Abstract
Background
Chronic kidney disease is characterized by stiffening, thinning, dilatation, and increased circumferential wall stress of large arteries, associated with increased cardiovascular risk. Kidney transplantation (KT) reverses many pathological features of chronic kidney disease and improves life expectancy; however, longitudinal studies exploring the impact of KT on recipient large arteries are scarce.
Methods and Results
This study was designed to appraise arterial changes following KT. Carotid‐femoral pulse wave velocity, carotid remodeling (circumferential wall stress and carotid internal diameter), and stiffness were measured in 161 consecutive recipients receiving either a living (n=49) or a deceased (n=112) donor allograft, at 3 and 12 months after transplantation. Mean pulse wave velocity decreased from 10.8 m/s (95% confidence interval, 10.5–11.2 m/s) (at month 3) to 10.1 m/s (95% confidence interval, 9.8–10.5 m/s) (at month 12) (P<0.001). After multivariate adjustment, pulse wave velocity reduction from month 3 to month 12 was significantly larger in the living donor allograft KT (P<0.001). Circumferential wall stress decreased, 70 kPa (95% confidence interval, 68–72 kPa) to 64 kPa (95% confidence interval, 62–67 kPa), as well as carotid internal diameter and carotid stiffness (P<0.001 for all). Reductions in circumferential wall stress, diameter, and stiffness were significantly larger in the living donor allograft KT (P<0.001). When deceased donor allograft patients were classified into standard and expanded criteria donors, changes in both pulse wave velocity and circumferential wall stress were blunted in expanded criteria donors. Changes were independent of graft function and blood pressure changes.
Conclusions
Large‐artery stiffness and maladaptive carotid artery remodeling of chronic kidney disease is partially reversed within 12 months of KT and appears unrelated to renal function. Improvements were independently associated with live organ donation. Our data suggest that expanded criteria donors may hamper vascular recovery.
Keywords: renal disease, stiffness, transplantation, vascular function, vascular remodeling
Subject Categories: Vascular Disease, Nephrology and Kidney
Clinical Perspective
What Is New?
Kidney transplantation can reverse the modifications of large arteries induced by chronic kidney disease, such as stiffening, dilatation, decreased thickness, and increased wall stress.
Improvement of vascular health in allograft recipients is more pronounced when transplantation is performed with living donors, independently of post‐transplant renal function.
In contrast, kidney transplantation with extended criteria deceased donors—those older than 60 or those older than 50 years with a history of cardiovascular disease—may hamper arterial recovery of the recipient.
What Are the Clinical Implications?
In patients with chronic kidney disease with significant cardiovascular risk factors and/or complications, living donor kidney transplantation may contribute to the improvement of post‐transplant cardiovascular morbidity and mortality.
Kidney recipients of extended criteria deceased donors may not benefit from this post‐transplant reversal of arterial modifications.
Introduction
Chronic kidney disease (CKD) accelerates aging of the arterial vascular tree, with complex changes producing large‐vessel stiffening and outward remodeling characterized as dilatation, decreased thickness, and increased wall stress.1, 2, 3, 4 These changes possibly explain the high cardiovascular mortality rate observed among patients with CKD and especially with end‐stage renal disease.5, 6, 7
Aortic stiffness has been used for several years as a strong independent predictive marker of cardiovascular mortality, in populations with diabetes mellitus and hypertension in particular.8, 9 Carotid to femoral pulse wave velocity (PWV) is a simple and reproducible method to measure aortic stiffness, and is highly predictive of cardiovascular mortality in patients with end‐stage renal disease10 and kidney transplant and CKD,3, 11 which is remarkable in the absence of predictive equations in these populations. We and others have shown that not only aortic stiffness but also internal diameter enlargement, thinning of the arterial wall, increased circumferential wall stress, and reduction of the arterial distensibility occur early during CKD and progress in parallel with the fall in renal function and influence prognosis.1, 2, 3, 4, 12
Kidney transplantation (KT) reduces cardiovascular risk in patients with end‐stage renal disease, with cardiovascular death rates reduced by ≈75% in transplant recipients when compared with matched patients undergoing dialysis still on the waiting list.13, 14 This important benefit is mainly explained by the reversal of renal dysfunction. However, the mechanism of cardiovascular risk improvement is yet to be adequately elucidated since transplant‐specific diseases, especially side effects of immunosuppressive drugs, may offset the decrease in cardiovascular risk.
Several but not all studies have suggested that PWV may fall after KT15, 16 which may represent a global improvement in vascular health translating into improvement in graft and patient survival.11, 17, 18 However, longitudinal data relating to large‐vessel vascular remodeling in a transplant population are lacking, especially concerning specific changes in vessel diameter, thickness and wall stress. We hypothesized that such changes can occur following KT. Therefore, the objective of this noninterventional, prospective study was to describe the arterial modifications following KT and to identify the key determinants of this process.
Methods
Design and Patients
This prospective single‐center study ran from January 2009 to the end of December 2012. Eligible patients were aged 18 to 70 years and recipients of a first living or cadaveric single KT. A body mass index <35 kg/m² was required. Immunosuppression regimens had to include a calcineurin inhibitor. Estimated glomerular filtration rate (GFR) at month 3 (M3), time of the first arterial parameters evaluation, had to be >30 mL/min per 1.73 m2. Noninclusion criteria were cardiac arrhythmia, symptomatic lower limb arteriopathy and dual‐kidney or combined organ transplantation.
A total of 307 KTs were performed at the transplantation department of Foch Hospital during this period. A total of 232 consecutive patients met the inclusion criteria (169 deceased donor [DD] allograft KT and 63 living donor [LD] allograft KT), of whom 192 were enrolled and 161 completed the study. Of note, 31 patients did not complete the study because of graft failure (n=12), death (n=2), or personal reasons (n=17). Enrolled patients underwent an extended evaluation including medical interview, clinical examination, blood samples, direct measured GFR through 51Cr‐EDTA urinary clearance, and study of arterial parameters (see below) at M3 and 12 months (M12) after transplantation.
Baseline clinical data included recipient's age and sex, cardiovascular risk factors and previous cardiovascular events, cause of CKD, and duration of dialysis therapy.
