Abstract
Background
Radiation therapy (RT) is a standard treatment for head and neck cancer; however, it is associated with inflammation, accelerated atherosclerosis, and cerebrovascular events (CVEs; stroke or transient ischemic attack). Human papillomavirus (HPV) is found in nearly half of head and neck cancers and is associated with inflammation and atherosclerosis. Whether HPV confers an increased risk of CVEs after RT is unknown.
Methods and Results
Using an institutional database, we identified all consecutive patients treated with RT from 2002 to 2012 for head and neck cancer who were tested for HPV. The outcome of interest was the composite of ischemic stroke and transient ischemic attack, and the association between HPV and CVEs was assessed using Cox proportional hazard models, competing risk analysis, and inverse probability weighting. Overall, 326 participants who underwent RT for head and neck cancer were tested for HPV (age 59±12 years, 75% were male, 9% had diabetes mellitus, 45% had hypertension, and 61% were smokers), of which 191 (59%) were tumor HPV positive. Traditional risk factors for CVEs were similar between HPV‐positive and ‐negative patients. Over a median follow‐up of 3.4 years, there were 18 ischemic strokes and 5 transient ischemic attacks (event rate of 1.8% per year). The annual event rate was higher in the HPV‐positive patients compared with the HPV‐negative patients (2.6% versus 0.9%, P=0.002). In a multivariable model, HPV‐positive status was associated with a >4 times increased risk of CVEs (hazard ratio: 4.4; 95% confidence interval, 1.5–13.2; P=0.008).
Conclusions
In this study, HPV‐positive status is associated with an increased risk of stroke or transient ischemic attack following RT for head and neck cancer.
Keywords: cancer and stroke, cerebrovascular disease/stroke, human papillomavirus, radiation therapy, transient ischemic attack, virus
Subject Categories: Cerebrovascular Disease/Stroke, Transient Ischemic Attack (TIA)
Clinical Perspective
What Is New?
Head and neck cancer patients treated with neck radiotherapy face a high risk of future cerebrovascular events, disproportionate to traditional risk factors, and human papillomavirus status appears to be associated with that risk.
What Are the Clinical Implications?
Determination of human papillomavirus tumor status may help guide clinicians in the assessment of stroke and transient ischemic attack risk following neck radiotherapy.
Introduction
Survival with head and neck cancer (HNCA) has significantly improved.1 Radiation therapy (RT) to the neck is a standard primary treatment for HNCA2; however, RT that involves the neck is associated with a 5‐ to 17‐fold increased risk of cerebrovascular events (CVEs; stroke or transient ischemic attack [TIA]).3, 4 The mechanisms for this increase in CVEs after RT that involves the neck are not completely understood but likely are related to inflammation and accelerated atherosclerosis in the carotid artery.4
The excess risk of CVEs after RT to the neck is only partially explained by traditional cardiovascular stroke risk factors5; therefore, research is needed to understand whether additional factors can explain the increased risk of CVEs after RT in this vulnerable population. Significant epidemiological and scientific data suggest that human papillomavirus (HPV) may be a significant risk factor for CVEs after RT: HPV infection is common and has been estimated at up to 11% to 12% worldwide, and oncogenic HPV has been recognized as a primary risk factor for the development of HNCA.5, 6, 7 Currently, well over one third of all HNCAs are linked to HPV, with oropharyngeal cancers testing positive in >70% of new cases alone.8 Preliminary data suggest that HPV may promote atherosclerosis and may be a risk factor for CVEs after RT. Specifically, inhibition of macrophage p53 and Rb (retinoblastoma protein) are linked to the oncogenicity of HPV and to inflammation and accelerated atherosclerosis.9, 10, 11, 12 Furthermore, data suggest that vaginal HPV status may be associated with an increased risk of cardiovascular events in women.13 Consequently, because HPV is increasingly recognized as a risk factor for HNCAs and HPV is linked to atherosclerosis and inflammation in the general population, we hypothesized that positive HPV tumor status in HNCA compared with negative HPV tumor status may be associated with an increased risk of CVEs among patients treated with neck RT. Specifically, we postulated that a robust clinical oncogenic HPV infection, as reflected by the development of HPV‐associated HNCA, is also likely associated with the disproportionately increased risk of CVEs after RT to the neck.
Methods
Study Patients
Using an institutional database, we retrospectively identified all patients with HNCA who underwent neck RT and who also had neck‐tumor–related HPV testing over an 11‐year period, from January 2002 to December 2012. Two teams of independent investigators performed all data collection, including cardiovascular and cancer‐specific variables, as well as event adjudication. Variables collected represented data available at the time of initiation of RT. We excluded patients who received neck RT before January 2002 because of inconsistent data availability. The study protocol was approved by our institutional human subjects research review committee, and the requirement for informed consent was waived.
