Abstract
Background
Obesity is key feature of the metabolic syndrome and is associated with high cardiovascular morbidity and mortality. Obesity is associated with macrovascular endothelial dysfunction, a determinant of outcome in patients with coronary artery disease. Here, we compared the influence of obesity on microvascular endothelial function to that of established cardiovascular risk factors such as diabetes mellitus, hypertension, hypercholesterolemia, and smoking in patients with suspected coronary artery disease.
Methods and Results
Endothelial function was assessed during postocclusive reactive hyperemia of the brachial artery and downstream microvascular beds in 108 patients who were scheduled for coronary angiography. In all patients, microvascular vasodilation was assessed using peripheral arterial tonometry; laser Doppler flowmetry and digital thermal monitoring were performed. Body mass index was significantly associated with decreased endothelium‐dependent vasodilatation measured with peripheral arterial tonometry (r=0.23, P=0.02), laser Doppler flowmetry (r=0.30, P<0.01), and digital thermal monitoring (r=0.30, P<0.01). In contrast, hypertension, hypercholesterolemia, and smoking had no influence on microvascular vasodilatation. Especially in diabetic patients, endothelial function was not significantly reduced (control versus diabetes mellitus, mean±SEM or median [interquartile range], peripheral arterial tonometry: 1.90±0.20 versus 1.67±0.20, P=0.19, laser Doppler flowmetry: 728% [interquartile range, 427–1110] versus 589% [interquartile range, 320–1067] P=0.28, and digital thermal monitoring: 6.6±1.0% versus 2.5±1.7%, P=0.08). In multivariate linear regression analysis, body mass index was the only risk factor that significantly attenuated endothelium‐dependent vasodilatation using all 3 microvascular function tests.
Conclusions
Higher body mass index is associated with reduced endothelial function in patients with suspected coronary artery disease, even after adjustment for treated diabetes mellitus, hypertension, hypercholesterolemia, and smoking.
Keywords: body mass index, digital thermal monitoring, endothelial function, laser Doppler flowmetry, microcirculation, peripheral arterial tonometry
Subject Categories: Endothelium/Vascular Type/Nitric Oxide, Diagnostic Testing, Obesity
Clinical Perspective
What Is New?
This observational study shows an association between body mass index and endothelial function, even in patients with treated metabolic risk factors.
What Are the Clinical Implications?
Body mass index strongly influences endothelial function throughout the spectrum of patients with and without treated metabolic risk factors.
This study sheds light on the relation of endothelial function and the occurrence of metabolic disease in obese patients.
Further studies are necessary to investigate the role of endothelial dysfunction as a predictor of outcome in patients with a high body mass index.
Introduction
During the past 3 decades, the prevalence of obesity has nearly doubled worldwide and is still increasing.1 Obesity is closely related to the occurrence of glucose intolerance, high blood pressure, dyslipidemia, and also cardiovascular disease (CVD). Endothelial dysfunction is a well‐known early step in the pathogenesis of CVD.2, 3 Endothelial function plays an important role in the regulation of vascular tone, thrombosis, and smooth muscle cell proliferation and when impaired, it can initiate and accelerate progression of atherosclerosis.4, 5
Endothelial function can be assessed with different methods that rely on the administration of stimuli of different vasodilator pathways such as mechanical stress, thermal provocation, electrical stimuli, or administration of vasoactive agents by iontophoresis.6 A blunted response is indicative of endothelial dysfunction and predictive for CVD and clinical prognosis.7, 8, 9, 10
Only recently, the cutaneous microcirculation has emerged as a clinically relevant model for microvascular reactivity and endothelial function.6, 11 In the microcirculation of the skin, postocclusive reactive hyperemia triggers several pathways for vascular reactivity. In addition to endothelial nitric oxide synthase activity, sensory nerves and endothelium‐derived hyperpolarizing factors play an important role in the vasomotor regulation of the microcirculation.12 Laser Doppler flowmetry (LDF) is frequently used to measure changes in blood cell flow (flux) in the skin microcirculation after endothelial stimulation.6 Recently, peripheral arterial tonometry (EndoPAT®; Itamar Medical Ltd, Caesarea, Israel) has been developed to measure changes in pulse wave amplitude within the fingertip.13 A new method to assess microvascular endothelial reactivity is digital thermal monitoring (DTM). By monitoring the digital skin temperature overshoot during hyperemia, vascular reactivity can be quantified.14
In the current study we aimed to determine the influence of obesity on endothelial function compared with other components of the metabolic syndrome and smoking in a population of patients with suspected coronary artery disease. Endothelial function was assessed simultaneously by LDF, EndoPAT, and DTM, and its relationship to obesity as well as other clinical risk factors and baseline characteristics was analyzed.
Methods
Study Design and Population
The study design was previously published and primarily focused on the relationship between radial artery spasm and endothelial function.15 In summary, patients at risk for coronary disease and referred for elective cardiac catheterization were eligible for study participation. Patients with ST‐segment elevation myocardial infarction or hemodynamic instability or renal disease with a glomerular filtration rate <30 were excluded. Also, patients with an intention to undergo transfemoral arterial access (ie, patients with coronary bypass grafts) were excluded. Between May 2014 and June 2015, patients were screened at the VU University Medical Center. After obtaining informed consent, baseline characteristics were noted. Also, a predefined set of established cardiovascular risk factors (diabetes mellitus, hypercholesterolemia, hypertension, body mass index [BMI], age, smoking, and sex) and cardiovascular history, were registered. Thereafter, 3 different endothelial function tests were performed at the same time and subsequently all patients underwent coronary angiography. In this secondary analysis, the relationship between BMI, other known cardiovascular risk factors, and endothelial function was studied. The study protocol was approved by the local ethics committee and performed in accordance with the Declaration of Helsinki.