The main characteristics of the kidney donor were also collected, including age, sex, cardiovascular risk factors, serum creatinine, and estimated GFR before organ donation. Kidney donors were classified as LDs, standard criteria donors (SCDs), or expanded criteria donors (ECDs), using classical criteria.19
Post‐transplant clinical events were collected, such as delayed graft function, biopsy‐proven acute rejections episodes, and diagnosis of new‐onset diabetes mellitus after transplantation. All medications, including immunosuppressant, antihypertensive, statin, and diabetic therapies were listed.
The protocol was approved by the local ethics committee, and all patients gave written informed consent before enrolment.
Arterial Parameters
Patients were studied in a quiet, temperature‐controlled room (22±1°C) room as previously described.1 Changes were calculated for every measured parameter between M3 and M12 (M12−M3, negative figures meaning decrease). Blood pressure was monitored with a standard oscillometric method and mean blood pressure (MBP) was derived from systolic and diastolic blood pressure using the 1/3 formula.
Aortic stiffness was estimated by the carotid to femoral PWV using the validated foot‐to‐foot velocity method (Sphygmocor, Atcor Medical). Briefly, pressure waveforms were obtained at the common carotid artery and the femoral artery with a pressure transducer. The transit time was measured between the feet of the 2 waveforms by intersecting tangents. The distance was measured between the 2 sites (carotid‐femoral). PWV was calculated as carotid‐femoral distance (m)/transit time (s), corrected (distance×0.8) according to published recommendations.20 Measurement of carotid‐femoral PWV was always performed by choosing the side opposite to renal allograft arterial anastomosis.
Common carotid artery pressure waveforms were recorded noninvasively by applanation tonometry (Sphygmocor, Atcor Medical) and local carotid artery pulse pressure was used for further calculations.
End‐diastolic internal diameter, change in internal diameter between systole and diastole, and intima‐media thickness (IMT) were measured on the right common carotid artery with a high‐precision echotracking system (Artlab system, Esaote), as previously described and validated. Carotid internal diameter (CID) was normalized to body surface area. Circumferential wall stress (CWS), given in kPa, was calculated according to the Lamé's equation as: (MBP.CID)/2IMT. Carotid distensibility was determined from systolic‐diastolic variations in arterial cross‐sectional area (ΔA) and local pulse pressure (ΔP) as described earlier, assuming the lumen to be circular. Cross‐sectional distensibility coefficient was calculated as ΔA/AΔP. Carotid stiffness was calculated as (distensibility coefficient)−1/2.
Biological Parameters
Blood and urine samples were collected at M3 and M12 to determine values of serum creatinine, glucose, glycated hemoglobin, lipid profile (high‐density lipoprotein/low‐density lipoprotein and triglyceride), C‐reactive protein, calcium, and phosphorus. Proteinuria was expressed as urinary protein:creatinine ratio (uPCR).
GFR was measured by the renal clearance of 51Cr‐EDTA, as mentioned. Briefly, 1.8 to 3.5 MBq of 51Cr‐EDTA (GE Healthcare) was injected intravenously as a single bolus. After allowing 1 hour for distribution of the tracer in the extracellular fluid, average renal 51Cr‐EDTA clearance was determined on 5 to 6 consecutive 30‐minute clearance periods.
Statistical Analyses
Data are expressed as mean±SD, mean (95% confidence interval [CI]), or median [interquartile range] as appropriate. Group comparisons were made using 2‐sample t test or Wilcoxon rank test as appropriate. Chi‐square test was used for discrete variables. Within‐group comparisons (change between M3 and M12) were made using the paired t test or the Wilcoxon signed rank nonparametric test as appropriate. Associations between arterial parameter changes (as dependent variable) and all other independent variables related to recipient characteristics, donor characteristics, transplantation procedure (eg, cold ischemia time), main post‐transplant events (delayed graft function, acute rejection, new‐onset diabetes mellitus after transplantation), and treatments (eg, renin‐angiotensin system blockers, immunosuppressive agents) were tested using stepwise multiple robust regressions. All tests were 2‐sided and P values <0.05 were deemed significant.
All statistical analyses were performed using NCSS 10 Statistical Software (2015) (NCSS, LLC).
Results
Demographics and Clinical Data
Of the 161 transplant recipients who completed the study, 49 (30.4%) received LD kidneys and 112 (69.6%) received cadaveric kidneys, 60 of whom were SCDs and 52 ECDs. The mean age was 50±11 years, with DD recipients tending to be older. A total of 68% of patients were men, with a similar preponderance in both LD and DD groups. The cause of end‐stage renal disease was also similar between groups, with the most frequent causes including primary glomerulonephritis (19%), polycystic kidney disease (22%), diabetic nephropathy (10%), and hypertensive nephropathy (10%) (Table 1).
Table 1.
