHI, reactive hyperemia index; SDB, sleep‐disordered breathing.
Figure 4.
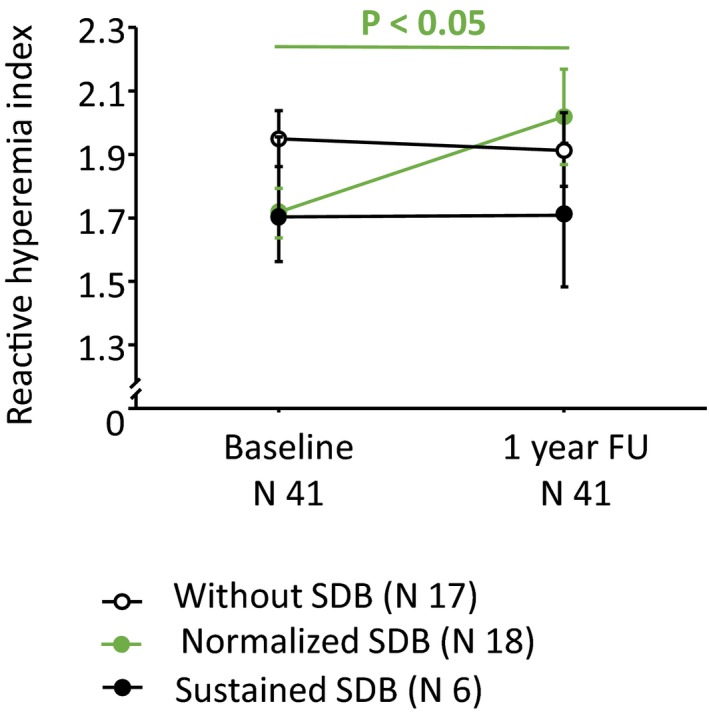
Peripheral endothelial function according to reactive hyperemia index (RHI) in the follow‐up cohort at baseline and at 1‐year follow‐up examination. SDB indicates sleep‐disordered breathing.
Discussion
The main findings of the present study are (1) the association of peripheral ED with presence of SDB in patients with acute stroke and (2) an improvement of peripheral endothelial function in a subset of study patients with recovery of SDB 1 year after ischemic stroke. Furthermore, we confirm earlier studies showing that SDB is frequently found in patients with acute ischemic stroke, particularly in those with moderate neurological deficit.
Influence of SDB on the Endothelial Function
We observed a strong association between presence of SDB and peripheral ED in the acute phase after ischemic stroke. Whereas ED belongs to established risk factors contributing to the development of cerebrovascular and cardiovascular diseases, ED was also found in around 30% of stroke patients without SBD in the acute phase after ischemic stroke. Increased sympathetic activation after stroke might lead to transient worsening of autonomic function.11 Our results support this hypothesis by observing an improvement of endothelial function in patients who recovered from SDB 1 year after the index stroke, but not in those patients with persistent SDB. Our data support an association between the SBD and ED, because the presence of SDB remained independently associated with the peripheral ED (adjusted harzard ratio, 3.4 [95% CI, 1.27–9.01]).
Peripheral vascular function has been analyzed in several studies before. Peripheral arterial tonometry techniques—based on the reactive hyperemia induced by forearm ischemia—enables noninvasive measurements of the peripheral endothelial function and correlates significantly with flow‐mediated dilation of the brachial artery.21 The measurement of endothelial function in the current study was performed on the index finger of the nonparetic arm. We therefore believe that the findings represent a systemic effect on endothelial function and are not biased by local effects of the paretic limb.
