Abstract
Background
Peripheral artery disease (PAD) is one of the most common clinical presentations of atherosclerosis, and its prevalence is still increasing. Despite improvement of health care, morbidity and mortality risks remain high, including the risk of amputation. GDF15 (growth differentiation factor 15) is a member of the transforming growth factor family that is involved in apoptosis and inflammation; therefore, GDF15 is a potential biomarker to identify patients at high risk of adverse clinical outcomes.
Methods and Results
Circulating GDF15 levels were measured using a multiplex immunoassay in patients with critical limb ischemia and PAD from 2 different patient cohorts that included patients with clinically manifest PAD: the JUVENTAS (Rejuvenating Endothelial Progenitor Cells via Transcutaneous Intra‐Arterial Supplementation) trial (n=160, 67 major events; critical limb ischemia) and the Athero‐Express Biobank (n=386, 64 major events; PAD). Kaplan–Meier curves demonstrated that high levels of GDF15 were associated with increased risk of major events, defined as major amputation (at or above the ankle joint) and all‐cause mortality, in both cohorts (highest versus lowest, JUVENTAS: hazard ratio: 4.01 [95% confidence interval, 2.05–7.84; P<0.0001]; Athero‐Express: hazard ratio: 3.27 [95% confidence interval, 1.64–6.54; P=0.0008]). In the JUVENTAS trial, this was more pronounced in women. Cox proportional multivariable regression models with median follow‐up of 3 years, corrected for common confounders, showed hazard ratios of 1.70 (95% confidence interval, 1.18–2.69; P=0.0053) and 1.57 (95% confidence interval, 1.02–2.41; P=0.041) per 2.78‐fold increase of GDF15 in JUVENTAS and Athero‐Express, respectively.
Conclusions
High GDF15 levels are associated with increased risk of major amputation and/or death in PAD patients. GDF15 levels could be of additive value to identify patients who are at high risk of amputation or death and could help guide treatment choices.
Keywords: biomarker, cardiovascular disease risk factors, cytokine, growth differentiation factor 15, follow‐up studies, mortality, peripheral artery disease, revascularization, secondary prevention, vascular biology, women
Subject Categories: Atherosclerosis, Peripheral Vascular Disease
Clinical Perspective
What Is New?
High GDF15 (growth differentiation factor 15) levels in blood are associated with increased risk of amputation or death in patients with peripheral artery disease (PAD).
In patients with severe PAD, GDF15 levels in blood appear to be more strongly associated with major events in women.
Circulating GDF15 is highly correlated with hemoglobin levels.
Circulating GDF15 levels do not correlate with specific plaque characteristics.
What Are the Clinical Implications?
GDF15 levels in blood can predict outcome in PAD patients.
Circulating GDF15 levels can improve prediction beyond traditional risk factors in patients with severe PAD.
Sex‐adjusted risk prediction might be needed for patients with severe PAD.
Peripheral artery disease (PAD) is one of the most common clinical presentations of atherosclerosis.1 In 2010, the global prevalence of PAD was 202 million, and this is ever increasing.2 Critical limb ischemia is the most severe form of PAD, and mortality rates reach up to 20% in the first 6 months after diagnosis.3 Despite improved health care for atherosclerosis and subsequent increased survival in PAD patients, morbidity and mortality risks in PAD patients are still high.2, 4 Patients with critical limb ischemia are at particularly high risk of major amputation. To improve patient management, it is important to identify those patients who are at exceedingly high risk of amputation and death.
GDF15 (growth differentiation factor 15) is a protein found in circulation that is a member of the transforming growth factor family. GDF15 is a promising prognostic biomarker for cardiovascular disease. GDF15 levels are a strong predictor of mortality and recurrent myocardial infarction in patients with acute coronary syndrome.5, 6, 7, 8, 9, 10 Furthermore, high levels of GDF15 are associated with the risk of developing adverse left ventricular remodeling and heart failure.11, 12, 13, 14, 15 In heart failure patients, both with preserved and reduced ejection fraction, high GDF15 levels identify patients at high risk of rehospitalization and death.14
The causal role of GDF15 in atherosclerosis has been studied in vitro and in animal models. GDF15 affects early plaque formation, progression, and plaque composition.16, 17 From a mechanistic point of view, GDF15 modulates CCR2 (C‐C motif chemokine receptor 2)–mediated macrophage chemotaxis, affects macrophage apoptosis,18 and inhibits the proliferation of endothelial cells.19 Macrophage accumulation and endothelial dysfunction play key roles in atherosclerosis and thus are important in PAD patients.
The prognostic value of GDF15 in PAD is currently unknown. Consequently, the aim of this study was to determine the association of GDF15 levels with major events, namely, major amputation and all‐cause mortality, in PAD patients. For this study, we used 2 independent cohorts: the JUVENTAS (Rejuvenating Endothelial Progenitor Cells via Transcutaneous Intra‐Arterial Supplementation) trial20 and the Athero‐Express Biobank study.21
Methods
JUVENTAS Trial
Patient population
The JUVENTAS trial was a single‐center (University Medical Center in Utrecht, Utrecht, the Netherlands), placebo‐controlled, double‐blinded, randomized trial on repeated intra‐arterial injections of bone marrow–derived mononuclear cells in patients with nonrevascularizable severe PAD (Fontaine classes IIb, III, and IV). Autologous erythrocytes were used as a placebo control. Patients with severe PAD (Fontaine classes IIb, III, and IV) who were not eligible for conventional revascularization were included in this study. The trial was registered at ClinicalTrials.gov (identifier NCT00371371). No effect by the treatment on primary outcome was observed.20
Patient selection
Patients with a life expectancy of <1 year were excluded.22 In total, 160 patients were enrolled in this trial between 2006 and 2012. The primary outcome was major amputation (through or above the ankle joint) or death at 6 months of follow‐up. For the present study, the follow‐up was extended, leading to a median follow‐up duration of 3 years.
Sample collection
Blood was drawn at inclusion in the trial, and serum was collected and stored at −80°C until further analysis.
Athero‐Express Biobank Study
Patient population
The Athero‐Express Biobank is an ongoing cohort study that includes patients undergoing carotid and iliofemoral endarterectomy in 2 Dutch hospitals (St. Antonius Hospital, Nieuwegein, and University Medical Center Utrecht). Blood and plaque specimens are collected from these patients, and follow‐up is for 3 years.21 The study was approved by the ethics boards of both hospitals, and informed consent was obtained from all patients.
Patient selection
All patients who underwent an iliac or femoral endarterectomy between 2002 and 2013 with available plasma samples were included in the current study (n=386). Standardized questionnaires and hospital patient records were used to collect clinical data. The Athero‐Express Biobank includes mainly patients with intermittent claudication.
Sample collection
The sample collection protocol was described in detail previously.21 Briefly, preoperative blood was drawn, and citrate plasma was collected and stored at −80°C until further analysis.
