Introduction
Aortic valve stenosis due to calcification of the valve leaflets is the most common valve disease in the developed world. It is the third leading cause of cardiovascular disease.1 Risk factors include male gender, smoking, diabetes mellitus, hypertension, high levels of circulating lipids, and metabolic syndrome.2 Calcification was earlier believed to be a passive degenerative process, but it is now recognized as an active disease process driven by the cells native to the aortic valve.3, 4 The only option for treatment is heart surgery with implantation of a valve prosthesis. The heart valve prostheses are either mechanical, requiring life‐long anticoagulation treatment, or based on biological material, which will degenerate and calcify after 10 to 15 years. Implantation of heart valve prostheses has been characterized as “replacing one disease with another.” Understanding the cellular and molecular processes behind valve calcification may possibly lead to nonsurgical treatment.
The aortic valve is composed of three fine collagenous leaflets attached to the fibrous ring at the outlet of the left ventricle. The leaflets in young healthy humans are a fraction of a millimeter thick, and in systole they are washed onto the walls of the ascending aorta by the jet of blood.5 The leaflets are composed of a dense extracellular matrix, usually delineated into 3 layers: lamina fibrosa, lamina spongiosa, and lamina ventricularis5 (Figure 1). All 3 layers are populated with valve interstitial cells (VICs), with the entire structure covered by valve endothelial cells (VECs). The 3 layers have different matrix compositions: lamina ventricularis is richer in elastin, lamina spongiosa in proteoglycans, and lamina fibrosa in collagen.6 During embryogenesis the endothelial cells covering the primordial valve cushions migrate inside the underlying matrix and undergo endothelial‐to‐mesenchymal transition to become the interstitial cells.7 Thus, the VICs have an endothelial origin.
Figure 1.
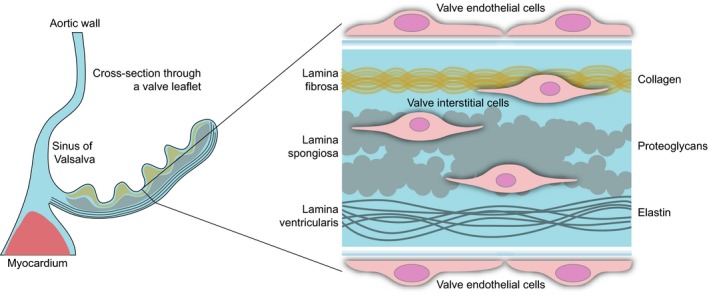
Simplified structure of the human aortic valve leaflet. On the left is a schematic cross section through the noncoronary leaflet of the aortic valve. The blowup on the right shows the trilayered organization of the extracellular matrix and the localization of the aortic valve endothelial cells (shortened throughout to VECs) and interstitial cells (VICs).
The gross pathology of valve calcification has some similarities with atherosclerosis. One of the early events is disruption of the endothelial layer on the aortic side of the leaflets.8 This is followed by slow reorganization of the valve matrix via metalloproteinases on top of subtle matrix changes occurring inevitably with age. This leads to valve thickening known as aortic valve sclerosis, which usually is asymptomatic. The leaflet is then infiltrated by immune cells; at the same time angiogenesis occurs along with deposition of lipids, proteoglycans, and cell debris, which will deform the leaflet.8, 9, 10 In the course of this process the valve matrix calcifies, and the aortic leaflets become stiff, causing aortic stenosis and obstruction of blood flow. With an orifice of under 1 cm2, versus 2.5 to 4.5 cm2 observed in a normal valve, the stenotic valve generates a pressure gradient of over 40 mm Hg, representing a severe degree of constriction.11
A hallmark of valve calcification is the inflammatory response. Valves are invaded by blood‐borne immune cells, but there is gathering evidence that the immune response in the resident cells plays a major role.12 Sterile inflammation may be triggered by circulating lipids in the form of low‐density lipoproteids, which infiltrate the valve matrix.6 A nonsterile inflammation might also occur, as bacterial infections of the oral cavity may predispose to valve calcification.13
Most of the calcification occurring in the aortic valve is diffuse and is believed to be secondary to the action of myofibroblasts. These cells stem from pathologically differentiated VICs as a result of paracrine action, most probably mediated by the transforming growth factor β‐1 (TGFβ1).14 The myofibroblasts contract the extracellular matrix, and the cell aggregates embedded in the reorganized matrix undergo apoptosis, which leads to the sedimentation of calcium salts around “nucleators” such as cleaved chains of collagen type I and elastin, bone sialoprotein, apoptotic bodies, and others.15
About 13% of calcified aortic valves at the time of surgery are estimated to contain true bone with osteoblasts and osteoclasts.8 Osteoblastic differentiation of VICs is similar to physiological osteogenesis and is largely orchestrated by Runt‐related transcription factor 2 (Runx2), a key transcriptional regulator of osteoblasts, as well as bone morphogenetic proteins (BMPs). Osteoblasts secrete a number of proteins highly expressed in bone tissue: osteocalcin, alkaline phosphatase, and osteopontin. Finally, calcium crystals are laid in the matrix of the valve, and osteoblasts organize them in structures similar to lamellar bone, while the osteoclasts (likely descendants of recruited monocytes) control its turnover by resorption.16
Valve Interstitial Cells: A Model to Study Aortic Valve Calcification
Key Sources of Cells
Animal Cells
Most research on aortic valve calcification is performed on cells from large animals. The primary source of cells due to the anatomical similarity to humans is the porcine aortic valve.17, 18 Other large animal models include sheep19, 20, 21 and cow.22, 23, 24 Recently VICs were isolated and cultured from mice.25 An advantage of animal cells over human cells is ease of access because cells can be isolated from all 4 heart valves for comparison, whereas in humans usually a single valve is obtained.20 The key disadvantage of animal cells is that whatever the result, experiments have to be replicated in human cells before we can proceed any further with the application of findings. To date, there is no head‐to‐head comparison of animal versus human VIC with regard to key pathophysiological processes such as osteoblastic and myofibroblastic differentiation. Another notion is that the most commonly used animals with native genotype do not develop aortic valve calcification. Pigs on high‐cholesterol diet develop valve sclerosis and calcific lesions.26 Some mouse strains are capable of induced valve calcification, but others are not. Genetic modifications may be required to induce the process roughly similar to that in humans,25 which confounds the model. Experiments with larger animals require expensive animal housing facilities and dedicated personnel or access to abattoirs.
Human Cells From Diseased Valves
Human cells can be accessible to researchers working in proximity to cardiac surgery units. Humans with stenotic aortic valves are far from a uniform population, as are the calcified valves themselves. The major variables, beside the patient age, sex, and concomitant diseases, are the amount of calcium, the degree of stenosis (and, hence, the pressure gradient), and the valvular anatomy.2 By default the aortic valve is tricuspid; however, congenital anomalies occur. The most common anomaly (around 1.5%) is a bicuspid valve, with extremely rare variants including monocuspid, quadricuspid, and pentacuspid. Among the valves operated for aortic stenosis, however, bicuspid and tricuspid valves are represented equally.27 Such overrepresentation indicates that a bicuspid aortic valve is a major risk factor for aortic stenosis and calcification. Whether it is a genetic defect that underlies both the bicuspid morphology and the increased ability for calcification, or altered morphology with different flow patterns predispose to calcification, the initiating mechanisms of calcification are probably quite different in bicuspid and tricuspid valves. Therefore, it is crucial to distinguish between the 2 variants and to avoid pooling them in the same experimental group.
Gender‐related differences between VICs are an interesting and yet little investigated area. It is known that being male is a risk factor for developing aortic stenosis.28 Transcriptome analysis performed on porcine VICs demonstrated substantial differences between males and females, where male VICs had higher expression of factors driving angiogenesis, lipid deposition, and inflammation, and the cells displayed a higher rate of proliferation and apoptosis.29 However, these findings require verification in human isolated VICs.
Human Control Cells
A major bottleneck is access to high‐quality control human material, which means healthy human valves. Cells obtained from cadavers provide 1 option; however, the quality of cadaver cells is more variable than that in cells isolated from fresh living tissue. There are a number of available alternatives:
Donor hearts that were considered unusable for transplantation.30, 31 This is an ideal source of control material but raises some complicated ethical questions. In Norway the donor card system contains an option to donate one's body to research, but few organ donors choose it.
Hearts removed from recipients of heart transplantation without a history of aortic valve disease.32, 33, 34 Hearts in this group often include those from patients with idiopathic dilated cardiomyopathy and postinfarction heart failure. It can be argued whether these patients had a genetic or epigenetic abnormality that also affects the biology of heart valves.
Noncalcified aortic valves removed because of valve insufficiency. Often this pathology is combined with aneurysm of the aortic root and the ascending portion of the aorta.35 In some cases the surgeon can reconstruct the aortic root without removing the valve, but many are still removed. This model has the same limitations as the previous one.
Pediatric valves removed due to congenital abnormality.36 This source has the same weakness as the previous 2 models.
Valves extracted during operation for aortic dissection.35, 37 These valves are perhaps closest to normal as long as the underlying predisposing cause of dissection is presumed not to affect the aortic valve. One problem is to obtain the valves: these are acute patients admitted at any time of the day or night.
Noncalcified cusps (or portions of the cusp) of the calcified valve.38 This is perhaps the easiest material to obtain. However, its limitations are obvious. Nevertheless, these cells can be used as an additional control for determining if the process in question is intrinsic or extrinsic to the valve.39 These cells can also be used as a reference for gene expression studies in the calcified portion.40
Optimal Conditions for Isolation and Culture of Valve Interstitial Cells
From the point of view of a laboratory technician, the VICs are similar to fibroblasts elsewhere in the body.41 This includes relative robustness and long survival time after the cessation of nutrient delivery, as in explanted valves or valves from cadavers. Following surgery the valve leaflets should be placed in saline, Ringer solution, or phosphate‐buffered saline, and for optimal results, the cells should be isolated ex tempore. The first step in isolating VICs is removal of VECs, usually performed by a 5‐minute exposure to collagenase II and swabbing.42 Despite the removal of VECs, they still represent a significant (up to 10%) contamination of a porcine VIC culture.43 The 2 major methods of further VIC isolation/enrichment are based on either outgrowth of cells from the sections onto the cell culture plastic44 or digestion of extracellular matrix with subsequent centrifugation and seeding of the cell suspension. In the latter, one can use collagenase I,36 collagenase II,45 or collagenase III.42
The culture of VICs is relatively routine using medium such as Dulbecco Modified Eagle Medium45 or M199.13 The optimal type of serum is fetal bovine, which is superior to bovine calf serum and newborn calf serum.18 Ten percent serum is usually supplemented to the above base medium. In 10% serum, VICs double every 30 hours,46 reach confluence in about 5 days,18 and should be passaged at 80% confluence. The concentration of serum is empiric: in 15% to 20% serum VICs engage in active proliferation,47 whereas at percentages below 10 they gradually become quiescent or differentiate into myofibroblasts.48 It is also possible to maintain proliferative ability in low serum by supplementing the medium with insulin—in this case the impact of growth factors naturally present in serum will be reduced.49 The authors of this study also suggest the use of fibroblast growth factor (FGF) to block the spontaneous myofibroblastic differentiation of human VICs.49 This is addressed below. VICs are stable for approximately 8 passages, but the majority of studies are performed between passages 2 and 5 due to the changing biology of the VICs.50
For some studies the isolation of VICs is not required. By stripping the endothelial cells from a healthy valve with collagenase, the investigator is left with a leaflet filled with VICs in situ, in their natural environment. These can then be exposed to various stressors or treatments, and the gene regulation can only be attributed to VICs.51 Instead of using a whole leaflet, it is also possible to use strips.52 In this review, however, we focus on studies performed with isolated VICs in culture and not explanted valves.
An important issue is associated infection in VICs. Mycoplasma is especially common in freshly isolated valves. Therefore, effective testing and quarantine measures are to be taken when harvesting a fresh batch of valvular cells. If infected, the cells should be discarded or, if especially valuable, may be treated with, for example, Plasmocin (InvivoGen, San Diego, CA) for 2 weeks to eradicate Mycoplasma. However, results obtained with these cells should be interpreted with caution. Whether the source of contamination is the patient or it occurs somewhere on the way to the bench in unclear.
Key Properties of Valvular Interstitial Cells
A number of experimental studies have addressed the biological properties of VIC and developed original methodology now forming the backbone of the VIC experimental repertoire. We chose the studies we deem most significant in this respect, and present a short list in chronological order in Table 1.
Table 1.