The association between ED and SDB has been shown in few studies.22, 23, 24 However, a recent study stated an association of moderate and severe SDB with increased arterial stiffness in patients 3 months after stroke, but did not find an association with endothelial dysfunction.15 Because repeated measurements in our study demonstrate a temporal course of an association, the delayed follow‐up in the recent study may explain the respective findings. Our observations are in line with previous reports showing a temporal imbalance of autonomic nervous regulation that attenuates within days after acute stroke.11 A recent meta‐analysis demonstrated a clinically relevant improvement of endothelial function after treatment of OSA with continuous positive airway pressure, which is also in accord with our findings.25
SDB and Autonomic Dysfunction
Consistent with previous studies,26, 27 our study showed that stroke patients with SDB had more often severe stroke compared with those without SDB, whereas both patient groups were comparable regarding demographic and comorbidity status as well as metabolic, cardiac, and inflammatory characteristics. The association of SDB with neurological impairment might be related to stroke‐associated autonomic vegetative imbalance. However, a previous study showed an association between severe stroke and progressive failure of cardiac autonomic function in 50 patients with ischemic stroke.28 In our present study, the Alberta Stroke Program Early CT score at baseline—as an estimation of stroke volume—was associated with a higher prevalence of SDB. Furthermore, we observed an association between SDB and peripheral ED, potentially serving as a downstream surrogate marker of autonomic function.
Of note, other factors than impaired central control of vegetative regulation may contribute to ED in acute stroke as well. Previously, we have shown a role of the l‐arginine/nitric oxide pathway in the peripheral endothelial function in patients with acute stroke, by observing elevated levels of asymmetric dimethylarginine, an endogenous inhibitor of nitric oxide synthesis, in parallel to stroke severity.13
SDB: A Potential Treatment Target After Stroke?
The question is whether patients should be treated for SDB in the acute phase of stroke. Based on the observed association in the present study, normalized breathing pattern may attenuate autonomic nervous imbalance and may hence prevent clinical complications such as arrhythmias, blood pressure peaks, or ED. Given the transient character of this neurovegetative imbalance, also, a temporary intervention may be effective.11
In contrast to these considerations, however, recent data put into question a potential benefit from augmented ventilation support therapy in the SDB. Considering Cheyne–Stoke respiration as a part of the compensatory mechanism in autonomic dysfunction,6 manipulating this breathing pattern could be detrimental. Indeed, a recent large, randomized treatment trial (SERVE‐HF [The Treatment of Predominant Central Sleep Apnoea by Adaptive Servo Ventilation in Patients With Heart Failure]) investigated the treatment of the SBD by adaptive servoventilation in patients with chronic HF with reduced ejection fraction showed an increased mortality in the intervention group.29 The investigators hypothesized that distraction from the compensatory breathing pattern by the adaptive servo ventilation might be responsible for the adverse outcome in the study.
Notably, all patients with sustained SDB at 1‐year FU in the present study suffered from a right hemispheric stroke. Both hemispheres are known to have a different influence on autonomic function, and increased sympathetic activity has been observed in right hemispheric stroke in experimental stroke models and in human stroke.30
There are some limitations of the present study. The study population was limited to patients with mild‐to‐moderate stroke. One could speculate that a more‐pronounced mechanistic interaction may have been observed in patients with even more‐severe stroke. Indeed, we detected only a small number of patients with severe SBD in this specific study population. This makes the founded changes in ED more remarkable. Furthermore, the detection of SDB was based on 1 parameter. The standard sleep apnea monitoring includes a minimum of 3 parameters: airflow, respiratory effort, and blood oxygenation.31 Impedance is a known technique for detection of the thoracic effort, but does not allow distinguishing between central and obstructive breathing disorders. Furthermore, the calculated AHI is an estimation because sleep time was anamnestic collected. However, screening is feasible and accurate.18, 32 Another limitation was a small number of patients available for FU assessment. One year after stroke, all of the patients were contacted either by telephone or by mail and invited to 1‐year FU. As a result, roughly 40% of the patients were able to come to the hospital. The analyses of patients who came to 1‐year FU in comparison with the rest of the entire study cohort (age, 70±12 years; body mass index, 28.6±4.9 kg/m2) revealed better physical performance (BI, 80±26 versus 64±35; P=0.016, and mRS, 2.0±1.2 versus 2.6±1.6; P=0.026, respectively) at baseline. Thus, moderate functional impairment and long‐term disability might be causal for the high rate of loss to FU in the present study.