HPV Identification
The decision to pursue testing was presumed related to the type of tumor and the data associating HPV with HNCA that evolved during the study period.2, 5, 14 HPV tumor status was determined by the chromogenic in situ hybridization technique during the initial study period. Specifically, chromogenic in situ hybridization probes (Ventana probes; Ventana Research) were used to test for the presence of 12 oncogenic HPV types (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, and 66).15 After 2009, when the chromogenic in situ hybridization high‐risk probes were no longer commercially available, polymerase chain reaction amplification of purified DNA using the SPF (short polymerase chain reaction fragment) primer system, which amplified a 65‐bp region of the conserved L1 viral capsid gene, became the primary method of testing for both low‐ and high‐risk HPV (Inno‐LiPA; Innogenetics).15, 16 In addition, during the same period, reflex testing for the presence of p16 was incorporated for cases of squamous cell origin because p16 protein expression is generally considered a surrogate for HPV infection (Genpoint; Dako).17 All tumor study results were verified by an experienced pathologist.
Outcomes
The primary outcome was the occurrence of a post‐RT CVE. This was specifically defined as a composite of ischemic stroke and TIA. We chose this outcome based on data showing an increase in the combination of stroke and TIA after neck RT.3, 4, 18 Death was also reported but was not the focus of this study.19 CVEs were adjudicated by investigators (blinded to HPV status and all other variables) using recommended standardized definitions for stroke and TIA from recent published consensus American Heart Association and American Stroke Association definitions of stroke.20 As such, ischemic stroke was defined as an episode of neurological dysfunction caused by focal cerebral, retinal, or spinal infarction. Available computed tomography and magnetic resonance neuroimaging reports were also reviewed for additional confirmation. TIA was defined as transient (<24 hours) stroke symptoms and the absence of objective cerebral, retinal, or spinal infarction, as seen with imaging or pathology.
Statistical Analysis
Continuous data are presented as mean±SD. Comparisons between groups (HPV‐positive versus ‐negative patients) and baseline characteristics were performed with the use of an independent sample t test for continuous variables, the Pearson χ2 test for categorical variables, and the Wilcoxon rank sum test for ordinal variables. There were no missing covariates. Annualized event rates as a function of tumor HPV status were calculated, and Kaplan–Meier event curves for CVEs were generated; time‐course comparisons were performed by log‐rank tests. We used Cox regression models to examine associations between the independent variables and the development of CVEs. Covariates included in the model were variables significant on univariate analysis as well as known predictors and confounders of CVEs.3, 20 Hazard ratios (HRs) for the association of HPV status with events were estimated using Cox proportional hazards. Time of follow‐up was calculated from the start of RT, and censoring criteria included death, first stroke or TIA, or last documented visit for those without events or death. For all analyses, a 2‐tailed P value of <0.05 was considered significant. In addition, we performed competing risk analyses using death as the competing risk, given the expected difference in survival based on HPV tumor status, to further assess the impact of HPV status on cerebrovascular outcomes. From these analyses, an HR for CVE risk was obtained, adjusting for age, male sex, race, baseline lipids, blood pressure, history of diabetes mellitus, smoking, and use of antihypertensive medications. Those participants with HPV testing performed were more likely to be nonsmokers, to be male, to have oropharyngeal cancer, and to have received platinum‐based or taxol chemotherapy. Inverse probability weighting was used to account for the nonrandomness of missing HPV genotype status. Statistical tests were performed using STATA version 14.1 (StataCorp).
Results
The final cohort included 326 patients treated with neck RT who were tested for HPV (Figure 1). The mean age of the entire cohort at the time of RT was 59±12 years (range: 20–83 years), and 75% were male. The characteristics of the study population by HPV status are summarized in Table 1. Overall, 191 (59%) were tumor HPV positive. In addition, 89% of all included participants were treated with chemotherapy plus RT, 53% were comanaged with surgery, and 3% were treated with RT alone. Patients with HPV infection were more likely to be male and to have oropharyngeal cancer and less likely to have laryngeal carcinoma as their cancer type. There was no difference in the prevalence of baseline coronary or cerebrovascular disease or of traditional cardiovascular risk factors among groups with and without HPV.
Figure 1.
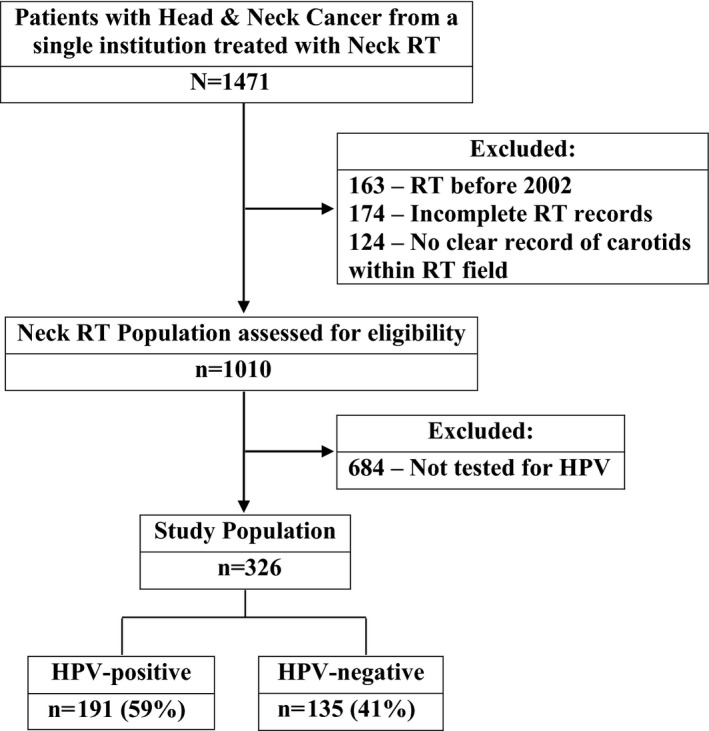
Study flow diagram. HPV indicates human papillomavirus; RT, radiation therapy.