Key Studies With Original Methodology Revealing Important Properties of Isolated, Cultured VICs
| Reference No. | Author, Year | VIC Source | Technique | Key Results |
|---|---|---|---|---|
| 20 | Merryman, 2006 | Ovine | VICs isolated from all 4 heart valves are tested for stiffness using a pipette aspirator | The left‐sided cells are stiffer, express more αSMA and heat shock protein 47, a chaperone of collagen |
| 17 | Butcher, 2006 | Porcine | Cocultures of VIC with VEC in proprietary bioreactor with tubular molds exposed to shear stress | VECs downregulate expression of αSMA in VICs and oppose proliferation of VICs in response to shear stress |
| 38 | Clark‐Greuel, 2007 | Ovine | Timeline of gene expression and calcium accumulation in response to TGFβ1 stimulation | Calcium deposits appear and alkaline phosphatase activity increases after 72 h; matrix metalloproteinase 9 expression is increased after 7 d |
| 18 | Bond, 2007 | Porcine | Comparison of different serum variants for VIC culture | Cells prefer fetal bovine serum of all sera, reach confluence within 5 d |
| 53 | Merryman, 2007 | Porcine | Study of inherent stiffness of aortic and pulmonary VIC and ability to contract collagen gels. | Aortic VICs are stiffer than pulmonary and contract gel matrix more quickly |
| 54 | Benton, 2008 | Porcine | Test of substrate coatings for VIC: plastic vs polyethylene glycol hydrogel, bare or coated with fibronectin or fibrin | VIC form nodules better on bare plastic and fibrin, but not on fibronectin; polyethylene glycol hydrogel, coated or uncoated, suppresses calcification |
| 55 | Yip, 2009 | Porcine | Comparison of substrate (matrix) of variable stiffness for culturing and osteogenic differentiation. | VIC proliferated better on compliant matrix and calcify more readily in osteogenic medium |
| 56 | Benton, 2009 | Porcine | Timeline and mechanism of nodule formation in response to TGFβ1 | The VICs are torn from culture plastic by contraction and clump together into a nodule |
| 48 | Gu, 2010 | Porcine | A comparison of 3 peptide coatings for their ability to induce nodule formation | RGD‐containing peptide is the most procalcific; RGD is common for fibrin, collagen, fibronectin, laminin |
| 57 | Hakuno, 2010 | Murine (rat) | A large study on periostin in valve disease; includes isolation of VIC from rats | |
| 58 | Bertacco, 2010 | Bovine | Proteomic analysis of the calcifying clones of VIC, 3D culture (collagen I sponges) | Calcification is triggered by exposure to lipopolysaccharide and inorganic phosphate. |
| 21 | Gwanmesia, 2010 | Ovine | Comparison of different coatings for VICs | VICs grown on fibronectin formed very few nodules and showed no apoptosis |
| 59 | Rodriguez, 2011 | Porcine | VIC cultured on hyaluronic acid of variable molecular weight | Culturing VICs on hyaluronic acid reduces nodule formation; optimal hyaluronan MW 64 kDa |
| 35 | Yu, 2011 | Human | Stimulation of control and calcified VICs with tumor necrosis factor α | TNFα induces calcification only in calcified VIC, the effect is mediated by bone morphogenetic protein 2 and nuclear factor κB |
| 31 | Ferdous, 2011 | Human | Healthy VIC and aortic smooth muscle cells are stretched in tubular molds with collagen. | Stretch triggers calcification in both cell types |
| 26 | Yip, 2011 | Porcine | Cultured VIC from normal and high‐cholesterol‐fed pigs | The aortic valves are stenotic in high‐cholesterol‐fed pigs, cells are thus a feasible model of human disease |
| 60 | Hutcheson, 2012 | Porcine | Steady stretch in 2D cultures using FlexCell®, and in 3D cultures in proprietary bioreactor | Steady stretch induces intracellular calcium accumulation, which triggers apoptosis in VIC |
| 61 | Wyss, 2012 | Porcine | Elastic modulus of VICs | Elastic modulus in cultured VICs increases over time and passaging, proportional to αSMA expression |
| 62 | Gould, 2012 | Porcine | A proprietary bioreactor for application of isotropic (circular molds) and anisotropic (oval molds) stretch to a 3D culture | VICs orient along the longer axis of anisotropy and remodel the matrix the same way; anisotropy also increases apoptosis and proliferation in VICs |
| 63 | Fisher, 2013 | Porcine | FlexCell® treatment of VIC alongside stimulation with TGFβ1 | Both TGFβ1 and the degree of stretch are proportional to the quantity of calcific nodules |
| 64 | Quinlan, 2012 | Porcine | Effect of stiffness of collagen‐coated polyacrylamide gel substrate on VIC proliferation | Loose gels: cuboidal cells, no stress fibers. Stiff gels: sprouting cells with stress fibers made of αSMA |
| 29 | McCoy, 2012 | Porcine | Microarray study of untreated VIC and ingenuity pathway analysis with emphasis on gender differences | Male VICs have higher expression of genes responsible for proliferation and apoptosis; functional implications are confirmed by cell behavior in culture |
| 65 | Monzack, 2012 | Porcine | Time‐dependent response of VICs to growth, myofibroblast and osteogenic medium | Cells in osteogenic medium have increased alkaline phosphatase activity, high proliferation, downregulation of αSMA. |
| 66 | Moraes, 2013 | Porcine | A proprietary bioreactor to stretch VICs over the polyurethane membranes; isolation of VIC from lamina ventricularis and lamina fibrosa | The VICs adhere better to polyurethane than plastic; stretch triggers myofibroblastic differentiated VIC from ventricularus express more αSMA than those from fibrosa, also in response to TGFβ1. |
| 67 | Richards, 2013 | Porcine | Cocultures of VIC and VEC, both attached, detached, and treated with osteogenic medium | Osteogenic medium triggers calcification only in attached VIC monocultures; VEC inhibit calcification driven by osteogenic medium |
| 68 | Ferdous, 2013 | Human | Long‐term (3 w) cyclic stretch using a proprietary bioreactor for tubular molds | Different stretch amplitudes are tested; 10% stretch was most procalcific. Arsenazo dye used for calcium. |
| 24 | Duan, 2013 | Bovine | VIC cultured in hyaluronic acid‐based hydrogels; VIC culture using hanging drop method | |
| 43 | Wang, 2013 | Porcine | Flow cytometry analysis of isolated VIC using markers specific for various cell types | Under 10% of isolated cells are positive for other markers than fibroblasts. Some of the cells are VEC; some myofibroblasts express endothelial markers too. |
| 69 | Gould, 2014 | Porcine | Isolation and coculture of VIC and VEC using peptide‐functionalized polyethylene glycol gels. | Harder substrates promote myofibroblast differentiation, softer ones purely fibroblastic. VECs in coculture inhibit myofibroblast differentiation of VIC via nitric oxide signaling. |
| 25 | El Husseini, 2014 | Murine (mouse) | Isolation and culture of murine VICs | |
| 49 | Latif, 2016 | Human | Optimal conditions for culture of human VIC | Dulbecco modified Eagle medium induces myofibroblast differentiation in VIC. Medium that does not contains 2% fetal calf serum, 50 ng/mL insulin, 10 nm/mL fibroblast growth factor. |
| 70 | Porras, 2017 | Porcine | Optimal conditions for culture of porcine VIC | As in previous study, to prevent myofibroblast differentiation one should use 2% fetal calf serum, 5.25 μg/mL insulin, 10 nm/mL fibroblast growth factor |
3D indicates 3‐dimensional; 2D, 2‐dimensional; αSMA, α smooth muscle actin; MW, molecular weight; TGFβ1, transforming growth factor β1; TNFα, tumor necrosis factor α; VEC, valve endothelial cells; VIC, valve interstitial cells.
Cell‐Specific Markers of VICs
A practical concern during isolation of VICs is cross‐contamination with VECs. Healthy human VICs can be distinguished from VECs by positive α‐smooth muscle actin (αSMA) and prolyl‐4‐hydrolase staining and/or absence of CD31 and von Willebrand factor.71 VECs are also positive for VE‐cadherin, whereas VICs are not.72 These markers can be used for separating live cells using, for example, magnetic assisted cell sorting.
After the VICs have been separated from VECs, it is important to be able to define the cell populations within the VIC culture. VICs have a differentiation potential reminiscent of mesenchymal stem cells: in the Petri dish they can differentiate into adipocytes, osteoblasts, and chondrocytes,73 depending on the molecular triggers and physical environment. In diseased valves the physical and biochemical milieu changes so radically over time that the resident cells can undergo differentiation into other mesenchymal cell types. Therefore, it is crucial to have a definition for what an actual VIC is. The most commonly used markers that define VICs, differentiating them from VECs, are αSMA, smooth muscle myosin, vimentin, and desmin.33, 47 However, expression levels need to be taken into account. At higher levels, αSMA is a marker of myofibroblastic differentiation; this is why it is imperative to use additional markers such as desmin, which is low in VICs isolated from healthy human valves and high in stenotic valve cusps.41 Another consideration is interspecies differences. Ovine valves express desmin and calponin but not vimentin or αSMA.19 Sixty‐four percent of healthy pig VICs express αSMA, 98% express vimentin, and 90% express smooth muscle myosin.52 The expression of αSMA in porcine VICs is generally low initially after isolation, but a small population of cells can express αSMA to the level of myofibroblasts.46
Interestingly, expression of αSMA increases in the course of primary isolation of cells, up to 4‐fold by 7 days. However, if the cells are appropriately passaged, the expression level remains low.46 Recently developed medium formulations allow keeping levels of αSMA consistently low, maintaining the native phenotype over a long time.49, 70 Finally, VICs are also a heterogeneous population when it comes to valve‐side–specific differences. Porcine VICs from the ventricular side, populating the “lamina ventricularis,” express more αSMA and respond with higher levels of αSMA to TGFβ1 stimulation .66
The observed heterogeneity of VICs has led to attempts at segregating these subpopulations. An early study described 2 types of cells isolated from porcine valves: secretory (containing vesicles) and contractile, where contraction was enhanced in response to angiotensin II, bradykinin, epinephrine, and isoproterenol.47 One may speculate that the latter population represented myofibroblasts. A later report outlined 3 cell types derived from both human and porcine valve cusps: fibroblasts, myofibroblasts, and cells resembling smooth muscle cells, which expressed both αSMA and smoothelin and displayed contractile properties.44 A recent study of bovine VICs revealed 4 unique cell populations with differential expression of αSMA, SM22, smooth muscle myosin, osteocalcin, and osteopontin.22 Fluorescence‐assisted cell sorting analysis of porcine VICs, obtained from different layers of the valve leaflet, also revealed 4 populations distinguished by cell type–specific markers: contaminating endothelial cells (about 10% positive cells), myofibroblasts, cells bearing known markers of embryonic stem cells, and progenitor cells. Some VECs expressed markers of myofibroblasts; thus, part of the latter might have endothelial origin—cells that underwent endothelial‐to‐mesenchymal transition. The myofibroblasts were mainly localized on the ventricular side, and the cells positive for markers of embryonic stem cells were found in the spongiosa.43 In all, there is probably a continuous spectrum of cells covering a smooth transition between more defined cell types such as the VICs, smooth muscle cells, osteoblasts, and potentially others as well. The reason for this might be the anisotropy of forces that the valve cusps are exposed to. Thus, cells with different properties are needed to maintain the distension, compression, shear stress, etc. There are no parts of our body that are exposed to so much beating continuously during our whole lives. Mammalian evolution had to develop an extraordinary structure in its light weight, robustness, and precision, and we are yet to understand how the aortic valve is built to these specifications using the cells we find in it.
Mechanical Properties of VICs
All VICs are robust cells, but they are different across the different valves, even though by gross appearance these cells appear similar.74 Porcine VICs isolated from the left side of the heart (mitral and aortic valves) have higher stiffness than the pulmonary and tricuspid VICs, as tested by an aspirator pipette, an effect attributed to higher expression of αSMA and heat shock protein 47.20 The observed differences in mechanical properties were further confirmed with an atomic force microscope.53 Porcine aortic VICs also display a greater ability to contract the extracellular matrix than their pulmonary counterparts. However, dermal fibroblasts contract even more efficiently.53 Interestingly, the mechanical stiffness of porcine VICs in culture increases with passaging and with culturing on harder substrates (stiffer collagen gels).61 The tougher character of the aortic VICs is understandable because the pressure gradient across the aortic valve is greater.
Choice of Substrate for VICs
Biology of VICs is highly dependent on the substrate they are maintained on as well as the configuration of the culture. Two‐dimensional cell cultures are the least representative of the in vivo situation; however, because of their simplicity they are widely employed to study VICs. Following isolation the VICs are spindle‐shaped, whereupon they assume a flattened sprouting shape and create a monolayer in the dish. VICs have been grown on glass, plastic alone, and plastic coated with collagen, elastin, fibronectin, and so on.66 In addition, the cells can be grown on elastic silicone membranes to facilitate studies of biomechanics. Porcine VICs grown on silicone rubber demonstrate an ability to compress and wrinkle the substrate.47 The latter creates an opportunity to study the interaction of the cells with the substrate and measures it by the extent of deformation.
A more advanced and “physiological” option is to study the cells in the 3‐dimensional (3D) environment. The most basic of these is a 3D collagen hydrogel culture, where the cells are essentially cast in a collagen solution, which is then allowed to stiffen/gel. This model allows for measurement of the contractile ability of the fibroblasts by studying the dynamics of gel compaction.18 Different densities of collagen gel have different effects on cell viability and proliferation. A comparison of collagen gels (at 1%, 2%, and 5%) demonstrated that the maximum effect on proliferation of healthy human VICs was observed in a 1% gel; however, these VICs also had an increased expression of αSMA, collagen I and III, and a number of matrix metalloproteinases (MMPs), possibly reflecting myofibroblastic differentiation.75 Such phenotypes may result in unwanted matrix remodeling, and cell behavior will be further from the physiological situation. Human VICs grown in 2% collagen displayed a more quiescent phenotype and gene expression pattern,75 and this density is optimal for studying gel compression.18, 76 A more sophisticated model to study the response of VICs to stretch involves casting a 3D collagen gel culture with VICs in elastic molds and subjecting them to stretch.62 For studying the interplay between VICs and VECs, collagen gels populated with VICs can be further seeded with VECs, thereby creating a coculture.17 As an alternative to casting cells in a collagen gel, they can be seeded on microfibrillar sponges made from collagen.58 The newest development in VIC collagen culture constructs is stacked layers of filter paper embedded in collagen and seeded with the cells. The porcine VICs not only proliferate in this environment but also show a phenotypic consistency characteristic of healthy cells.77
Collagen is not the only option for creating a 3D culture of VICs. The porcine VICs thrive in a polyethylene glycol hydrogel uncoated or coated with fibronectin or fibrin just as well as on the tissue culture plastic.48, 54 Polyethylene glycol hydrogels can be dynamically stiffened: after the cells have been seeded, the cross‐linker is added, and the elastic modulus can be brought to the desired modality.78
In another study a polyacrylamide gel of variable stiffness was coated with collagen and seeded with porcine VICs. The cells grown on looser gels displayed a cuboidal shape, with the absence of stress fibers, but VICs maintained on stiffer matrices expressed αSMA and sprouting morphology.64
Culturing bovine VICs on hyaluronic acid–based hydrogels yielded viability greater than 75% over 14 days but affected cell spreading, keeping them in a spherical shape for several days. The advantage of matrices made of hyaluronic acid is their improved stability over time compared with collagen.24
The choice of substrate for VICs is important because the aortic valve is a very attractive target for bioengineering and 1 of the structures that possibly will be bioprinted. However, it needs to be self‐renewing and self‐maintaining. When we know more about the substrate preference of VICs, we may preseed the bioprinted valves with the mesenchymal stem cells from the same patient and hopefully expect them to differentiate into the fully functional VICs.
Pathological Differentiation of VICs
Today's understanding of aortic valve calcification builds on the concept of diffuse calcification secondary to myofibroblast differentiation of VICs (sometimes called the dystrophic calcification) and ossification carried out in a regulated fashion by osteoblasts, also stemming from resident interstitial cells. The relative contribution of each of these 2 processes is at present unknown. For now, this dichotomy leads research into 2 main courses; pursuing 1 or another is for now a matter of preference or belief.
Myofibroblastic Differentiation
Activation of interstitial cells (fibroblasts) and myofibroblastic transformation are normal regenerative processes throughout the body.79 As shown above, myofibroblasts are claimed to be present among the interstitial cells populating the aortic valves normally.44 Myofibroblasts can be defined as fibroblasts that assume some of the properties of smooth muscle cells and are able to contract the extracellular matrix. Myofibroblast‐specific factors are often listed as follows: αSMA, smooth muscle myosin, and vimentin.52 Strikingly, these are the same factors that are often used to distinguish the VICs themselves. However, myofibroblasts usually present with much higher levels of αSMA, and they are much more active at contraction than the healthy VICs. Recent studies point out that native VICs should not be positive for αSMA, and it is a sign of unwanted phenotype change. By maintaining the culture in low serum and additionally stimulating cells with FGF‐2, human VICs in culture can be prevented from activation and spontaneous myofibroblast differentiation.49
The most commonly used approach to differentiate VICs into myofibroblasts is to stimulate them with TGFβ1. The first measurable effect of TGFβ1 on VICs is increased expression of αSMA as assessed by quantitative PCR, immunostaining, or Western blot. αSMA organizes into bundles, which are often called “stress fibers.” The next major event in myofibroblast differentiation in vitro is formation of nodules, a process more pronounced when the cells are cultured on laminin and fibrin80 but that can also be present on plastic, where 5 ng/mL of TGFβ1 is sufficient for maximal effect.81 The nodules form over the course of several days. The process is more or less rapid depending on the substrate and is driven by αSMA, which literally tears the cells from the surface.56 Finally, after nodule formation and compaction, the cells within the nodule undergo dystrophic changes and apoptosis, leading to leak of factors that stimulate nucleation of calcium crystals.79 The process of calcification in TGFβ1‐treated cultures takes days. The first calcium deposits and nodules are observed after 72 hours and increase with time. Ovine VICs in the nodule become apoptotic from around day 14.38 The calcification in culture is usually quantified after 21 days.