Conclusions
This study explores an interaction between ischemic stroke, SDB, and peripheral ED (Figure 5). This is an important observation, which identifies a modifiable risk factor, and even a potential therapeutic target. Acute ischemic stroke is accompanied by a high prevalence of SDB, probably attributed to loss of cortical control and autonomic nervous system imbalance. Whereas SDB was transient in a subset of stroke patients, peripheral ED—as a surrogate marker of autonomic dysfunction—was associated with the presence of SDB after acute ischemic stroke and during the clinical course. Further studies are needed to analyze an impact of SDB in patients with ischemic stroke.
Figure 5.
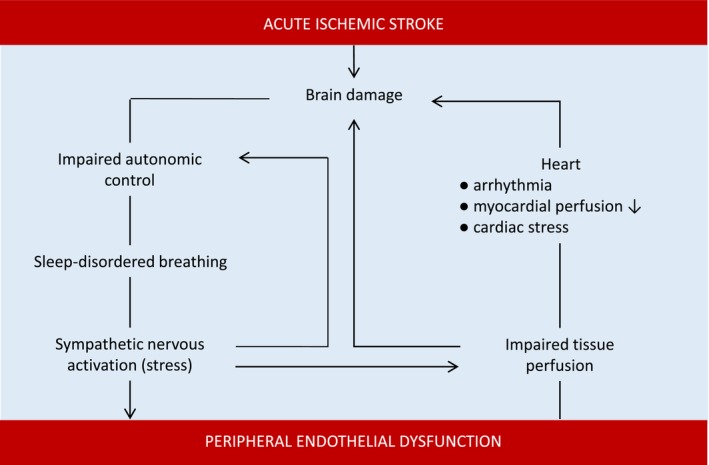
Interaction between acute ischemic stroke, sleep‐disordered breathing, and peripheral endothelial dysfunction.
Sources of Funding
This work was supported by the grant from the German Federal Ministry of Education and Research (Center for Stroke Research Berlin; 01GEO0801).
Disclosures
None.
(J Am Heart Assoc. 2017;6:e006010 DOI: 10.1161/JAHA.117.006010.)28893762
References
- 1. Hermann DM, Bassetti CL. Role of sleep‐disordered breathing and sleep‐wake disturbances for stroke and stroke recovery. Neurology. 2016;87:1407–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Floras JS. Sleep apnea and cardiovascular risk. J Cardiol. 2014;63:3–8. [DOI] [PubMed] [Google Scholar]
- 3. Stone KL, Blackwell TL, Ancoli‐Israel S, Barrett‐Connor E, Bauer DC, Cauley JA, Ensrud KE, Hoffman AR, Mehra R, Stefanick ML, Varosy PD, Yaffe K, Redline S; Osteoporotic Fractures in Men (MrOS) Study Research Group . Sleep‐disordered breathing and risk of stroke in older community‐dwelling men. Sleep. 2016;39:531–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ifergane G, Ovanyan A, Toledano R, Goldbart A, Abu‐Salame I, Tal A, Stavsky M, Novack V. Obstructive sleep apnea in acute stroke: a role for systemic inflammation. Stroke. 2016;47:1207–1212. [DOI] [PubMed] [Google Scholar]
- 5. Birchfield RI, Sieker HO, Heyman A. Alterations in blood gases during natural sleep and narcolepsy; a correlation with the electroencephalographic stages of sleep. Neurology. 1958;8:107–112. [DOI] [PubMed] [Google Scholar]
- 6. Heyman A, Birchfield RI, Sieker HO. Effects of bilateral cerebral infarction on respiratory center sensitivity. Neurology. 1958;8:694–700. [DOI] [PubMed] [Google Scholar]
- 7. Nopmaneejumruslers C, Kaneko Y, Hajek V, Zivanovic V, Bradley TD. Cheyne‐Stokes respiration in stroke: relationship to hypocapnia and occult cardiac dysfunction. Am J Respir Crit Care Med. 2005;171:1048–1052. [DOI] [PubMed] [Google Scholar]
- 8. Roquer J, Segura T, Serena J, Castillo J. Endothelial dysfunction, vascular disease and stroke: the ARTICO study. Cerebrovasc Dis. 2009;27(Suppl 1):25–37. [DOI] [PubMed] [Google Scholar]
- 9. Dütsch M, Burger M, Dörfler C, Schwab S, Hilz MJ. Cardiovascular autonomic function in poststroke patients. Neurology. 2007;69:2249–2255. [DOI] [PubMed] [Google Scholar]
- 10. Korpelainen JT, Sotaniemi KA, Myllylä VV. Autonomic nervous system disorders in stroke. Clin Auton Res. 1999;9:325–333. [DOI] [PubMed] [Google Scholar]