Table 1.
Baseline Characteristics by HPV Status
| Variable | Overall Cohort | HPV Positive | HPV Negative | P Value |
|---|---|---|---|---|
| (n=326) | (n=191) | (n=135) | ||
| Demographics | ||||
| Age, y | 58.8±11.9 | 59.3±10.6 | 58.1±13.5 | 0.36 |
| Male | 245 (74.9) | 152 (79.2) | 93 (68.4) | 0.021 |
| BMI, kg/m2 | 27.6±5.4 | 28.0±5.3 | 27.0±5.4 | 0.12 |
| Traditional CVD risk factors | ||||
| Hypertension | 146 (44.6) | 92 (48.2) | 55 (40.4) | 0.17 |
| Diabetes mellitus | 30 (9.2) | 20 (10.5) | 11 (8.1) | 0.47 |
| Dyslipidemia | 102 (31.2) | 65 (34.0) | 37 (27.2) | 0.23 |
| Mean LDLa | 105.6±35.5 | 105.4±37.3 | 105.8±34.1 | 0.92 |
| Smoking historyb | 199 (60.9) | 118 (61.8) | 81 (60.0) | 0.73 |
| Current smoking | 69 (21.1) | 36 (18.8) | 33 (24.3) | 0.24 |
| Carotid artery disease | 7 (2.1) | 5 (2.6) | 2 (1.5) | 0.48 |
| Coronary artery disease | 26 (8.0) | 17 (8.9) | 10 (7.4) | 0.62 |
| Peripheral arterial disease | 12 (3.7) | 5 (2.6) | 7 (5.1) | 0.25 |
| Prior CVE | 19 (5.8) | 12 (6.3) | 7 (5.1) | 0.67 |
| Prior CHF | 10 (3.1) | 6 (3.1) | 4 (2.9) | 0.92 |
| Chronic kidney disease | 9 (2.8) | 5 (2.6) | 4 (2.9) | 1.0 |
| Atrial fibrillation | 11 (3.4) | 8 (4.2) | 3 (2.2) | 0.33 |
| Mean ASCVD 10‐y risk, % | 9.3±10.5 | 9.0±10.1 | 9.7±11.1 | 0.58 |
| Radiation characteristics | ||||
| Mean radiation dose, mSv | 70.2±19.9 | 71.6±16.4 | 68.3±23.8 | 0.14 |
| Proton RT | 13 (4.0) | 6 (3.1) | 7 (5.1) | 0.40 |
| Cancer treatmentsc | ||||
| Chemotherapy | 288 (89.4) | 172 (90.1) | 121 (89.0) | 0.85 |
| Neck dissection | 132 (40.4) | 82 (42.9) | 52 (38.2) | 0.39 |
| RT alone | 10 (3.1) | 5 (2.6) | 5 (3.7) | 0.75 |
| Type of HNCA | ||||
| Nasopharyngeal | 15 (4.6) | 9 (4.7) | 6 (4.4) | 0.9 |
| Oropharyngeal | 235 (71.9) | 152 (79.6) | 83 (61.0) | 0.001 |
| Hypopharyngeal | 18 (5.5) | 8 (4.2) | 10 (7.4) | 0.23 |
| Laryngeal | 17 (5.2) | 6 (3.1) | 11 (8.1) | 0.047 |
| Other | 58 (17.7) | 28 (14.7) | 30 (22.1) | 0.11 |
| Advanced disease at presentationd | 60 (18.3) | 33 (17.3) | 27 (19.9) | 0.57 |
| Medications | ||||
| Antihypertensive | 153 (46.9) | 91 (47.6) | 62 (45.9) | 0.82 |
| Antiplatelet | 75 (23.0) | 43 (22.5) | 32 (23.7) | 0.89 |
| Lipid lowering | 103 (31.6) | 67 (35.1) | 36 (26.7) | 0.12 |
Values are mean (%) and continuous variables are mean ±SD. Antihypertensive medications include aldosterone antagonists, angiotensin‐converting enzyme inhibitors, angiotensin receptor blockers, beta blockers, calcium channel blockers, diuretics, and long‐acting nitrates. Antiplatelet medications include aspirin and clopidogrel. Lipid‐lowering medications are ezetimibe, niacin, and statins. ASCVD, atherosclerotic cardiovascular disease; BMI, body mass index; CHF, congestive heart failure; CVD, cerebrovascular disease; CVE, cerebrovascular event; HNCA, head and neck cancer; HPV, human papillomavirus; LDL, low‐density lipoprotein; RT, radiation therapy.
Mean LDL is the most recent LDL available before RT.
Smoking history indicates current or prior smoking.
Percentage of full cohort treated.
Advanced disease is stage IV.