Endothelial Function Measurements
All endothelial function measurements were performed at the same time by trained researchers in a quiet and temperature‐controlled room. The patients were resting during the entire measurement period of 15 minutes. To determine postocclusive reactive hyperemia, a blood pressure cuff was inflated at the upper arm to a pressure at least 30 mm Hg above the systolic arterial blood pressure. The microvascular measurements were started 5 minutes before the occlusion period and were stopped 5 minutes after the occlusion period (during postocclusive reactive hyperemia). The maximum hyperemic response is reached after 5 minutes of vascular occlusion. This time point is recommended for EndoPAT measurements.16
EndoPAT Measurements
The EndoPAT® device (Itamar Medical Ltd) was used to measure the arterial pulse wave amplitude with pneumatic probes placed on the index finger of each hand, capable of sensing volume changes in the digit with each arterial pulsation. The contralateral hand was used as a reference to correct for potential systemic changes. The fingertip probes have a rigid external casing containing inflatable chambers with uniform applied pressure field across the finger to prevent venous pooling. Volume changes in the fingertip were recorded digitally as pulse amplitude that can be tracked over time. Data are presented as Reactive Hyperemia Index (RHI); this is the post‐to‐preocclusion PAT signal ratio in the occluded side, divided by the same ratio of the (nonoccluded) control arm as a correction for possible systemic effects.17
Laser Doppler Flowmetry
Single‐point LDF (Moor Instruments, Devon, UK) was measured at the upper phalanx of the right index finger. The signal was quantified as the product of average blood cell velocity and concentration. Because it does not provide an exact measure of flow (mL/min), flux is often used.6 The flux was determined at baseline and the peak (5 minutes after cuff deflation). Peak minus baseline and the percentage increase between baseline and peak were calculated. This percentage was used as a measure of endothelial function.
Digital Thermal Monitoring
Continuous DTM was performed during the entire measurement of 15 minutes with the GOBI‐1417 camera (XenICs NV, Leuven, Belgium) at the distal phalanx of the thumb and the proximal phalanx of the index finger (D2). DTM data were expressed in temperature rebound (peak temperature minus baseline temperature) compared with baseline temperature (change in %).14
Statistical Analysis
Baseline characteristics are presented as percentage for categorical variables and as mean± SD in case of a normal distribution and median (interquartile range, IQR) otherwise for the continuous variables. The baseline table is divided into 3 BMI groups: normal (BMI <25), overweight (BMI 25–30), and obese (BMI >30). Differences between these groups were analyzed using χ2 testing for categorical variables and ANOVA for continuous variables. Test results are presented as mean±SEM or median in case of a skewed distribution. Postocclusive reactive hyperemic values of each of the tests (EndoPAT, LDF, DTM) were compared between groups based on presence of risk factors using independent samples t tests for normally distributed random variables and the Mann–Whitney U test for variables that were not normally distributed. Separate comparisons were performed for each endothelial test and risk factor. Normality was assessed using separate QQ‐plots of the postocclusive reactive hyperemic values made in the subgroup with and without the risk factor. All statistical tests were 2‐tailed, and a P value of <0.05 was considered as statistically significant. Multivariate analyses (linear regression) were performed for each endothelial function test to relate the endothelial function measurement to the 7 predefined cardiovascular risk factors (age, sex, diabetes mellitus, hypertension, hypercholesterolemia, smoking, and BMI). Furthermore, to find a set of independent risk factors associated with endothelial function, a forward selection procedure was used. Consistency of measurements obtained with the 3 tests was quantified using Spearman's correlation coefficient. All statistical analyses were performed with SPSS for Windows version 22.0 (SPSS, Inc, Chicago, IL).
Results
A total of 796 patients referred for elective intervention were screened from May 2014 to June 2015. Because of the time burden of extensive endothelial testing, only 1 patient per day (the first in the morning) was asked to participate, resulting in 164 eligible patients with informed consent. In 35 patients, not all endothelial tests could be performed because of logistical reasons, leaving 129 patients with all 3 different tests performed. Nineteen of the EndoPAT measurements and 2 of the LDF measurements were too noisy to analyze, leaving 108 of the 129 patients with all 3 tests available for the final analysis. The mean age of the study sample was 63±11 years and 68% of the patients were male. Of the female subjects, the mean age was 65±11 (ranging from 47 to 82 years) and all women were presumed to be postmenopausal. Baseline patient characteristics are presented in Table 1. The mean postocclusive reactive hyperemia–induced vascular response was 1.86±0.07% and 5.9±0.9% measured by RHI and DTM, respectively (±SEM). The LDF values had a skewed distribution and the median vascular response was 680% (IQR, 424–1090). The Spearman correlation coefficient between the 3 methods was 0.01 (P=0.32), 0.38 (P<0.01), and 0.26 (P=0.01) for EndoPAT and DTM, EndoPAT and LDF, and LDF and DTM, respectively.