- 11. Orlandi G, Fanucchi S, Strata G, Pataleo L, Landucci Pellegrini L, Prontera C, Martini A, Murri L. Transient autonomic nervous system dysfunction during hyperacute stroke. Acta Neurol Scand. 2000;102:317–321. [DOI] [PubMed] [Google Scholar]
- 12. Mochmann HC, Scheitz JF, Petzold GC, Haeusler KG, Audebert HJ, Laufs U, Schneider C, Landmesser U, Werner N, Endres M, Witzenbichler B, Nolte CH; TRELAS Study Group . Coronary angiographic findings in acute ischemic stroke patients with elevated cardiac troponin: the troponin elevation in acute ischemic stroke (TRELAS) study. Circulation. 2016;133:1264–1271. [DOI] [PubMed] [Google Scholar]
- 13. Scherbakov N, Sandek A, Martens‐Lobenhoffer J, Kung T, Turhan G, Liman T, Ebinger M, von Haehling S, Bode‐Böger SM, Endres M, Doehner W. Endothelial dysfunction of the peripheral vascular bed in the acute phase after ischemic stroke. Cerebrovasc Dis. 2012;33:37–46. [DOI] [PubMed] [Google Scholar]
- 14. Kunz AB, Kraus J, Young P, Reuss R, Wipfler P, Oschmann P, Blaes F, Dziewas R. Biomarkers of inflammation and endothelial dysfunction in stroke with and without sleep apnea. Cerebrovasc Dis. 2012;33:453–460. [DOI] [PubMed] [Google Scholar]
- 15. Cereda CW, Tamisier R, Manconi M, Andreotti J, Frangi J, Pifferini V, Bassetti CL. Endothelial dysfunction and arterial stiffness in ischemic stroke: the role of sleep‐disordered breathing. Stroke. 2013;44:1175–1178. [DOI] [PubMed] [Google Scholar]
- 16. Knops M, Werner CG, Scherbakov N, Fiebach J, Dreier JP, Meisel A, Heuschmann PU, Jungehülsing GJ, von Haehling S, Dirnagl U, Anker SD, Doehner W. Investigation of changes in body composition, metabolic profile and skeletal muscle functional capacity in ischemic stroke patients: the rationale and design of the Body Size in Stroke Study (BoSSS). J Cachexia Sarcopenia Muscle. 2013;4:199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pexman JH, Barber PA, Hill MD, Sevick RJ, Demchuk AM, Hudon ME, Hu WY, Buchan AM. Use of the Alberta Stroke Program Early CT Score (ASPECTS) for assessing CT scans in patients with acute stroke. AJNR Am J Neuroradiol. 2001;22:1534–1542. [PMC free article] [PubMed] [Google Scholar]
- 18. Mueller A, Fietze I, Voelker R, Eddicks S, Glos M, Baumann G, Theres H. Screening for sleep‐related breathing disorders by transthoracic impedance recording integrated into a Holter ECG system. J Sleep Res. 2006;15:455–462. [DOI] [PubMed] [Google Scholar]
- 19. Somers VK, White DP, Amin R, Abraham WT, Costa F, Culebras A, Daniels S, Floras JS, Hunt CE, Olson LJ, Pickering TG, Russell R, Woo M, Young T. Sleep apnea and cardiovascular disease: an American Heart Association/American College of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing. J Am Coll Cardiol. 2008;52:686–717. [DOI] [PubMed] [Google Scholar]
- 20. McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez‐Sanchez MA, Jaarsma T, Køber L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Rønnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A; ESC Committee for Practice Guidelines . ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2012;33:1787–1847. [DOI] [PubMed] [Google Scholar]