HPV Status and Cerebrovascular Outcomes
From the final cohort, there were 23 CVEs over median follow‐up of 3.4 years (range: 1–14 years) yielding a total event rate of 7.0% and an annual event of 1.8% per year. There were 18 CVEs (15 strokes and 3 TIAs) in HPV‐positive patients compared with 5 events in the HPV‐negative patients (3 strokes and 2 TIAs). The annual rate for CVEs was 2.6% per year in HPV‐positive persons compared with 0.9% per year in the HPV‐negative persons (unadjusted P=0.01). Median time to event among HPV‐positive participants was 3.4 years compared with 4.2 years in those who were HPV negative (P=0.876). In a Kaplan–Meier analysis stratified by tumor HPV status, HPV positivity was associated with events that began ≈2 years after RT and persisted throughout the duration of follow‐up (Figure 2). A representative set of images from an HPV‐positive participant that had a TIA after RT is shown in Figure 3.
Figure 2.

Cerebrovascular event‐free survival by human papillomavirus (HPV) status.
Figure 3.
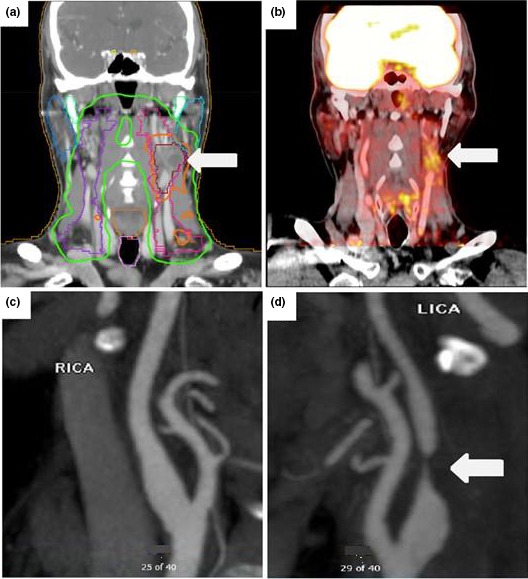
A series of images from a patient positive for human papillomavirus (HPV) who had radiation therapy to the left side of the neck for hypopharyngeal cancer and developed neurological complications. A, Radiation dose map computed tomography images showing dose exposure fields with an arrow pointing to the highest exposure area, which overlaps the left carotid artery. B, Surveillance positron emission tomography images taken 3.5 years after radiation therapy demonstrate increased inflammation (white arrow) around the left carotid. Subsequently, the patient reported several episodes of transient vision loss in the left eye and was diagnosed with a transient ischemic attack; computed tomography angiogram images demonstrate a normal right carotid and critical atherosclerotic stenosis in the proximal left carotid (C and D, arrows) at the same site as the area of exposure. LICA indicates left internal carotid artery; RICA, right internal carotid artery.
We observed no significant difference in baseline covariates between those with and without events other than HPV status (19 [83%] versus 4 [1.3%], P<0.001) and history of a CVE (15 [4.9%] versus 4 [17.4%], P=0.014; Table 2). In a multivariable model containing HPV status and prior CVEs, tumor HPV status was associated with a >4‐fold increased risk of a subsequent CVE after neck RT (adjusted HR: 4.58 [95% confidence interval (CI), 1.54–13.7]; P=0.006; Table 3). The addition of known predictors or confounders of CVEs did not change the association between HPV status and events. For example, a model containing both hypertension and history of CVE before the model showed an unchanged association (adjusted HR: 4.41 [95% CI, 1.50–13.1]; P=0.008). Over the duration of follow‐up (median 3.4 years), there were 76 deaths (27%). HPV‐positive status was associated with reduced all‐cause mortality (36 [22%] versus 41 [36%], P=0.02; adjusted HR: 0.68 [95% CI, 0.46–0.99]; P=0.04). Accounting for the competing risk of death, HPV status remained positively associated with subsequent CVEs (adjusted HR: 10.3 [95% CI, 1.61–65.5]; P=0.01). Testing for HPV was performed in only one third of the entire population. Compared with participants who did not have HPV, those with HPV testing were more likely to be nonsmokers, to be male, to have oropharyngeal cancer, and to have received platinum‐based or taxol chemotherapy. When inverse probability weighting was used to account for differential rates of missing HPV genotype status, no study findings differed (HPV status remained a significant predictor of CVEs in both unadjusted models (HR: 7.04 [95% CI, 2.0–24.4]; P=0.002) and multivariate models (HR: 8.57 [95% CI, 2.5–29.4]; P<0.001).
Table 2.
