Abstract
Background
Current guidelines recommend early P2Y12 inhibitor administration in non‐ST‐elevation myocardial infarction, but it is unclear if precatheterization use is associated with longer delays to coronary artery bypass grafting (CABG) or higher risk of post‐CABG bleeding and transfusion. This study examines the patterns and outcomes of precatheterization P2Y12 inhibitor use in non‐ST‐elevation myocardial infarction patients who undergo CABG.
Methods and Results
Retrospective analysis was done of 20 304 non‐ST‐elevation myocardial infarction patients in the ACTION (Acute Coronary Treatment and Intervention Outcomes Network) Registry (2009–2014) who underwent catheterization within 24 hours of admission and CABG during the index hospitalization. Using inverse probability‐weighted propensity adjustment, we compared time from catheterization to CABG, post‐CABG bleeding, and transfusion rates between patients who did and did not receive precatheterization P2Y12 inhibitors. Among study patients, 32.9% received a precatheterization P2Y12 inhibitor (of these, 2.2% were given ticagrelor and 3.7% prasugrel). Time from catheterization to CABG was longer among patients who received precatheterization P2Y12 inhibitor (median 69.9 hours [25th, 75th percentiles 28.2, 115.8] versus 43.5 hours [21.0, 71.8], P<0.0001), longer for patients treated with prasugrel (median 114.4 hours [66.5, 155.5]) or ticagrelor (90.4 hours [48.7, 124.5]) compared with clopidogrel (69.3 [27.5, 114.6], P<0.0001). Precatheterization P2Y12 inhibitor use was associated with a higher risk of post‐CABG major bleeding (75.7% versus 73.4%, adjusted odds ratio 1.33, 95% confidence interval 1.22‐1.45, P<0.0001) and transfusion (47.6% versus 35.7%, adjusted odds ratio 1.51, 95% confidence interval 1.41‐1.62, P<0001); these relationships did not differ among patients treated with clopidogrel, prasugrel, or ticagrelor.
Conclusions
Precatheterization P2Y12 inhibitor use occurs commonly among non‐ST‐elevation myocardial infarction patients who undergo early catheterization and in‐hospital CABG. Despite longer delays to surgery, the majority of pretreated patients proceed to CABG <3 days postcatheterization. Precatheterization P2Y12 inhibitor use is associated with higher risks of postoperative bleeding and transfusion.
Keywords: coronary artery bypass graft surgery, Non‐ST‐segment elevation acute coronary syndrome, P2Y12
Subject Categories: Percutaneous Coronary Intervention
Clinical Perspective
What Is New?
The benefits of precatheterization P2Y12 inhibitors that were demonstrated in older studies may not be applicable in contemporary practice in which a large percentage of patients undergo early catheterization.
The use of P2Y12 inhibitors before catheterization in non‐ST‐elevation myocardial infarction patients may be associated with increased delays to surgery and increased postoperative bleeding and transfusion in patients who require coronary artery bypass grafting during the same hospitalization.
What Are the Clinical Implications?
When non‐ST‐elevation myocardial infarction patients present to hospitals where early catheterization is available, it may be reasonable to start P2Y12 inhibitors when the decision to perform percutaneous intervention is made.
Patients treated with P2Y12 inhibitors who undergo coronary artery bypass grafting before the recommended washout period may have higher rates of postoperative bleeding and transfusion.
Introduction
Platelet P2Y12 receptor inhibitors have been a cornerstone of treatment for patients with acute coronary syndrome; both clopidogrel and higher potency P2Y12 inhibitors (prasugrel and ticagrelor) were shown to significantly reduce the risk of major adverse cardiovascular events.1, 2, 3 Yet, the optimal timing of administration of P2Y12 inhibitors has been a moving target for patients with non‐ST‐elevation myocardial infarction (NSTEMI). Earlier American College of Cardiology/American Heart Association guidelines had provided a class 1 recommendation (level of evidence A) for P2Y12 inhibitors to be administered as soon as possible in patients presenting with NSTEMI.4 Nevertheless, data from this time period5 showed that only 57% of NSTEMI patients received P2Y12 inhibitors within the first 24 hours of admission. The benefits of precatheterization P2Y12 inhibitor use with faster‐onset, higher‐potency agents such as prasugrel and ticagrelor are less certain, with some studies suggesting lack of benefit and higher bleeding risk with early treatment.6 In 2014, the American College of Cardiology/American Heart Association guidelines changed their recommendation to “before stenting” instead of “as soon as possible.” This change reflected, in part, a trend toward much earlier coronary angiography in current practice7 as well as concerns for revascularization delays in patients who are subsequently discovered to need coronary artery bypass grafting (CABG).