Table 1.
Baseline Characteristics
| Overall (n=108) | BMI <25 (n=37) | BMI 25 to 30 (n=43) | BMI >30 (n=28) | |
|---|---|---|---|---|
| Patient characteristics | ||||
| Male, n (%) | 73 (68) | 21 (57) | 31 (72) | 21 (75) |
| Mean age (y) ±SD | 63±11 | 63±11 | 63±11 | 63±10 |
| Length (cm) ±SD | 175±9 | 173±10 | 176±9 | 174±10 |
| Weight (kg) ±SD | 83±16 | 69±9 | 84±9 | 101±14a |
| BMI ±SD | 27±4 | 23±1 | 27±1 | 33±3a |
| Systolic blood pressure±SD | 140±21 | 137±20 | 141±19 | 142±24 |
| Diastolic blood pressure±SD | 78±11 | 78±9 | 79±11 | 78±12 |
| Cardiovascular history and coronary status | ||||
| Previous MI, n (%) | 21 (19) | 8 (22) | 7 (16) | 6 (21) |
| Previous PCI, n (%) | 31 (29) | 8 (22) | 12 (28) | 11 (39) |
| PAD, n (%) | 3 (3) | 2 (5) | 1 (2) | 0 (0) |
| Indication ACS, n (%) | 8 (7) | 2 (5) | 4 (9) | 2 (7) |
| Angiographic CAD | 80 (74%) | 26 (70%) | 33 (77%) | 21 (75%) |
| No CHD history or angiographic CAD | 21 (19%) | 9 (24%) | 8 (19%) | 4 (14%) |
| Cardiovascular risk factors | ||||
| Current smoking, n (%) | 14 (13) | 6 (16) | 6 (14) | 2 (7) |
| Diabetes mellitus, n (%) | 18 (17) | 0 (0) | 4 (9) | 14 (50)a |
| Hypertension, n (%) | 47 (44) | 14 (38) | 15 (35) | 18 (64)a |
| Hypercholesterolemia, n (%) | 38 (35) | 9 (24) | 17 (40) | 12 (43) |
Differences between the 3 groups were analyzed using χ2 testing for categorical variables and ANOVA for continuous variables. ACS indicates acute coronary syndrome; BMI, body mass index; CAD, coronary artery disease; CHD, coronary heart disease; MI, myocardial infarction; PAD, peripheral artery disease; PCI, percutaneous coronary intervention.
P value for group difference <0.05.
BMI, Metabolic Risk Factors, and Endothelial Function
There was a significant correlation between BMI and endothelial function as determined by all 3 tests (EndoPAT: r=−0.23, P = 0.02, LDF: r=−0.30, P<0.01, and DTM: r=−0.30, P<0.01, Figures 1 and 2). None of the other predefined risk factors were related to a significant decrease in endothelial function in our population, especially the metabolic risk factors (control compared with diabetes mellitus: 1.90±0.20 versus 1.67±0.20, P=0.19, 728% [IQR 427–1110] versus 589% [IQR 320–1067] P=0.28, and 6.6±1.0% versus 2.5±1.7%, P=0.08). Control compared with hypertension: 1.91±0.09 versus 1.80±0.10, P=0.42, 785% (IQR 517–1349) versus 648% (IQR 391–1033) P=0.11, and 6.9±1.3% versus 4.5±1.0%, P=0.17. Control compared with hypercholesterolemia: 1.84±0.08 versus 1.91±0.12, P=0.58, 668% (IQR 425–1046) versus 771% (IQR 384–1179) P=0.42, and 7.1±1.2% versus 3.7±1.1%, P=0.06. In a linear multivariate analysis, using all predefined risk factors in a forward selection (sex, age, smoking, diabetes mellitus, hypertension, hypercholesterolemia, and BMI), again only high BMI was found to be associated with impaired endothelial function (RHI: B=−0.51, 95% CI [confidence interval] [−0.86, −0.15]; LDF: B=−44, 95% CI [−74, −14] and DTM: B=−0.51, 95% CI [−0.97, −0.05]). When all risk factors were combined into a multivariable linear regression model, BMI still remained independently related to endothelial function (Table 2).
Figure 1.
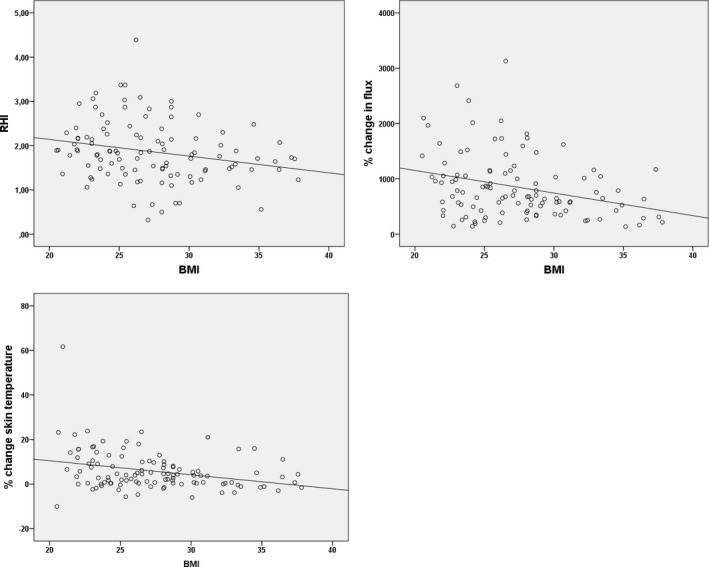
Scatter plots of correlation BMI and RHI, flux change (LDF), and temperature change (DTM). BMI indicates body mass index; DTM, digital thermal monitoring; LDF, laser Doppler flowmetry; RHI, Reactive Hyperemic Index.