Immediately after surgery, the plaque was processed. The segment with the culprit lesion (smallest lumen) or the segment with the largest plaque diameter (in case of total occlusion) was chosen to be fixed in 4% formaldehyde, decalcified, and embedded in paraffin.
Histological assessment
Histological slides were evaluated using a previously validated protocol.23, 24 In short, per‐patient staining was performed with CD68 (macrophages), α‐actin (smooth muscle cells), picrosirius red (collagen), elastin, hematoxylin and eosin, and Mallory's phosphotungstic acid–hematoxylin.
Intraplaque hemorrhage was determined by hematoxylin and eosin and Mallory's staining. Luminal thrombosis and intraplaque hemorrhage were considered plaque thrombosis.
Collagen and calcification were semiquantitatively scored and separated into binary groups of no or minor and moderate or heavy staining. The size of the lipid core was assessed with the use of polarized light. Smooth muscle cells and macrophages were automatically quantified as percentages of plaque area (AnalySiS version 3.2; Soft Imaging GmbH).
GDF15 Multiplex Immunoassay
GDF15 levels in plasma were determined by multiplex immunoassay.
For Athero‐Express samples, a Human Magnetic Luminex Assay (R&D Systems) was used, according to the manufacturer's protocol. Citrate plasma samples were diluted 1:1 with Calibrator Diluent RD6‐52 (R&D systems). For JUVENTAS samples, serum measurements were performed using an in‐house developed and validated multiplex immunoassay based on Luminex technology (xMAP; Luminex), using MagPlex microspheres (as described by van Balkom et al.25). The assay contained a standard curve based on custom‐made control peptides and controls for heterophylic antibodies. Acquisition was performed on the Bio‐Plex system (Bio‐Rad Laboratories) with the Bio‐Plex 3D reader (Luminex FlexMAP 3D) in combination with xPONENT software version 4.2 (Luminex). Data were analyzed by 5‐parameter curve fitting using Bio‐Plex Manager software, version 6.1.1 (Bio‐Rad).
Outcomes
The primary outcome of the present study was major amputation‐free survival (AFS), for which an event was defined as either amputation through or above the ankle joint or all‐cause mortality.
Statistical Analysis
Continuous data are reported as mean±SD when normally distributed and as median and interquartile range (IQR) otherwise. GDF15 plasma (Athero‐Express) or serum (JUVENTAS) values were log‐transformed and intercept‐normalized per plate median; therefore, values are reported as lnGDF15. Independent‐samples Student t tests were used to compare means of continuous data. Survival analysis was performed using the Kaplan–Meier method or Cox proportional hazards regression. For Cox regression, hazard ratios (HRs) are presented as tertiles or unstandardized β for lnGDF15 and thus reflect the HR per ln increase in GDF15. Multivariable Cox regression was used to create simple (age and sex) and full adjusted models based on the literature and on clinical relevance (age, sex, body mass index, estimated glomerular filtration rate, diabetes mellitus, smoking, hypertension, and history of coronary artery disease). The proportional hazards assumptions for all presented Cox models were evaluated by plotting Schoenfeld residuals. In no case were these proportional hazards assumptions violated. To estimate a dose‐response relationship, a restricted cubic spline (3 knots) was fit in the time‐to‐event analysis. To investigate the possibility of risk stratification, multivariable logistic regression models were created to predict major events at 1 year after inclusion. Receiver operating characteristic curves were presented to summarize predictive performance of different models, Net reclassification improvement was used to compare models. To analyze the plaque characteristics in the Athero‐Express Biobank, χ2 testing was used. A P value <0.05 was considered statistically significant. All analyses were performed with R software (version 3.1.1).
Results
Baseline Patient Characteristics
In the JUVENTAS trial, median age was 67 years (range: 56–76 years), and 68% of participants were male. A history of other cardiovascular diseases, such as myocardial infarction, was abundantly present (Table 1). In the Athero‐Express Biobank, median age was 69 years (range: 62–75 years), and 73% of participants were male. Most patients had a history of cerebrovascular accident or coronary artery disease (Table 1).
Table 1.
Baseline Characteristics of JUVENTAS and Athero‐Express
| Amputation Free Survival | JUVENTAS | Athero‐Express | ||||||
|---|---|---|---|---|---|---|---|---|
| No Event | Event | Total | P Value | No Event | Event | Total | P Value | |
| n=93 | n=67 | n=160 | n=323 | n=63 | n=386 | |||
| Sex (male), n (%) | 56 (60) | 53 (79) | 108 (68) | 0.03 | 235 (73) | 46 (73) | 281 (73) | 0.99 |
| Age, y, median (IQR) | 62.0 (52–72) | 71.0 (61.5–79.0) | 67.0 (56.0–76.0) | 0.002 | 68.0 (62.0–74.0) | 71.0 (68.0–78.0) | 69 (62.2–75.0) | 0.0003 |
| BMI, kg/m2, median (IQR) | 26.3 (23.5–28.8) | 25.7 (24.1–28.1) | 26.17 (23.7–28.7) | 0.74 | 26.4 (23.6–28.4) | 25.3 (23.3–28.0) | 26.2 (23.5–28.4) | 0.43 |
| Smoking (current), n (%) | 29 (31) | 13 (19) | 42 (26) | 0.14 | 124 (38) | 23 (37) | 147 (38) | 0.81 |
| Systolic BP, mm Hg, mean (SD) | 130.0 (18.6) | 132.5 (20.8) | 131 (19.5) | 0.42 | 147.0 (22.6) | 150 (32.9) | 147.5 (24.5) | 0.54 |
| Diastolic BP, mm Hg, mean (SD) | 73.5 (9.7) | 72.1 (10.2) | 72.9 (9.1) | 0.39 | 77.4 (11.7) | 72.9 (17.2) | 76.7 (12.8) | 0.07 |
| Creatinine, μmol/L, median (IQR) | 87.0 (75.0–107.0) | 105.0 (75.0–146.5) | 90.0 (74.8–114.5) | 0.04 | 85.0 (70.0–99.5) | 90.0 (65.0–119.0) | 85.0 (70.0–101.0) | 0.03 |
| eGFR, MDRD, mL/min/1.73 cm2, mean (SD) | 72.3 (23.8) | 66.1 (32.0) | 69.7 (27.6) | 0.18 | 80.7 (25.5) | 73.1 (31.5) | 79.5 (26.7) | 0.08 |
| Cholesterol, mmol/L, mean (SD) | 4.4 (1.1) | 4.1 (1.1) | 4.3 (1.1) | 0.15 | 4.6 (1.1) | 4.8 (1.4) | 4.6 [1.1) | 0.56 |
| HDL, mmol/L, mean (SD) | 1.3 (0.4) | 1.1 (0.4) | 1.2 (0.4) | 0.02 | 1.2 (0.4) | 1.1 (0.4) | 1.2 (0.4) | 0.32 |
| Triglycerides, mmol/L, mean (SD) | 1.6 (0.9) | 1.7 (1.1) | 1.7 (1.0) | 0.59 | 1.7 (1.0) | 2.1 (1.1) | 1.8 (1.0) | 0.07 |
| Hemoglobin, mmol/L, mean (SD) | 8.3 (1.0) | 7.8 (1.0) | 8.1 (1.1) | 0.004 | 8.6 (0.9) | 8.2 (1.3) | 8.5 (1.0) | 0.02 |
| History of CVA, n (%) | 2 (2) | 10 (15) | 12 (8) | 0.006 | 17 (5) | 6 (9) | 23 (6) | 0.27 |
| History of CAD, n (%) | 30 (32) | 36 (54) | 66 (41) | 0.02 | 134 (42) | 29 (46) | 163 (42) | 0.53 |
| Diabetes mellitus, n (%) | 30 (32) | 30 (44) | 60 (38) | 0.15 | 86 (27) | 20 (31) | 106 (27) | 0.50 |
| ABI, median (IQR) | 0.54 (0.41–0.77) | 0.47 (0.27–0.64) | 0.51 (0.38–0.74) | 0.17 | 0.59 (0.45–0.70) | 0.50 (0.39–0.60) | 0.57 (0.44–0.70) | 0.9 |
| Fontaine class (II, III, IV) | 0.004 | 0.18 | ||||||
| II, n (%) | 8 (9) | 0 (0) | 8 (5) | 202 (63) | 31 (49) | 233 (60) | ||
| III, n (%) | 35 (38) | 16 (24) | 41 (27) | 78 (24) | 18 (29) | 96 (25) | ||
| IV, n (%) | 50 (53) | 51 (76) | 101 (67) | 43 (13) | 14 (22) | 57 (15) | ||
ABI indicates ankle brachial index; BMI, body mass index; BP, blood pressure; CAD, coronary artery disease; CVA, cerebrovascular accident; eGFR, estimated glomerular filtration rate; HDL, high‐density lipoprotein; IQR, interquartile range; MDRD, Modification of Diet in Renal Disease.