Using contemporary data from the National Cardiovascular Data Registry, we examined patterns of precatheterization P2Y12 inhibitor use among NSTEMI patients undergoing early catheterization within 24 hours of admission, as these are the patients for whom we have most clinical equipoise between precatheterization P2Y12 inhibitor administration or deferral until when the coronary anatomy is known. Prior studies conducted when early catheterization was not routine showed a benefit to early P2Y12 inhibitor use, and thus, precatheterization P2Y12 inhibitors are routinely recommended for patients who anticipate a delay in catheterization.8 This study was designed to compare the duration from catheterization to CABG, postoperative bleeding, and transfusion use between patients who underwent in‐hospital CABG with and without precatheterization P2Y12 inhibitor use, and to further assess associated outcomes stratified by P2Y12 inhibitor type.
Methods
Data Source
The ACTION (Acute Coronary Treatment and Intervention Outcomes Network) Registry is a quality improvement initiative of the American College of Cardiology that collects detailed clinical, process‐of‐care, and outcomes data of patients admitted with acute myocardial infarction (MI) in the United States. Data abstraction is performed retrospectively via review of medical records by trained data collectors using standardized definitions. Hospital participation is voluntary and either requires approval from the local institutional review board or a waiver of approval if participation is deemed essential as a part of a hospital's quality improvement process. Because the data are anonymized and collected retrospectively, individual patient consent is not required.
Study Population
A total of 324 451 consecutive patients were admitted with NSTEMI at 540 hospitals in the ACTION Registry from July 2009 through December 2014. We focused on patients who underwent early cardiac catheterization within 24 hours of admission (Figure 1) and excluded patients in whom P2Y12 inhibitor therapy was contraindicated or blinded, patients who were transferred out to another hospital (United States privacy laws preclude tracking of outcomes beyond hospital discharge), patients with missing records of either time of catheterization, time of P2Y12 inhibitor administration, or time of CABG. We first described the rate of precatheterization P2Y12 inhibitor use in this overall NSTEMI study population. We then compared patient characteristics and outcomes among patients who underwent CABG during the index hospitalization. Patients who received P2Y12 inhibitors after catheterization were excluded because this small group may represent complex surgical decision making or treatment availability gaps.
Figure 1.
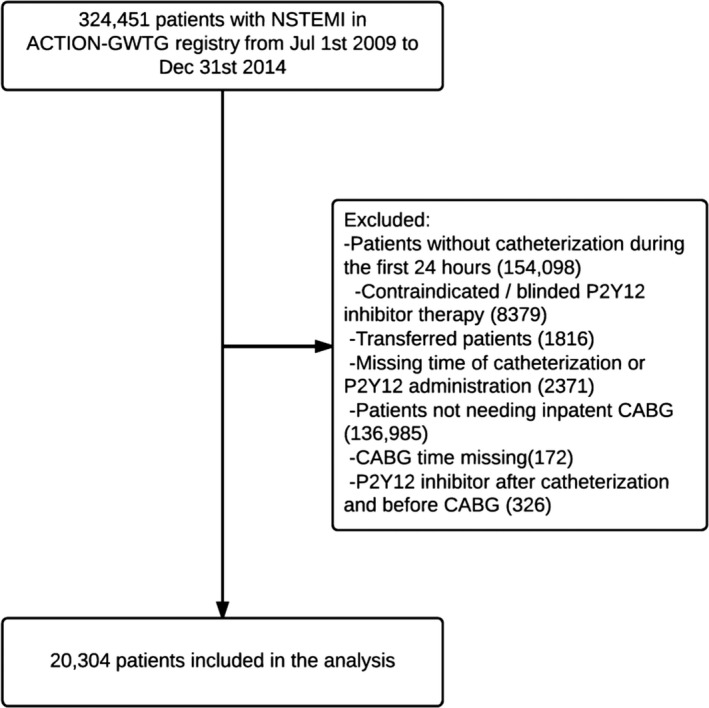
Study flow diagram showing sequential exclusions leading to the final analysis population. ACTION indicates Acute Coronary Treatment and Intervention Outcomes Network; CABG, coronary artery bypass grafting; GWTG, Get With The Guidelines; NSTEMI, non‐ST‐elevation myocardial infarction.