TGFβ1 belongs to the transforming growth factor superfamily, which signals through the cell surface TGFβ receptor. The signal is further conveyed via the canonical pathway involving small mothers against decapentaplegic (SMADs), or a noncanonical pathway involving mitogen‐activated protein kinase p38. SMAD2 and 3 are activated in both porcine and calcified human VICs in response to TGFβ1 stimulation.42 The procalcific effects include enhanced binding of low‐density lipoproteins to glycosaminoglycan chains; this effect is higher the longer the chains are.42 Nodule formation and alkaline phosphatase (ALP) activity in porcine VICs are mediated by the noncanonical pathway of TGFβ1.82 Mitogen‐activated kinase p42/44 (ERK) activated downstream of the TGFβ1 receptor triggers expression of cadherin‐11, which enables nodule formation by binding to αSMA. Blocking ERK phosphorylation or knocking down cadherin‐11 expression with small interfering RNA (siRNA) abolishes nodule formation.79 Other members of the TGFβ superfamily can also cause calcification as shown by the observation that healthy human VICs treated with TGFβ1, TGFβ3, BMP‐2, ‐4, or ‐7 show increased ALP activity and osteocalcin expression comparable to that triggered by osteogenic medium.32
TGFβ1 treatment is not absolutely necessary to achieve nodule formation. Instead, a combination of low serum concentration (1% or even 0.5%) and an appropriate extracellular matrix is enough to stimulate this response.21, 48 Porcine VICs display dramatic nodule formation after just 5 days of culture on fibrin‐coated or untreated plastic in 1% serum. Ovine VICs form nodules after just 24 hours in 0.5% serum.21 The characteristic of human VIC culture in low‐serum conditions is the associated increased apoptosis, which is believed to be integral to calcification.39
One possible reason substrate is important for nodule formation is the tensile stress experienced by VICs. Growing VICs on a hard/stiff substrate in 3D (thicker collagen gel matrix) is essential for αSMA expression.46 Tissue culture on plastic (stiff compliance) favors calcification, whereas cultures of porcine VICs on softer polyethylene glycol hydrogel calcify considerably less on treatment with TGFβ1.54 Myofibroblast differentiation of porcine VICs is activated on stiff collagen matrices in the presence of osteogenic medium and the absence of TGFβ1. These cells express αSMA in stress fibers and display high elastic modulus. The same cells on lower‐compliance gels aggregate and differentiate into osteoblasts.61 Porcine VICs cultured on stiff polyacrylamide matrices were avidly stimulated by TGFβ1 to express stress fibers.64 Of note, tensile stress is not unique in this ability to stimulate myofibroblast differentiation. Shear stress applied to VICs in collagen also triggers expression of αSMA.17
Furthermore, the effect varies depending on the extracellular matrix coating of the surface. For example, fibronectin efficiently inhibits nodule formation and expression of both Runx2 and αSMA in porcine VICs, whereas fibrin, on the other hand, promotes calcification.54 Collagen, fibronectin, and laminin oppose the nodule formation in porcine cells, with fibronectin also opposing it in ovine VICs.21 Integrin receptors that interact with specific peptides of the extracellular matrix are believed to explain this difference; the RGDS peptide was most procalcific of the ones tested using porcine VICs.48 The results obtained on 2D‐cultures were reproduced in a pilot 3D culture experiment with polyethylene glycol hydrogel coated with different peptides; again, the RGDS peptide was procalcific.48 Further development of this model, with dynamically stiffened hydrogels, showed that progressive decline in αSMA expression correlated with the increase in gel stiffness, which is contradictory to the studies involving collagen hydrogels.78
We regard calcium accumulation and its possible inhibition as the primary end points when studying mechanisms of calcification. An important point about models of myofibroblastic differentiation is that calcium accumulation is usually not measured in these studies. Indeed, data on calcium deposition measured by alizarin red are scarce. The main end points are the nodule formation or expression of procalcific genes. This may be a major weakness of these studies, and they should be interpreted with caution.
Osteoblastic Differentiation
Practically any interstitial cells can differentiate into osteoblasts when stimulated with appropriate stimuli. This explains a large versatility of ectopic calcification that may occur after tissue injury in most parts of the body. The VICs are no exception. In cell cultures the earliest signs of calcification are usually flagged by increased activity of ALP and activation of the osteoblastic factors Runx2 and BMP2.36 Runx2 is the master transcriptional regulator of the osteoblastic lineage, and its expression manifests commitment of the cells. The late and the ultimate indicator of calcification is calcium as detected by alizarin red,54 Von Kossa staining,58, 73 or arsenazo dye.68 The osteoblastic pathway does not normally include activation of αSMA or the contraction of extracellular matrix, although there is a certain degree of overlap.61
Standard osteogenic medium contains β‐glycerophosphate, dexamethasone, and ascorbic acid. β‐Glycerophosphate is an organic donor of phosphate groups, necessary for the formation of calcium phosphate crystals.13 Osteogenic medium applied to human VICs for 21 days induces increased ALP activity and osteocalcin32 as well as Runx2 expression.40 Effects similar to osteogenic medium can be achieved in human VICs by adenosine triphosphate.71 Osteogenic medium can also be supplemented with 50 ng/mL of BMP2.39
The variability described cell specific markers of VIC with respect to VICs after isolation is also observed in their response to osteogenic medium, and heterogeneity is found even among VICs isolated from the same valve. Porcine VICs were shown to form 4 types of colonies, with only 1 of them being responsive to calcification.73 The proportion of calcifying colonies decreased with passage number; therefore, experiments with osteogenic medium should be carried out on low‐passage VICs. In response to osteogenic medium the cells form calcifying colonies as well as nodules grossly similar to those triggered by TGFβ1. However, the cells within the nodule present with a cuboidal morphology characteristic of osteoblasts.73 Flow cytometry established that the porcine VICs most active in calcium accumulation were positive for the transporter ABCG2 (ATP‐binding cassette, sub‐family G, member 2), a side population progenitor cell marker.43
The response of VICs to osteogenic medium is dependent on the underlying surface as observed with the response to TGFβ1. Only soft collagen matrices promoted osteoblastic differentiation, in contrast to the stiffer matrices, whereas the opposite was observed with TGFβ1‐induced aggregation of VICs into nodules. VICs cultured in stiff matrices also expressed higher levels of TGFβ receptor.73 Again, calcium accumulation occurs in both models but by different mechanisms: apoptosis in TGFβ1‐treated porcine cultures or via osteoblast differentiation in osteogenic medium‐treated cells.55
Interesting data have been obtained when porcine VICs were compared side by side to murine calvarian preosteoblasts MC3T3 and embryonic derived fibroblasts C3H10T for their capacity to respond to osteogenic medium.83 The end points were ALP, osteocalcin, and αSMA expression, respectively. The porcine VICs downregulated αSMA and osteocalcin but upregulated ALP, whereas MC3T3 cells downregulated αSMA but upregulated ALP and osteocalcin. In murine embryonic fibroblasts C3H10T, osteogenic medium upregulated all 3 markers.83
Overlap Between Myofibroblast and Osteoblast Pathways
The 2 pathways of valve calcification are not necessarily mutually exclusive in the same cell population. It is unknown whether the given cell assumes characteristics of both myofibroblast and osteoblast, or 2 cells from the same source, treated with the same cocktail, react differently to it. In any event, on treatment with osteogenic medium, murine embryonic fibroblasts, C3H10T,83 and porcine VICs in 3D collagen matrices expressed increased levels of αSMA indicative of myofibroblast differentiation.61 Cloyd and co‐authors treated porcine VICs and murine primary neonatal calvarian osteoblasts with a combination of osteogenic medium and TGFβ1. The derived nodules were analyzed after 21 days. The nodules formed by the VICs were rich in collagen type 1 and stained positive for calcium, similarly to the nodules produced in mouse osteoblast culture. However, the expression of osteocalcin was lower in all cells treated with the combination of osteogenic medium and TGFβ1.84 The authors stated that this combination of treatments resulted in a myofibroblastic and not osteoblastic phenotype.
Role of Mechanical Stress in Differentiation of VICs
As with most fibroblasts, VIC phenotype is dependent on mechanical stimulation. Valve leaflets are exposed to the highest pressure gradients and jet speeds in the entire circulatory system, and the mechanobiology of VICs is crucial for their phenotype. Models of mechanical stress of isolated VICs can be employed alone or in combination with pathological differentiation (osteogenic medium and TGFβ1 stimulation).
A popular in vitro model for studying stretch in VICs is FlexCell® bioreactor by FlexCell International (Burlington, NC). The magnitude of stretch required to induce calcification is an open question. Some believe that 10% is actually a physiological distention for the aortic valve, and for an “overstretch” one should go up to 15%.68 Porcine VICs grow eagerly on both collagen‐ and laminin‐coated Bioflex plates and respond to stretch by expressing collagen III. The effect is both time and intensity dependent.45 Furthermore, the FlexCell treatment of human VICs from stenotic valves at 1 Hz, 10% distention for 72 hours upregulates osteocalcin, osteopontin, ALP, tenascin‐C, and biglycan.85 Shorter stretching times in porcine VICs showed intracellular accumulation of calcium that leads to apoptosis; calcium influx is also proportional to the degree of strain.60 The osteoblastic differentiation triggered by stretch can be further augmented by combining it with TGFβ1. The result is a marked enhancement of nodule formation in porcine VICs proportional to the degree of stretch and TGFβ1 concentration. For the granules to calcify, TGFβ1 has to be added first, followed by the applied stress.63 It should be noted that for these experiments the authors chose BioFlex plates that had been coated with Pronectin, a “positively charged, protein polymer which incorporates multiple copies of the RGD cell attachment epitope derived from human fibronectin between repeated structural peptide units” (Sanyo Chemical Industries, Kyoto, Japan). It should be reiterated that fibronectin inhibits calcification in the experiments where stretch is not applied.21, 54
Mechanical stress in the valve leaflet is anisotropic: it is different for the cells on 2 opposite sides as well as for cells near the free edge versus cells near the aortic wall. Porcine VICs subjected to anisotropic stress (oval molds) in 3D collagen gels align along the longer axis of anisotropy and remodel the surrounding matrix in the same way. Anisotropic stress, compared with a uniform one, also promotes VIC apoptosis and proliferation.62 This finding demonstrates how the leaflet morphology can affect cell function; this may contribute to the tendency of abnormally shaped bicuspid valves to calcify faster than their tricuspid counterparts.
Stretch is not the only mechanical stress modality that influences VICs. Pressure is indispensable for the calcification of bone, a principle known as the Wolff law. For example, in vivo bending stress applied to the rat tibia increases the proliferation of preosteoblasts86 and ALP activity in the bone.87 To date there is limited information on the effect of pressure on calcification of VICs. Porcine valve leaflets stripped of endothelium were subjected to pressure in a custom‐built bioreactor, and mRNA was analyzed by microarray. A number of inflammatory factors, mainly centering around tumor necrosis factor alpha (TNFα), were upregulated in the compressed cells.51
Key Findings Regarding Aortic Valve Calcification Obtained Using Valvular Interstitial Cells
VICs provide an attractive model for studying aortic valve calcification, and it has been around for at least 20 years. In this section we highlight the most interesting findings obtained using these cells. The most prominent findings are summarized in chronological order in Table 2 and are also represented in graphical form in Figure 2.
Table 2.