In JUVENTAS, at last available follow‐up (median follow‐up time of 3 years), 67 of 160 patients (42%) experienced a major event (41 patients underwent a major amputation; 40 patients died, of which 14 underwent an amputation before death; see Table 1 for baseline characteristics). In Athero‐Express, at 3 years after inclusion, 64 of 386 patients experienced a major event (13 patients underwent a major amputation; 54 patients died, of which 3 underwent an amputation before death; Table 1).
Patients Who Experienced a Major Event Had Higher GDF15 Levels
The median GDF15 level was 1793 pg/mL (IQR: 1043–2943 pg/mL) in JUVENTAS and 1574 pg/mL (IQR: 1070–2102 pg/mL) in Athero‐Express. GDF15 levels were significantly higher in patients who experienced a major event (amputation or death) compared with patients who remained event‐free, both in JUVENTAS (2346 pg/mL [IQR: 1466 to 3191 pg/mL] versus 1398 pg/mL [IQR: 890.4–2569 pg/mL], respectively; P=0.0002) and Athero‐Express (1957 pg/mL [IQR: 1315–3395 pg/mL] versus 1458 pg/mL [IQR: 1032–1971 pg/mL], respectively; P=0.0003; Figure 1). GDF15 levels increased with age and Fontaine classification in both JUVENTAS and Athero‐Express (Figure S1).
Figure 1.
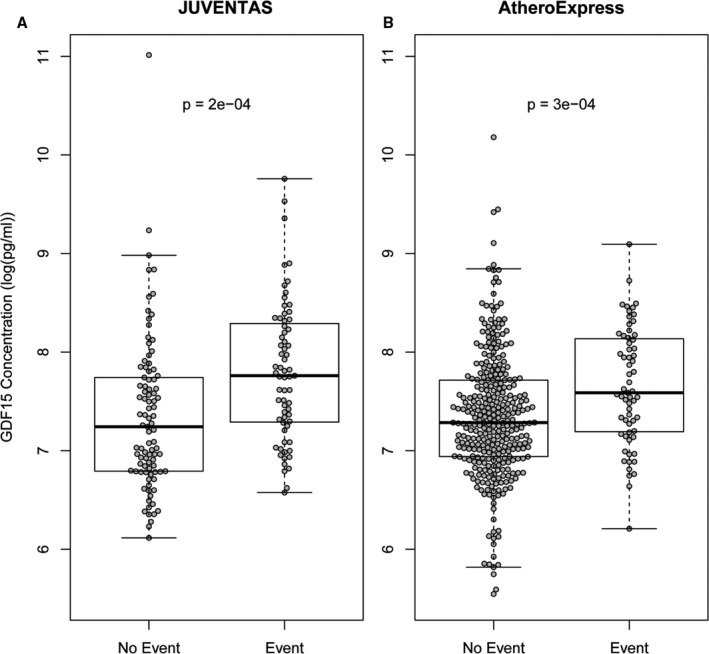
Box plots of log‐transformed and intercept‐normalized GDF15 levels in patients with no event or with an event in the JUVENTAS and Athero‐Express cohorts. A, Median GDF15 levels in the JUVENTAS trial were 2346 pg/mL (IQR: 1466–3191 pg/mL) for patients with an event and 1398 pg/mL (IQR: 890.4–2569 pg/mL) for patients without an event (P=0.0002). B, Median GDF15 levels in the Athero‐Express Biobank were 1957 pg/mL (IQR: 1315–3395 pg/mL) for patients with an event and 1458 pg/mL (IQR: 1032–1971 pg/mL) for patients without an event (P=0.0003). IQR indicates interquartile range; GDF15, growth differentiation factor 15; JUVENTAS, Rejuvenating Endothelial Progenitor Cells via Transcutaneous Intra‐Arterial Supplementation Trial.
High GDF15 Levels Are Associated With Increased Risk of Amputation or Death
We divided the JUVENTAS and Athero‐Express patients in tertiles based on GDF15 levels (low, medium, and high) for the Kaplan–Meier curves of the estimated cumulative incidence rate of major events, defined as amputation or death (Figure 2A and 2B). Unadjusted Cox proportional hazards analysis was used to compare the different tertiles. AFS was significantly lower in patients in the high versus low GDF15 tertiles, in both JUVENTAS (HR: 4.01; 95% confidence interval [CI], 2.05–7.84; P=0.000005) and Athero‐Express (HR: 3.27, 95% CI, 1.64–6.54; P=0.00077; Table 2). Of note, in JUVENTAS, GDF15 proved to be more strongly associated with AFS in women (high versus low, HR: 10.1; 95% CI, 2.14–47.81) than in men (high versus low, HR: 2.69; 95% CI, 1.28–5.66; Figures S2 and S3 and Table S1). In Athero‐Express, there was no differential effect of GDF15 levels on AFS between the sexes (Figures S2 and S3 and Table S1).
Figure 2.