Definitions and Study End Points
Early catheterization was defined as coronary angiography within 24 hours after admission. Precatheterization P2Y12 inhibitor use was defined as the administration of any dose (loading dose or lower) of clopidogrel, prasugrel, ticagrelor, or ticlopidine at any time between admission and coronary angiography or the use of home P2Y12 inhibitors (prescribed medication that the patient takes routinely at home preadmission). Time to CABG was defined as the time from cardiac catheterization to CABG. Post‐CABG bleeding was defined as intracranial hemorrhage, witnessed bleeding occurring after CABG, or an absolute hemoglobin drop of ≥4 g/dL (baseline hemoglobin to nadir hemoglobin) where the nadir occurred after CABG. Post‐CABG transfusion was defined as transfusion after the date and time of CABG during the same hospitalization.
Statistical Analysis
Study patients were divided into 2 groups, those who received precatheterization P2Y12 inhibitors and those who did not. Baseline demographic, clinical, and procedural characteristics for each group are presented as counts and percentages for categorical variables and medians with 25th and 75th percentiles for continuous variables. Chi‐squared statistics were then used to compare categorical variables and Wilcoxon rank‐sum test to compare continuous variables between the 2 groups. To examine the association of precatheterization P2Y12 inhibitor use with the time from catheterization to CABG, an inverse probability‐weighted Cox proportional hazard regression model was used to adjust for potential demographic and clinical confounders. At a particular point in time postcatheterization, the model quantifies the chance that a patient who has not yet undergone CABG will undergo CABG in the next increment of time, and the risk ratio (RR) expresses the magnitude of this quantity between patients who did and did not receive pre‐catheterization P2Y12 inhibitors at that point in time. A value of RR <1 indicates a longer time from catheterization to CABG.
For binary outcomes (post‐CABG bleeding and transfusion), inverse probability‐weighted logistic regression models with generalized estimating equations were used to compare outcomes between patients with and without precatheterization P2Y12 inhibitor use, adjusting for potential demographic and clinical confounders. For the adjusted regression models, each patient receiving precatheterization P2Y12 inhibitors was inversely weighted in the analysis according to the estimated probability of receiving precatheterization P2Y12 inhibitors, and each patient who did not receive a precatheterization P2Y12 inhibitor was inversely weighted by the estimated probability of not receiving treatment. The probability of receiving precatheterization P2Y12 inhibitors was estimated from a logistic model that included the following covariates: year of presentation, demographic parameters (age, sex, race, insurance status), medical history (smoking status, hypertension, diabetes mellitus, dyslipidemia, dialysis, chronic lung disease, prior MI, prior percutaneous coronary intervention, prior heart failure, prior CABG, atrial fibrillation or flutter, prior stroke, peripheral arterial disease), presenting signs and symptoms (electrocardiographic findings, location first evaluated, body mass index, signs of heart failure, cardiogenic shock, cocaine use, cardiac arrest), home medications (aspirin, warfarin), left ventricular ejection fraction, time from presentation until arrival at the catheterization lab, hospital characteristics (region, teaching hospital, total bed number), initial lab tests (creatinine, hemoglobin, international normalized ratio, and troponin). A good balance of covariates was observed between groups in the inverse probability‐weighted population (Figures S1 and S2). Then, a sensitivity analysis compared outcomes between precatheterization P2Y12 inhibitor use versus nonuse after excluding patients who were on home P2Y12 inhibitors.