Clinical and Demographic Data of Study Population
| All Patients (N=161) | Living Donors (n=49) | Deceased Donors (n=112) | P Value | Standard Criteria Donors (n=60) | Expanded Criteria Donors (n=52) | P Value | |
|---|---|---|---|---|---|---|---|
| Recipient age, y | 50 (49–52) | 48 (44–51) | 51 (49–53) | 0.1 | 47 (45–50) | 56 (54–58) | <0.001 |
| Men/women | 110/51 | 37/49 | 73/112 | 0.1 | 40/60 | 33/52 | 0.7 |
| BMI, kg/m2 | 24 (24–25) | 25 (23–26) | 24 (23–25) | 0.3 | 24 (23–25) | 24 (23–25) | 0.7 |
| Time on dialysis, mo | 30 [10–51] | 7 [1–24] | 37 [21–66] | <0.001 | 39 [23–64] | 36 [19–70] | 0.9 |
| Pre‐KT diabetes mellitus, % | 12 | 10 | 12 | 0.7 | 7 | 19 | 0.05 |
| Pre‐KT dyslipidemia, % | 47 | 43 | 51 | 0.5 | 38 | 60 | 0.03 |
| Pre‐KT former tobacco, % | 22 | 26 | 24 | 0.9 | 22 | 23 | 0.9 |
| Preemptive KT, No. | 10 | 9 | 1 | <0.001 | 2 | 0 | 0.2 |
| Donor age, y | 51 (49–53) | 50 (47–53) | 52 (49–54) | 0.4 | 42 (40–45) | 63 (61–65) | <0.001 |
| DGF, % | 14 | 0 | 22 | <0.001 | 17 | 25 | 0.2 |
| Acute rejection, % | 9 | 7 | 11 | 0.3 | 15 | 6 | 0.1 |
| NODAT, % | 19 | 10 | 23 | 0.05 | 18 | 29 | 0.2 |
| ARB or ACEI, % | 67 | 65 | 69 | 0.6 | 65 | 73 | 0.005 |
| mGFR M3, mL/min per 1.73 m2 | 53 [43–64] | 52 [44–60] | 55 [44–66] | 0.8 | 58 [49–70] | 51 [39–61] | 0.05 |
| mGFR M12, mL/min per 1.73 m2 | 54 [45–68] | 57 [46–65] | 52 [42–69] | 0.3 | 60 [46–71] | 47 [38–60] | 0.001 |
| uPCR M3, g/mmol | 0.03 [0.02–0.04] | 0.03 [0.02–0.04] | 0.02 [0.02–0.04] | 0.8 | 0.02 [0.02–0.04] | 0.02 [0.02–0.05] | 0.5 |
| uPCR M12, g/mmol | 0.02 [0.01–0.03] | 0.02 [0.01–0.03] | 0.02 [0.01–0.04] | 0.9 | 0.02 [0.01–0.03] | 0.02 [0.01–0.04] | 0.4 |
Recipient age, donor age, and body mass index (BMI) values are expressed as mean (95% confidence interval). Time on dialysis, measured glomerular filtration rate (mGFR), and urine protein/creatinine ratio (uPCR) values are expressed as median [interquartile range]. ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; DGF, delayed graft function; KT, kidney transplantation; M3, month 3 after kidney transplantation; M12, month 12 after kidney transplantation; NODAT, new‐onset diabetes mellitus after transplantation.
Renal function (measured GFR) was adequate at M3 (54; 95% CI, 43–64 mL/min per 1.73 m2) and did not change appreciably during the study, with no difference between the LD and DD groups at either M3 or M12. Similarly, proteinuria was minimal (uPCR, 0.03; 95% CI, 0.02–0.04 g/mmol) and did not alter during the study. None of the biological variables were independently associated with arterial changes, except for a weak association between uPCR at M12 and PWV change.
In the LD group (n=49), the median duration of dialysis treatment before transplantation was shorter compared with the DD group (P<0.001). There were no episodes of delayed graft function (P<0.001) and the cold ischemia time was markedly reduced: 1.4 hours (95% CI, 1.0–1.7) versus 16.6 hours (95% CI, 12.2–22.6) (P<0.001), when compared with the DD group.
The standard immunosuppressive regimen included corticosteroids (96%), mycophenolate mofetil (100%), and tacrolimus or cyclosporin (91% and 9%, respectively). Induction immunosuppressive therapy included polyclonal antilymphocyte antibodies (Thymoglobulin) in 43% of all patients, anti–interleukin 2 receptor monoclonal antibodies in 43%, and polyvalent intravenous immunoglobulins in 27%. There was a tendency to a lower incidence of new‐onset diabetes mellitus after transplantation in the LD group and a higher incidence in the ECD group.
Other medications were widely used and included angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers (69%), calcium channel blockers (56%), statins (43%), and β‐blockers (40%). The mean number of antihypertensive agents used per patient was 1.8±1.1. No difference was identified between the LD and DD groups.
Arterial Stiffness: PWV and Carotid Stiffness
PWV decreased from 10.8 (95% CI, 10.5–11.2) to 10.2 (95% CI, 9.8–10.5) (P<0.001) during the study. This occurred in parallel with a small albeit significant decrease in MBP (−3 mm Hg; 95% CI, −4 to −1). Once adjusted for changes in MBP, changes in PWV remained virtually identical (data not shown). The fall in PWV was more marked (P<0.001) in the LD group (−1.4 m/s; 95% CI, −1.8 to −1.0) than in the DD group (−0.4 m/s; 95% CI, −0.6 to −0.1). Carotid stiffness showed similar changes to PWV between M3 and M12 (−0.2 m/s; 95% CI, −0.4 to 0.0) (P<0.05), with changes again greater (P<0.001) in the LD group (Figure 1A, Table 2).
Figure 1.
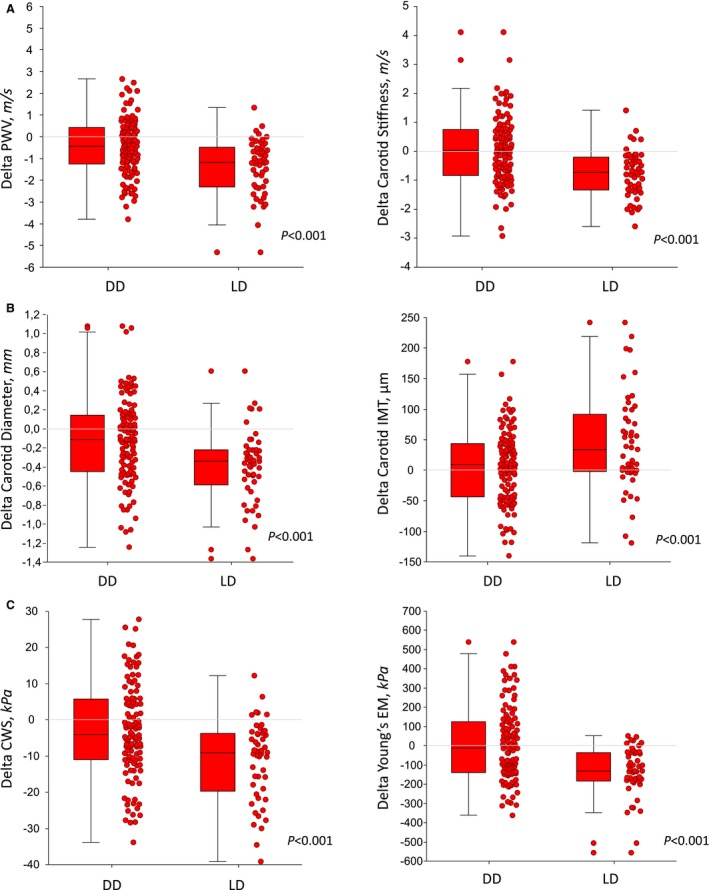
Changes in measured and calculated parameters between 3 and 12 months post transplant in 161 recipients according to donor source (deceased donor [DD] vs live donor [LD]). A, Pulse wave velocity (PWV) and carotid stiffness. B, Carotid internal diameter and intima‐media thickness. C, Circumferential wall stress (CWS) and Young's elastic modulus (EM). Box plots represent median, interquartile range, and adjacent values. Comparisons are for between‐group changes.