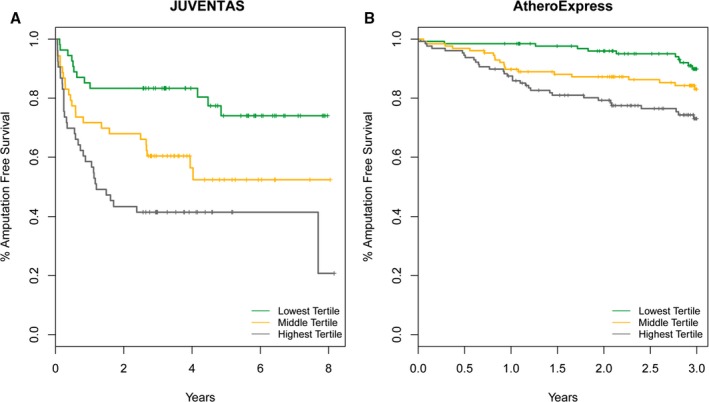
Kaplan–Meier curves representing the estimated cumulative incidence rates of major amputation or death in patients with low, medium, and high GDF15 levels. A, In JUVENTAS, patients in the middle tertile showed an HR of 2.41 (95% CI, 1.20–4.85; P=0.014) compared with the lowest tertile, and patients in the highest tertile showed an HR of 4.01 (95% CI, 2.05–7.84; P=0.000048) compared with the lowest tertile. B, In Athero‐Express, patients in the middle tertile showed an HR of 2.19 (95% CI, 1.07–4.49; P=0.033) compared with the lowest tertile, and the highest tertile showed an HR of 3.27 (95% CI, 1.64–6.54; P=0.00077) compared with the lowest tertile. CI indicates confidence interval; HR, hazard ratio; GDF15, growth differentiation factor 15; JUVENTAS, Rejuvenating Endothelial Progenitor Cells via Transcutaneous Intra‐Arterial Supplementation Trial.
Table 2.
Cox Proportional Hazards Ratios Between Low and Middle and Low And High Tertiles in JUVENTAS and Athero‐Express
| JUVENTAS | Athero‐Express | |||||
|---|---|---|---|---|---|---|
| Model | HR | 95% CI | P Value | HR | 95% CI | P Value |
| Middle tertile | 2.41 | 1.20–4.85 | 0.014 | 2.19 | 1.07–4.49 | 0.033 |
| Highest tertile | 4.01 | 2.05–7.84 | 0.000005 | 3.27 | 1.64–6.54 | 0.00077 |
CI indicates confidence interval; HR, hazard ratio; JUVENTAS, Rejuvenating Endothelial Progenitor Cells via Transcutaneous Intra‐Arterial Supplementation Trial.
Circulating GDF15 levels are highly correlated with the traditional risk factors, including age and eGFR (estimated glomerular filtration rate). Interestingly, GDF15 was also strongly correlated with blood hemoglobin levels (Figure S1). The unadjusted univariate HR for continuous GDF15 levels for the risk of death or major amputation was 1.62 (95% CI, 1.27–2.06; P=0.00012) in JUVENTAS and 1.95 (95% CI, 1.38–2.76; P=0.00016) in Athero‐Express (Table 3). The multivariate analysis, corrected for age, sex, body mass index, eGFR, diabetes mellitus, smoking, hypertension, and history of coronary artery disease showed an HR for continuous GDF15 of 1.78 (95% CI, 1.18–2.69; P=0.0053) for JUVENTAS and 1.57 (95% CI, 1.02–2.41; P=0.041) for Athero‐Express. After adjustment of the full model for hemoglobin, HRs were marginally lower in both cohorts (1.64 versus 1.70 in JUVENTAS and 1.39 versus 1.57 in Athero‐Express; Table 2).
Table 3.
Cox Proportional Multivariable Hazards Regression Models for Major Events in JUVENTAS and Athero‐Express
| JUVENTAS | Athero‐Express | |||||
|---|---|---|---|---|---|---|
| Model | HR | 95% CI | P Value | HR | 95% CI | P Value |
| Unadjusted | 1.62 | 1.27–2.06 | 0.00012 | 1.95 | 1.38–2.76 | 0.00016 |
| Age plus sex | 1.46 | 1.1–1.95 | 0.0093 | 1.73 | 1.19–2.51 | 0.0044 |
| Fulla | 1.70 | 1.14–2.52 | 0.0053 | 1.57 | 1.02–2.41 | 0.041 |
| Full plus hemoglobin | 1.64 | 1.07–2.52 | 0.023 | 1.39 | 0.89–2.18 | 0.15 |
CI indicates confidence interval; HR, hazard ratio; JUVENTAS, Rejuvenating Endothelial Progenitor Cells via Transcutaneous Intra‐Arterial Supplementation Trial.
Full model is corrected for age, sex, body mass index, estimated glomerular filtration rate, diabetes mellitus, smoking, hypertension, and coronary artery disease history.
GDF15 Levels Alone Can Predict Outcome in Both Cohorts and Can Even Improve Prediction Beyond Traditional Risk Factors in JUVENTAS
GDF15 levels alone performed equally as well in predicting outcome as 9 traditional risk factors combined, as shown by the C‐statistics for the receiver operative characteristic curves in Athero‐Express (66.1% AUC; CI, 57.1–75.2, versus 67.6% AUC; CI, 57.2–78.1; fixed end point at 1 year, eg, as in PREVENT III26; Figure S4). In JUVENTAS, GDF15 even has additive value beyond the 9 traditional risk factors (74.4% AUC; CI, 66.3–82.6, versus 70.8% AUC; CI, 62.4–79.2; Figure S4).
GDF15 Levels Do Not Correlate With Plaque Characteristics
In Athero‐Express, plaque specimens were studied to characterize plaque phenotype. We did not observe differences in plaque phenotype in patients who had different GDF15 levels (low, medium, high) and who experienced an event or remained event‐free (Table 4).
Table 4.
Plaque Characteristics in Athero‐Express
| GDF15, n (%) | |||||||
|---|---|---|---|---|---|---|---|
| Low | Medium | High | |||||
| Event | No Event | Event | No Event | Event | No Event | ||
|
Calcification P=0.17 |
No/minor | 38 (18) | 4 (9) | 27 (13) | 6 (13) | 28 (13) | 11 (24) |
| Moderate/heavy | 37 (17) | 4 (9) | 45 (21) | 11 (24) | 40 (18) | 9 (20) | |
|
Collagen P=0.22 |
No/minor | 11 (5) | 1 (2) | 12 (6) | 3 (7) | 16 (8) | 6 (13) |
| Moderate/heavy | 64 (30) | 7 (16) | 57 (27) | 14 (31) | 53 (25) | 14 (31) | |
|
Smooth muscle cells P=0.26 |
No/minor | 18 (9) | 2 (4) | 22 (10) | 7 (16) | 17 (8) | 4 (9) |
| Moderate/heavy | 56 (26) | 6 (13) | 49 (23) | 10 (22) | 51 (24) | 16 (36) | |
|
Macrophages P=0.05 |
No/minor | 65 (31) | 8 (18) | 56 (26) | 17 (38) | 57 (27) | 15 (33) |
| Moderate/heavy | 9 (4) | 0 (0) | 15 (7) | 0 (0) | 10 (5) | 5 (11) | |
|
Intraplaque fat P=0.42 |
<10% | 62 (29) | 7 (16) | 59 (27) | 13 (29) | 51 (24) | 16 (36) |
| >10% | 14 (7) | 1 (2) | 13 (6) | 4 (9) | 17 (8) | 4 (9) | |
|
Intraplaque hemorrhage P=0.44 |
No | 36 (17) | 5 (11) | 39 (18) | 9 (20) | 38 (18) | 9 (20) |
| Yes | 39 (18) | 3 (7) | 33 (15) | 8 (18) | 31 (14) | 11 (24) | |
Percentages are given per event or no event. GDF15 indicates growth differentiation factor 15.