Baseline Characteristics by Cerebrovascular Event Development
| Variable | Overall Cohort | No Event | Stroke/TIA | P Value |
|---|---|---|---|---|
| (n=326) | (n=303) | (n=23) | ||
| Demographics | ||||
| Age, y | 58.8±11.9 | 58.5±11.6 | 62.4±12.0 | 0.13 |
| Male | 245 (74.9) | 227 (74.7) | 18 (78.3) | 0.70 |
| BMI, kg/m2 | 27.6±5.4 | 27.6±5.4 | 27.9±5.3 | 0.74 |
| Traditional CVD risk factors | ||||
| Hypertension | 146 (44.6) | 133 (43.8) | 14 (60.9) | 0.11 |
| Diabetes mellitus | 30 (9.2) | 29 (9.5) | 2 (8.7) | 0.89 |
| Dyslipidemia | 102 (31.2) | 96 (31.6) | 6 (26.1) | 0.65 |
| Mean LDLa | 105.6±35.5 | 104.7±35.7 | 115.5±31.4 | 0.16 |
| Smoking historyb | 199 (60.9) | 118 (61.8) | 81 (60.0) | 0.73 |
| Current smoking | 69 (21.1) | 63 (20.7) | 6 (26.1) | 0.54 |
| Carotid artery disease | 7 (2.1) | 6 (2.0) | 1 (4.3) | 0.45 |
| Coronary artery disease | 26 (8.0) | 25 (8.2) | 2 (8.7) | 0.94 |
| Peripheral arterial disease | 12 (3.7) | 11 (3.6) | 1 (4.3) | 0.59 |
| Prior CVE | 19 (5.8) | 15 (4.9) | 4 (17.4) | 0.014 |
| Prior CHF | 10 (3.1) | 9 (3.0) | 1 (4.3) | 0.71 |
| Chronic kidney disease | 9 (2.8) | 7 (2.3) | 2 (8.7) | 0.13 |
| Atrial fibrillation | 11 (3.4) | 9 (3.0) | 2 (8.7) | 0.14 |
| Mean ASCVD 10‐y risk, % | 9.3±10.5 | 9.1±10.4 | 12.3±11.9 | 0.24 |
| Radiation characteristics | ||||
| Mean radiation dose, mSv | 70.2±19.9 | 69.9±19.9 | 74.9±19.4 | 0.25 |
| Proton RT | 13 (4.0) | 13 (4.3) | 0 (0) | 0.61 |
| Cancer treatmentsc | ||||
| Chemotherapy | 288 (89.4) | 273 (89.8) | 20 (87.0) | 0.72 |
| Neck dissection | 132 (40.4) | 128 (42.1) | 6 (26.1) | 0.13 |
| RT alone | 10 (3.1) | 8 (2.6) | 2 (8.7) | 0.15 |
| Type of HNCA | ||||
| Nasopharyngeal | 15 (4.6) | 13 (4.3) | 2 (8.7) | 0.33 |
| Oropharyngeal | 235 (71.9) | 218 (71.7) | 17 (73.9) | 0.82 |
| Hypopharyngeal | 18 (5.5) | 18 (5.9) | 0 (0) | 0.63 |
| Laryngeal | 17 (5.2) | 15 (4.9) | 2 (8.7) | 0.43 |
| Other | 58 (17.7) | 55 (18.1) | 3 (13.0) | 0.78 |
| Advanced disease at presentationd | 60 (18.3) | 58 (19.1) | 2 (8.7) | 0.27 |
| Medications | ||||
| Antihypertensive | 153 (46.9) | 138 (45.5) | 15 (65.2) | 0.08 |
| Antiplatelet | 75 (23.0) | 69 (22.8) | 6 (26.1) | 0.80 |
| Lipid lowering | 103 (31.6) | 99 (32.7) | 4 (17.4) | 0.16 |
Values are mean (%) and continuous variables are mean ±SD. Antihypertensive medications include aldosterone antagonists, angiotensin‐converting enzyme inhibitors, angiotensin receptor blockers, beta blockers, calcium channel blockers, diuretics, and long‐acting nitrates. Antiplatelet medications include aspirin and clopidogrel. Lipid‐lowering medications are ezetimibe, niacin, and statins. ASCVD indicates atherosclerotic cardiovascular disease; BMI, body mass index; CHF, congestive heart failure; CVD, cerebrovascular disease; CVE, cerebrovascular event; HNCA, head and neck cancer; HPV, human papillomavirus; LDL, low‐density lipoprotein; RT, radiation therapy; TIA, transient ischemic attack.
Mean LDL is the most recent LDL available before RT.
Smoking history indicates current or prior smoking.
Percentage of full cohort treated.
Advanced disease is stage IV.
Table 3.
Cox Regression Analysis for Post–Radiation Therapy CVEs
| Characteristic | HR (95% CI) | P Value |
|---|---|---|
| HPV status | 4.82 (1.6–14.3) | 0.005 |
| HPV status, age, male sex | 4.37 (1.5–13.1) | 0.008 |
| HPV status, prior CVE | 4.58 (1.5–13.7) | 0.006 |
| HPV status, prior CVE, hypertension | 4.43 (1.5–13.2) | 0.008 |
| HPV status, prior CVE, age | 4.41 (1.5–13.1) | 0.008 |
| HPV status, prior CVE, age, male sex | 4.33 (1.4–13.1) | 0.009 |
| HPV status, prior CVE, age, male sex, neck dissection, radiation dose, oropharyngeal cancer, laryngeal cancer | 5.36 (1.7–16.7) | 0.004 |
CI indicates confidence interval; CVE, cerebrovascular event; HPV, human papillomavirus; HR, hazard ratio.