Figure 2.
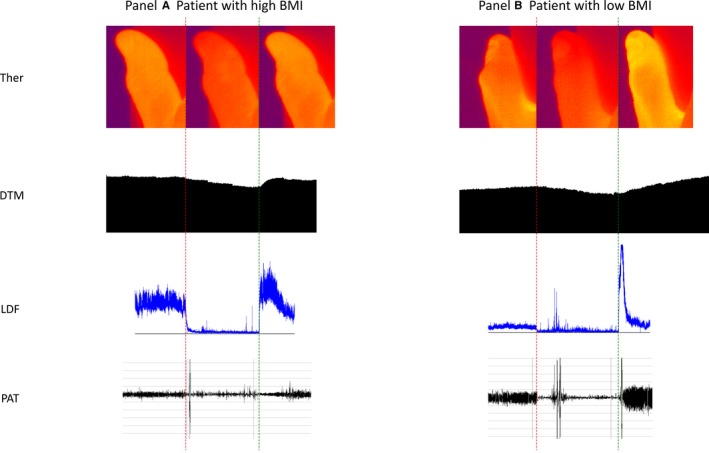
Examples of microvascular endothelial test in a patient with high and low BMI: (A) representing a patient with a high BMI (34.9 kg/m2) and (B) representing a patient with a low BMI (20.6 kg/m2). The red dotted line indicates time of occlusion, the green dotted line hyperemia. Upper to lower panel: thermography (Ther) of the thumb followed by DTM, LDF, and PAT curves. BMI indicates body mass index; DTM, digital thermal monitoring; LDF, laser Doppler flowmetry; PAT, peripheral arterial tonometry.
Table 2.
Univariable and Multivariable Linear Regression With Microvascular Function as Dependent Variable (Separate for Each of the 3 Tests)
| Test | Risk Factor | Univariable βa | 95% CI | P Value | Multivariable βa | 95% CI | P Value | ||
|---|---|---|---|---|---|---|---|---|---|
| Lower | Upper | Lower | Upper | ||||||
| PAT | Female vs male | −0.11 | −0.21 | 0.06 | 0.28 | −0.14 | −0.23 | 0.04 | 0.16 |
| Age | −0.16 | −0.24 | 0.02 | 0.11 | −0.20 | −0.28 | 0.00 | 0.05 | |
| Smoking | −0.10 | −0.20 | 0.07 | 0.32 | −0.19 | −0.26 | 0.01 | 0.06 | |
| Diabetes mellitus | −0.13 | −0.22 | 0.04 | 0.19 | −0.01 | −0.15 | 0.14 | 0.91 | |
| Hypertension | −0.08 | −0.19 | 0.08 | 0.42 | 0.03 | −0.12 | 0.16 | 0.80 | |
| Hchol | 0.05 | −0.10 | 0.17 | 0.58 | 0.11 | −0.05 | 0.21 | 0.24 | |
| BMI | −0.23 | −0.29 | −0.03 | 0.02b | −0.31 | −0.37 | −0.31 | <0.01b | |
| LDF | Female vs male | −0.03 | −0.22 | 0.16 | 0.77 | −0.09 | −163 | 59 | 0.35 |
| Age | −0.01 | −0.20 | 0.18 | 0.91 | 0.02 | −107 | 128 | 0.86 | |
| Smoking | 0.04 | −0.22 | 0.16 | 0.66 | −0.03 | −127 | 96 | 0.78 | |
| Diabetes mellitus | −0.09 | −0.15 | 0.27 | 0.28 | 0.06 | −86 | 160 | 0.56 | |
| Hypertension | −0.20 | −0.35 | 0.03 | 0.11 | −0.16 | −210 | 27 | 0.13 | |
| Hchol | 0.07 | −0.07 | 0.31 | 0.42 | 0.12 | −43 | 179 | 0.23 | |
| BMI | −0.30 | −0.52 | −0.10 | <0.01b | −0.33 | −317 | −62 | <0.01b | |
| DTM | Female vs male | 0.19 | 0.0 | 3.4 | 0.05 | 0.13 | −0.5 | 2.9 | 0.17 |
| Age | 0.03 | −1.5 | 2.0 | 0.76 | 0.02 | −1.6 | 2.0 | 0.84 | |
| Smoking | −0.03 | −2.0 | 1.5 | 0.79 | −0.06 | −2.3 | 1.2 | 0.51 | |
| Diabetes mellitus | −0.17 | −3.2 | 0.2 | 0.08 | −0.02 | −2.0 | 1.8 | 0.88 | |
| Hypertension | −0.14 | −2.9 | 0.5 | 0.17 | −0.07 | −2.4 | 1.2 | 0.51 | |
| Hchol | −0.18 | −3.3 | 0.1 | 0.06 | −0.14 | −2.9 | 0.5 | 0.17 | |
| BMI | −0.30 | −4.3 | −1.0 | <0.01b | −0.24 | −4.1 | −0.2 | 0.03b | |
BMI indicates body mass index; CI, confidence interval; DTM, digital thermal monitoring; Hchol, hypercholesterolemia; LDF, laser Doppler flowmetry; PAT, peripheral arterial tonometry.