Dose‐Response Model Illustrates That Increased Levels of GDF15 Exponentially Increase Relative Hazard
We attempted to create an unbiased estimate of the relationship between GDF15 levels and the risk of a major event for PAD patients. We used the pooled data from both cohorts and observed an exponential increase in the relative hazard of amputation or death with increased GDF15 levels (Figure 3). Consequently, with higher GDF15 levels, there is an exponentially higher risk of an event.
Figure 3.
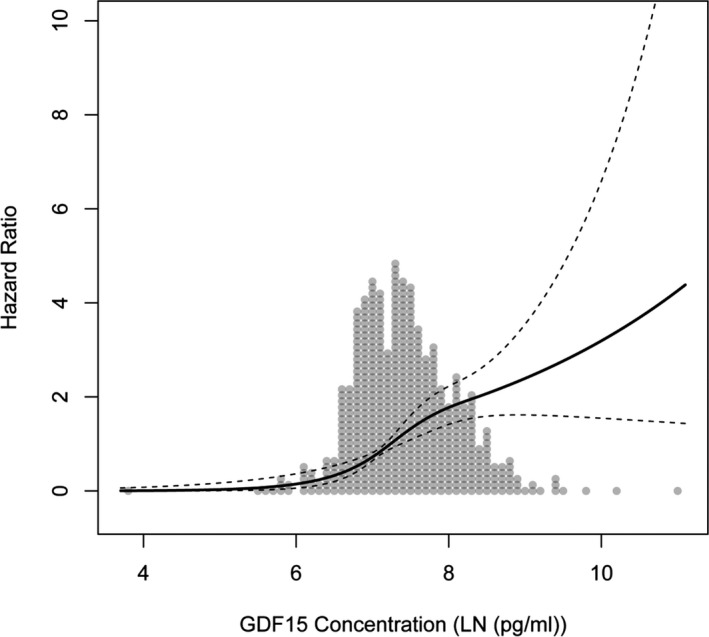
Dose‐response curve of the hazard ratio for amputation or death to log‐transformed (LN) and intercept‐normalized GDF15 (growth differentiation factor 15) levels based on both JUVENTAS and Athero‐Express data. An increase in GDF15 levels led to an exponential increase in HR. GDF15 indicates growth differentiation factor 15; HR, hazard ratio.
Discussion
In this study, we showed that high levels of GDF15 are associated with major amputation and all‐cause mortality in patients with PAD. This was true for patients with severe non‐revascularizable PAD and critical limb ischemia (JUVENTAS) and for those with milder PAD (Athero‐Express). Patients in the highest tertile of GDF15 levels had >3 times higher risk of major amputation or death compared with the lowest tertile in both cohorts. In JUVENTAS, high GDF15 levels were more predictive in women than in men. We did not observe this sex difference in Athero‐Express. We could not find any difference in estimated glomerular filtration rate, Fontaine classification, hemoglobin, history of stroke, history of coronary artery disease, or age that could explain this sex discrepancy between JUVENTAS and Athero‐Express.
GDF15 colocalizes with macrophages in carotid artery plaques,16, 18 and oxidized low‐density lipoprotein has been shown to increase GDF15 production by human macrophages ex vivo. In addition, atherosclerotic mouse models show a causal role of GDF15 in the chemotaxis of macrophages to the plaque specifically during the early phase of atherosclerosis.16 In our Athero‐Express plaques of PAD patients, we did not observe a difference in the amount of macrophages; however, we were looking at an advanced stage of atherosclerosis. Furthermore, GDF15 was shown to promote cholesterol efflux in an in vitro human foam cell model27; though, we did not observe differences in GDF15 levels in relation to fat content in the plaque. During plaque progression and in the more advanced stages of atherosclerosis, data on GDF15 are conflicting because GDF15 has been shown to be protective,28, 29 whereas it was also shown that the absence of GDF15 can positively affect plaque stability.16 Mechanistic studies are needed to examine the role of GDF15 in plaque initiation and progression in PAD patients.
GDF15 has been reported to be involved in apoptosis in various disease models. In cultured adult rat cardiomyocytes, GDF15 seems to have an anti‐apoptotic effect.30 Also in cardiac ischemia/reperfusion injury in mice, GDF15 functions as an anti‐apoptotic cytokine for cardiomyocytes.31 In contrast, GDF15 appears to have a pro‐apoptotic effect in numerous cancer types, such as glioblastoma,32 prostate cancer,33 and colorectal cancer.34 In a mouse model of atherosclerosis, GDF15 showed to be involved in apoptosis of mononuclear cells in the atherosclerotic plaque.16 Whether GDF15 plays a role in apoptosis of mononuclear cells in the plaques of PAD patients, and thus the cellular composition of the plaque and its characteristics, is currently unknown. We did not observe a relationship between circulating GDF15 levels and the number of macrophages in the plaques of the Athero‐Express cohort.
Levels of GDF15 were highly correlated with disease severity (Fontaine classification), and levels were higher in the JUVENTAS cohort compared with the Athero‐Express cohort; this could be a reflection of the severity of tissue hypoxia. It is known that in vitro hypoxia can induce GDF15 expression in human umbilical vein endothelial cells35 and in human glioblastoma cells.36 Increased levels of GDF15 can promote angiogenesis35, 37; therefore, GDF15 might be an important activator of local angiogenesis. In contrast, it also has been shown that GDF15 can inhibit CCN2‐induced angiogenesis38 and the proliferation of endothelial cells,19 highlighting that additional factors and conditions are involved in the regulation of angiogenesis and that GDF15 does not simply reflect the angiogenic activity. GDF15 levels rather seem to reflect a cumulative risk factor burden and precisely follow established risk factors for events in PAD, such as age,1 renal damage,39 and diabetes mellitus.40
Interestingly, we observed that GDF15 levels are strongly negatively correlated with blood hemoglobin. A low blood hematocrit, the fractional hemoglobin volume of blood, was previously shown to be an independent predictor of AFS in the PREVENT III prediction model.26 At present, no mechanistic explanation has been offered with regard to this association. GDF15 has been linked to anemia in various pathogenic conditions, such as chronic heart failure,13, 41 thalassemia,42 and pyruvate kinase deficiency,43 and in kidney44 or heart41 allograft recipients. It has been described that an insufficient erythropoiesis can lead to an increase in GDF15 levels. GDF15, however, is also involved in the inhibition of hepcidin (a key regulator of the entry of iron into the blood stream) and thus iron uptake. Whether high GDF15 levels are the cause or consequence of a decrease in hemoglobin remains to be elucidated. The fact that the pathways of GDF15 and erythropoiesis are related might explain that both are related to prognosis in PAD.