Table 2.
Baseline Values and Changes of Arterial Parameters During the Study
| Variable | All Patients (N=161) | LDs (n=49) | DDs (n=112) | P Value | ECDs (N=52) | SCDs (n=60) | P Value |
|---|---|---|---|---|---|---|---|
| Baseline arterial parameters (at M3) | |||||||
| MBP, mm Hg | 98 (97–100) | 99 (96–102) | 98 (96–100) | 0.5 | 99 (96–102) | 97 (94–100) | 0.4 |
| PWV, m/s | 10.8 (10.5–11.2) | 11.2 (10.5–11.9) | 10.7 (10.3–11.0) | 0.4 | 10.6 (10.1–11.2) | 10.7 (10.2–11.2) | 0.7 |
| Carotid stiffness, m/s | 6.6 (6.4–6.8) | 6.7 (6.3–7.0) | 6.6 (6.3–6.9) | 0.5 | 6.8 (6.3–7.2) | 6.4 (6.0–6.8) | 0.2 |
| Carotid diameter, mm | 6.4 (6.2–6.5) | 6.2 (6.0–6.5) | 6.4 (6.3–6.4) | 0.2 | 6.6 (6.4–6.8) | 6.3 (6.1–6.5) | <0.05 |
| IMT, μm | 601 (586–616) | 583 (552–613) | 610 (593–627) | 0.1 | 622 (598–646) | 599 (575–623) | 0.2 |
| CWS, kPa | 70 (68–72) | 71 (66–75) | 70 (67–73) | 0.6 | 71 (67–75) | 69 (64–73) | 0.4 |
| Young's EM, kPa | 476 (441–512) | 466 (410–522) | 481 (436–527) | 0.8 | 508 (436–579) | 457 (398–517) | 0.4 |
| Variable | All Patients | LDs | DDs | P Value | ECDs | SCDs | P Value |
|---|---|---|---|---|---|---|---|
| Change in arterial parameters from M3 to M12 | |||||||
| MBP, mm Hg | −3 (−4 to −1)† | −4 (−7 to −1)* | −2 (−4 to 0)* | 0.3 | −3 (−0.6 to 0)* | −1 (−4 to 1) | 0.3 |
| PWV, m/s | −0.7 (−0.9 to −0.5)‡ | −1.4 (−1.8 to −1)‡ | −0.4 (−0.6 to 0.1)† | <0.001 | 0.1 (−0.4 to 0.4) | −0.7 (−1 to −0.4)‡ | <0.01 |
| Carotid stiffness, m/s | −0.2 (−0.4 to 0.0)* | −0.9 (−1.1 to −0.6)‡ | 0.1 (−0.2 to 0.3) | <0.001 | 0.1 (−0.2 to 0.4) | 0.1 (−0.3 to 0.4) | 0.7 |
| Carotid diameter, mm | −0.2 (−0.3 to −0.1)‡ | −0.4 (−0.5 to −0.3)‡ | −0.1 (−0.2 to 0)† | <0.001 | −0.1 (−0.2 to 0.1) | −0.2 (−0.3 to −0.1)‡ | 0.1 |
| IMT, μm | 17 (7–28)† | 44 (20–66)‡ | 6 (−6 to 17) | <0.001 | 1 (−13 to 16) | 10 (−8 to 27) | 0.5 |
| CWS, kPa | −6 (−8 to −4)‡ | −11 (−15 to −11)‡ | −3 (−6 to −1)† | <0.001 | −3 (−7 to 1) | −4 (−7 to 0)† | 0.8 |
| Young's EM, kPa | −47 (−79 to −14)† | −145 (−187 to −102)‡ | −2 (−43 to 38) | <0.001 | 6 (−52 to 65) | −10 (−68 to 48) | 0.7 |
Values are expressed as mean (95% confidence interval). CWS indicates circumferential wall stress; DDs, deceased donors; ECDs, extended criteria donors; EM, elastic modulus; IMT, intima‐media thickness; LDs, living donors; M3, month 3 after kidney transplantation; M12, month 12 after kidney transplantation; MBP, mean arterial blood pressure; PWV, pulse wave velocity; SCDs, standard criteria donors.
P: statistical difference between groups (LD:DD; ECD:SCD); *P<0.05, † P<0.01, and ‡ P<0.001 for change within groups.
Carotid Remodeling: CID and IMT
The fall in CID was significant overall (P<0.001), but again more marked in the LD group: −0.4 mm (95% CI, −0.5 to −0.3) versus −0.1 mm (95% CI, −0.2 to 0.0) (P<0.01). IMT rose significantly during the study (17 μm; 95% CI, 7–28 [P<0.01]), an effect largely driven by the LD group (44 μm; 95% CI, 20–66), as there was no appreciable change in the DD group (Figure 1B). Other indices of carotid stiffness were also modified. We observed a 10% fall in Young's elastic modulus (Figure 1C) (−47 kPa; 95% CI, −79 to −14 [P<0.01]), with a 31% fall in the LD group (P<0.001). No changes were found in the DD group. Circumferential wall stress integrates both the changes in diameter and IMT, and decreased by 9% overall (P<0.001), which mainly related to a 17% reduction in the LD group, compared with a 4% reduction in the DD group (P<0.001 for difference) (Table 2).