Linear regression is expressed as a standardized coefficient. In the multivariable analyses, the coefficients are corrected for all 6 other risk factors.
Statistically significant.
Medication and Endothelial Function
The use of vasoactive medication had a minimal effect on the test results (Table 3). Only patients using angiotensin‐converting enzyme inhibitor or angiotensin II receptor inhibitors had significantly reduced DTM (3.9±0.8% versus 8.2±1.6%, P=0.01) and in patients using calcium blocker, LDF was reduced (588% [IQR 341–919] versus 785% [IQR 525–1161], P=0.05). All other test results were not influenced by the use of vasoactive medication. Also in patients without any vasoactive drug (n=6), no difference in endothelial function was found compared with patients on medication (2.05±0.22 versus 1.85±0.07, P=0.50, 804% [IQR 504–1968] versus 680% [IQR 423–1077], P=0.46, 8.4±4.3% versus 5.7±0.9%, P=0.48 for RHI, LDF, and DTM, respectively). All patients with hypertension or diabetes mellitus were medically treated for this condition, and 90% of patients with hypercholesterolemia received statin therapy.
Table 3.
Vasoactive Drugs and Endothelial Function
| RHI±SEM | P Value | LDF (IQR) | P Value | DTM±SEM | P Value | |
|---|---|---|---|---|---|---|
| ACE/ARB+ | 1.89±0.10 | 0.65 | 646 (386–1044) | 0.11 | 3.9±0.8 | 0.01a |
| ACE/ARB− | 1.83±0.09 | 835 (517–1428) | 8.2±1.6 | |||
| BBl+ | 1.90±0.08 | 0.39 | 698 (423–1161) | 0.54 | 5.6±1.1 | 0.58 |
| BBl− | 1.77±0.11 | 649 (425–967) | 6.7±1.3 | |||
| CaBl+ | 1.74±0.13 | 0.29 | 588 (341–919) | 0.05a | 4.7±1.1 | 0.38 |
| CaBl− | 1.91±0.08 | 785 (525–1161) | 6.4±1.1 | 0.05 | ||
| Stat+ | 1.87±0.08 | 0.85 | 677 (425–1052) | 0.61 | 6.1±1.1 | 0.72 |
| Stat− | 1.84±0.13 | 788 (367–1199) | 5.3±1.3 | |||
| Nitra+ | 1.62±0.13 | 0.07 | 589 (326–908) | 0.16 | 5.1±1.7 | 0.63 |
| Nitra− | 1.93±0.08 | 770 (479–1139) | 6.1±1.0 |
ACE/ARB indicates angiotensin‐converting enzyme inhibitor or angiotensin II receptor blocker; BBl, beat blocker; CaBl, calcium blocker; DTM, digital thermal monitoring; IQR, interquartile range; LDF, laser Doppler flowmetry; Nitra, long‐acting nitrate; RHI, reactive hyperemia index; Stat, statin.
Statistically significant.
Cardiovascular History, Coronary Artery Disease, and Endothelial Function
Compared with patients with angiographic coronary artery disease, defined as >50% stenosis of a coronary segment obtained by coronary angiogram after the endothelial function tests, patients without previous or angiographic coronary artery disease showed no difference in endothelial function (RHI 1.92±0.07 versus 1.63±0.14, P=0.09, LDF 698% [IQR 426–1152] versus 582% [IQR 303–950] P=0.16, DTM 6.4±1.0% versus 4.0±1.2%, P=0.28). In comparison to patients with stable coronary artery disease (n=80), patients with the indication acute coronary syndrome (n=7) had significantly reduced values of endothelial function, as measured with EndoPAT: 1.36±0.19 versus 1.97±0.08, respectively, P=0.03, but not with the other endothelial function tests.
Discussion
In our current study on patients with suspected significant coronary artery disease, BMI was independently and strongly associated with microvascular endothelial dysfunction. Diabetes mellitus, hypertension, hypercholesterolemia, and smoking did not significantly influence endothelial function in this population. In patients with a high BMI, a significant impairment of the hyperemic flow response was found with all 3 microvascular endothelial function tests.
Several studies have been published reporting factors influencing endothelial function. However, the outcomes vary significantly, possibly as a result of different study populations, different stimuli used for probing endothelial function, and different methods used for analyzing vascular response.18, 19 Also several studies showed a high coefficient of variability when performing repeated measurements with EndoPAT on the same day.20, 21 LDF and DTM have lower coefficients of variability when same‐day repeated testing is done.14, 22
Obese patients, especially those with visceral adiposity, are known to have low‐grade inflammation, which can lead to insulin resistance and type 2 diabetes mellitus.23 Only recently perivascular adipose tissue, which is increased in obesity, has been shown to be of influence in microvascular function. In addition to the lamina intima, media, and adventitia, it is described as the fourth vessel wall layer, and this tissue has an important role in vasomotor regulation by excreting vasoactive adipocytokines.24 Under normal circumstances, perivascular adipose tissue functions in a paracrine fashion, directly reducing smooth muscle tone25 and enhancing insulin‐dependent vasodilatation. In obese mice, perivascular adipose tissue–dependent vascular relaxation is attenuated, partly as a result of reduced nitric oxide (NO) bioavailability.26, 27 Change of perivascular adipose tissue function in healthy obese women has been shown to lead to a decreased insulin‐dependent microvascular vasodilator capacity,28 which in turn is linked to NO‐dependent vasodilatation.29 In 1 study, obese patients had a significant lower microvascular hyperemic response to postocclusive reactive hyperemia compared with a healthy control population. This difference diminished after lowering the BMI by gastric bypass surgery, both in patients with and without type 2 diabetes mellitus.30 In line with these results, our study showed that a high BMI is an independent predictor of reduced endothelial function in patients with suspected and established vascular disease. Importantly, we have found that the relationship between BMI and microvascular endothelial dysfunction remains, even taking in account treated obesity‐related cardiometabolic risk factors such as hypertension, hypercholesterolemia, and diabetes mellitus.