The prognostic value of GDF15 has been reported extensively for various cardiovascular diseases5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15; however, the current study is the first to report the role of GDF15 in the prediction of major amputation or death in patients with PAD. The dose‐response curve shows that risk of amputation or death increases exponentially with increased levels of GDF15. Furthermore, C‐statistics of the receiver operating characteristic curves showed that GDF15 could be of additive value as a classification parameter.
GDF15 levels could be used in the clinic to predict major events and thus may be useful in improving risk stratification of PAD patients. Patients with high GDF15 levels should be monitored more closely. Furthermore, GDF15 levels could be helpful in deciding whether an affected limb should be amputated.
Conclusion
We showed for the first time, to our knowledge, that GDF15 levels predict major amputation or death in PAD patients. Circulating GDF15 levels could be implemented in clinical practice as a biomarker to identify patients with PAD and critical limb ischemia who are at high risk of major events and to guide treatment choices.
Sources of Funding
We acknowledge the support from the Netherlands CardioVascular Research Initiative: An initiative with support of the Dutch Heart Foundation, CVON2014‐11 RECONNECT.
Disclosures
None.
Supporting information
Figure S1. A, Correlation between lnGDF15 and hemoglobin, age and estimated glomerular filtration rate based on pooled data from JUVENTAS and Athero‐Express. B, Box plots of lnGDF15 levels in male and female participants who smoke or who have diabetes mellitus, hypertension, history of coronary artery disease, or history of stroke or of Fontaine classification based on pooled data from JUVENTAS and Athero‐Express. lnGDF15 indicates log‐transformed and intercept‐normalized GDF15 (growth differentiation factor 15).
Figure S2. Box plots of log‐transformed and intercept‐normalized GDF15 (growth differentiation factor 15) levels in male and female participants with no event or with an event in the JUVENTAS and Athero‐Express cohorts.
Figure S3. Kaplan–Meier curves representing the estimated cumulative incidence rates of major amputation or death in patients with low, medium, or high GDF15 (growth differentiation factor 15) levels, stratified by sex in (A) JUVENTAS and (B) Athero‐Express.
Figure S4. Receiver operating characteristic curves showing additive value of GDF15 (growth differentiation factor 15) in JUVENTAS as a classification parameter. In JUVENTAS, GDF15 showed an added value as a classification parameter in the full model plus hemoglobin, with a C‐statistic of 74.4% vs 70.8% (with vs without GDF). Net reclassification improvement index (NRI) was 0.3459 (range: 0.0055–0.6863; P=0.04642); the integrated discrimination improvement index (IDI) was 0.0327 (range: 0.0059–0.0596; P=0.01683). In Athero‐Express, GDF15 did not show an improved classification with a C‐statistic of 69.1% vs 67.6% (with vs without GDF). NRI was 0.1593 (range: −0.2187 to 0.5374; P=0.40876), and IDI was 8e‐04 (range: −2e‐04 to 0.0018; P=0.11431).
Table S1. Cox Proportional Hazards Regression Models for Major Events in JUVENTAS and Athero‐Express Stratified by Sex
Acknowledgments
We would like to sincerely thank Arjan Schoneveld, Petra van der Kraak, Evelyn Velema, Sander van de Weg, Danny Elbersen and Noortje van den Dungen for their expert technical assistance.
(J Am Heart Assoc. 2017;6:e006225 DOI: 10.1161/JAHA.117.006225.)28855167
References
- 1. Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FGR. Inter‐Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J Vasc Surg. 2007;45:S5–S67. [DOI] [PubMed] [Google Scholar]
- 2. Fowkes FGR, Rudan D, Rudan I, Aboyans V, Denenberg JO, Mcdermott MM, Norman PE, Sampson UKA, Williams LJ, Mensah GA, Criqui MH. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. 2013;382:1329–1340. [DOI] [PubMed] [Google Scholar]
- 3. Teraa M, Conte MS, Moll FL, Verhaar MC. Critical limb ischemia: current trends and future directions. J Am Heart Assoc. 2016;5:e002938 DOI: 10.1161/JAHA.115.002938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yiu W, Conte MS. Primary stenting in femoropopliteal occlusive disease—what is the appropriate role?. Circ J. 2015;79:704–711. [DOI] [PubMed] [Google Scholar]
- 5. Zhang S, Dai D, Wang X, Zhu H, Jin H, Zhao R, Jiang L, Lu Q, Yi F, Wan X, Cui H. Growth differentiation factor‐15 predicts the prognoses of patients with acute coronary syndrome: a meta‐analysis. BMC Cardiovasc Disord. 2016;16:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wollert KC, Kempf T, Peter T, Olofsson S, James S, Johnston N, Lindahl B, Horn‐Wichmann R, Brabant G, Simoons ML, Armstrong PW, Califf RM, Drexler H, Wallentin L. Prognostic value of growth‐differentiation factor‐15 in patients with non–ST‐elevation acute coronary syndrome. Circulation. 2007;115:962–971. [DOI] [PubMed] [Google Scholar]
- 7. Kempf T, Bjorklund E, Olofsson S, Lindahl B, Allhoff T, Peter T, Tongers J, Wollert KC, Wallentin L. Growth‐differentiation factor‐15 improves risk stratification in ST‐segment elevation myocardial infarction. Eur Heart J. 2007;28:2858–2865. [DOI] [PubMed] [Google Scholar]
- 8. Brown DA, Breit SN, Buring J, Fairlie WD, Bauskin AR, Liu T, Ridker PM. Concentration in plasma of macrophage inhibitory cytokine‐1 and risk of cardiovascular events in women: a nested case‐control study. Lancet. 2002;359:2159–2163. [DOI] [PubMed] [Google Scholar]
- 9. Lin J‐F, Wu S, Hsu S‐Y, Yeh K‐H, Chou H‐H, Cheng S‐T, Wu T‐Y, Hsu W‐T, Yang C‐C, Ko Y‐L. Growth‐differentiation factor‐15 and major cardiac events. Am J Med Sci. 2014;347:305–311. [DOI] [PubMed] [Google Scholar]
- 10. Bonaca MP, Morrow DA, Braunwald E, Cannon CP, Jiang S, Breher S, Sabatine MS, Kempf T, Wallentin L, Wollert KC. Growth differentiation factor‐15 and risk of recurrent events in patients stabilized after acute coronary syndrome. Arterioscler Thromb Vasc Biol. 2011;31:203–210. [DOI] [PubMed] [Google Scholar]