Key Findings Obtained Using VIC Regarding Calcific Aortic Valve Disease
| Reference No. | Author, Date | VIC Source | Factor | Key Result, End Point |
|---|---|---|---|---|
| 19 | Jian, 2002 | Ovine | Serotonin | Serotonin induces TGFβ1, and they both trigger matrix remodeling. |
| 32 | Osman, 2006 | Human, noncalcified | TGFβ family cytokines, statins | TGFβ family cytokines increase osteoblast differentiation; atorvastatin inhibits it. |
| 71 | Osman, 2006 | Human, noncalcified | Adenosine triphosphate, statins | Adenosine triphosphate activates osteoblast differentiation; atorvastatin inhibits this effect. |
| 40 | Osman, 2007 | Human, calcified | β1‐, β2‐, β3‐Adrenoreceptors | β1‐Adrenoreceptor mRNA is upregulated during osteoblast differentiation; salmeterol (selective β2‐agonist) reduces osteoblastic differentiation. |
| 76 | Cushing, 2008 | Porcine | Fibroblast growth factor 2 | Fibroblast growth factor 2, via mitogen‐associated protein kinases, inhibits myofibroblast differentiation induced by TGFβ1. |
| 81 | Kennedy, 2009 | Porcine | Nitric oxide signaling | Nodule formation induced by TGFβ1 is inhibited by nitric oxide donor via cyclic guanosine monophosphate signalling. |
| 30 | Yang, 2009 | Human, calcified and noncalcified | LPS and peptidoglycan | LPS and peptidoglycan stimulate osteoblast differentiation via toll‐like receptors 2 and 4. |
| 88 | Yang, 2009 | Human, calcified and noncalcified | BMP2 | BMP2 induces initial stages of osteoblast differentiation via canonical and noncanonical pathways. |
| 56 | Benton, 2009 | Porcine | Statins | Pravastatin inhibits myofibroblast differentiation via Rho kinase, HMG‐CoA, and myosin light chain kinase. |
| 89 | Nigam, 2009 | Ovine | Notch1 | Notch cleavage inhibitor and siRNA both cause osteoblast differentiation in sheep VIC through BMP2. |
| 58 | Bertacco, 2010 | Bovine | l‐Arginine LPS | LPS increases osteoblast differentiation in calcifying VIC; l‐arginine diminishes this effect, possibly via nitric oxide signaling. |
| 21 | Gwanmesia, 2010 | Ovine | Vascular endothelial growth factor | Vascular endothelial growth factor treatment combined with fibronectin coating prevents calcified nodule formation, calcification, and apoptosis. |
| 59 | Rodriguez, 2011 | Porcine | Hyaluronan | Culturing VICs on hyaluronic acid reduces nodule formation; adding it to the medium reduces calcification. |
| 90 | Chen, 2011 | Porcine | Wnt3, β‐catenin | TGFβ1 and Wnt3A synergistically induce myofibroblast differentiation via β‐catenin. |
| 35 | Yu, 2011 | Human, calcified and noncalcified | Tumor necrosis factor α, BMP2 | Tumor necrosis factor α induces osteoblast differentiation only in calcified VICs through BMP2 and NFkB signalling. |
| 91 | Carthy, 2012 | Human, noncalcified | Versican | Versican is secreted by VICs in wound assay; blocking its receptor CD44 decreases stress fiber (αSMA formation in migrating VIC and inhibits collagen gel contraction. |
| 26 | Yip, 2011 | Porcine, normal and high‐cholesterol‐fed pigs | C‐natriuretic peptide | C‐natriuretic peptide inhibits both osteoblastic and myofibroblastic differentiation of VIC; simvastatin upregulates C‐natriuretic peptide mRNA. |
| 52 | Witt, 2012 | Porcine | Sphingosine | Sphingosine increases nodule formation in a concentration‐dependent manner, acting via S1P2 receptor, RhoA, and ROCK kinases, executed by calcium release from internal cellular stores. |
| 92 | Yanagawa, 2012 | Porcine | MicroRNA 141, | MicroRNA 141 inhibits TGFβ1‐induced nodule formation and alkaline phosphatase activity by inhibition of BMP2 and Runx2 expression. |
| 93 | Xu, 2013 | Porcine | β‐Catenin, Wnt3 | Wnt3a increases VIC proliferation, the mechanism involves β‐catenin |
| 82 | Hutcheson, 2012 | Porcine | Serotonin, 5‐HT2b (serotonin receptor) | Antagonists of 5‐HT2b counteract myofibroblast differentiation induced by TGFβ1, likely by blocking noncanonical and enhancing canonical TGFβ1 signaling. |
| 94 | Song, 2012 | Human, calcified and noncalcified | Biglycan | VICs from calcified valves have increased biglycan expression; biglycan induces osteoblast differentiation via toll‐like receptor 2 and ERK. Biglycan expression and calcification are stimulated by oxidized low‐density lipopolysaccharides. |
| 95 | Zeng, 2012 | Human, calcified and noncalcified | LPS, toll‐like receptor 4, Notch | LPS via toll‐like receptor 4 activates inflammatory phenotype in VIC. In calcified VIC Notch1 sensitizes toll‐like receptor 4 to LPS by means of NFκB. |
| 96 | Nadlonek, 2012 | Human, noncalcified | γ‐Radiation | Irradiation of cultured VICs increases osteoblast differentiation. |
| 79 | Hutcheson, 2013 | Porcine | Cadherin‐11 | Cadherin‐11 is activated by TGFβ1 via phosphorylation of ERK. Cadherin‐11 is essential for calcified nodule formation as it increases intercellular tension. |
| 97 | Branchetti, 2013 | Human, calcified | DNA damage and repair mechanisms, antioxidants | DNA repair mechanisms are compromised in calcified VIC; cells are vulnerable to H2O2 ‐induced damage. Catalase adenovirus transfection reverses this. |
| 50 | Poggio, 2013 | Human, calcified and noncalcified | Bone morphogenetic protein 4 | Bone morphogenetic protein 4 triggers osteoblast differentiation only in noncalcified VIC, to levels higher than osteogenic medium alone. |
| 67 | Richards, 2013 | Porcine, VIC and VEC | Nitric oxide signaling from VEC to VIC | Osteogenic medium causes osteoblast differentiation in attached VIC 3D monocultures. This is inhibited by VEC by means of nitric oxide signaling. |
| 98 | Zeng, 2013 | Human, calcified and noncalcified | LPS, Notch1 | LPS stimulates cleavage and nuclear translocation of Notch1 intracellular domain which then leads to osteoblast differentiation through ERK and NFκB pathways. |
| 34 | Nadlonek, 2013 | Human, noncalcified | Interleukin‐1β | Interleukin‐1β induces an inflamatory phenotype in VIC via NFκB. |
| 39 | Zhang, 2014 | Human, noncalcified | MicroRNA 30b | BMP2 triggers osteoblastic differentiation in VIC and inhibits expression of microRNA 30b. MicroRNA 30b suppresses osteoblastic differentiation and apoptosis. |
| 72 | Farrar, 2014 | Porcine, VIC and VEC | TNFα | TNFα stimulates endothelial‐to‐mesenchymal transition in VEC, TNFα‐treated VECs have similar gene expression profile to TNFα‐treated VICs. |
| 99 | Galeone, 2013 | Human, calcified and noncalcified | TNF‐related apoptosis‐inducing ligand (TRAIL) | Calcified VICs express TRAIL receptors. Adding TRAIL to osteogenic medium increases calcified nodule formation and apoptosis. |
| 69 | Gould, 2014 | Porcine, VIC and VEC | Role of VEC | VECs in coculture inhibit myofibroblast differentiation in VIC through nitric oxide signaling. |
| 25 | El Husseini, 2014 | Human, noncalcified; murine from wild type and P2Y2−/− | AKT kinase, P2Y2 receptor |
Both AKT kinase and P2Y2 receptor via NFκB pathway inhibit expression of interleukin 6, which is necessary for mineralization. Cells from P2Y2 −/− mice are prone to osteoblast differentiation. |
| 100 | Zhang, 2014 | Human, from noncalcified areas of calcified valves | Transcription factor Twist | Osteogenic medium upregulates Twist. Overexpression of Twist decreased other calcification genes, and Twist siRNA triggers osteoblast differentiation. |
| 101 | Carrion, 2014 | Human, noncalcified | Long noncoding RNA HOTAIR | HOTAIR is downregulated by stretch via Wnt signaling; siRNA to HOTAIR upregulates BMP2 and alkaline phosphatase expression. |
| 102 | Zeng, 2014 | Human, noncalcified | Oxidized low‐density lipoproteins, LPS, Notch1 | Oxidized low‐density lipoproteins augment osteoblastic differentiation triggered by LPS through NFκB and Notch1 cleavage. |
| 103 | Witt, 2014 | Porcine, Human, noncalcified | Polyunsaturated fatty acids | Several polyunsaturated fatty acids reversibly inhibit myofibroblast activation via Rho kinase and ROCK kinase. |
| 104 | Song, 2014 | Human, noncalcified | Biglycan | Biglycan is a ligand for toll‐like receptors 2 and 4 in activation of inflammation in VIC; effect is mediated by NFκB and ERK. |
αSMA indicates α smooth muscle actin; 3D, 3‐dimensional; BMP2, bone morphogenetic protein 2; ERK, extracellular signal‐regulated kinase; LPS, lipopolysaccharide; NFκB, nuclear factor κB; TGFβ1, transforming growth factor β1; TNFα, tumor necrosis factor α; VEC, valve endothelial cells; VIC, valve interstitial cells.
Figure 2.
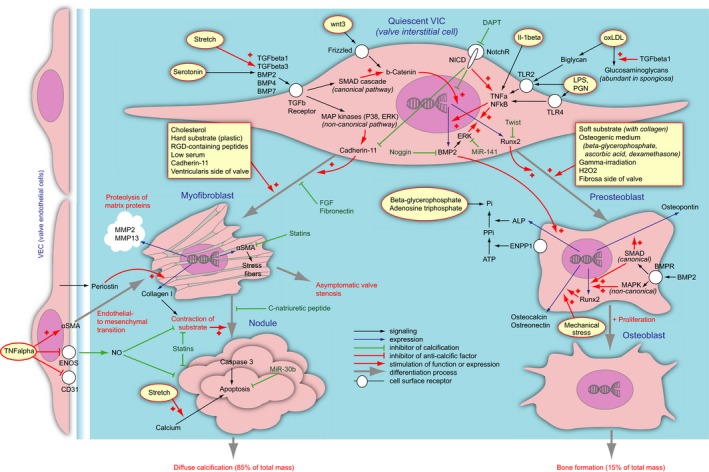
A current understanding of the pathological differentiation of valvular interstitial cells in aortic valve calcification. The cell types are given in blue. Quiescent valvular interstitial cels (VICs) as an effect of exogenous stimuli (given in yellow fields) differentiate into myofibroblasts (left) or preosteoblasts (right). Differentiation process is shown as gray arrows. The myofibroblasts can further assemble themselves into nodules, which undergo apoptosis and provide substrate for diffuse calcification (bottom left). The process is negatively regulated by valve endothelial cells (far left). The preosteoblasts can further differentiate into osteoblasts, which in turn synthesize ordinary bone (bottom right). The processes are orchestrated by a complex network of factors. The ligands stimulate surface receptors (white circles), which further relay to the signaling networks (black arrows). The signals can be inhibitory (stump arrows) or stimulatory (arrows with a “+”). The signal can constitute stimulation of expression of certain factors, a process shown as blue arrows. Generally the procalcific stimuli are shown with red arrows, and anticalcific are given in green. Due to the scheme complexity, several factors appear in multiple places on the scheme. ALP indicates alkaline phosphatase; aSMA, α‐smooth muscle actin; BMP, bone morphogenetic protein; BMPR, bone morphogenetic protein receptor; DAPT, inhibitor of γ‐secretase; ENOS, endothelial nitric oxide synthase; ENPP, ectonucleotide pyrophosphatase/phosphodiesterase 1; ERK, extracellular signal‐regulated kinase; FGF, fibroblast growth factor; IL, interleukin; LPS, lipopolysaccharide; MAPK, mitogen‐associated protein kinase; MMP, matrix metalloproteinase; NFkB Nuclear factor κB; NICD, Notch intracellular domain; NO, nitric oxide; NotchR, Notch receptor; oxLDL, oxidized low‐density lipoproteids; PGN, peptidoglycan; Runx2, runt‐related transcriptional factor 2; SMAD, small mothers against decapentaplegic; TGF, transforming growth factor; TLR, toll‐like receptor; TNF, tumor necrosis factor.
Role of Nonsterile Inflammation
Even though the direct link between bacterial infection and aortic valve calcification is not established, bacteria have been found in aortic valve cusps on autopsy. This puts bacterial toxins, such as lipopolysacharide (LPS) and peptidoglycan, on the tentative list of potential culprits.98 LPS is a Gram‐negative bacterial toxin, and peptidoglycan is the prevailing component of Gram‐positive bacterial cell walls. The innate immune system recognizes these 2 compounds via the toll‐like receptors TLR2 and TLR4. Healthy human VICs isolated from explanted hearts were found to express both of these receptors. Stimulation of VICs with LPS triggered a nuclear factor κB (NFκB) signaling response, involving nuclear translocation of p65 subunit, secretion of interleukins IL‐6 and IL‐8, resulting in increased expression of both BMP2 and Runx2.33 This effect was abolished by the administration of siRNA against TLR2 and 4.30 VICs from calcified human valves had a stronger response to LPS stimulation in terms of IL‐8, monocyte chemoattractant protein‐1, and intercellular adhesion molecule 1 (ICAM‐1) expression than healthy ones.95 Microarray analysis revealed that LPS affected human VICs in several ways similarly to β‐glycerophosphate (a critical component of osteogenic medium). For instance, LPS activated genes of BMP2, MMP2, platelet‐derived growth factor, and FGF, and the latter 2 were activated by β‐glycerophosphate as well.13 BMP2 was upregulated in human VICs following treatment with LPS, and peptidoglycan stimulated ALP activity via toll‐like receptors TLR2 and TLR4. This effect can be blocked by Noggin (a BMP2 signaling inhibitor).30 The signaling immediately downstream of BMP2 includes the canonical pathway involving the SMADs, in which SMAD1 activates expression of osteopontin, and the noncanonical pathway, in which ERK activates expression of Runx2. The phosphorylation of ERK following LPS stimulation was higher and more sustained in human VICs obtained from already calcified aortic valves.88 TLR4 stimulation in healthy human VICs also activated Il‐1β, a known proinflammatory cytokine. It further activated ICAM‐1, MCP‐1, IL‐6 and ‐8, and ultimately NFκB. ERK inhibition did not affect the calcification caused by IL‐1β, suggesting that the 2 pathways are parallel to each other.34
As indicated above, all VICs are not equal, and bacterial toxins do not trigger calcification uniformly in all of them. The response of VICs to both LPS and peptidoglycan was found to be higher in human aortic VICs compared with those isolated from the pulmonary valve, which was reflected by increased transcription of BMP2 and Runx2,30 although the expression of TLR4 was similar in human VICs from all 4 cardiac valve sites.74 VICs from all 4 sites reacted with LPS by upregulation of ICAM‐1 and MCP‐1, but BMP2 was induced only in aortic VICs.74 Furthermore, VICs from diseased human aortic valves exhibited a higher baseline expression of TLR2, TLR4, and BMP2.30, 88 A study of different cell populations among the bovine VICs revealed 4 distinct colony types, and the calcific response to LPS (ALP activity) exclusively happened in cells expressing osteocalcin but not αSMA, SM22, SMM, or osteopontin.22 In the calcifying clones, a downregulation of the l‐arginine‐metabolizing enzymes was found. Supplementation with l‐arginine and dimethylarginine countered ALP activity in the clones. The effect was replicated in 3D cultures using collagen I sponges.58
An important mediator of inflammation is TNFα. Human VICs isolated from calcified and noncalcified (aortic regurgitation, aortic dissection patients) valves revealed that TNFα triggered calcification in the calcified VICs via NFκB signaling to BMP2. Blocking NFκB signaling abolished this effect.35 It is plausible that TNFα signaling is the link between LPS and BMP2 in other studies as well. TNFα‐related apoptosis‐inducing ligand is a member of the TNFα superfamily of ligands and is expressed by T‐lymphocytes, macrophages, and VICs. Expression of TNFα‐related apoptosis‐inducing ligand death receptors DR4, DR5, and the decoy receptors DcR1 and DcR2 is increased in calcified human VICs. Osteogenic medium increased DR4 receptor expression, and adding TNFα‐related apoptosis‐inducing ligand to the osteogenic medium augmented human VIC calcification.99
Regarding the bulk of studies on LPS mentioned in this article, the main end point in many of them was protein expression of BMP2 in a short (days) time frame. Whether it is an adequate reflection of calcific response in VICs can be questioned. After all, as stated above, the primary end point in studying valve calcification must be qualitative and quantitative assessment of calcium.
Role of Wnt in Osteoblastic Differentiation of VIC
Wnt (wingless‐related integration site) is a secreted glycoprotein that signals through several pathways, one of them called “canonical,”105 which involves the second messenger β‐catenin. In the absence of signal, β‐catenin forms a complex with glycogen synthase kinase β, Axin, and APC (destruction complex), which leads to β‐catenin being degraded. However, on stimulation of the Wnt pathway through Wnt ligands, β‐catenin is stabilized by disruption of the distruction complex. The Wnt signaling pathway has multifaceted roles in differentiation, proliferation, and death as well as development and homeostasis. Wnt/β‐catenin signaling is known to play a critical role in cardiac development, in particular regulating cardiac valve formation, and also in regeneration and repair in frogs and fish.106, 107, 108 On the flip side Wnt/β‐catenin signaling is implicated in cancer and a number of diseases that are outside the scope of this review.109 The role of Wnt signaling has been studied for over a decade in the regulation of osteoblastic differentiation in bone and regulation of bone mass, as well as its involvement in disorders of bone.110 There is emerging evidence for the role of Wnt/β‐catenin signaling in calcification of aortic valves.111
In healthy VICs, β‐catenin is found in the cytoplasm, but Wnt3a ligand stimulates its nuclear translocation. A similar effect was observed in VICs treated with TGFβ1, and the blocking of β‐catenin translocation was shown to cancel the ensuing myofibroblast differentiation. In stenotic human valves αSMA, β‐catenin, TGFβ1, Wnt3a, and SMADs, 2/3 colocalize in the same areas.90 Porcine VICs stimulated with Wnt3 increased levels of β‐catenin both in the cytoplasm and in the nucleus and increased proliferative activity.93 In addition, clues can be found to the role of Wnt by looking at the associated risk factors of aortic valve disease, which include elevated levels of low‐density lipoprotein (LDL) and familial hypercholesterolemia, where patients develop vascular disease, coronary artery disease, and aortic valve lesions that calcify over time.112 The work of Rajamannan and colleagues further implicates the participation of the Wnt pathway via the low‐density receptor‐related protein Lrp5, which is a component of the Wnt receptor (comprised of LPR5/6 and Frizzled). Using a rabbit model they investigated the role of cholesterol and statins. They showed that a high‐cholesterol diet induced bone formation in aortic valves but that the administration of atorvastatin along with cholesterol suppressed the observed formation of bone.111
The Statin Paradox
There are many histopathological similarities between aortic valve calcification and atherosclerosis, which have led to a number of attempts at comparing the two diseases. It was shown that a high‐cholesterol diet in pigs leads to increased αSMA expression in aortic valve leaflets.46 Human VICs were used by the Yacoub group to investigate the effect of HMG‐CoA reductase inhibitors/statins on the osteogenic response. They showed that atorvastatin inhibited both ALP activity and cytokine expression triggered by TGFβ1, TGFβ3, TNFα, and BMP4. This effect was also alleviated by atorvastatin in healthy human VICs.71 In addition to inhibiting the osteoblast pathway, statins exerted an effect on myofibroblastic differentiation. In cultured porcine VICs simvastatin completely inhibited collagen gel contraction, even when the cells were stimulated with TGFβ1.80 Nodule formation by cultured porcine VICs was inhibited by pravastatin, acting via Rho kinase, HMG‐CoA, and myosin light chain kinase, inhibiting expression of αSMA. However, although the formation of nodules was suppressed, the already formed nodules were not resolved.56 The effect of statins (simvastatin at least) on nodule formation could possibly be mediated by the C‐natriuretic peptide, which is expressed by VECs populating the ventricular side of the valve.26
The data on statins remain highly controversial as 3 randomized clinical trials using statins for treatment of aortic valve calcification—SALTIRE (Scottish Aortic Stenosis and Lipid Lowering Trial, Impact on Regression), SEAS (Simvastatin and Ezetimibe in Aortic Stenosis), and ASTRONOMER (Aortic Stenosis Progression Observation: Measuring Effects of Rosuvastatin)—failed to demonstrate any beneficial effects.113 Instead, we now have a statin paradox—although vascular calcification is reduced by statin use, bone mineralization and aortic valve calcification are actually increased.6 A recent study by Monzack and colleagues showed that in porcine VICs treated with osteogenic medium or under reduced serum conditions (where the cells expressed high levels of αSMA), statins actually increased ALP activity and the expression of osteocalcin.65 Statins are now the front line in prevention of atherosclerosis, but the present conclusion is that they have no effect in preventing aortic valve calcification. Possibly the statins might inhibit development in healthy valves, but once the calcification process has started, the statins cannot stop it.