Not only is increased BMI a plausible cause for endothelial dysfunction, but it has also been posed that endothelial dysfunction itself may initiate other features of metabolic syndrome. There is evidence that microvascular dysfunction precedes insulin resistance, type 2 diabetes mellitus, and high blood pressure.31, 32, 33, 34
The weak correlation between the 3 tests in our study may reflect the different entities of microvascular function. While the peripheral arterial tonometry output is primarily determined by the effect of total increase of pulsatile arterial digital blood volume (dilatation of arterioles), the output of the other 2 tests reflects changes in the capillary blood flow of the outer layer of the skin.35 The temperature of the skin is highly dependent on skin capillary blood volume and might rather reflect the (temperature) exchange capacity of the superficially located capillary endothelium than blood (cell) flow in deeper situated arterioles as detected by LDF. Despite different types of information obtained using these tests of microvascular function, we have shown here that obesity reduces microvascular responses measured with all techniques, indicating that obesity impairs both arteriolar and capillary function.
Our population was small, heterogeneous, and consisted of patients with and without established CVD. It is possible that treatment of vascular disease and risk factors might have influenced endothelial function. Indeed, most patients received vasoactive medication at the time of the measurements. However, the independent relation of BMI with endothelium‐dependent vasodilatation supports the hypothesis that microvascular dysfunction is not reversed by treatment of risk factors in obese patients and remains influenced by adipose tissue, even in patients without diabetes mellitus or other metabolic risk factors.28 The sample size may have been too small to detect differences in endothelial function in patients with and without metabolic risk factors. In particular, comparison between groups based on risk factors with low prevalence may have been limited by low power. For this reason we also reported standardized regression coefficients that can be used to judge the strength of association of risk factors with endothelium‐dependent vasodilatation. Again BMI and to a lesser extent diabetes mellitus and hypertension showed small‐to‐moderate associations with endothelium‐dependent vasodilatation that were consistent for the 3 tests used. In addition, there have been several small studies that found a significant influence of metabolic factors on endothelial function.36, 37 In our population, metabolic risk factors were well treated, potentially obscuring the relation between these risk factors and endothelial function.
In patients without established CVD, microvascular dysfunction potentially indicates a very early stage of atherosclerosis and it might be the first sign of cardiovascular risk, even before the appearance of classical metabolic risk factors. Especially in so‐called metabolically healthy obese persons, microvascular function testing might identify subjects at risk for development of metabolic risk factors and cardiovascular events. These apparently healthy persons may benefit more from early (lifestyle) intervention. Such intervention studies are mandated and will shed light on the value of endothelial function testing in addition to known metabolic risk factors.
Conclusion
High BMI is associated with endothelial dysfunction in patients with suspected coronary artery disease, even in patients with treated metabolic risk factors.
Disclosures
None.
(J Am Heart Assoc. 2017;6:e006082 DOI: 10.1161/JAHA.117.006082.)28912211
References
- 1. Stevens GA, Singh GM, Lu Y, Danaei G, Lin JK, Finucane MM, Bahalim AN, McIntire RK, Gutierrez HR, Cowan M, Paciorek CJ, Farzadfar F, Riley L, Ezzati M, Mhurchu CN, Rodgers A, Pan W, Gu D, Woodward M, Ezzati M, Lopez A, Rodgers A, Whitlock G, Lewington S, Sherliker P, Clarke R, Emberson J, Halsey J, Qizilbash N, Collins R, Peto R, Wormser D, Kaptoge S, Di Angelantonio E, Wood A, Pennells L, Thompson A, Sarwar N, Kizer J, Lawlor D, Nordestgaard B, Finucane M, Stevens G, Cowan M, Danaei G, Lin J, Paciorek C, Singh G, Gutierrez H, Lu Y, Bahalim A, Danaei G, Finucane M, Lin J, Singh G, Paciorek C, Cowan M, Farzadfar F, Stevens G, Lim S, Riley L, Ezzati M, Ahmad O, Boschi‐Pinto C, Lopez A, Murray C, Lozano R, Inoue M, Molarius A, Seidell J, Sans S, Tuomilehto J, Kuulasmaa K, Monteiro C, Moura E, Conde W, Popkin B, Flegal K, Carroll M, Ogden C, Curtin L, Beccuti G, Pannain S, Mozaffarian D, Hao T, Rimm E, Willett W, Hu F, Gortmaker S, Swinburn B, Levy D, Carter R, Mabry P, Finegood D, Huang T, Marsh T, Moodie M. National, regional, and global trends in adult overweight and obesity prevalences. Popul Health Metr. 2012;10:751–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kwaśniewska M, Kozińska J, Dziankowska‐Zaborszczyk E, Kostka T, Jegier A, Rębowska E, Orczykowska M, Leszczyńska J, Drygas W. The impact of long‐term changes in metabolic status on cardiovascular biomarkers and microvascular endothelial function in middle‐aged men: a 25‐year prospective study. Diabetol Metab Syndr. 