We then compared the rates of post‐CABG bleeding and transfusion between patients who underwent CABG within 5 days of the precatheterization exposure to P2Y12 inhibitors with those who underwent CABG later than 5 days. Additionally, within the group of patients who received precatheterization P2Y12 inhibitors, time from catheterization to CABG and post‐CABG bleeding and transfusion rates were compared among patients treated with clopidogrel, prasugrel, and ticagrelor. When patients received more than one P2Y12 inhibitor before catheterization, a mutually exclusive assignment model was implemented using the following hierarchy: prasugrel, ticagrelor, then clopidogrel. A multivariable Cox proportional hazard model adjusted for patients' ACTION bleeding score9 was used to assess the association between each P2Y12 inhibitor type and study outcomes, referenced to clopidogrel.
Finally, to determine the likelihood of CABG being deferred to a subsequent hospitalization for patients who had precatheterization P2Y12 inhibitor use, we examined a subset of Medicare‐insured patients with linked inpatient claims data from which we can ascertain postdischarge CABG. Of the 9277 linked Medicare patients who were admitted with NSTEMI between July 2009 and October 2012, 959 patients underwent CABG during the index hospitalization and were therefore excluded, leaving 8318 patients who were included in this secondary analysis population. Deferred CABG was defined as surgery performed after discharge from the index NSTEMI hospitalization and was identified by the following ICD‐9 procedure codes in subsequent inpatient claims: 3610, 3611, 3612, 3613, 3614, 3615, 3617, 3618, 3619, 362, 3630, 3633, 3634, 3635, 3636, 3637, 3638, 3639, 3691, and 3699. We determined the cumulative incidence rate of readmission with CABG within 60 days postdischarge from the index MI hospitalization.
Results
From the starting population of 324 451 consecutive NSTEMI patients, 170 353 patients (52.5%) underwent early cardiac catheterization within 24 hours of hospital admission (Figure 1). We excluded 8379 patients in whom P2Y12 inhibitor therapy was contraindicated or blinded, 1816 patients transferred out to another hospital, and 2371 patients were missing either time of catheterization or time of P2Y12 inhibitor administration. In this overall NSTEMI population, 59.9% received precatheterization P2Y12 inhibitor. We then focused our study population on the 20 802 patients who underwent in‐hospital CABG and further excluded 172 patients with missing information on the timing of CABG and 326 patients who received P2Y12 inhibitor after catheterization but before CABG.
Our final study population included 20 304 NSTEMI patients who underwent early cardiac catheterization and in‐hospital CABG at 485 hospitals across the United States. Among these, 32.9% had precatheterization P2Y12 inhibitor use. This rate decreased from 39.6% in 2009 to 29.9% in 2014. Patients who received precatheterization P2Y12 inhibitors were more likely younger and of nonwhite race compared with patients who did not receive pretreatment (Table 1). They were also more likely to have a history of prior MI, percutaneous coronary intervention, heart failure, and stroke and had a higher prevalence of hypertension, diabetes mellitus, dyslipidemia, and smoking history than patients who did not receive precatheterization P2Y12 inhibitors. At presentation, patients who received precatheterization P2Y12 inhibitors were more likely to have electrocardiographic changes, but there were no significant differences in initial cardiac biomarkers levels. Patients who received precatheterization P2Y12 inhibitor were more likely to be treated at teaching hospitals.
Table 1.