LD Versus SCD Versus ECD
When the DD group was further divided into SCDs and ECDs, it became evident that most of the improvement found in this group was related to changes in the SCD subgroup. There was no significant difference between the ECD and SCD groups for sex balance, time on dialysis, rate of delayed graft function, rate of new‐onset diabetes mellitus after transplantation, acute rejection rate, and BMI either at M3 or M12. However, and by construction, the ECD group was older (56±11 years versus 47±11 years, P=0.0001) and received kidneys from older donors (56±9 versus 42±10, P=0.0001) than the SCD group. GFR was significantly lower in the ECD than in the LD and SCD groups at M3 and M12 (P<0.01). Patients receiving SCD kidneys had significant reductions for PWV (P<0.001), CID (P<0.001), and CWS (P<0.05), and favorable trends in carotid stiffness, IMT, or Young's elastic modulus. By contrast, no parameter improved in the ECD group, despite a significant fall in MBP (P<0.05). When compared with the LD group, the improvement in PWV, carotid diameter, IMT, and CWS was observed to be “stepped,” suggesting that the improvement in the SCD group was midway between the LD and ECD groups (Figure 2). These differences were unaltered by adjustment for potential confounding factors (Tables 1 and 2).
Figure 2.
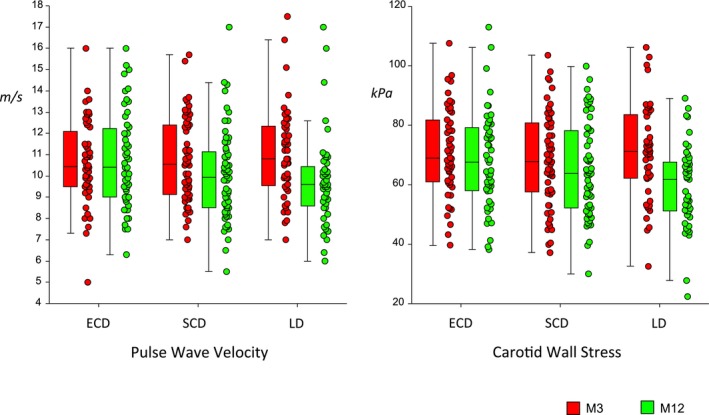
Measured pulse wave velocity and circumferential wall stress at 3 months (red) and at 12 months (green) post transplant in 161 recipients according to donor source. The deceased donor group is further subdivided into standard criteria donor (SCD) and expanded criteria donor (ECD) groups. Box plots represent median, interquartile range, and adjacent values. Comparisons are for within‐group differences. LD indicates living donor; M3, month 3; M12, month 12.
Correlations
Five variables consistently and highly significantly correlated with changes in the 5 parameters studied (PWV, carotid stiffness, CID, IMT, and CWS, N=161) during the study. These variables were: (1) basal (M3) measurements, (2) donor source (LD/DD) or (3) characteristics of the transplanted kidney (LD/SCD/ECD), (4) cold ischemia time, and (5) time on dialysis. Basal values at M3 were similarly correlated, with the direction of change supportive of a greater change in patients with worse initial pathology. Reduction in MBP correlated with improvement of PWV (P<0.001), carotid stiffness (P<0.001), CID (P<0.05), and Young's elastic modulus (P<0.001) (Table 3, Tables S1 through S5).
Table 3.
Multivariate Analysis of Changes in Measured Parameters According to Donor Source (LDs or DDs)
| Independent Variable | Beta Coefficient (95% CI) | R (Standardized Coefficient) | P Value | Partial R 2 |
|---|---|---|---|---|
| PWV (R 2=0.33) | ||||
| Donor source (LDs/DDs) | −0.85 (−0.24 to −0.46) | −0.36 | <0.001 | 10.5 |
| Basal PWV | −0.24 (−0.33 to −0.15) | −0.35 | <0.001 | 16.2 |
| Δ MBP | 0.03 (0.01–0.05) | 0.24 | 0.001 | 6.3 |
| Log uProt/uCreat | 0.24 (0.06–0.43) | 0.14 | 0.011 | 4.1 |
| Carotid CWS (R 2=0.29) | ||||
| Donor source (LDs/DDs) | −7.1 (−11.1 to −3.1) | −0.27 | <0.001 | 9.6 |
| Basal CWS | −0.35 (−0.47 to −0.23) | −0.45 | <0.001 | 22.5 |
| Carotid IMT (R 2=0.09) | ||||
| Donor source (LDs/DDs) | 26.9 (6.2–47.6) | 0.20 | 0.02 | 4.1 |
| Basal IMT | −0.11 (−0.21 to −0.00) | −0.16 | <0.05 | 2.6 |
| Carotid stiffness (R 2=0.38) | ||||
| Donor source (LDs/DDs) | −0.73 (−1.06 to −0.41) | −0.29 | <0.001 | 11.8 |
| Basal carotid stiffness | −0.30 (−0.41 to −0.20) | −0.37 | <0.001 | 18.1 |
| Δ MBP | 0.03 (0.02–0.05) | 0.29 | <0.001 | 11.5 |
| Δ BMI | 0.11 (0.02–0.19) | 0.17 | 0.02 | 4.2 |
| Carotid diameter (R 2=0.26) | ||||
| Donor source (LDs/DDs) | −0.27 (−0.41 to −0.14) | −0.31 | <0.001 | 11.3 |
| Δ BMI | 0.06 (0.03–0.09) | 0.26 | 0.001 | 8.3 |
| Basal carotid diameter | −0.11 (−0.18 to −0.04) | −0.23 | <0.005 | 6.4 |
| Δ MBP | 0.01 (0.00–0.01) | 0.20 | 0.01 | 5.2 |
Δ indicates change; BMI, body mass index; CWS, circumferential wall stress; DDs, deceased donors; IMT, intima‐media thickness; LDs, living donors; MBP, mean arterial blood pressure; PWV, pulse wave velocity; uProt/uCreat, urine protein/urine creatinine ratio.
Multivariate analysis including those covariates identified specifically and consistently that donor source (LD versus DD, Table 3), or transplanted kidney characteristics (LD/SCD/ECD, Table 4), and basal values at M3 were the primary independent determinants of isobaric change among the significant variables. Donor source and transplanted kidney characteristics were highly associated with improvement (P<0.001) of all variables including increase in IMT (P<0.05) (Tables 3 and 4).
Table 4.