- 11. Wollert KC, Kempf T. Growth differentiation factor 15 in heart failure: an update. Curr Heart Fail Rep. 2012;9:337–345. [DOI] [PubMed] [Google Scholar]
- 12. Anand IS, Kempf T, Rector TS, Tapken H, Allhoff T, Jantzen F, Kuskowski M, Cohn JN, Drexler H, Wollert KC. Serial measurement of growth‐differentiation factor‐15 in heart failure: relation to disease severity and prognosis in the Valsartan Heart Failure Trial. Circulation. 2010;122:1387–1395. [DOI] [PubMed] [Google Scholar]
- 13. Kempf T, von Haehling S, Peter T, Allhoff T, Cicoira M, Doehner W, Ponikowski P, Filippatos GS, Rozentryt P, Drexler H, Anker SD, Wollert KC. Prognostic utility of growth differentiation factor‐15 in patients with chronic heart failure. J Am Coll Cardiol. 2007;50:1054–1060. [DOI] [PubMed] [Google Scholar]
- 14. Chan MMY, Santhanakrishnan R, Chong JPC, Chen Z, Tai BC, Liew OW, Ng TP, Ling LH, Sim D, Leong KTG, Yeo PSD, Ong H‐Y, Jaufeerally F, Wong RC, Chai P, Low AF, Richards AM, Lam CSP. Growth differentiation factor 15 in heart failure with preserved vs. reduced ejection fraction. Eur J Heart Fail. 2016;18:81–88. [DOI] [PubMed] [Google Scholar]
- 15. Jankovic‐Tomasevic R, Pavlovic SU, Jevtovic‐Stoimenov T, Apostolovic S, Stanojevic D, Jovanovic I, Koracevic G, Djordjevic‐Radojkovic D, Damjanovic M, Salinger‐Martinovic S, Pavlovic M. Prognostic utility of biomarker growth differentiation factor‐15 in patients with acute decompensated heart failure. Acta Cardiol. 2016;71:587–595. [DOI] [PubMed] [Google Scholar]
- 16. De Jager SC, Bermúdez B, Bot I, Koenen RR, Bot M, Kavelaars A, de Waard V, Heijnen CJ, Muriana FJG, Weber C, van Berkel TJC, Kuiper J, Lee S‐J, Abia R, Biessen EAL. Growth differentiation factor 15 deficiency protects against atherosclerosis by attenuating CCR2‐mediated macrophage chemotaxis. J Exp Med. 2011;208:217–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bonaterra GA, Zügel S, Thogersen J, Walter SA, Haberkorn U, Strelau J, Kinscherf R. Growth differentiation factor‐15 deficiency inhibits atherosclerosis progression by regulating interleukin‐6‐dependent inflammatory response to vascular injury. J Am Heart Assoc. 2012;1:e002550 DOI: 10.1161/JAHA.112.002550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schlittenhardt D, Schober A, Strelau J, Bonaterra GA, Schmiedt W, Unsicker K, Metz J, Kinscherf R. Involvement of growth differentiation factor‐15/macrophage inhibitory cytokine‐1 (GDF‐15/MIC‐1) in oxLDL‐induced apoptosis of human macrophages in vitro and in arteriosclerotic lesions. Cell Tissue Res. 2004;318:325–333. [DOI] [PubMed] [Google Scholar]
- 19. Adela R, Banerjee SK. GDF‐15 as a target and biomarker for diabetes and cardiovascular diseases: a translational prospective. J Diabetes Res. 2015;2015:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Teraa M, Sprengers RW, Schutgens REG, van der Tweel I, Doevendans PA, Mali WPTM, Moll FL, Verhaar MC. Effect of repetitive intra‐arterial infusion of bone marrow mononuclear cells in patients with no‐option limb ischemia. Circulation. 2015;131:851–860. [DOI] [PubMed] [Google Scholar]
- 21. Verhoeven BAN, Velema E, Schoneveld AH, de Vries JPPM, de Bruin P, Seldenrijk CA, de Kleijn DPV, Busser E, van der Graaf Y, Mol F, Pasterkamp G. Athero‐express: differential atherosclerotic plaque expression of mRNA and protein in relation to cardiovascular events and patient characteristics. Rationale and design. Eur J Epidemiol. 2004;19:1127–1133. [DOI] [PubMed] [Google Scholar]
- 22. Sprengers RW, Moll FL, Teraa M. Rationale and design of the JUVENTAS trial for repeated intra‐arterial infusion of autologous bone marrow‐derived mononuclear cells in patients with critical limb ischemia. J Vasc Surg. 2010;51:1564–1568. [DOI] [PubMed] [Google Scholar]
- 23. Hellings WE, Pasterkamp G, Vollebregt A, Seldenrijk CA, De Vries JPM, Velema E, De Kleijn DPV, Moll FL. Intraobserver and interobserver variability and spatial differences in histologic examination of carotid endarterectomy specimens. J Vasc Surg. 2007;46:1147–1154. [DOI] [PubMed] [Google Scholar]
- 24. Vrijenhoek JEP, Nelissen BGL, Velema E. High reproducibility of histological characterization by whole virtual slide quantification; an example using carotid plaque specimens. PLoS One. 2014;9:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Van Balkom BWM, Gremmels H, Ooms LSS, Toorop RJ, Dor FJMF, de Jong OG, Michielsen LA, de Borst GJ, de Jager W, Abrahams AC, van Zuilen AD, Verhaar MC. Proteins in preservation fluid as predictors of delayed graft function in kidneys from donors after circulatory death. Clin J Am Soc Nephrol. 2017;12:817–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schanzer A, Mega J, Meadows J, Samson RH, Bandyk DF, Conte MS. Risk stratification in critical limb ischemia: derivation and validation of a model to predict amputation‐free survival using multicenter surgical outcomes data. J Vasc Surg. 2008;48:1464–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wu J‐F, Wang Y, Zhang M, Tang Y‐Y, Wang B, He P‐P, Lv Y‐C, Ouyang X‐P, Yao F, Tan Y‐L, Tang S‐L, Tang D‐P, Cayabyab FS, Zheng X‐L, Zhang D‐W, Zeng G‐F, Tang C‐K. Growth differentiation factor‐15 induces expression of ATP‐binding cassette transporter A1 through PI3‐K/PKCζ/SP1 pathway in THP‐1 macrophages. Biochem Biophys Res Commun. 2014;444:325–331. [DOI] [PubMed] [Google Scholar]
- 28. Preusch MR, Baeuerle M, Albrecht C, Blessing E, Bischof M, Katus HA, Bea F. GDF‐15 protects from macrophage accumulation in a mousemodel of advanced atherosclerosis. Eur J Med Res. 2013;18:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Johnen H, Kuffner T, Brown DA, Wu BJ, Stocker R, Breit SN. Increased expression of the TGF‐b superfamily cytokine MIC‐1/GDF15 protects ApoE(−/−) mice from the development of atherosclerosis. Cardiovasc Pathol. 2012;21:499–505. [DOI] [PubMed] [Google Scholar]