2015;7:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Barbosa JAA, Rodrigues AB, Mota CCC, Barbosa MM, Silva ACSE. Cardiovascular dysfunction in obesity and new diagnostic imaging techniques: the role of noninvasive image methods. Vasc Health Risk Manag. 2011;7:287–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Widlansky ME, Gokce N, Keaney JF, Vita JA. The clinical implications of endothelial dysfunction. J Am Coll Cardiol. 2003;42:1149–1160. [DOI] [PubMed] [Google Scholar]
- 5. Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362:801–809. [DOI] [PubMed] [Google Scholar]
- 6. Roustit M, Cracowski JL. Assessment of endothelial and neurovascular function in human skin microcirculation. Trends Pharmacol Sci. 2013;34:373–384. [DOI] [PubMed] [Google Scholar]
- 7. Kruger A, Stewart J, Sahityani R, O'Riordan E, Thompson C, Adler S, Garrick R, Vallance P, Goligorsky MS. Laser Doppler flowmetry detection of endothelial dysfunction in end‐stage renal disease patients: correlation with cardiovascular risk. Kidney Int. 2006;70:157–164. [DOI] [PubMed] [Google Scholar]
- 8. IJzerman RG, de Jongh RT, Beijk MA, van Weissenbruch MM, Delemarre‐van de Waal HA, Serné EH, Stehouwer CD. Individuals at increased coronary heart disease risk are characterized by an impaired microvascular function in skin. Eur J Clin Invest. 2003;33:536–542. [DOI] [PubMed] [Google Scholar]
- 9. Yamamoto‐Suganuma R, Aso Y. Relationship between post‐occlusive forearm skin reactive hyperaemia and vascular disease in patients with Type 2 diabetes—a novel index for detecting micro‐ and macrovascular dysfunction using laser Doppler flowmetry. Diabet Med. 2009;26:83–88. [DOI] [PubMed] [Google Scholar]
- 10. Halcox JPJ, Schenke WH, Zalos G, Mincemoyer R, Prasad A, Waclawiw MA, Nour KRA, Quyyumi AA. Prognostic value of coronary vascular endothelial dysfunction. Circulation. 2002;106:653–658. [DOI] [PubMed] [Google Scholar]
- 11. Gutterman DD, Chabowski DS, Kadlec AO, Durand MJ, Freed JK, Ait‐Aissa K, Beyer AM. The human microcirculation. Circ Res. 2016;118:157–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lorenzo S, Minson CT. Human cutaneous reactive hyperaemia: role of BKCa channels and sensory nerves. J Physiol. 2007;585:295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kuvin JT, Patel AR, Sliney KA, Pandian NG, Sheffy J, Schnall RP, Karas RH, Udelson JE. Assessment of peripheral vascular endothelial function with finger arterial pulse wave amplitude. Am Heart J. 2003;146:168–174. [DOI] [PubMed] [Google Scholar]
- 14. Ahmadi N, McQuilkin GL, Akhtar MW, Hajsadeghi F, Kleis SJ, Hecht H, Naghavi M, Budoff M. Reproducibility and variability of digital thermal monitoring of vascular reactivity. Clin Physiol Funct Imaging. 2011;31:422–428. [DOI] [PubMed] [Google Scholar]
- 15. van der Heijden D, van Leeuwen M, Janssens G, Hermie J, Lenzen M, Ritt M, van de Ven P, Kiemeneij F, van Royen N. Endothelial dysfunction and the occurrence of radial artery spasm during transradial coronary procedures: the ACRA‐Spasm study. EuroIntervention. 2016;12:1263–1270. [DOI] [PubMed] [Google Scholar]
- 16. Faizi AK, Kornmo DW, Agewall S. Evaluation of endothelial function using finger plethysmography. Clin Physiol Funct Imaging. 2009;29:372–375. [DOI] [PubMed] [Google Scholar]
- 17. Bonetti PO, Pumper GM, Higano ST, Holmes DR, Kuvin JT, Lerman A. Noninvasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. J Am Coll Cardiol. 2004;44:2137–2141. [DOI] [PubMed] [Google Scholar]
- 18. Philpott AC, Lonn E, Title LM, Verma S, Buithieu J, Charbonneau F, Anderson TJ. Comparison of new measures of vascular function to flow mediated dilatation as a measure of cardiovascular risk factors. Am J Cardiol. 2009;103:1610–1615. [DOI] [PubMed] [Google Scholar]