Role of Notch
Notch is a key signaling pathway in embryonic development, ensuring cross talk between different types of cells and their physiological differentiation.114 Notch is particularly important during cardiac valvulogenesis as well as in the pathogenesis of aortic valve calcification. In a seminal study Garg and co‐authors showed that Notch1 haploinsufficiency results in aortic valve calcification.115 Mutations in Notch1 are associated with bicuspid aortic valves and consequent valve calcification. Later Notch1 has been shown to repress osteogenic pathways in aortic valve cells.26 However, the exact mechanisms of Notch1 action in aortic valve calcification remain unknown, and the existing evidence is rather controversial. Some reports show that Notch activation prevents osteogenic differentiation but that the Notch ligand Jag1 may promote osteogenic differentiation.116, 117
The lab of Srivastava cultured both sheep VICs and endocardial cells from mice. Using a transgenic model with a heterozygous knockout of Notch1 they showed that these mice developed valve stenosis if fed with the high‐fat (“Western”) diet. Inhibition of Notch1 with a siRNA or using its inhibitor DAPT increased Runx2 expression; however, this effect was abolished when siRNA against BMP2 was used simultaneously.89
It would seem that Notch is a clear anticalcification factor. However, Zeng and colleagues showed that Notch1 increased the sensitivity of TLR4 to LPS stimulation in human VICs through the activation of NFκB signaling, effectively linking TLR4 and NFκB. Notch1 intracellular domain cleavage (required for Notch1 signal transduction) was proportional to the dose of LPS. The effect was inhibited by DAPT, an inhibitor of γ‐secretase, an enzyme that cleaves the Notch1 intracellular domain from the membrane domain.95 A follow‐up study showed that Notch1 maintained the phosphorylation of NFκB and ERK (mediator of the noncanonical BMP2 signaling) via MEK1/2 kinase. Surprisingly, ERK and NFκB activation were found to be upstream of BMP2 activation, and they could activate them without Notch1, but to a lesser degree.98 Notch cleavage, subsequent ALP activation, and BMP2 expression were also triggered by a combination of LPS and oxidized LDL, higher than the LPS alone. Also NFκB activation gave an equivalent response.102
New data on the role of Notch in aortic valve calcification have been obtained recently with the help of Notch1 +/− mice. As outlined above, an important aspect of myofibroblast activation and contraction is the interaction between αSMA and cadherin‐11.79 Notch1 +/− mice display aortic valve mineralization. Paradoxically, the VICs from these mice have a decrease of both Runx2 and αSMA, and a stark increase of cadherin‐11. Notch1 +/− VICs are hypersensitive to mechanical stress, as stretch stimulates an increased response in αSMA levels compared with wild‐type cells. This response is likely to explain the valve calcification observed in these mice in vivo. Thus, Notch1 harnesses the myofibroblastic differentiation of VICs and prevents dystrophic mineralization.118
In line with the reports mentioned above it has been shown that inhibition of Notch activity by γ‐secretase inhibitor in rat aortic VICs causes significant downregulation of transcription factor Sox9 along with several cartilage‐specific genes. In porcine VICs Notch inhibition resulted in accelerated calcification, whereas stimulation of Notch signaling attenuated the calcific process. The addition of Sox9 to the medium was able to prevent calcification that occurs at Notch inhibition.119 Altogether, it seems that Notch has a complex role in aortic valve calcification, which should be interpreted with respect to the process timeline, the species, and the end point employed in the study.
Cross‐Talk Between Valve Interstitial and Valve Endothelial Cells
VECs probably play a major role in aortic valve calcification, and they deserve a separate review, although at present there is a scarcity of studies. Only recently has it been possible to isolate and reliably characterize VECs. Studies now utilize VECs in coculture systems with VICs and are unveiling multiple effects of endothelium on the underlying interstitial cells. VECs harnessed the αSMA expression in porcine VICs, both in control cultures and in those subjected to shear stress.17 It was later demonstrated that nodule formation, an important step of the myofibroblast pathway, was inhibited with the nitric oxide donor DETA‐NONOate,81 implying that the effect may be due to the nitric oxide produced by the VECs. In a key study on VIC‐VEC interaction Richards and colleagues demonstrated that porcine VECs prevented VICs from calcification with osteogenic medium, reflected in normal expression of Runx2, αSMA, and osteocalcin.67 The effect was proven by treating a VIC monoculture with DETA‐NONOate and blocked by the nitric oxide signaling blocker l‐NAME.67 In human valves, VECs were found to express more endothelial nitric oxide synthase on the ventricular side, where calcification is not known to occur.67 Similar to the osteogenic differentiation, porcine VECs in coculture with VICs also inhibited myofibroblast differentiation on hard substrates (peptide‐functionalized polyethylene glycol) in the absence of TGFβ1. The role of VECs was again traced to nitric oxide production, as the above effect was negated with the use of l‐NAME.69 Mice with homozygous deletion of endothelial nitric oxide synthase are born with bicuspid aortic valves in 30% of the cases, and the majority of these calcify by 18 months of age. The bicuspid valves had increased expression of caspase 3, a marker and mediator of apoptosis, and osterix, a cofactor of osteoblast differentiation.120
The myofibroblasts in aortic valves have commonly been thought to be derived from VICs. However, a study by Wang and colleagues used flow cytometry to demonstrate that a proportion of porcine valve myofibroblasts bear endothelial cell markers.43 Farrar and colleagues investigated whether porcine VECs undergo endothelial‐to‐mesenchymal transition and contribute to the myofibroblast population.72 VECs were seeded on transwell membranes on top of a collagen gel and stimulated with TNFα. After 6 days it was shown that a portion of the VECs had undergone mesenchymal transformation and migrated into the collagen gel, rearranging the collagen fibrils. These cells expressed low levels of CD31 and high levels of αSMA. These invading VECs were in many ways similar to VICs: their response to TNFα showed an upregulation of MMP9, TGFβ1, BMP4, Notch1, and ICAM‐1 and a downregulation of nitric oxide synthase.72
Endothelial‐to‐mesenchymal transition in VECs has recently been reproduced in cells isolated from sheep after treatment with TGFβ and osteogenic medium. The transition was confirmed by a decrease in endothelial markers and an upregulation of αSMA and MMP‐2 after 7 days of treatment. By day 14 the cells expressed osteopontin, osteocalcin, and Runx2 as well as increased ALP activity. After 21 days of exposure to osteogenic medium, the VEC cultures were positive for calcium deposition. When VICs were directly cocultured with VECs, TGFβ‐induced endothelial‐to‐mesenchymal transition and osteogenic medium‐induced calcification were attenuated. However, VECs did not oppose the myofibroblastic or osteogenic differentiation of VICs. The authors showed staining of human aortic valves in which the same cells were positive for CD31 and αSMA, which they interpreted as cells undergoing endothelial‐to‐mesenchymal transition.121
Recently it has been shown in a mouse model that VEC‐specific Notch1 signaling regulates endothelial‐to‐mesenchymal transition in aortic valves through promoting expression of TNFα in VECs. TNFα interacts with its receptors Tnfr1 and Tnfr2 on VICs and induces apoptosis in VICs.122
Thus, there is accumulating evidence that VICs do not act alone, but VECs and VICs interact to ensure the proper development and maintenance of the aortic valve. Probably a disruption of this interaction could contribute to valve pathology and subsequent calcification.
Role of Sterile Inflammation
Sterile inflammation, chiefly triggered by uncontained cellular debris or irritant particles, is currently a hot topic in heart research. Sterile inflammation is by definition an immune response to signals of damage instead of signals of foreignness. The concept challenges a lot of preexisting knowledge but is continuously gaining ground in the area of valve research. A number of intrinsic inflammatory ligands have been shown to contribute to the pathological differentiation of VICs by initiating an inflammatory reaction.
Oxidized Low‐Density Lipoproteins
Oxidized low‐density lipoproteins (oxLDL) are known to accumulate in atherosclerotic plaques as well as in the calcified valves and to take part in tissue degeneration and calcification.6 OxLDL stimulation of healthy human VICs increased expression of inorganic phosphate transporters, which play a role in accumulation of calcium phosphate during mineralization.123 OxLDL treatment of porcine VICs triggered expression of Runx2 and osteocalcin and calcification via the receptor for advanced glycation end products and MAP kinases p38 and c‐Jun N‐kinase. Blocking the receptor for advanced glycation end products with targeted siRNA abolished the calcific response exerted by oxLDL.124
Biglycan
Biglycan is an extracellular proteoglycan whose expression is correlated with the degree of stenosis.85 It is elevated in stenotic human VICs compared with the healthy ones. Biglycan expression in human VICs is stimulated by oxLDLs. Biglycan binds to TLR2 receptor, where the signal is relayed via ERK kinase to BMP2 and ALP.94 Thus, it is a link between triggers of nonsterile inflammation and the ensuing osteoblast differentiation of VICs. The molecular events triggered by exogenous biglycan in human VICs mimic those exerted by LPS: activation of ICAM‐1, MCP1, and IL‐6 with a likely involvement of NFκB. The effect was attenuated by siRNA against TLR2 and TLR4 and blocked by the ERK inhibitor PD98059.104
High‐Mobility Group Box 1
The high‐mobility group box 1 (HMGB1) is a nuclear protein that orchestrates the inflammatory response in atherosclerosis and is thought to be secreted by macrophages, other immune cells, or by leakage from dying resident cells. The receptor for HMGB1 is TLR4, and the signal is further conveyed by NFκB and MAP kinases. Inside the human VICs HMGB1 undergoes translocation from the nucleus to the cytoplasm and out into the extracellular space to exert its inflammatory effect. This process is dependent on osteopontin, a known marker of calcification.125 Osteopontin expression is increased in calcified human valves, concurrent with large amounts of HMGB1 in the extracellular space and in some VECs. Osteopontin promotes proliferation of healthy VECs and inhibits proliferation of VECs from calcified valves. It also stimulates VECs to secrete HMGB1.125 Treatment of human VICs with HMGB1 induces a dose‐dependent calcific response: it induces expression of MCP1, BMP2, TNFα, and IL‐6 as well as calcium deposition.37 Thus, the HMGB might engage a similar response as the LPS, but in absence of microorganisms, which elevates the relevance of studies of TLR2 and TLR4 in calcific aortic valve disease.
Intracellular Adhesion Molecule‐1
ICAM‐1 is an indicator of cellular stress and inflammation. It interacts with integrins on leukocytes and enhances migration of leukocytes into the tissues. However, ICAM‐1 may have an independent role in calcification of VICs. Exposure of human VICs to lymphocyte function‐associated molecule‐1, a ligand of ICAM, elicited an osteogenic response through mechanisms involving BMP2, NFκB, and Notch1. Inhibition of γ‐secretase, an enzyme necessary for Notch1 cleavage (and subsequent signaling), blocks the osteogenic response to ICAM‐1.126
Cyclooxygenase 2
Cyclooxygenase 2 (Cox2) is an enzyme that plays a crucial role in synthesis of leukotrienes and prostaglandins and is a target for most nonsteroidal anti‐inflamatory drugs. Cox2 has a role in bone formation, namely in mechanotransduction stimulating osteoblastogenesis.127 In healthy murine aortic valves Cox2 expression is confined to the endothelial cells. Mice with a genetic ablation of Klotho manifest early onset of aging and nodular calcification of the aortic valve and are used as model animals. VICs in calcific nodules in Klotho‐deficient mice had high levels of Cox2, similar to human histology specimens. Porcine VICs treated with osteogenic medium and selecoxib, a selective Cox2 inhibitor, showed decreased osteocalcin and bone sialoprotein expression compared with osteogenic medium alone. Selecoxib also alleviated aortic valve calcification in Klotho‐deficient mice.128
Interleukin‐6
IL‐6 is a cytokine secreted by many different cells in the course of an immune response. It is increasingly expressed in VICs from calcified human valves and in vitro promotes calcification in cultured human VICs. P2Y2 nucleotide receptor was shown to inhibit the expression of IL‐6 by means of phosphorylation of AKT, which then represses the NFκB pathway. Calcified valves were found to express less AKT; furthermore, VICs from P2Y2‐knockout mice showed increased mineralization.25
MicroRNAs and Epigenetic Mechanisms
MicroRNAs (miRNAs) are emerging as the new regulators of genes at the translational level. Each miRNA regulates many transcripts, and a single transcript often has binding sites for several miRNAs.129 MiRNA profiling was used to compare human VICs isolated from calcified and noncalcified valves, both of bicuspid and tricuspid morphology. The profile of cells from bicuspid aortic valves was quite different from the profile of tricuspid counterparts. Furthermore, a single miRNA, MiR‐141, was found to inhibit nodule formation, ALP activity, Runx2, and BMP2 expression induced by TGFβ1 in porcine VICs.64 Another miRNA, MiR‐30b, was downregulated in calcified valve regions and in human calcified VICs treated with BMP2. The potential target genes for this miRNA include transcripts of Runx2, SMAD1, and Caspase 3. Treatment of VICs stimulated with osteogenic medium (supplemented with BMP2) with MiR‐30b precursor reduced ALP activity, Runx2 and osteocalcin expression. MiR‐30b also reduced apoptosis in human VICs cultured for 96 hours in low‐serum medium.39
Mature miRNAs are short, 22 to 23 nucleotides in length, whereas long noncoding RNAs are longer than 200 nucleotides. A long noncoding RNA, HOTAIR, has been shown to play a role in aortic calcification. The expression of this long noncoding RNA is lower in diseased bicuspid valves than tricuspid, and it is downregulated by stretch and Wnt agonists. HOTAIR targets are, among others, Periostin, MMP‐2, ‐10, and ‐12, collagen 1α1, BMP‐1, ‐4, ‐6, and BMP receptor 2. Thus, HOTAIR may be the endogenous inhibitor of ectopic mineralization and a potential therapeutic drug target.101
Another regulatory mechanism is epigenetic modification, for example, by DNA methylation, which is responsible for repressing transcription of a huge variety of factors. One example is 5‐lipoxygenase, which is involved in leukotriene synthesis, which promotes inflammation. Calcified human VICs showed decreased methylation at the 5‐lipoxygenase promoter site and, consequently, increased expression of the 5‐lipoxygenase mRNA, which may contribute to inflammation.130
Miscellaneous Factors
What follows is a listing of factors that are active at different points of the proposed mechanisms of calcification. At present it is impossible to outline the most important ones.