Multivariate Analysis of Changes in Measured Parameters According to Transplanted Kidney Characteristics (LDs, SCDs, ECDs)
| Independent Variable | Beta Coefficient (95% CI) | R (Standardized Coefficient) | P Value | Partial R 2, % |
|---|---|---|---|---|
| PWV (R 2=0.37) | ||||
| ECDs vs SCDs | 0.73 (0.48–0.98) | 0.44 | <0.001 | 17.3 |
| LDs vs SCDs | −0.63 (−0.89 to −0.38) | −0.37 | <0.001 | 13.2 |
| Basal PWV | −0.21 (−0.30 to −0.13) | −0.33 | <0.001 | 14.2 |
| Δ MBP | 0.04 (0.02–0.06) | 0.27 | <0.001 | 10.4 |
| Carotid CWS (R 2=0.29) | ||||
| ECDs vs SCDs | 3.33 (1.01–5.66) | 0.23 | 0.012 | 4.9 |
| LDs vs SCDs | −0.63 (−0.89 to −0.37) | −0.33 | <0.001 | 9.7 |
| Basal CWS | −0.32 (−0.42 to −0.21) | −0.46 | <0.001 | 22.7 |
| Carotid IMT (R 2=0.09) | ||||
| ECDs vs SCDs | −10.55 (−23.93 to 2.84) | −0.14 | 0.2 | 1.5 |
| LDs vs SCDs | 17.95 (4.14–31.75) | 0.24 | <0.05 | 4.1 |
| Basal IMT | −0.36 (−0.46 to −0.25) | −0.18 | <0.05 | 3.2 |
| Carotid stiffness (R 2=0.36) | ||||
| ECDs vs SCDs | 0.43 (0.22–0.64) | 0.31 | <0.001 | 9.6 |
| LDs vs SCDs | −0.63 (−0.89 to −0.37) | −0.37 | <0.001 | 13.1 |
| Basal carotid stiffness | −0.32 (−0.42 to −0.21) | −0.41 | <0.001 | 19.7 |
| Δ MBP | 0.03 (0.02–0.05) | 0.27 | <0.001 | 10.0 |
| Carotid diameter (R 2=0.28) | ||||
| ECDs vs SCDs | 0.19 (0.11–0.27) | 0.38 | <0.001 | 12.1 |
| LDs vs SCDs | −0.19 (−0.27 to −0.11) | −0.38 | <0.001 | 12.4 |
| Basal carotid diameter | −0.13 (−0.20 to −0.06) | −0.26 | 0.001 | 8.5 |
| Δ MBP | 0.01 (0–0.01) | 0.21 | 0.007 | 5.6 |
| Δ BMI | 0.05 (0.02–0.08) | 0.25 | 0.001 | 8.0 |
Δ indicates change; BMI, body mass index; CI, confidence interval; CWS, circumferential wall stress; ECDs, extended criteria donors; IMT, intima‐media thickness; LDs, living donors; MBP, mean arterial blood pressure; PWV, pulse wave velocity; SCDs, standard criteria donors.
Discussion
This study demonstrates improved aortic stiffness and reversal of maladaptive vascular remodeling in transplant recipients in the first year following KT. We demonstrated that: (1) both large‐artery stiffening and maladaptive outward remodeling of CKD can significantly improve within the first year after KT, and (2) the donor source (living versus cadaveric) and kidney characteristics (living, standard criteria, or expanded criteria) are the main independent determinants of changes in arterial properties.
These changes have to be interpreted in the context of large‐artery changes in CKD. We have previously shown that early stages in CKD (2–5) are marked by maladaptive remodeling of large arteries associating increased stiffness, dilatation, and thinning, resulting in increased wall stress. We have further shown that the intensity of this maladaptive remodeling was associated with the evolution of CKD (notably wall stress) and morbid cardiovascular events (notably aortic stiffness and carotid dilatation).1, 2, 3, 4 Although previous studies have identified significant and potentially favorably prognostic modifications in aortic stiffness16, 21 and/or carotid IMT22 within several months of KT, these studies have been either cross‐sectional or have primarily addressed the relationship between carotid IMT and inflammation.
The major finding of this study is that KT reverses the maladaptive remodeling of large arteries particularly if the transplanted kidney comes from an LD. These patients showed a 13% improvement in both PWV and carotid stiffness, 6% increase in ICD, 7% increase in IMT, and a 17% reduction in CWS. These changes are important since they correspond grossly to 10/15 years of normal vascular aging.23, 24, 25 This improvement persisted unchanged after adjustment for dialysis duration and other potential confounders. In addition, when the DD data were further split into SCD and ECD, it was evident that all effective improvement in vascular compliance and remodeling in the DD group were observed in the SCD group and not in the ECD group. The lack of change in this latter group is notable, since ECD patients were similar to their SCD counterparts in respect to time on dialysis, rate of delayed graft function, and episodes of acute rejection. Measured GFR was lower in the ECD group (Table 1) but the difference in arterial changes between groups was still significant after adjustment for measured graft function. The patients in the ECD group were both older and received older kidneys. Vascular health deteriorates with age, and it is possible that the greater age of ECD recipients reduced the capacity for vessel changes. In addition, we have previously shown that donor age has an important impact on recipient aortic stiffness in the post‐transplant period26 which, together with the deleterious effect of brain death and cold ischemia on kidney function, can clearly influence the capacity for vascular aging reversal and, potentially, long‐term renal and cardiovascular prognosis. Two recent, large epidemiological studies demonstrated that: (1) kidney allografts from live donors or young cadaveric donors were associated with better overall post‐transplant mortality,27, 28 and (2) ECD allografts correlated with a higher risk of graft failure.29
The contrasting findings between the 2 groups (ECD versus SCD) in this study raise the issue of optimization of kidney allocation. In the context of a shortage of organ supply, a growing number of patients receive kidney allografts from donors aged older than 60 years and/or with vascular comorbidities. Although this strategy does offer a significant benefit for quality of life and survival compared with long‐term dialysis therapy,30 as shown here it is associated with suboptimal correction of major cardiovascular risk markers. It is not possible to address this issue with any certainty from the current data; however, our results do support the promotion of live donation for KT, and also suggest that an awareness of long‐term cardiovascular prognosis is a relevant consideration when accepting kidneys for donation.