Discussion
This study provides the first clinical evidence that tumor HPV infection in HNCA patients is associated with a higher risk of ischemic stroke and TIA following RT. Overall, HPV‐positive tumor status conferred a >4‐fold increased risk of CVEs following RT that involved the neck. This result remained, even after accounting for more traditional CVE risk factors. Separation in event curves was apparent within 3.5 years of post‐RT follow‐up. These observations are of clinical importance because of the increasing prevalence of HPV‐associated HNCA and survival of patients as well as the limited number of available tools for ischemic stroke and TIA risk stratification among patients treated with RT. To our knowledge, this report is the first linking HPV status with the risk of stroke and TIA following RT for HNCA.
The predictors of CVEs among RT‐treated participants are incompletely understood. Data from the general population suggest that traditional established risk factors for CVEs, such as hypertension, diabetes mellitus, dyslipidemia, active smoking, and atrial fibrillation, account for nearly 90% of all stroke events.21 In contrast, in an analysis of >50 000 RT‐treated HNCA patients, traditional risk factors (hypertension and diabetes mellitus) were observed in only 30% of stroke events.22 Consequently, there are likely other risk factors that may at least partially explain the >500% increased risk of stroke events faced by HNCA patients treated with RT.3, 4 In this study, we found that HPV status was predictive of stroke and TIA following RT, even after adjustment for traditional risk factors and RT dose. This is important, particularly because HPV is prevalent among those who go on to develop HNCAs and is a potentially modifiable risk factor.23
Although there are no available prior studies investigating the relationship between HPV status and CVEs in RT‐treated HNCA patients, multiple investigators have reported parallel findings in other populations that are supportive of the current results.12, 24, 25 Data from the National Health Insurance Research Database of Taiwan showed a >50% increase in the risk of ischemic stroke for women treated with RT involving the neck for cervical cancer.24 In that study, the investigators postulated that the elevated stroke risk was primarily related to systemic injury caused by RT. In addition, analysis from the US National Health and Nutrition Examination Survey data set revealed a >2‐fold increased risk of cardiovascular disease, including stroke, among young women with vaginal HPV infection without known cancer.13 Furthermore, recent pathologic data from Lawson et al demonstrated a >50% prevalence of oncogenic HPV infection in the atheromatous coronary plaques of donors deceased from acute myocardial infarction.25 Taken together, these studies provide support for the underlying association of HPV with the accelerated development of atherosclerosis leading stroke and TIA following RT, as noted in this study.
Several potential mechanisms may explain these findings. Consistent animal data suggest that the HPV‐inhibited macrophage p53 and Rb tumor suppressor genes play important roles in the development of atherosclerosis.12, 26 Specifically, p53 inhibition has been shown to accelerate atherosclerotic growth through extracellular proliferation, reduction of apoptotic cellular activity, and direct increases in atherosclerotic lesion area.12, 26, 27 The inhibition of Rb has also been linked to increases in atherosclerotic lesion size as well as smooth muscle cell proliferation.27 Furthermore, inflammation has been postulated as a common pathway of viral‐mediated injury and atherosclerosis.25, 28 Caspases, which are protein enzymes that are vital to the process of vascular inflammation and cellular apoptosis, have been linked with the development of atherosclerosis, and recent reports have suggested that HPV E6 oncoproteins may promote nuclear localization of active caspase 8, leading to increased inflammation and probable atherosclerosis.29, 30 Furthermore, it may be reasonable to postulate that the downstream vascular effects of RT‐induced endothelial damage and medial fibrosis may be enhanced by oncogenic HPV‐induced cellular proliferation, allowing for stimulation of the proliferation of damaged vascular cells and leading to accelerated atherosclerosis4, 31; however, further studies are needed.
Our study has limitations. Data on tumor cell type were only inconsistently reported and thus was not included in this analysis. HPV testing was performed at the discretion of the treating medical oncologist; therefore, only a subset of the entire cohort had HPV status available. Nevertheless, when inverse probability weighting was used to account for these missing HPV genotypic data, all study findings remained significant. As with other studies examining the prevalence of HPV‐associated HNCAs, oropharyngeal carcinoma accounted for a majority of the patients included and thus represents a large proportion of the cohort studied.5, 7, 10 Given the retrospective nature of the study, available follow‐up within the cohort was nonuniform, which may have limited the ability to capture additional events. In addition, although observed events were adjudicated by the study team, the specific etiology of observed stroke events was not consistently available across all participants, given reliance on the documentation of non–study members. Relative quantities of alcohol consumption were not consistently available across participants. Although the number of persons included in this analysis was small, the annual CVE rate was significant, consistent with previous neck RT reports.3, 4
In conclusion, HNCA patients treated with neck RT face a significant downstream risk of CVEs. The presence of HPV‐infection appears to be strongly associated with that risk. Determination of HPV tumor status may help guide clinicians in the assessment of stroke and TIA risk following neck RT. Future studies of HNCA patients treated with RT are needed to evaluate potential stroke prevention strategies based on HPV status.
Sources of Funding
Dr Seidelmann is supported by NIH grant number 2T32HL094301‐06 and Dr Addison is supported by NIH/NHLBI 5T32HL076136. Dr Neilan is supported by a kind gift from the Kohlberg Foundation; an American Heart Association Fellow to Faculty Award (12FTF12060588); the National Institutes of Health/National Heart, Lung, and Blood Institute (1R01HL130539‐01A1; 1R01HL137562‐01A1); and the National Institutes of Health/Harvard Center for AIDS Research (P30 AI060354).