Baseline Characteristics: Baseline Demographic and Clinical Characteristics, Medications and Laboratory Tests, as Well as Hospital Characteristics of the 2 Study Groups
| No Precatheterization P2Y12 Inhibitor (n=13 624) | Received Precatheterization P2Y12 Inhibitor | P Value | ||||
|---|---|---|---|---|---|---|
| All (6680) | Clopidogrel (6288) | Prasugrel (245) | Ticagrelor (147) | |||
| Year of presentation | <0.0001 | |||||
| 2009 | 620 (4.6) | 407 (6.1) | 403 (6.4) | 4 (1.6) | 0 | |
| 2010 | 1522 (11.8) | 952 (14.3) | 936 (14.9) | 16 (6.5) | 0 | |
| 2011 | 2147 (15.8) | 1158 (17.3) | 1121 (17.8) | 37 (15.1) | 0 | |
| 2012 | 2596 (19.1) | 1210 (18.1) | 1142 (18.2) | 68 (27.8) | 0 | |
| 2013 | 3170 (23.3) | 1428 (21.4) | 1327 (21.1) | 67 (27.4) | 34 (23.1) | |
| 2014 | 3569 (26.2) | 1525 (22.8) | 1359 (21.6) | 53 (21.6) | 113 (76.9) | |
| Demographics | ||||||
| Age, y | 64.0 (56.0‐72.0) | 63.0 (56.0‐71.0) | 63.0 (56.0‐71.0) | 60.0 (52.0‐67.0) | 64.0 (57.0‐70.0) | <0.0001 |
| Males | 10 206 (74.9) | 4961 (74.3) | 4680 (74.4) | 174 (71.0) | 107 (72.8) | 0.32 |
| Body mass index, kg/m2 | 29.2 (25.9‐33.3) | 29.2 (25.9‐33.2) | 29.2 (25.9‐33.2) | 29.3 (27.0‐33.9) | 29.2 (26.1‐33.1) | 0.77 |
| Race/Ethnicity | <0.0001 | |||||
| White | 11 518 (84.5) | 5444 (81.5) | 5512 (81.5) | 199 (81.2) | 123 (83.7) | |
| Black | 945 (6.9) | 620 (9.3) | 580 (9.2) | 24 (9.8) | 16 (10.9) | |
| Asian | 245 (1.8) | 115 (1.7) | 111 (1.8) | 1 (0.4) | 3 (2.0) | |
| Hispanic | 739 (5.4) | 419 (6.3) | 396 (6.3) | 18 (7.4) | 5 (3.4) | |
| Other | 125 (0.9) | 60 (0.9) | 58 (0.9) | 2 (0.8) | 0 | |
| Baseline characteristics | ||||||
| Prior MI | 1955 (14.4) | 1694 (25.4) | 1563 (24.9) | 102 (41.6) | 29 (19.7) | <0.0001 |
| Prior heart failure | 664 (4.9) | 516 (7.7) | 483 (7.7) | 19 (7.8) | 14 (9.5) | <0.0001 |
| Prior stroke | 673 (4.9) | 550 (8.2) | 530 (8.4) | 11 (4.5) | 9 (6.1) | <0.0001 |
| Prior PCI | 1793 (13.2) | 1955 (29.3) | 4488 (71.4) | 116 (47.4) | 113 (76.9) | <0.0001 |
| Prior CABG | 237 (1.7) | 246 (3.7) | 237 (3.8) | 6 (2.5) | 3 (2.0) | <0.0001 |
| Atrial fibrillation/flutter | 603 (4.4) | 277 (4.2) | 264 (4.2) | 12 (4.9) | 1 (0.7) | 0.36 |
| Cerebrovascular disease | 1180 (8.6) | 893 (13.4) | 866 (13.8) | 15 (6.1) | 12 (8.2) | <0.0001 |
| Peripheral arterial disease | 955 (7.0) | 756 (11.3) | 719 (11.4) | 25 (10.2) | 12 (8.2) | <0.0001 |
| Current/recent smoker | 4507 (33.0) | 2384 (35.7) | 2247 (35.7) | 92 (37.6) | 45 (30.1) | 0.0002 |
| Hypertension | 10 128 (74.3) | 5236 (78.4) | 4932 (78.4) | 194 (79.2) | 110 (74.8) | <0.0001 |