- 19. Hamburg NM, Keyes MJ, Larson MG, Vasan RS, Schnabel R, Pryde MM, Mitchell GF, Sheffy J, Vita JA, Benjamin EJ. Cross‐sectional relations of digital vascular function to cardiovascular risk factors in the Framingham Heart Study. Circulation. 2008;117:2467–2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liu J, Wang J, Jin Y, Roethig HJ, Unverdorben M. Variability of peripheral arterial tonometry in the measurement of endothelial function in healthy men. Clin Cardiol. 2009;32:700–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Onkelinx S, Cornelissen V, Goetschalckx K, Thomaes T, Verhamme P, Vanhees L. Reproducibility of different methods to measure the endothelial function. Vasc Med. 2012;17:79–84. [DOI] [PubMed] [Google Scholar]
- 22. Keymel S, Sichwardt J, Balzer J, Stegemann E, Rassaf T, Kleinbongard P, Kelm M, Heiss C, Lauer T. Characterization of the non‐invasive assessment of the cutaneous microcirculation by laser Doppler perfusion scanner. Microcirculation. 2010;17:358–366. [DOI] [PubMed] [Google Scholar]
- 23. Lopes HF, Corrêa‐Giannella ML, Consolim‐Colombo FM, Egan BM. Visceral adiposity syndrome. Diabetol Metab Syndr. 2016;8:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kagota S, Iwata S, Maruyama K, Wakuda H, Shinozuka K. Functional relationship between arterial tissue and perivascular adipose tissue in metabolic syndrome. Yakugaku Zasshi. 2016;136:693–697. [DOI] [PubMed] [Google Scholar]
- 25. Greenstein AS, Khavandi K, Withers SB, Sonoyama K, Clancy O, Jeziorska M, Laing I, Yates AP, Pemberton PW, Malik RA, Heagerty AM. Local inflammation and hypoxia abolish the protective anticontractile properties of perivascular fat in obese patients. Circulation. 2009;119:1661–1670. [DOI] [PubMed] [Google Scholar]
- 26. Meijer RI, Bakker W, Alta CLAF, Sipkema P, Yudkin JS, Viollet B, Richter EA, Smulders YM, Van Hinsbergh VWM, Serné EH, Eringa EC. Perivascular adipose tissue control of insulin‐induced vasoreactivity in muscle is impaired in db/db mice. Diabetes. 2013;62:590–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Aghamohammadzadeh R, Unwin RD, Greenstein AS, Heagerty AM. Effects of obesity on perivascular adipose tissue vasorelaxant function: nitric oxide, inflammation and elevated systemic blood pressure. J Vasc Res. 2016;52:299–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Meijer RI, Serné EH, Korkmaz HI, van der Peet DL, de Boer MP, Niessen HWM, van Hinsbergh VWM, Yudkin JS, Smulders YM, Eringa EC. Insulin‐induced changes in skeletal muscle microvascular perfusion are dependent upon perivascular adipose tissue in women. Diabetologia. 2015;58:1907–1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bradley EA, Richards SM, Keske MA, Rattigan S. Local NOS inhibition impairs vascular and metabolic actions of insulin in rat hindleg muscle in vivo. Am J Physiol Endocrinol Metab. 2013;305:E745–E750. [DOI] [PubMed] [Google Scholar]
- 30. Rossi M, Nannipieri M, Anselmino M, Pesce M, Muscelli E, Santoro G, Ferrannini E. Skin vasodilator function and vasomotion in patients with morbid obesity: effects of gastric bypass surgery. Obes Surg. 2011;21:87–94. [DOI] [PubMed] [Google Scholar]
- 31. Wong TY, Klein R, Sharrett AR, Schmidt MI, Pankow JS, Couper DJ, Klein BEK, Hubbard LD, Duncan BB. Retinal arteriolar narrowing and risk of diabetes mellitus in middle‐aged persons. JAMA. 2002;287:2528–2533. [DOI] [PubMed] [Google Scholar]
- 32. Hedman A, Reneland R, Lithell HO. Alterations in skeletal muscle morphology in glucose‐tolerant elderly hypertensive men: relationship to development of hypertension and heart rate. J Hypertens. 2000;18:559–565. [DOI] [PubMed] [Google Scholar]
- 33. Karaca Ü, Schram MT, Houben AJHM, Muris DMJ, Stehouwer CDA. Microvascular dysfunction as a link between obesity, insulin resistance and hypertension. Diabetes Res Clin Pract. 2014;103:382–387. [DOI] [PubMed] [Google Scholar]
- 34. Yudkin JS, Eringa E, Stehouwer CDA. “Vasocrine” signalling from perivascular fat: a mechanism linking insulin resistance to vascular disease. Lancet. 2005;365:1817–1820. [DOI] [PubMed] [Google Scholar]
- 35. Coffman JAYD. Effects of endothelium‐derived nitric and digital blood flow in humans. Am J Physiol. 1994;267:2087–2090. [DOI] [PubMed] [Google Scholar]
- 36. Walther G, Obert P, Dutheil F, Chapier R, Lesourd B, Naughton G, Courteix D, Vinet A. Metabolic syndrome individuals with and without type 2 diabetes mellitus present generalized vascular dysfunction: cross‐sectional study. Arterioscler Thromb Vasc Biol. 2015;35:1022–1029. [DOI] [PubMed] [Google Scholar]
- 37. Tomsa A, Klinepeter Bartz S, Krishnamurthy R, Krishnamurthy R, Bacha F. Endothelial function in youth: a biomarker modulated by adiposity‐related insulin resistance. J Pediatr. 2016;178:1216–1227. [DOI] [PubMed] [Google Scholar]