Disclosures
None.
(J Am Heart Assoc. 2017;6:e006453 DOI: 10.1161/JAHA.117.006453.)28855164
References
- 1. Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010–2020. J Natl Cancer Inst. 2011;103:117–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Haddad RI, Shin DM. Recent advances in head and neck cancer. N Engl J Med. 2008;359:1143–1154. [DOI] [PubMed] [Google Scholar]
- 3. Dorresteijn LD, Kappelle AC, Boogerd W, Klokman WJ, Balm AJ, Keus RB, van Leeuwen FE, Bartelink H. Increased risk of ischemic stroke after radiotherapy on the neck in patients younger than 60 years. J Clin Oncol. 2002;20:282–288. [DOI] [PubMed] [Google Scholar]
- 4. Plummer C, Henderson RD, O'Sullivan JD, Read SJ. Ischemic stroke and transient ischemic attack after head and neck radiotherapy: a review. Stroke. 2011;42:2410–2418. [DOI] [PubMed] [Google Scholar]
- 5. Mork J, Lie AK, Glattre E, Hallmans G, Jellum E, Koskela P, Møller B, Pukkala E, Schiller JT, Youngman L, Lehtinen M, Dillner J. Human papillomavirus infection as a risk factor for squamous‐cell carcinoma of the head and neck. N Engl J Med. 2001;344:1125–1131. [DOI] [PubMed] [Google Scholar]
- 6. Chaturvedi AK, Engels EA, Pfeiffer RM, Hernandez BY, Xiao W, Kim E, Jiang B, Goodman MT, Sibug‐Saber M, Cozen W, Liu L, Lynch CF, Wentzensen N, Jordan RC, Altekruse S, Anderson WF, Rosenberg PS, Gillison ML. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29:4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Forman D, de Martel C, Lacey CJ, Soerjomataram I, Lortet‐Tieulent J, Bruni L, Vignat J, Ferlay J, Bray F, Plummer M, Franceschi S. Global burden of human papillomavirus and related diseases. Vaccine. 2012;30(suppl 5):F12–F23. [DOI] [PubMed] [Google Scholar]
- 8. O'Sullivan B, Huang SH, Su J, Garden AS, Sturgis EM, Dahlstrom K, Lee N, Riaz N, Pei X, Koyfman SA, Adelstein D, Burkey BB, Friborg J, Kristensen CA, Gothelf AB, Hoebers F, Kremer B, Speel EJ, Bowles DW, Raben D, Karam SD, Yu E, Xu W. Development and validation of a staging system for HPV‐related oropharyngeal cancer by the International Collaboration on Oropharyngeal cancer Network for Staging (ICON‐S): a multicentre cohort study. Lancet Oncol. 2016;17:440–451. [DOI] [PubMed] [Google Scholar]
- 9. Werness BA, Levine AJ, Howley PM. Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science. 1990;248:76–79. [DOI] [PubMed] [Google Scholar]
- 10. Ndiaye C, Mena M, Alemany L, Arbyn M, Castellsagué X, Laporte L, Bosch FX, de Sanjosé S, Trottier H. HPV DNA, E6/E7 mRNA, and p16INK4a detection in head and neck cancers: a systematic review and meta‐analysis. Lancet Oncol. 2014;15:1319–1331. [DOI] [PubMed] [Google Scholar]
- 11. Gallo R, Padurean A, Jayaraman T, Marx S, Roque M, Adelman S, Chesebro J, Fallon J, Fuster V, Marks A, Badimon JJ. Inhibition of intimal thickening after balloon angioplasty in porcine coronary arteries by targeting regulators of the cell cycle. Circulation. 1999;99:2164–2170. [DOI] [PubMed] [Google Scholar]
- 12. Van Vlijmen BJ, Gerritsen G, Franken AL, Boesten LS, Kockx MM, Gijbels MJ, Vierboom MP, van Eck M, van De Water B, van Berkel TJ, Havekes LM. Macrophage p53 deficiency leads to enhanced atherosclerosis in APOE*3‐Leiden transgenic mice. Circ Res. 2001;88:780–786. [DOI] [PubMed] [Google Scholar]
- 13. Kuo HK, Fujise K. Human papilloma virus and cardiovascular disease among U.S. women in the National Health and Nutrition Examination Survey, 2003 to 2006. J Am Coll Cardiol. 2011;58:2001–2006. [DOI] [PubMed] [Google Scholar]
- 14. 2008 National Comprehensive Cancer Network Guidelines. Head and neck cancer guidelines. Available at: http://www.sld.cu/galerias/pdf/sitios/cirugiamaxilo/nccn-head-and-neck.pdf. Accessed November 29, 2016.