| Dyslipidemia | 8515 (62.5) | 4598 (68.8) | 4330 (68.9) | 180 (73.5) | 88 (59.9) | <0.0001 |
| Diabetes mellitus | 4936 (36.2) | 2684 (40.2) | 2519 (40.0) | 105 (42.9) | 60 (40.8) | <0.0001 |
| Home aspirin | 5505 (40.4) | 3268 (48.9) | 3065 (48.7) | 136 (55.5) | 67 (45.6) | <0.0001 |
| Home P2Y12 inhibitor | 0 (0) | 1934 (29.0) | 1795 (28.6) | 116 (47.4) | 23 (15.7) | <0.0001 |
| Presentation characteristics | ||||||
| Electrocardiographic findings | <0.0001 | |||||
| ST depression or transient ST elevation | 3978 (29.2) | 2057 (30.8) | 1937 (30.8) | 77 (31.4) | 43 (29.3) | |
| T‐wave inversion | 1761 (12.9) | 1065 (15.9) | 1002 (15.9) | 37 (15.1) | 26 (17.7) | |
| None | 7885 (57.9) | 3558 (53.3) | 3349 (53.3) | 131 (53.5) | 78 (53.1) | |
| Signs of heart failure | 1603 (11.8) | 812 (12.2) | 774 (12.3) | 23 (9.4) | 15 (10.2) | 0.42 |
| Cardiogenic shock | 259 (1.9) | 105 (1.6) | 98 (1.6) | 3 (1.2) | 4 (2.7) | 0.10 |
| Initial hemoglobin, g/dL | 14.4 (13.2‐15.5) | 14.2 (12.9‐15.4) | 14.2 (12.9‐15.4) | 14.1 (12.7‐15.2) | 14.3 (13.0‐15.6) | <0.0001 |
| Creatinine clearance, mL/mina | 76.2 (61.1‐91.6) | 75.9 (59.6‐91.8) | 75.4 (59.4‐91.6) | 80.6 (62.3‐93.6) | 79.4 (61.3‐95.9) | 0.18 |
| Initial troponin (ratio over institution upper limit of normal) | 3.5 (0.9‐17.9) | 3.6 (0.9‐18.2) | 3.6 (0.9‐18.3) | 3.4 (1.0‐12.2) | 5.6 (1.1‐23.0) | 0.32 |
| Ejection fraction, % | ||||||
| ≥50 | 7971 (59.2) | 3781 (57.4) | 3565 (57.5) | 135 (55.8) | 81 (55.5) | 0.01 |
| 40 to 50 | 3007 (22.3) | 1501 (22.8) | 1396 (22.5) | 67 (27.7) | 38 (26.0) | |
| 25 to 40 | 1975 (14.7) | 1083 (16.4) | 1030 (16.6) | 34 (14.1) | 19 (13.0) | |
| <25 | 473 (3.5) | 212 (3.2) | 198 (3.2) | 6 (2.5) | 8 (5.5) | |
| No. of diseased vessels, %b | ||||||
| 1 | 474 (3.5) | 253 (3.8) | 238 (3.8) | 10 (4.1) | 5 (3.4) | 0.09 |
| 2 | 2755 (20.0) | 1437 (21.5) | 1340 (21.3) | 67 (27.4) | 30 (20.4) | |
| 3 | 10 342 (75.4) | 4966 (74.3) | 4690 (74.6) | 166 (67.8) | 110 (74.8) | |
| Hospital characteristics | ||||||
| Teaching | 2590 (19.0) | 2304 (34.5) | 2153 (34.2) | 68 (27.8) | 83 (56.5) | <0.0001 |
| Total number of beds | 395.0 (271.0‐567.0) | 431.0 (306.0‐647.0) | 430 (306‐646) | 432 (295‐622) | 476 (356‐749) | <0.0001 |
Continuous numbers are presented as medians with 25th to 75th percentiles in parentheses. Categorical values are presented as counts with percentages in parentheses. CABG indicates coronary artery bypass grafting; MI, myocardial infarctiom; PCI, percutaneous coronary intervention.
Creatinine clearance calculated by the Modification of Diet in Renal Disease (MDRD) formula.
1% of the data are missing.