The fact that renal function did not relate to vascular stiffness has been observed in several trials, especially when GFR was measured directly, rather than by equation.1 Renal function was stable during the study, although patients with clear graft failure were excluded, thus minimizing any potential effect on GFR. The duration of observation might also be too short, and longer‐term analysis of this cohort may shed light on whether relatively mild renal dysfunction influences the return of vascular properties to normal, and whether one donor subgroup is more likely to experience clinical sequelae than another.
Study Limitations
The main limitations to this study are that it is observational by nature and thus causality cannot be ascertained. The difference in waiting time on dialysis between the LD and DD groups was a potential confounder, although such a difference was not evident between the SCD and ECD groups, and results persisted after adjustment for this variable. More specific biological markers of CKD such as mineral metabolism parameters or vasculotoxic uremic toxins might have a specific influence on vascular changes. However, most are strongly linked with renal function31 and it is not clear whether any have a significant independent direct effect on arterial wall properties. Finally, we must underline the fact that our study population was characterized by a particularly low (12%) prevalence of pretransplant diabetes mellitus, when compared with the usual kidney transplant populations. In other words, the results of this study concern an intermediate cardiovascular risk population but the fact that the basal values of arterial stiffness were correlated with the magnitude of the post‐transplant improvement suggest that our results may also apply to patients with more severe vascular disease.
Conclusions
Our data demonstrate that the maladaptive vascular remodeling observed in patients with CKD can improve within 12 months of KT. This improvement is independent of BP and renal function and appears most evident with live donation.
Sources of Funding
This study was supported by a grant from the French Ministry of Health (PHRC 2010—AOM10008—MIN 00‐08).
Disclosures
None.
Supporting information
Table S1. Univariate Analysis of PWV Change
Table S2. Univariate Analysis of Carotid Stiffness Change
Table S3. Univariate Analysis of CWS Change
Table S4. Univariate Analysis of Carotid IMT Change
Table S5. Univariate Analysis of Carotid Diameter Change
(J Am Heart Assoc. 2017;6:e006078 DOI: 10.1161/JAHA.117.006078.)28889098
References
- 1. Briet M, Bozec E, Laurent S, Fassot C, London GM, Jacquot C, Froissart M, Houillier P, Boutouyrie P. Arterial stiffness and enlargement in mild‐to‐moderate chronic kidney disease. Kidney Int. 2006;69:350–357. [DOI] [PubMed] [Google Scholar]
- 2. Briet M, Collin C, Karras A, Laurent S, Bozec E, Jacquot C, Stengel B, Houillier P, Froissart M, Boutouyrie P; Nephrotest Study Group . Arterial remodeling associates with CKD progression. J Am Soc Nephrol. 2011;22:967–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Karras A, Haymann JP, Bozec E, Metzger M, Jacquot C, Maruani G, Houillier P, Froissart M, Stengel B, Guardiola P, Laurent S, Boutouyrie P, Briet M; Nephrotest Study Group . Large artery stiffening and remodeling are independently associated with all‐cause mortality and cardiovascular events in chronic kidney disease. Hypertension. 2012;60:1451–1457. [DOI] [PubMed] [Google Scholar]
- 4. Briet M, Boutouyrie P, Laurent S, London GM. Arterial stiffness and pulse pressure in CKD and ESRD. Kidney Int. 2012;82:388–400. [DOI] [PubMed] [Google Scholar]
- 5. Muntner P, Bowling CB, Gao L, Rizk D, Judd S, Tanner RM, McClellan W, Warnock DG. Age‐specific association of reduced estimated glomerular filtration rate and albuminuria with all‐cause mortality. Clin J Am Soc Nephrol. 2011;6:2200–2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. [DOI] [PubMed] [Google Scholar]
- 7. van der Velde M, Matsushita K, Coresh J, Astor BC, Woodward M, Levey A, de Jong P, Gansevoort RT; Chronic Kidney Disease Prognosis Consortium . Lower estimated glomerular filtration rate and higher albuminuria are associated with all‐cause and cardiovascular mortality. A collaborative meta‐analysis of high‐risk population cohorts. Kidney Int. 2011;79:1341–1352. [DOI] [PubMed] [Google Scholar]
- 8. Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker‐Boudier H; European Network for Non‐invasive Investigation of Large Arteries . Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588–2605. [DOI] [PubMed] [Google Scholar]
- 9. Cruickshank K, Riste L, Anderson SG, Wright JS, Dunn G, Gosling RG. Aortic pulse‐wave velocity and its relationship to mortality in diabetes and glucose intolerance: an integrated index of vascular function. Circulation. 2002;106:2085–2090. [DOI] [PubMed] [Google Scholar]
- 10. Guerin AP, Blacher J, Pannier B, Marchais SJ, Safar ME, London GM. Impact of aortic stiffness attenuation on survival of patients in end‐stage renal failure. Circulation. 2001;103:987–992. [DOI] [PubMed] [Google Scholar]
- 11. Verbeke F, Marechal C, Van LS, Van BW, Devuyst O, Van Bortel LM, Jadoul M, Vanholder R. Aortic stiffness and central wave reflections predict outcome in renal transplant recipients. Hypertension. 2011;58:833–838. [DOI] [PubMed] [Google Scholar]
- 12. Townsend RR, Wimmer NJ, Chirinos JA, Parsa A, Weir M, Perumal K, Lash JP, Chen J, Steigerwalt SP, Flack J, Go AS, Rafey M, Rahman M, Sheridan A, Gadegbeku CA, Robinson NA, Joffe M. Aortic PWV in chronic kidney disease: a CRIC ancillary study. Am J Hypertens. 2010;23:282–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, Agodoa LY, Held PJ, Port FK. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. 1999;341:1725–1730. [DOI] [PubMed] [Google Scholar]