- 15. Lee WT, Tubbs RR, Teker AM, Scharpf J, Strome M, Wood B, Lorenz RR, Hunt J. Use of in situ hybridization to detect human papillomavirus in head and neck squamous cell carcinoma patients without a history of alcohol or tobacco use. Arch Pathol Lab Med. 2008;132:1653–1656. [DOI] [PubMed] [Google Scholar]
- 16. Seme K, Lepej SZ, Lunar MM, Iscić‐Bes J, Planinić A, Kocjan BJ, Vince A, Poljak M. Digene HPV Genotyping RH Test RUO: comparative evaluation with INNO‐LiPA HPV Genotyping Extra Test for detection of 18 high‐risk and probable high‐risk human papillomavirus genotypes. J Clin Virol. 2009;46:176–179. [DOI] [PubMed] [Google Scholar]
- 17. Schlecht NF, Brandwein‐Gensler M, Nuovo GJ, Li M, Dunne A, Kawachi N, Smith RV, Burk RD, Prystowsky MB. A comparison of clinically utilized human papillomavirus detection methods in head and neck cancer. Mod Pathol. 2011;24:1295–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Smith GL, Smith BD, Buchholz TA, Giordano SH, Garden AS, Woodward WA, Krumholz HM, Weber RS, Ang KK, Rosenthal DI. Cerebrovascular disease risk in older head and neck cancer patients after radiotherapy. J Clin Oncol. 2008;26:5119–5125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen‐Tân PF, Westra WH, Chung CH, Jordan RC, Lu C, Kim H, Axelrod R, Silverman CC, Redmond KP, Gillison ML. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sacco RL, Kasner SE, Broderick JP, Caplan LR, Connors JJ, Culebras A, Elkind MS, George MG, Hamdan AD, Higashida RT, Hoh BL, Janis LS, Kase CS, Kleindorfer DO, Lee JM, Moseley ME, Peterson ED, Turan TN, Valderrama AL, Vinters HV; American Heart Association Stroke Council, Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular and Stroke Nursing; Council on Epidemiology and Prevention; Council on Peripheral Vascular Disease; Council on Nutrition, Physical Activity and Metabolism . An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:2064–2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. O'Donnell MJ, Xavier D, Liu L, Zhang H, Chin SL, Rao‐Melacini P, Rangarajan S, Islam S, Pais P, McQueen MJ, Mondo C, Damasceno A, Lopez‐Jaramillo P, Hankey GJ, Dans AL, Yusoff K, Truelsen T, Diener HC, Sacco RL, Ryglewicz D, Czlonkowska A, Weimar C, Wang X, Yusuf S; INTERSTROKE investigators . Risk factors for ischemic and intracerebral hemorrhagic stroke in 22 countries (the INTERSTROKE): a case‐control study. Lancet. 2010;376:112–123. [DOI] [PubMed] [Google Scholar]
- 22. Chu CN, Chen SW, Bai LY, Mou CH, Hsu CY, Sung FC. Increase in stroke risk in patients with head and neck cancer: a retrospective cohort study. Br J Cancer. 2011;105:1419–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kim JJ, Goldie SJ. Health and economic implications of HPV vaccination in the United States. N Engl J Med. 2008;359:821–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tsai SJ, Huang YS, Tung CH, Lee CC, Lee MS, Chiou WY, Lin HY, Hsu FC, Tsai CH, Su YC, Hung SK. Increased risk of ischemic stroke in cervical cancer patients: a nationwide population‐based study. Radiat Oncol. 2013;8:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lawson JS, Glenn WK, Tran DD, Ngan CC, Duflou JA, Whitaker NJ. Identification of human papilloma viruses in atheromatous coronary artery disease. Front Cardiovasc Med. 2015;2:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Merched AJ, Williams E, Chan L. Macrophage‐specific p53 expression plays a crucial role in atherosclerosis development and plaque remodeling. Arterioscler Thromb Vasc Biol. 2003;23:1608–1614. [DOI] [PubMed] [Google Scholar]
- 27. Guevara NV, Kim HS, Antonova EI, Chan L. The absence of p53 accelerates atherosclerosis by increasing cell proliferation in vivo. Nat Med. 1999;5:335–339. [DOI] [PubMed] [Google Scholar]
- 28. Joshi FR, Rajani NK, Abt M, Woodward M, Bucerius J, Mani V, Tawakol A, Kallend D, Fayad ZA, Rudd JH. Does vascular calcification accelerate inflammation? A substudy of the dal‐PLAQUE Trial. J Am Coll Cardiol. 2016;67:69–78. [DOI] [PubMed] [Google Scholar]
- 29. Usui F, Shirasuna K, Kimura H, Tatsumi K, Kawashima A, Karasawa T, Hida S, Sagara J, Taniguchi S, Takahashi M. Critical role of caspase‐1 in vascular inflammation and development of atherosclerosis in western diet‐fed apolipoprotein E‐deficient mice. Biochem Biophys Res Commun. 2012;425:162–168. [DOI] [PubMed] [Google Scholar]
- 30. Manzo‐Merino J, Massimi P, Lizano M, Banks L. The human papillomavirus (HPV) E6 oncoproteins promotes nuclear localization of active caspase 8. Virology. 2014;45:146–152. [DOI] [PubMed] [Google Scholar]
- 31. Fonkalsrud EW, Sanchez M, Zerubavel R, Mahoney A. Serial changes in arterial structure following radiation therapy. Surg Gynecol Obstet. 1977;145:395–400. [PubMed] [Google Scholar]