Periostin is found in VICs from healthy bovine aortic valves, but expression increases following exposure to LPS. Periostin is chiefly expressed in VICs of the lamina ventricularis, less in the fibrosa, and is coexpressed with elastin.57 Periostin is secreted by macrophages and myofibroblasts, and it stimulates expression of MMP‐2 and ‐9 in human VICs. Wild‐type mice fed with the Western diet develop aortic stenosis, but periostin‐knockout mice do not. In addition, they express lower levels of αSMA, collagen 1, and MMP‐2 and ‐13.57 Periostin may thus play a stimulatory role in the development of valve calcification.
Jian and colleagues showed that whereas healthy aortic valves did not express MMP‐2 or ALP, diseased valves expressed both, as well as tenascin‐C, an extracellular matrix glycoprotein found in atherosclerotic plaques. Ovine VICs grown on tenascin‐C showed an increased expression of MMP2.36 Its possible role in calcification is at present unknown.
Immunohistochemistry has revealed increased expression of β1‐, β2‐, and β3‐adrenoreceptors in the calcified area of the human valve along with RANK, one of the markers of osteoclasts. Osteogenic medium applied to VICs harvested from the calcified area stimulated increased expression of β1‐adrenoreceptor. Treatment of these cells with salmeterol (selective β2 agonist) reduced ALP activity and osteocalcin expression.40 A possible pathophysiological role is not known.
Versican is a proteoglycan that is upregulated after tissue injury and binds CD44 receptor. In a wound assay using healthy human VICs, versican was secreted and orchestrated gel contraction to repair the wound. Blocking the CD44 receptor with an antibody disabled the gel contraction property of the VICs.91 Gel contraction is one of the key functional end points for myofibroblasts, thus implying the role of versican in myofibroblast differentiation of VICs.
C‐Natriuretic peptide is expressed in healthy VICs and is downregulated in VICs from calcified valves. Experiments using porcine VICs showed that C‐natriuretic peptide inhibited nodule formation and gel contraction and in addition the osteoblastic differentiation potential.26 C‐Natriuretic peptide also increased cell elasticity properties as measured by micropipette aspiration.61
Another factor that promotes nodule formation in porcine VICs is sphingosine‐1. The small molecule JTE‐013, which blocks the S1P2 sphingosine receptor, abolishes this effect. The addition of sphingosine‐1 to a culture of VICs activates both RhoA and ROCK kinases and triggers influx of calcium leading to cell contraction. These results obtained in VICs were verified using strips of porcine valves stimulated with sphingosine‐1.52
Serotonin is an active vasodilator activating endothelial nitric oxide synthase. Agonists of the serotonin 2b receptor (norfenfluramine and pergolide) were found to promote myofibroblastic differentiation of porcine VICs and ensuing calcification.82 Production of TGFβ1 in ovine VICs is stimulated by serotonin in a dose‐dependent manner through G‐coupled receptor signaling.19 Serotonin receptor antagonists block the noncanonical signaling pathway of TGFβ1 in porcine VICs, simultaneously enhancing the canonical one, inhibiting nodule formation and expression of αSMA and smooth muscle myosin.82 This is in agreement with heart valve damage due to TGFβ1 signaling caused by high levels of serotonin in the circulation secondary to carcinoids, serotonin‐producing tumors.19
TGFβ1 stimulation in a 3D collagen culture of porcine VICs also stimulates faster and more powerful contraction of the extracellular matrix.53 This effect is counteracted by FGF‐2. The antifibrotic effect of FGF‐2 on VICs is mediated by SMADs and ERK. The concept of FGF‐2 treatment against leaflet fibrosis was suggested by experiments in excised pig valve leaflets in the form of reduced αSMA expression.76 FGF is also suggested as a medium supplement to reduce myofibroblast activation in freshly isolated VICs.49
The role of BMP2 has been amply referred to above in terms of nonsterile inflammation. However, there are several BMP isoforms, including BMP4. Addition of recombinant BMP4 to strips cut from healthy human aortic valve subjected to cyclic stretch induced expression of αSMA and calcification. BMP4 antagonist Noggin abolished the effect of BMP4.50 Stretching healthy human VICs in tubular molds of collagen gel for 3 weeks at 15% resulted in a modest increase of BMP2 and BMP4 mRNA and BMP2 protein.68 Microarray studies of human sclerotic, stenotic, and control aortic valves showed an increased expression of BMP4 in both diseased groups. The additive effect of mechanical stress and BMP4 is reminiscent of a combination of stretch and TGFβ1.50
Even though BMP2 has a net procalcific effect in valve mineralization, some of its targets may actually have an opposite, beneficial effect. As stated above, the BMPs belong to the transforming growth factor superfamily and rely on SMADs for their canonical signaling pathway. The SMADs fall into an activating and inhibitory group.131 SMAD6 is an inhibitory SMAD activated by BMP2, and SMAD6‐knockout mice have aortic valve calcification. These mice also display reduced levels of SMAD6 in their valve leaflets. Treatment of murine VICs with TNFα elicited an osteogenic response and reduced expression of SMAD6. Knocking down SMAD6 in murine VICs led to mineralization in absence of other stimuli.132
Twist‐related protein (TWIST) inhibits Runx2 function in preosteoblasts by adherence to its DNA‐binding domain and recruitment of histone deacetylases.133 Calcified human VICs express less TWIST than the healthy ones. Immunohistochemistry shows that Runx2 and Twist expression areas are nonoverlapping. Overexpression of TWIST in VICs decreased expression of Runx2, osteocalcin, osteopontin, and ALP, whereas the knockdown of Twist with siRNA had the opposite effect.100
Hyaluronan is one of the abundant components of the extracellular matrix in connective tissues, including the aortic valve leaflets. Porcine VICs grown on collagen were found to secrete hyaluronan, and adding exogenous hyaluronan at the mean molecular weight of 64 kDa to the medium reduced nodule formation, although the higher and lower molecular hyaluronan did not have this effect. Digestion of hyaluronan in situ in the porcine valve specimens led to increased apoptosis, proliferation, and αSMA expression in resident VICs.59
Inhibitors of aortic valve calcification can come in many forms, but none are more attractive than the food supplements. A study of polyunsaturated fatty acids with fish oil showed that docosahexaenoic acid and arachidonic acid dose‐dependently inhibited nodule formation in both human and porcine VIC cultures. This inhibition was reversible, as the nodule formation increased again after the polyunsaturated fatty acid supplementation was discontinued.103
Radiotherapy is known to produce valve disease: over 60% of patients undergoing radiation therapy in the mediastinal region developed calcific aortic stenosis over the next 20 years. Aortic valves from irradiated patients express more BMP2 than cells that received no radiation. γ‐Irradiation of healthy human VICs with 10 Gy induced expression of BMP2, Runx2, osteopontin, and ALP.96
DNA damage and repair are a routine activities in all cells, but if the balance is tipped toward damage, the cells may undergo apoptosis. Human VICs from sclerotic and stenotic aortic valves have increased oxidative DNA damage compared with the healthy ones, impaired DNA repair enzymes, and decreased expression of superoxide dismutase, catalase, and other antioxidants. Adenovirus delivery of catalase alleviates the oxidative damage and the calcific response.97
Conclusion
VICs represent a relevant model for studies of aortic valve calcification, especially when complemented with VECs. The most relevant models are 3D. The ideal source of cells is human valves, both calcified and healthy ones, obtained during surgery. The cells require no specific culturing techniques compared with most fibroblasts; however, several things are to be kept in mind. The population of VICs is quite heterogeneous with respect both to capacity to differentiate and to morphology already present at isolation. The phenotype relevant for the physiological situation changes with passaging, and the cells should be used at as early a passage as possible. Also, we should be conscious at the choice of substrate, as its physical properties and chemical composition heavily influence the biology of VICs.
The key concept of the cellular mechanism leading to aortic valve calcification is the differentiation of resident interstitial cells into cell types foreign to the valve itself: osteoblasts and myofibroblasts (although the studies indicate that myofibroblasts may be present in some quantities even in the healthy valves). It is not known which mechanism prevails or which comes first and which follows. The conclusions are widely drawn based on autopsy findings, and the time‐course of the disease is basically unknown. The concepts of ossification driven by osteoblasts and dystrophic calcification secondary to formation of nodules by contraction of myofibroblasts (the current view) may be changed or entirely replaced by more accurate theories.
The future of aortic valve research is likely to elucidate the mechanisms underlying myofibroblast transformation and osteogenesis but also to go into previously unknown areas: circulating nucleic acids, epigenetics, unorthodox pathogens, radiation, and others. This will require that the models used are representative of the clinical and physiological situation. Unfortunately, the plethora of factors that may influence the phenotype of VICs represent important limitations of using VICs to clarify the molecular and cellular mechanisms of heart valve calcification. At the end of the day, one must create the maximally representative model for human disease, and many conflicting results can be explained by different protocols, culture conditions, and choice of cell source. After all, the VICs in culture are not identical to VICs in the living valve. Consequently, although VICs are the backbone of experimental models, findings in cultured VICs must be verified in cultured whole leaflets, in vivo animal models, and ultimately in humans.
Sources of Funding
This work was supported by South‐Eastern Norway Regional Health Authority (grant 2013109), the National Association (Norway), the University of Oslo, The Norwegian Research Council, the Government of Russian Federation (grant 074‐U01), and the Russian Foundation of Basic Research (grant 17‐04‐01318).
Disclosures
None.
Acknowledgments
The authors wish to acknowledge the valuable help from Professor Jonathan Butcher and his laboratory at Cornell University for providing crucial practical knowledge about the handling of VIC.
J Am Heart Assoc. 2017;6:e006339 DOI: 10.1161/JAHA.117.006339.28912209
References
- 1. Iung B, Baron G, Butchart EG, Delahaye F, Gohlke‐Bärwolf C, Levang OW, Tornos P, Vanoverschelde J‐L, Vermeer F, Boersma E. A prospective survey of patients with valvular heart disease in Europe: the Euro Heart Survey on Valvular Heart Disease. Eur Heart J. 2003;24:1231–1243. [DOI] [PubMed] [Google Scholar]
- 2. O'Brien KD. Epidemiology and genetics of calcific aortic valve disease. J Investig Med. 2007;55:284–291. [DOI] [PubMed] [Google Scholar]
- 3. Goldbarg SH, Elmariah S, Miller MA, Fuster V. Insights into degenerative aortic valve disease. J Am Coll Cardiol. 2007;50:1205–1213. [DOI] [PubMed] [Google Scholar]
- 4. Pomerance A. Ageing changes in human heart valves. Br Heart J. 1967;29:222–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Misfeld M, Sievers HH. Heart valve macro‐ and microstructure. Philos Trans R Soc Lond B Biol Sci. 2007;362:1421–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Helske S, Kupari M, Lindstedt KA, Kovanen PT. Aortic valve stenosis: an active atheroinflammatory process. Curr Opin Lipidol. 2007;18:483–491. [DOI] [PubMed] [Google Scholar]
- 7. Butcher JT, Markwald RR. Valvulogenesis: the moving target. Philos Trans R Soc Lond B Biol Sci. 2007;362:1489–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mohler ER III. Mechanisms of aortic valve calcification. Am J Cardiol. 2004;94:1396–1402, A1396. [DOI] [PubMed] [Google Scholar]
- 9. Chen JH, Simmons CA. Cell‐matrix interactions in the pathobiology of calcific aortic valve disease: critical roles for matricellular, matricrine, and matrix mechanics cues. Circ Res. 2011;108:1510–1524. [DOI] [PubMed] [Google Scholar]
- 10. Cawley PJ, Otto CM. Prevention of calcific aortic valve stenosis—fact or fiction? Ann Med. 2009;41:100–108. [DOI] [PubMed] [Google Scholar]
- 11. Zigelman CZ, Edelstein PM. Aortic valve stenosis. Anesthesiol Clin. 2009;27:519–532. [DOI] [PubMed] [Google Scholar]
- 12. Akat K, Borggrefe M, Kaden JJ. Aortic valve calcification: basic science to clinical practice. Heart. 2009;95:616–623. [DOI] [PubMed] [Google Scholar]
- 13. Babu AN, Meng X, Zou N, Yang X, Wang M, Song Y, Cleveland JC, Weyant M, Banerjee A, Fullerton DA. Lipopolysaccharide stimulation of human aortic valve interstitial cells activates inflammation and osteogenesis. Ann Thorac Surg. 2008;86:71–76. [DOI] [PubMed] [Google Scholar]
- 14. Xu S, Liu AC, Gotlieb AI. Common pathogenic features of atherosclerosis and calcific aortic stenosis: role of transforming growth factor‐β. Cardiovasc Pathol. 2010;19:236–247. [DOI] [PubMed] [Google Scholar]
- 15. Farzaneh‐Far A, Proudfoot D, Shanahan C, Weissberg PL. Vascular and valvar calcification: recent advances. Heart. 2001;85:13–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Miller JD, Weiss RM, Heistad DD. Calcific aortic valve stenosis: methods, models, and mechanisms. Circ Res. 2011;108:1392–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Butcher JT, Nerem RM. Valvular endothelial cells regulate the phenotype of interstitial cells in co‐culture: effects of steady shear stress. Tissue Eng. 2006;12:905–915. [DOI] [PubMed] [Google Scholar]
- 18. Bond WS, Roberts EL, Warnock JN. Evaluation of porcine aortic valve interstitial cell activity using different serum types in two‐ and three‐dimensional culture. Tissue Eng. 2007;13:343–349. [DOI] [PubMed] [Google Scholar]