- 21. Kuvin JT, Patel AR, Sliney KA, Pandian NG, Sheffy J, Schnall RP, Karas RH, Udelson JE. Assessment of peripheral vascular endothelial function with finger arterial pulse wave amplitude. Am Heart J. 2003;146:168–174. [DOI] [PubMed] [Google Scholar]
- 22. Wang J, Yu W, Gao M, Zhang F, Gu C, Yu Y, Wei Y. Impact of obstructive sleep apnea syndrome on endothelial function, arterial stiffening, and serum inflammatory markers: an updated meta‐analysis and metaregression of 18 studies. J Am Heart Assoc. 2015;4:e002454. doi: 10.1161/JAHA.115.002454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Itzhaki S, Lavie L, Pillar G, Tal G, Lavie P. Endothelial dysfunction in obstructive sleep apnea measured by peripheral arterial tone response in the finger to reactive hyperemia. Sleep. 2005;28:594–600. [DOI] [PubMed] [Google Scholar]
- 24. Nieto FJ, Herrington DM, Redline S, Benjamin EJ, Robbins JA. Sleep apnea and markers of vascular endothelial function in a large community sample of older adults. Am J Respir Crit Care Med. 2004;169:354–360. [DOI] [PubMed] [Google Scholar]
- 25. Schwarz EI, Puhan MA, Schlatzer C, Stradling JR, Kohler M. Effect of CPAP therapy on endothelial function in obstructive sleep apnoea: a systematic review and meta‐analysis. Respirology. 2015;20:889–895. [DOI] [PubMed] [Google Scholar]
- 26. Good DC, Henkle JQ, Gelber D, Welsh J, Verhulst S. Sleep‐disordered breathing and poor functional outcome after stroke. Stroke. 1996;27:252–259. [DOI] [PubMed] [Google Scholar]
- 27. Aaronson JA, van Bennekom CA, Hofman WF, van Bezeij T, van den Aardweg JG, Groet E, Kylstra WA, Schmand B. Obstructive sleep apnea is related to impaired cognitive and functional status after stroke. Sleep. 2015;38:1431–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hilz MJ, Moeller S, Akhundova A, Marthol H, Pauli E, De Fina P, Schwab S. High NIHSS values predict impairment of cardiovascular autonomic control. Stroke. 2011;42:1528–1533. [DOI] [PubMed] [Google Scholar]
- 29. Eulenburg C, Wegscheider K, Woehrle H, Angermann C, d'Ortho MP, Erdmann E, Levy P, Simonds AK, Somers VK, Zannad F, Teschler H, Cowie MR. Mechanisms underlying increased mortality risk in patients with heart failure and reduced ejection fraction randomly assigned to adaptive servoventilation in the SERVE‐HF study: results of a secondary multistate modelling analysis. Lancet Respir Med. 2016;4:873–881. [DOI] [PubMed] [Google Scholar]
- 30. Hachinski VC, Oppenheimer SM, Wilson JX, Guiraudon C, Cechetto DF. Asymmetry of sympathetic consequences of experimental stroke. Arch Neurol. 1992;49:697–702. [DOI] [PubMed] [Google Scholar]
- 31. Collop NA, Anderson WM, Boehlecke B, Claman D, Goldberg R, Gottlieb DJ, Hudgel D, Sateia M, Schwab R; Portable Monitoring Task Force of the American Academy of Sleep Medicine . Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. Portable Monitoring Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2007;3:737–747. [PMC free article] [PubMed] [Google Scholar]
- 32. Scharf C, Cho YK, Bloch KE, Brunckhorst C, Duru F, Balaban K, Foldvary N, Liu L, Burgess RC, Candinas R, Wilkoff BL. Diagnosis of sleep‐related breathing disorders by visual analysis of transthoracic impedance signals in pacemakers. Circulation. 2004;110:2562–2567. [DOI] [PubMed] [Google Scholar]