The median time from catheterization to CABG (25th, 75th percentile) was 69.9 hours (28.2‐115.8) among patients with precatheterization P2Y12 inhibitor use, which was significantly longer than patients without precatheterization P2Y12 inhibitors (43.5 hours [21.0‐71.8], P<0.001). Among patients with precatheterization P2Y12 inhibitor use, 5317 patients (79.6%) underwent CABG within 5 days postcatheterization. After adjusting for baseline demographic and clinical characteristics, precatheterization P2Y12 inhibitor use was independently associated with longer duration from catheterization to CABG (RR 0.70, 95% confidence interval [CI] 0.66‐0.73, P<0.0001), suggesting a 30% lower likelihood that a patient with precatheterization P2Y12 inhibitor use would undergo CABG at a given time point during the index hospitalization compared with patients who did not receive precatheterization P2Y12 inhibitor. The median length of stay of CABG patients with precatheterization P2Y12 inhibitor use was longer than that of patients without it: 10.0 (8.0‐13.0) days versus 8.0 (7.0‐11.0) days, P<0.0001. To determine the likelihood of CABG being deferred to a subsequent hospitalization for patients who had precatheterization P2Y12 inhibitor use, we used linked data from Medicare inpatient claims and observed a 1.3% cumulative incidence of readmission with CABG within 60 days postdischarge from the index NSTEMI hospitalization.
Patients with precatheterization P2Y12 inhibitor use were more likely to have post‐CABG bleeding (75.7% versus 73.5%, P=0.0003) or to receive post‐CABG blood transfusion (47.6% versus 35.7%, P<0.0001). After adjustment for baseline characteristics, precatheterization P2Y12 inhibitor use continued to be associated with higher risks of post‐CABG bleeding (OR 1.33, 95% CI 1.22‐1.45) and post‐CABG blood transfusion (OR 1.51, 95% CI 1.41‐1.62) (Table 2). Patients with precatheterization P2Y12 inhibitor use were also more likely to require surgical or procedural intervention for treatment of bleeding (34% versus 23%, P<0.001). Within the precatheterization P2Y12 inhibitor patient group, the rates of post‐CABG bleeding and transfusion were higher in those undergoing CABG within 5 days of the precatheterization dose of P2Y12 inhibitor than those undergoing CABG >5 days afterwards (76.8% versus 71.5% for bleeding and 48.8% versus 43.0% for transfusion, respectively).
Table 2.
Study End Points: Unadjusted Time From Catheterization to CABG and Incidence of Post‐CABG Bleeding/Transfusion as Well as Adjusted Hazard and Odds Ratios
| Time From Catheterization to CABG | |||||
|---|---|---|---|---|---|
| Unadjusted | Adjusted | ||||
| Precatheterization P2Y12 Inhibitor | No Precatheterization P2Y12 Inhibitor | Unadjusted P Value | Adjusted Risk Ratioa (95% CI) | Adjusted P Value | |
| Time from catheterization to CABGb | 69.9 h (28.2‐115.8) | 43.5 h (21.0‐71.8) | <0.0001 | 0.70 (0.66‐0.73) | <0.0001 |
| Post‐CABG Adverse Events | |||||
|---|---|---|---|---|---|
| Unadjusted | Adjusted | ||||
| Precatheterization P2Y12 Inhibitor | No Precatheterization P2Y12 Inhibitor | Unadjusted P Value | Adjusted Odds Ratio (95% CI) | Adjusted P Value | |
| Post‐CABG major bleedingc | 5057 (75.7) | 10 011 (73.5) | 0.0003 | 1.33 (1.22‐1.45) | <0.0001 |
| Post‐CABG transfusionc | 3178 (47.6) | 4865 (35.7) | <0.0001 | 1.51 (1.41‐1.62) | <0.0001 |
CABG indicates coronary artery bypass grafting; CI, confidence interval; HR, hazard ratio.
Risk ratio compares time from catheterization to CABG with HR <1 denoting longer delays to CABG.
Time from catheterization to CABG is presented as median (25th, 75th percentiles).
Post‐CABG major bleeding and transfusion presented as counts with percentages (%).
A sensitivity analysis that excluded the 861 patients who were on a P2Y12 inhibitor at home immediately before MI presentation showed similar results: precatheterization P2Y12 inhibitor use continued to be associated with longer delays from catheterization to CABG (RR 0.65, 95% CI 0.62‐0.69; adjusted RR 0.69, 95% CI 0.66‐0.73, P<0.001). Precatheterization P2Y12 inhibitor use was also associated with increased risk of post‐CABG bleeding (OR 1.19, 95% CI 1.10‐1.29; adjusted OR 1.33, 95% CI 1.22‐1.46, P<0.0001) and transfusion (OR 1.54, 95% CI 1.44‐1.64; adjusted OR 1.52, 95% CI 1.41‐1.62, P<0.0001).