- 19. Jian B, Xu J, Connolly J, Savani RC, Narula N, Liang B, Levy RJ. Serotonin mechanisms in heart valve disease I: serotonin‐induced up‐regulation of transforming growth factor‐β1 via G‐protein signal transduction in aortic valve interstitial cells. Am J Pathol. 2002;161:2111–2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Merryman WD, Youn I, Lukoff HD, Krueger PM, Guilak F, Hopkins RA, Sacks MS. Correlation between heart valve interstitial cell stiffness and transvalvular pressure: implications for collagen biosynthesis. Am J Physiol Heart Circ Physiol. 2006;290:H224–H231. [DOI] [PubMed] [Google Scholar]
- 21. Gwanmesia P, Ziegler H, Eurich R, Barth M, Kamiya H, Karck M, Lichtenberg A, Akhyari P. Opposite effects of transforming growth factor‐β1 and vascular endothelial growth factor on the degeneration of aortic valvular interstitial cell are modified by the extracellular matrix protein fibronectin: implications for heart valve engineering. Tissue Eng Part A. 2010;16:3737–3746. [DOI] [PubMed] [Google Scholar]
- 22. Rattazzi M, Iop L, Faggin E, Bertacco E, Zoppellaro G, Baesso I, Puato M, Torregrossa G, Fadini GP, Agostini C, Gerosa G, Sartore S, Pauletto P. Clones of interstitial cells from bovine aortic valve exhibit different calcifying potential when exposed to endotoxin and phosphate. Arterioscler Thromb Vasc Biol. 2008;28:2165–2172. [DOI] [PubMed] [Google Scholar]
- 23. Renato M, Bertacco E, Franchin C, Arrigoni G, Rattazzi M. Proteomic analysis of interstitial aortic valve cells acquiring a pro‐calcific profile. Methods Mol Biol. 2013;1005:95–107. [DOI] [PubMed] [Google Scholar]
- 24. Duan B, Hockaday LA, Kapetanovic E, Kang KH, Butcher JT. Stiffness and adhesivity control aortic valve interstitial cell behavior within hyaluronic acid based hydrogels. Acta Biomater. 2013;9:7640–7650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. El Husseini D, Boulanger MC, Mahmut A, Bouchareb R, Laflamme MH, Fournier D, Pibarot P, Bosse Y, Mathieu P. P2Y2 receptor represses IL‐6 expression by valve interstitial cells through Akt: implication for calcific aortic valve disease. J Mol Cell Cardiol. 2014;72:146–156. [DOI] [PubMed] [Google Scholar]
- 26. Yip CY, Blaser MC, Mirzaei Z, Zhong X, Simmons CA. Inhibition of pathological differentiation of valvular interstitial cells by C‐type natriuretic peptide. Arterioscler Thromb Vasc Biol. 2011;31:1881–1889. [DOI] [PubMed] [Google Scholar]
- 27. Roberts WC, Ko JM. Frequency by decades of unicuspid, bicuspid, and tricuspid aortic valves in adults having isolated aortic valve replacement for aortic stenosis, with or without associated aortic regurgitation. Circulation. 2005;111:920–925. [DOI] [PubMed] [Google Scholar]
- 28. Garg V. Molecular genetics of aortic valve disease. Curr Opin Cardiol. 2006;21:180–184. [DOI] [PubMed] [Google Scholar]
- 29. McCoy CM, Nicholas DQ, Masters KS. Sex‐related differences in gene expression by porcine aortic valvular interstitial cells. PLoS One. 2012;7:e39980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yang X, Fullerton DA, Su X, Ao L, Cleveland JC Jr, Meng X. Pro‐osteogenic phenotype of human aortic valve interstitial cells is associated with higher levels of toll‐like receptors 2 and 4 and enhanced expression of bone morphogenetic protein 2. J Am Coll Cardiol. 2009;53:491–500. [DOI] [PubMed] [Google Scholar]
- 31. Ferdous Z, Jo H, Nerem RM. Differences in valvular and vascular cell responses to strain in osteogenic media. Biomaterials. 2011;32:2885–2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Osman L, Yacoub MH, Latif N, Amrani M, Chester AH. Role of human valve interstitial cells in valve calcification and their response to atorvastatin. Circulation. 2006;114:I547–I552. [DOI] [PubMed] [Google Scholar]
- 33. Meng X, Ao L, Song Y, Babu A, Yang X, Wang M, Weyant MJ, Dinarello CA, Cleveland JC Jr, Fullerton DA. Expression of functional toll‐like receptors 2 and 4 in human aortic valve interstitial cells: potential roles in aortic valve inflammation and stenosis. Am J Physiol. 2008;294:C29–C35. [DOI] [PubMed] [Google Scholar]
- 34. Nadlonek N, Lee JH, Reece TB, Weyant MJ, Cleveland JC Jr, Meng X, Fullerton DA. Interleukin‐1 beta induces an inflammatory phenotype in human aortic valve interstitial cells through nuclear factor kappa beta. Ann Thorac Surg. 2013;96:155–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yu Z, Seya K, Daitoku K, Motomura S, Fukuda I, Furukawa K. Tumor necrosis factor‐α accelerates the calcification of human aortic valve interstitial cells obtained from patients with calcific aortic valve stenosis via the BMP2‐Dlx5 pathway. J Pharmacol Exp Ther. 2011;337:16–23. [DOI] [PubMed] [Google Scholar]
- 36. Jian B, Jones PL, Li Q, Mohler ER III, Schoen FJ, Levy RJ. Matrix metalloproteinase‐2 is associated with tenascin‐C in calcific aortic stenosis. Am J Pathol. 2001;159:321–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang B, Li F, Zhang C, Wei G, Liao P, Dong N. High‐mobility group box‐1 protein induces osteogenic phenotype changes in aortic valve interstitial cells. J Thorac Cardiovasc Surg. 2016;151:255–262. [DOI] [PubMed] [Google Scholar]
- 38. Clark‐Greuel JN, Connolly JM, Sorichillo E, Narula NR, Rapoport HS, Mohler ER III, Gorman JH III, Gorman RC, Levy RJ. Transforming growth factor‐β1 mechanisms in aortic valve calcification: increased alkaline phosphatase and related events. Ann Thorac Surg. 2007;83:946–953. [DOI] [PubMed] [Google Scholar]
- 39. Zhang M, Liu X, Zhang X, Song Z, Han L, He Y, Xu Z. MicroRNA‐30b is a multifunctional regulator of aortic valve interstitial cells. J Thorac Cardiovasc Surg. 2014;147:1073–1080.e1072. [DOI] [PubMed] [Google Scholar]
- 40. Osman L, Chester AH, Sarathchandra P, Latif N, Meng W, Taylor PM, Yacoub MH. A novel role of the sympatho‐adrenergic system in regulating valve calcification. Circulation. 2007;116:I282–I287. [DOI] [PubMed] [Google Scholar]
- 41. Olsson M, Rosenqvist M, Nilsson J. Expression of HLA‐DR antigen and smooth muscle cell differentiation markers by valvular fibroblasts in degenerative aortic stenosis. J Am Coll Cardiol. 1994;24:1664–1671. [DOI] [PubMed] [Google Scholar]
- 42. Osman N, Grande‐Allen KJ, Ballinger ML, Getachew R, Marasco S, O'Brien KD, Little PJ. Smad2‐dependent glycosaminoglycan elongation in aortic valve interstitial cells enhances binding of LDL to proteoglycans. Cardiovasc Pathol. 2013;22:146–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wang H, Sridhar B, Leinwand LA, Anseth KS. Characterization of cell subpopulations expressing progenitor cell markers in porcine cardiac valves. PLoS One. 2013;8:e69667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cimini M, Rogers KA, Boughner DR. Smoothelin‐positive cells in human and porcine semilunar valves. Histochem Cell Biol. 2003;120:307–317. [DOI] [PubMed] [Google Scholar]
- 45. Ku CH, Johnson PH, Batten P, Sarathchandra P, Chambers RC, Taylor PM, Yacoub MH, Chester AH. Collagen synthesis by mesenchymal stem cells and aortic valve interstitial cells in response to mechanical stretch. Cardiovasc Res. 2006;71:548–556. [DOI] [PubMed] [Google Scholar]
- 46. Pho M, Lee W, Watt DR, Laschinger C, Simmons CA, McCulloch CA. Cofilin is a marker of myofibroblast differentiation in cells from porcine aortic cardiac valves. Am J Physiol Heart Circ Physiol. 2008;294:H1767–H1778. [DOI] [PubMed] [Google Scholar]
- 47. Messier RH Jr, Bass BL, Aly HM, Jones JL, Domkowski PW, Wallace RB, Hopkins RA. Dual structural and functional phenotypes of the porcine aortic valve interstitial population: characteristics of the leaflet myofibroblast. J Surg Res. 1994;57:1–21. [DOI] [PubMed] [Google Scholar]
- 48. Gu X, Masters KS. Regulation of valvular interstitial cell calcification by adhesive peptide sequences. J Biomed Mater Res A. 2010;93:1620–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Latif N, Quillon A, Sarathchandra P, McCormack A, Lozanoski A, Yacoub MH, Chester AH. Modulation of human valve interstitial cell phenotype and function using a fibroblast growth factor 2 formulation. PLoS One. 2015;10:e0127844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Poggio P, Sainger R, Branchetti E, Grau JB, Lai EK, Gorman RC, Sacks MS, Parolari A, Bavaria JE, Ferrari G. Noggin attenuates the osteogenic activation of human valve interstitial cells in aortic valve sclerosis. Cardiovasc Res. 2013;98:402–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Warnock JN, Nanduri B, Pregonero Gamez CA, Tang J, Koback D, Muir WM, Burgess SC. Gene profiling of aortic valve interstitial cells under elevated pressure conditions: modulation of inflammatory gene networks. Int J Inflamm. 2011;2011:176412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Witt W, Jannasch A, Burkhard D, Christ T, Ravens U, Brunssen C, Leuner A, Morawietz H, Matschke K, Waldow T. Sphingosine‐1‐phosphate induces contraction of valvular interstitial cells from porcine aortic valves. Cardiovasc Res. 2012;93:490–497. [DOI] [PubMed] [Google Scholar]
- 53. Merryman WD, Liao J, Parekh A, Candiello JE, Lin H, Sacks MS. Differences in tissue‐remodeling potential of aortic and pulmonary heart valve interstitial cells. Tissue Eng. 2007;13:2281–2289. [DOI] [PubMed] [Google Scholar]
- 54. Benton JA, Kern HB, Anseth KS. Substrate properties influence calcification in valvular interstitial cell culture. J Heart Valve Dis. 2008;17:689–699. [PMC free article] [PubMed] [Google Scholar]
- 55. Yip CY, Chen JH, Zhao R, Simmons CA. Calcification by valve interstitial cells is regulated by the stiffness of the extracellular matrix. Arterioscler Thromb Vasc Biol. 2009;29:936–942. [DOI] [PubMed] [Google Scholar]
- 56. Benton JA, Kern HB, Leinwand LA, Mariner PD, Anseth KS. Statins block calcific nodule formation of valvular interstitial cells by inhibiting α‐smooth muscle actin expression. Arterioscler Thromb Vasc Biol. 2009;29:1950–1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hakuno D, Kimura N, Yoshioka M, Mukai M, Kimura T, Okada Y, Yozu R, Shukunami C, Hiraki Y, Kudo A, Ogawa S, Fukuda K. Periostin advances atherosclerotic and rheumatic cardiac valve degeneration by inducing angiogenesis and MMP production in humans and rodents. J Clin Invest. 2010;120:2292–2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bertacco E, Millioni R, Arrigoni G, Faggin E, Iop L, Puato M, Pinna LA, Tessari P, Pauletto P, Rattazzi M. Proteomic analysis of clonal interstitial aortic valve cells acquiring a pro‐calcific profile. J Proteome Res. 2010;9:5913–5921. [DOI] [PubMed] [Google Scholar]
- 59. Rodriguez KJ, Piechura LM, Masters KS. Regulation of valvular interstitial cell phenotype and function by hyaluronic acid in 2‐D and 3‐D culture environments. Matrix Biol. 2011;30:70–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hutcheson JD, Venkataraman R, Baudenbacher FJ, Merryman WD. Intracellular Ca(2+) accumulation is strain‐dependent and correlates with apoptosis in aortic valve fibroblasts. J Biomech. 2012;45:888–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wyss K, Yip CY, Mirzaei Z, Jin X, Chen JH, Simmons CA. The elastic properties of valve interstitial cells undergoing pathological differentiation. J Biomech. 2012;45:882–887. [DOI] [PubMed] [Google Scholar]
- 62. Gould RA, Chin K, Santisakultarm TP, Dropkin A, Richards JM, Schaffer CB, Butcher JT. Cyclic strain anisotropy regulates valvular interstitial cell phenotype and tissue remodeling in three‐dimensional culture. Acta Biomater. 2012;8:1710–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Fisher CI, Chen J, Merryman WD. Calcific nodule morphogenesis by heart valve interstitial cells is strain dependent. Biomech Model Mechanobiol. 2013;12:5–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Quinlan AM, Billiar KL. Investigating the role of substrate stiffness in the persistence of valvular interstitial cell activation. J Biomed Mater Res A. 2012;100:2474–2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Monzack EL, Masters KS. A time course investigation of the statin paradox among valvular interstitial cell phenotypes. Am J Physiol Heart Circ Physiol. 2012;303:H903–H909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Moraes C, Likhitpanichkul M, Lam CJ, Beca BM, Sun Y, Simmons CA. Microdevice array‐based identification of distinct mechanobiological response profiles in layer‐specific valve interstitial cells. Integr Biol (Camb). 2013;5:673–680. [DOI] [PubMed] [Google Scholar]
- 67. Richards J, El‐Hamamsy I, Chen S, Sarang Z, Sarathchandra P, Yacoub MH, Chester AH, Butcher JT. Side‐specific endothelial‐dependent regulation of aortic valve calcification: interplay of hemodynamics and nitric oxide signaling. Am J Pathol. 2013;182:1922–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ferdous Z, Jo H, Nerem RM. Strain magnitude‐dependent calcific marker expression in valvular and vascular cells. Cells Tissues Organs. 2013;197:372–383. [DOI] [PubMed] [Google Scholar]
- 69. Gould ST, Matherly EE, Smith JN, Heistad DD, Anseth KS. The role of valvular endothelial cell paracrine signaling and matrix elasticity on valvular interstitial cell activation. Biomaterials. 2014;35:3596–3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Porras AM, van Engeland NC, Marchbanks E, McCormack A, Bouten CV, Yacoub MH, Latif N, Masters KS. Robust generation of quiescent porcine valvular interstitial cell cultures. J Am Heart Assoc. 2017;6:e005041 DOI: 10.1161/JAHA.116.005041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Osman L, Chester AH, Amrani M, Yacoub MH, Smolenski RT. A novel role of extracellular nucleotides in valve calcification: a potential target for atorvastatin. Circulation. 2006;114:I566–I572. [DOI] [PubMed] [Google Scholar]
- 72. Farrar EJ, Butcher JT. Heterogeneous susceptibility of valve endothelial cells to mesenchymal transformation in response to TNFα. Ann Biomed Eng. 2014;42:149–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Chen JH, Yip CY, Sone ED, Simmons CA. Identification and characterization of aortic valve mesenchymal progenitor cells with robust osteogenic calcification potential. Am J Pathol. 2009;174:1109–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Venardos N, Nadlonek NA, Zhan Q, Weyant MJ, Reece TB, Meng X, Fullerton DA. Aortic valve calcification is mediated by a differential response of aortic valve interstitial cells to inflammation. J Surg Res. 2014;190:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Dreger SA, Thomas P, Sachlos E, Chester AH, Czernuszka JT, Taylor PM, Yacoub MH. Potential for synthesis and degradation of extracellular matrix proteins by valve interstitial cells seeded onto collagen scaffolds. Tissue Eng. 2006;12:2533–2540. [DOI] [PubMed] [Google Scholar]