- 30. Heger J, Schiegnitz E, Von Waldthausen D, Anwar MM, Piper HM, Euler G. Growth differentiation factor 15 acts anti‐apoptotic and pro‐hypertrophic in adult cardiomyocytes. J Cell Physiol. 2010;224:120–126. [DOI] [PubMed] [Google Scholar]
- 31. Kempf T, Eden M, Strelau J, Naguib M, Willenbockel C, Tongers J, Heineke J, Kotlarz D, Xu J, Molkentin JD, Niessen HW, Drexler H, Wollert KC. The transforming growth factor‐{beta} superfamily member growth‐differentiation factor‐15 protects the heart from ischemia‐reperfusion injury. Circ Res. 2006;98:351–360. [DOI] [PubMed] [Google Scholar]
- 32. Zhang Z, Wu L, Wang J, Li G, Feng D, Zhang B, Li L, Yang J, Ma L, Qin H. Opposing effects of PI3K/Akt and Smad‐dependent signaling pathways in NAG‐1‐induced glioblastoma cell apoptosis. PLoS One. 2014;9:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Liu T, Bauskin AR, Zaunders J, Brown DA, Pankurst S, Russell PJ, Breit SN. Macrophage inhibitory cytokine 1 reduces cell adhesion and induces apoptosis in prostate cancer cells. Cancer Res. 2003;63:5034–5040. [PubMed] [Google Scholar]
- 34. Baek SJ, Kim K‐S, Nixon JB, Wilson LC, Eling TE. Cyclooxygenase inhibitors regulate the expression of a TGF‐{beta} superfamily member that has proapoptotic and antitumorigenic activities. Mol Pharmacol. 2001;59:901–908. [PubMed] [Google Scholar]
- 35. Song H, Yin D, Liu Z. GDF‐15 promotes angiogenesis through modulating p53/HIF‐1α signaling pathway in hypoxic human umbilical vein endothelial cells. Mol Biol Rep. 2012;39:4017–4022. [DOI] [PubMed] [Google Scholar]
- 36. Albertoni M, Shaw PH, Nozaki M, Godard S, Tenan M, Hamou M‐F, Fairlie DW, Breit SN, Paralkar VM, de Tribolet N, Van Meir EG, Hegi ME. Anoxia induces macrophage inhibitory cytokine‐1 (MIC‐1) in glioblastoma cells independently of p53 and HIF‐1. Oncogene. 2002;21:4212–4219. [DOI] [PubMed] [Google Scholar]
- 37. Wang L, Liu Y, Li W, Song Z. Growth differentiation factor 15 promotes cell viability, invasion, migration, and angiogenesis in human liver carcinoma cell line HepG2. Clin Res Hepatol Gastroenterol. 2017;2017:1–7. [DOI] [PubMed] [Google Scholar]
- 38. Whitson RJ, Lucia MS, Lambert JR. Growth differentiation factor‐15 (GDF‐15) suppresses in vitro angiogenesis through a novel interaction with connective tissue growth factor (CCN2). J Cell Biochem. 2013;114:1424–1433. [DOI] [PubMed] [Google Scholar]
- 39. Otaki Y, Watanabe T, Takahashi H, Yamaura G, Nishiyama S, Arimoto T, Shishido T, Miyamoto T, Kubota I. Renal tubular damage is associated with poor clinical outcome in patients with peripheral artery disease who underwent endovascular therapy. Int J Cardiol. 2016;220:376–381. [DOI] [PubMed] [Google Scholar]
- 40. Spreen MI, Gremmels H, Teraa M, Sprengers RW, Verhaar MC, Statius van Eps RG, de Vries J‐PPM, Mali WPTM, van Overhagen H. Diabetes is associated with decreased limb survival in patients with critical limb ischemia: pooled data from two randomized controlled trials. Diabetes Care. 2016;39:2058–2064. [DOI] [PubMed] [Google Scholar]
- 41. Przybyłowski P, Wasilewski G, Bachorzewska‐Gajewska H, Golabek K, Dobrzycki S, Małyszko J. Growth differentiation factor 15 is related to anemia and iron metabolism in heart allograft recipients and patients with chronic heart failure. Transplant Proc. 2014;46:2852–2855. [DOI] [PubMed] [Google Scholar]
- 42. Tanno T, Bhanu NV, Oneal PA, Goh S‐H, Staker P, Lee YT, Moroney JW, Reed CH, Luban NLC, Wang R‐H, Eling TE, Childs R, Ganz T, Leitman SF, Fucharoen S, Miller JL. High levels of GDF15 in thalassemia suppress expression of the iron regulatory protein hepcidin. Nat Med. 2007;13:1096–1101. [DOI] [PubMed] [Google Scholar]
- 43. Finkenstedt A, Bianchi P, Theurl I, Vogel W, Witcher DR, Wroblewski VJ, Murphy AT, Zanella A, Zoller H. Regulation of iron metabolism through GDF15 and hepcidin in pyruvate kinase deficiency. Br J Haematol. 2009;144:789–793. [DOI] [PubMed] [Google Scholar]
- 44. Malyszko J, Koc‐Zorawska E, Malyszko JS, Glowinska I, Mysliwiec M, Macdougall IC. GDF15 is related to anemia and hepcidin in kidney allograft recipients. Nephron Clin Pract. 2013;123:112–117. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. A, Correlation between lnGDF15 and hemoglobin, age and estimated glomerular filtration rate based on pooled data from JUVENTAS and Athero‐Express. B, Box plots of lnGDF15 levels in male and female participants who smoke or who have diabetes mellitus, hypertension, history of coronary artery disease, or history of stroke or of Fontaine classification based on pooled data from JUVENTAS and Athero‐Express. lnGDF15 indicates log‐transformed and intercept‐normalized GDF15 (growth differentiation factor 15).
Figure S2. Box plots of log‐transformed and intercept‐normalized GDF15 (growth differentiation factor 15) levels in male and female participants with no event or with an event in the JUVENTAS and Athero‐Express cohorts.
Figure S3. Kaplan–Meier curves representing the estimated cumulative incidence rates of major amputation or death in patients with low, medium, or high GDF15 (growth differentiation factor 15) levels, stratified by sex in (A) JUVENTAS and (B) Athero‐Express.
Figure S4. Receiver operating characteristic curves showing additive value of GDF15 (growth differentiation factor 15) in JUVENTAS as a classification parameter. In JUVENTAS, GDF15 showed an added value as a classification parameter in the full model plus hemoglobin, with a C‐statistic of 74.4% vs 70.8% (with vs without GDF). Net reclassification improvement index (NRI) was 0.3459 (range: 0.0055–0.6863; P=0.04642); the integrated discrimination improvement index (IDI) was 0.0327 (range: 0.0059–0.0596; P=0.01683). In Athero‐Express, GDF15 did not show an improved classification with a C‐statistic of 69.1% vs 67.6% (with vs without GDF). NRI was 0.1593 (range: −0.2187 to 0.5374; P=0.40876), and IDI was 8e‐04 (range: −2e‐04 to 0.0018; P=0.11431).
Table S1. Cox Proportional Hazards Regression Models for Major Events in JUVENTAS and Athero‐Express Stratified by Sex