- 14. Ojo AO, Hanson JA, Meier‐Kriesche H, Okechukwu CN, Wolfe RA, Leichtman AB, Agodoa LY, Kaplan B, Port FK. Survival in recipients of marginal cadaveric donor kidneys compared with other recipients and wait‐listed transplant candidates. J Am Soc Nephrol. 2001;12:589–597. [DOI] [PubMed] [Google Scholar]
- 15. Zoungas S, Kerr PG, Chadban S, Muske C, Ristevski S, Atkins RC, McNeil JJ, McGrath BP. Arterial function after successful renal transplantation. Kidney Int. 2004;65:1882–1889. [DOI] [PubMed] [Google Scholar]
- 16. Covic A, Goldsmith DJ, Gusbeth‐Tatomir P, Buhaescu I, Covic M. Successful renal transplantation decreases aortic stiffness and increases vascular reactivity in dialysis patients. Transplantation. 2003;76:1573–1577. [DOI] [PubMed] [Google Scholar]
- 17. Bahous SA, Stephan A, Blacher J, Safar M. Cardiovascular and renal outcome in recipients of kidney grafts from living donors: role of aortic stiffness. Nephrol Dial Transplant. 2012;27:2095–2100. [DOI] [PubMed] [Google Scholar]
- 18. Dahle DO, Eide IA, Asberg A, Leivestad T, Holdaas H, Jenssen TG, Fagerland MW, Pihlstrom H, Mjoen G, Hartmann A. Aortic stiffness in a mortality risk calculator for kidney transplant recipients. Transplantation. 2015;99:1730–1737. [DOI] [PubMed] [Google Scholar]
- 19. Kasiske BL, Zeier MG, Chapman JR, Craig JC, Ekberg H, Garvey CA, Green MD, Jha V, Josephson MA, Kiberd BA, Kreis HA, McDonald RA, Newmann JM, Obrador GT, Vincenti FG, Cheung M, Earley A, Raman G, Abariga S, Wagner M, Balk EML. KDIGO clinical practice guideline for the care of kidney transplant recipients: a summary. Kidney Int. 2010;77:299–311. [DOI] [PubMed] [Google Scholar]
- 20. Van Bortel LM, Laurent S, Boutouyrie P, Chowienczyk P, Cruickshank JK, De Backer T, Filipovsky J, Huybrechts S, Mattace‐Raso FU, Protogerou AD, Schillaci G, Segers P, Vermeersch S, Weber T; Artery Society, European Society of Hypertension Working Group on Vascular Structure and Function, European Network for Noninvasive Investigation of Large Arteries . Expert consensus document on the measurement of aortic stiffness in daily practice using carotid‐femoral pulse wave velocity. J Hypertens. 2012;30:445–448. [DOI] [PubMed] [Google Scholar]
- 21. Verbeke F, Van BW, Honkanen E, Wikstrom B, Jensen PB, Krzesinski JM, Rasmussen M, Vanholder R, Rensma PL. Prognostic value of aortic stiffness and calcification for cardiovascular events and mortality in dialysis patients: outcome of the calcification outcome in renal disease (CORD) study. Clin J Am Soc Nephrol. 2011;6:153–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yilmaz MI, Sonmez A, Saglam M, Cayci T, Kilic S, Unal HU, Karaman M, Cetinkaya H, Eyileten T, Gok M, Oguz Y, Vural A, Mallamaci F, Zoccali C. A longitudinal study of inflammation, CKD‐mineral bone disorder, and carotid atherosclerosis after renal transplantation. Clin J Am Soc Nephrol. 2015;10:471–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Reference Values for Arterial Stiffness' Collaboration . Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: ‘establishing normal and reference values’. Eur Heart J. 2010;31:2338–2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Engelen L, Ferreira I, Stehouwer CD, Boutouyrie P, Laurent S; Reference Values for Arterial Measurements Collaboration . Reference intervals for common carotid intima‐media thickness measured with echotracking: relation with risk factors. Eur Heart J. 2013;34:2368–2380. [DOI] [PubMed] [Google Scholar]
- 25. Engelen L, Bossuyt J, Ferreira I, van Bortel LM, Reesink KD, Segers P, Stehouwer CD, Laurent S, Boutouyrie P; Reference Values for Arterial Measurements Collaboration . Reference values for local arterial stiffness. Part A: carotid artery. J Hypertens. 2015;33:1981–1996. [DOI] [PubMed] [Google Scholar]
- 26. Delahousse M, Chaignon M, Mesnard L, Boutouyrie P, Safar ME, Lebret T, Pastural‐Thaunat M, Tricot L, Kolko‐Labadens A, Karras A, Haymann JP. Aortic stiffness of kidney transplant recipients correlates with donor age. J Am Soc Nephrol. 2008;19:798–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Molnar MZ, Streja E, Kovesdy CP, Shah A, Huang E, Bunnapradist S, Krishnan M, Kopple JD, Kalantar‐Zadeh K. Age and the associations of living donor and expanded criteria donor kidneys with kidney transplant outcomes. Am J Kidney Dis. 2012;59:841–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chavalitdhamrong D, Gill J, Takemoto S, Madhira BR, Cho YW, Shah T, Bunnapradist S. Patient and graft outcomes from deceased kidney donors age 70 years and older: an analysis of the Organ Procurement Transplant Network/United Network of Organ Sharing database. Transplantation. 2008;85:1573–1579. [DOI] [PubMed] [Google Scholar]
- 29. Pascual J, Zamora J, Pirsch JD. A systematic review of kidney transplantation from expanded criteria donors. Am J Kidney Dis. 2008;52:553–586. [DOI] [PubMed] [Google Scholar]
- 30. Merion RM, Ashby VB, Wolfe RA, Distant DA, Hulbert‐Shearon TE, Metzger RA, Ojo AO, Port FK. Deceased‐donor characteristics and the survival benefit of kidney transplantation. JAMA. 2005;294:2726–2733. [DOI] [PubMed] [Google Scholar]
- 31. Neirynck N, Eloot S, Glorieux G, Barreto DV, Barreto FC, Liabeuf S, Lenglet A, Lemke HD, Massy ZA, Vanholder R. Estimated glomerular filtration rate is a poor predictor of the concentration of middle molecular weight uremic solutes in chronic kidney disease. PLoS One. 2012;7:e44201. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Univariate Analysis of PWV Change
Table S2. Univariate Analysis of Carotid Stiffness Change
Table S3. Univariate Analysis of CWS Change
Table S4. Univariate Analysis of Carotid IMT Change
Table S5. Univariate Analysis of Carotid Diameter Change