- 76. Cushing MC, Mariner PD, Liao JT, Sims EA, Anseth KS. Fibroblast growth factor represses Smad‐mediated myofibroblast activation in aortic valvular interstitial cells. FASEB J. 2008;22:1769–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Sapp MC, Fares HJ, Estrada AC, Grande‐Allen KJ. Multilayer three‐dimensional filter paper constructs for the culture and analysis of aortic valvular interstitial cells. Acta Biomater. 2015;13:199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Mabry KM, Lawrence RL, Anseth KS. Dynamic stiffening of poly(ethylene glycol)‐based hydrogels to direct valvular interstitial cell phenotype in a three‐dimensional environment. Biomaterials. 2015;49:47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Hutcheson JD, Chen J, Sewell‐Loftin MK, Ryzhova LM, Fisher CI, Su YR, Merryman WD. Cadherin‐11 regulates cell‐cell tension necessary for calcific nodule formation by valvular myofibroblasts. Arterioscler Thromb Vasc Biol. 2013;33:114–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Monzack EL, Gu X, Masters KS. Efficacy of simvastatin treatment of valvular interstitial cells varies with the extracellular environment. Arterioscler Thromb Vasc Biol. 2009;29:246–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Kennedy JA, Hua X, Mishra K, Murphy GA, Rosenkranz AC, Horowitz JD. Inhibition of calcifying nodule formation in cultured porcine aortic valve cells by nitric oxide donors. Eur J Pharmacol. 2009;602:28–35. [DOI] [PubMed] [Google Scholar]
- 82. Hutcheson JD, Ryzhova LM, Setola V, Merryman WD. 5‐HT(2B) antagonism arrests non‐canonical TGF‐β1‐induced valvular myofibroblast differentiation. J Mol Cell Cardiol. 2012;53:707–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Monzack EL, Masters KS. Can valvular interstitial cells become true osteoblasts? A side‐by‐side comparison. J Heart Valve Dis. 2011;20:449–463. [PMC free article] [PubMed] [Google Scholar]
- 84. Cloyd KL, El‐Hamamsy I, Boonrungsiman S, Hedegaard M, Gentleman E, Sarathchandra P, Colazzo F, Gentleman MM, Yacoub MH, Chester AH, Stevens MM. Characterization of porcine aortic valvular interstitial cell ‘calcified’ nodules. PLoS One. 2012;7:e48154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Lehmann S, Walther T, Kempfert J, Rastan A, Garbade J, Dhein S, Mohr FW. Mechanical strain and the aortic valve: influence on fibroblasts, extracellular matrix, and potential stenosis. Ann Thorac Surg. 2009;88:1476–1483. [DOI] [PubMed] [Google Scholar]
- 86. Yan YX, Gong YW, Guo Y, Lv Q, Guo C, Zhuang Y, Zhang Y, Li R, Zhang XZ. Mechanical strain regulates osteoblast proliferation through integrin‐mediated ERK activation. PLoS One. 2012;7:e35709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Boppart MD, Kimmel DB, Yee JA, Cullen DM. Time course of osteoblast appearance after in vivo mechanical loading. Bone. 1998;23:409–415. [DOI] [PubMed] [Google Scholar]
- 88. Yang X, Meng X, Su X, Mauchley DC, Ao L, Cleveland JC Jr, Fullerton DA. Bone morphogenic protein 2 induces Runx2 and osteopontin expression in human aortic valve interstitial cells: role of Smad1 and extracellular signal‐regulated kinase 1/2. J Thorac Cardiovasc Surg. 2009;138:1008–1015. [DOI] [PubMed] [Google Scholar]
- 89. Nigam V, Srivastava D. Notch1 represses osteogenic pathways in aortic valve cells. J Mol Cell Cardiol. 2009;47:828–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Chen JH, Chen WL, Sider KL, Yip CY, Simmons CA. β‐Catenin mediates mechanically regulated, transforming growth factor‐β1‐induced myofibroblast differentiation of aortic valve interstitial cells. Arterioscler Thromb Vasc Biol. 2011;31:590–597. [DOI] [PubMed] [Google Scholar]
- 91. Carthy JM, Boroomand S, McManus BM. Versican and CD44 in in vitro valvular interstitial cell injury and repair. Cardiovasc Pathol. 2012;21:74–82. [DOI] [PubMed] [Google Scholar]
- 92. Yanagawa B, Lovren F, Pan Y, Garg V, Quan A, Tang G, Singh KK, Shukla PC, Kalra NP, Peterson MD, Verma S. miRNA‐141 is a novel regulator of BMP‐2‐mediated calcification in aortic stenosis. J Thorac Cardiovasc Surg. 2012;144:256–262. [DOI] [PubMed] [Google Scholar]
- 93. Xu S, Gotlieb AI. Wnt3a/β‐catenin increases proliferation in heart valve interstitial cells. Cardiovasc Pathol. 2013;22:156–166. [DOI] [PubMed] [Google Scholar]
- 94. Song R, Zeng Q, Ao L, Yu JA, Cleveland JC, Zhao KS, Fullerton DA, Meng X. Biglycan induces the expression of osteogenic factors in human aortic valve interstitial cells via toll‐like receptor‐2. Arterioscler Thromb Vasc Biol. 2012;32:2711–2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Zeng Q, Jin C, Ao L, Cleveland JC Jr, Song R, Xu D, Fullerton DA, Meng X. Cross‐talk between the toll‐like receptor 4 and Notch1 pathways augments the inflammatory response in the interstitial cells of stenotic human aortic valves. Circulation. 2012;126:S222–S230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Nadlonek NA, Weyant MJ, Yu JA, Cleveland JC Jr, Reece TB, Meng X, Fullerton DA. Radiation induces osteogenesis in human aortic valve interstitial cells. J Thorac Cardiovasc Surg. 2012;144:1466–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Branchetti E, Sainger R, Poggio P, Grau JB, Patterson‐Fortin J, Bavaria JE, Chorny M, Lai E, Gorman RC, Levy RJ, Ferrari G. Antioxidant enzymes reduce DNA damage and early activation of valvular interstitial cells in aortic valve sclerosis. Arterioscler Thromb Vasc Biol. 2013;33:e66–e74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Zeng Q, Song R, Ao L, Weyant MJ, Lee J, Xu D, Fullerton DA, Meng X. Notch1 promotes the pro‐osteogenic response of human aortic valve interstitial cells via modulation of ERK1/2 and nuclear factor‐κB activation. Arterioscler Thromb Vasc Biol. 2013;33:1580–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Galeone A, Brunetti G, Oranger A, Greco G, Di Benedetto A, Mori G, Colucci S, Zallone A, Paparella D, Grano M. Aortic valvular interstitial cells apoptosis and calcification are mediated by TNF‐related apoptosis‐inducing ligand. Int J Cardiol. 2013;169:296–304.24148916 [Google Scholar]
- 100. Zhang XW, Zhang BY, Wang SW, Gong DJ, Han L, Xu ZY, Liu XH. Twist‐related protein 1 negatively regulated osteoblastic transdifferentiation of human aortic valve interstitial cells by directly inhibiting runt‐related transcription factor 2. J Thorac Cardiovasc Surg. 2014;148:1700–1708.e1701. [DOI] [PubMed] [Google Scholar]
- 101. Carrion K, Dyo J, Patel V, Sasik R, Mohamed SA, Hardiman G, Nigam V. The long non‐coding HOTAIR is modulated by cyclic stretch and WNT/β‐CATENIN in human aortic valve cells and is a novel repressor of calcification genes. PLoS One. 2014;9:e96577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Zeng Q, Song R, Ao L, Xu D, Venardos N, Fullerton DA, Meng X. Augmented osteogenic responses in human aortic valve cells exposed to oxLDL and TLR4 agonist: a mechanistic role of Notch1 and NF‐κB interaction. PLoS One. 2014;9:e95400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Witt W, Buttner P, Jannasch A, Matschke K, Waldow T. Reversal of myofibroblastic activation by polyunsaturated fatty acids in valvular interstitial cells from aortic valves. Role of RhoA/G‐actin/MRTF signalling. J Mol Cell Cardiol. 2014;74C:127–138. [DOI] [PubMed] [Google Scholar]
- 104. Song R, Ao L, Zhao KS, Zheng D, Venardos N, Fullerton DA, Meng X. Soluble biglycan induces the production of ICAM‐1 and MCP‐1 in human aortic valve interstitial cells through TLR2/4 and the ERK1/2 pathway. Inflamm Res. 2014;63:703–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Canalis E. Notch signaling in osteoblasts. Sci Signal. 2008;1:pe17. [DOI] [PubMed] [Google Scholar]
- 106. Gessert S, Kuhl M. The multiple phases and faces of Wnt signaling during cardiac differentiation and development. Circ Res. 2010;107:186–199. [DOI] [PubMed] [Google Scholar]
- 107. Hurlstone AF, Haramis AP, Wienholds E, Begthel H, Korving J, Van Eeden F, Cuppen E, Zivkovic D, Plasterk RH, Clevers H. The Wnt/β‐catenin pathway regulates cardiac valve formation. Nature. 2003;425:633–637. [DOI] [PubMed] [Google Scholar]
- 108. Ozhan G, Weidinger G. Wnt/β‐catenin signaling in heart regeneration. Cell Regen (Lond). 2015;4:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Clevers H, Nusse R. Wnt/β‐catenin signaling and disease. Cell. 2012;149:1192–1205. [DOI] [PubMed] [Google Scholar]
- 110. Yavropoulou MP, Yovos JG. The role of the Wnt signaling pathway in osteoblast commitment and differentiation. Hormones (Athens). 2007;6:279–294. [DOI] [PubMed] [Google Scholar]
- 111. Rajamannan NM, Subramaniam M, Caira F, Stock SR, Spelsberg TC. Atorvastatin inhibits hypercholesterolemia‐induced calcification in the aortic valves via the Lrp5 receptor pathway. Circulation. 2005;112:I229–I234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Stewart BF, Siscovick D, Lind BK, Gardin JM, Gottdiener JS, Smith VE, Kitzman DW, Otto CM. Clinical factors associated with calcific aortic valve disease. Cardiovascular health study. J Am Coll Cardiol. 1997;29:630–634. [DOI] [PubMed] [Google Scholar]
- 113. Butcher JT, Mahler GJ, Hockaday LA. Aortic valve disease and treatment: the need for naturally engineered solutions. Adv Drug Deliv Rev. 2011;63:242–268. [DOI] [PubMed] [Google Scholar]
- 114. Andersson ER, Sandberg R, Lendahl U. Notch signaling: simplicity in design, versatility in function. Development. 2011;138:3593–3612. [DOI] [PubMed] [Google Scholar]
- 115. Garg V, Muth AN, Ransom JF, Schluterman MK, Barnes R, King IN, Grossfeld PD, Srivastava D. Mutations in Notch1 cause aortic valve disease. Nature. 2005;437:270–274. [DOI] [PubMed] [Google Scholar]
- 116. Deregowski V, Gazzerro E, Priest L, Rydziel S, Canalis E. Notch 1 overexpression inhibits osteoblastogenesis by suppressing Wnt/β‐catenin but not bone morphogenetic protein signaling. J Biol Chem. 2006;281:6203–6210. [DOI] [PubMed] [Google Scholar]
- 117. Zhu F, Sweetwyne MT, Hankenson KD. PKCδ is required for Jagged‐1 induction of human mesenchymal stem cell osteogenic differentiation. Stem Cells. 2013;31:1181–1192. [DOI] [PubMed] [Google Scholar]
- 118. Chen J, Ryzhova LM, Sewell‐Loftin MK, Brown CB, Huppert SS, Baldwin HS, Merryman WD. Notch1 mutation leads to valvular calcification through enhanced myofibroblast mechanotransduction. Arterioscler Thromb Vasc Biol. 2015;35:1597–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Acharya A, Hans CP, Koenig SN, Nichols HA, Galindo CL, Garner HR, Merrill WH, Hinton RB, Garg V. Inhibitory role of Notch1 in calcific aortic valve disease. PLoS One. 2011;6:e27743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. El Accaoui RN, Gould ST, Hajj GP, Chu Y, Davis MK, Kraft DC, Lund DD, Brooks RM, Doshi H, Zimmerman KA, Kutschke W, Anseth KS, Heistad DD, Weiss RM. Aortic valve sclerosis in mice deficient in endothelial nitric oxide synthase. Am J Physiol Heart Circ Physiol. 2014;306:H1302–H1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Hjortnaes J, Shapero K, Goettsch C, Hutcheson JD, Keegan J, Kluin J, Mayer JE, Bischoff J, Aikawa E. Valvular interstitial cells suppress calcification of valvular endothelial cells. Atherosclerosis. 2015;242:251–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Wang Y, Wu B, Farrar E, Lui W, Lu P, Zhang D, Alfieri CM, Mao K, Chu M, Yang D, Xu D, Rauchman M, Taylor V, Conway SJ, Yutzey KE, Butcher JT, Zhou B. Notch‐TNF signalling is required for development and homeostasis of arterial valves. Eur Heart J. 2017;38:675–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Nadlonek NA, Lee JH, Weyant MJ, Meng X, Fullerton DA. Ox‐LDL induces PiT‐1 expression in human aortic valve interstitial cells. J Surg Res. 2013;184:6–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Li F, Zhao Z, Cai Z, Dong N, Liu Y. Oxidized low‐density lipoprotein promotes osteoblastic differentiation of valvular interstitial cells through RAGE/MAPK. Cardiology. 2015;130:55–61. [DOI] [PubMed] [Google Scholar]
- 125. Passmore M, Nataatmadja M, Fung YL, Pearse B, Gabriel S, Tesar P, Fraser JF. Osteopontin alters endothelial and valvular interstitial cell behaviour in calcific aortic valve stenosis through HMGB1 regulation. Eur J Cardiothorac Surg. 2015;48:e20–e29. [DOI] [PubMed] [Google Scholar]
- 126. Wang D, Zeng Q, Song R, Ao L, Fullerton DA, Meng X. Ligation of ICAM‐1 on human aortic valve interstitial cells induces the osteogenic response: a critical role of the Notch1‐NF‐κB pathway in BMP‐2 expression. Biochem Biophys Acta. 2014;1843:2744–2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Kreke MR, Sharp LA, Lee YW, Goldstein AS. Effect of intermittent shear stress on mechanotransductive signaling and osteoblastic differentiation of bone marrow stromal cells. Tissue Eng Part A. 2008;14:529–537. [DOI] [PubMed] [Google Scholar]
- 128. Wirrig EE, Gomez MV, Hinton RB, Yutzey KE. COX2 inhibition reduces aortic valve calcification in vivo. Arterioscler Thromb Vasc Biol. 2015;35:938–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Baek D, Villen J, Shin C, Camargo FD, Gygi SP, Bartel DP. The impact of microRNAs on protein output. Nature. 2008;455:64–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Nagy E, Back M. Epigenetic regulation of 5‐lipoxygenase in the phenotypic plasticity of valvular interstitial cells associated with aortic valve stenosis. FEBS Lett. 2012;586:1325–1329. [DOI] [PubMed] [Google Scholar]
- 131. Euler‐Taimor G, Heger J. The complex pattern of SMAD signaling in the cardiovascular system. Cardiovasc Res. 2006;69:15–25. [DOI] [PubMed] [Google Scholar]
- 132. Li X, Lim J, Lu J, Pedego TM, Demer L, Tintut Y. Protective role of Smad6 in inflammation‐induced valvular cell calcification. J Cell Biochem. 2015;116:2354–2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Bialek P, Kern B, Yang X, Schrock M, Sosic D, Hong N, Wu H, Yu K, Ornitz DM, Olson EN, Justice MJ, Karsenty G. A twist code determines the onset of osteoblast differentiation. Dev Cell. 2004;6:423–435. [DOI] [PubMed] [Google Scholar]