Among patients with precatheterization P2Y12 inhibitor use, 6288 (94.1%) were treated with clopidogrel, 245 (3.7%) with prasugrel, and 147 (2.2%) with ticagrelor. For patients who received P2Y12 inhibitors in the hospital, the median (25th, 75th percentile) time from P2Y12 inhibitor administration to catheterization was 7.5 hours (2.6, 14.6) for clopidogrel, compared to 4.0 hours (0.9, 11.4) for prasugrel and 8.4 hours (1.7, 14.4) for ticagrelor. The median time from catheterization to CABG was significantly longer in patients treated with prasugrel (114.4 hours [66.5, 155.4]), followed by ticagrelor (90.3 hours [48.6, 124.5]), then clopidogrel (69.3 hours [27.4, 114.6]) (Figure 2). After adjustment for patients' ACTION bleeding score, patients treated with prasugrel or ticagrelor before catheterization had longer duration from catheterization to CABG when compared with patients treated with clopidogrel. On the other hand, there was no difference in post‐CABG bleeding or transfusion between patients pretreated with clopidogrel, prasugrel, or ticagrelor (Table 3). Among patients who received prasugrel, 214 (87.4%) underwent CABG within 7 days postcatheterization. Among patients who received ticagrelor and clopidogrel, 107 (72.8%) and 5057 (80.7%), respectively, underwent CABG within 5 days postcatheterization.
Figure 2.
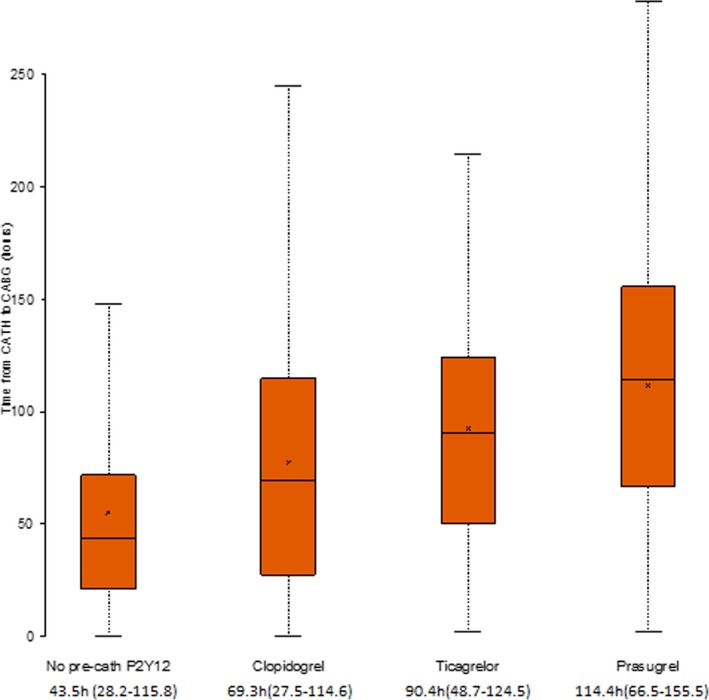
Time from catheterization to coronary artery bypass grafting (CABG) in patients with no precatheterization P2Y12 inhibitors and those with precatheterization clopidogrel, prasugrel, and ticagrelor. Box plot with the lower and upper ends of each bar representing the first and third quartiles and the band representing the median and the x‐axis representing the mean. The lowest whisker is the lowest limit, and the upper whisker is the highest limit of the data range; cath indicates catheterization.
Table 3.
Study End Points in Patients Taking Clopidogrel, Prasugrel, and Ticagrelor
| Clopidogrel | Prasugrel | Ticagrelor | P Value | |
|---|---|---|---|---|
| n=6288 | n=245 | n=147 | ||
| Time from catheterization to CABG, ha | 69.3 (27.5‐114.6) | 114.4 (66.5‐155.5) | 90.4 (48.7‐124.5) | <0.0001 |
| Length of stay, da | 10.0 (8.0‐13.0) | 11.0 (9.0‐14.0) | 11.0 (9.0‐14.0) | <0.0001 |
| Post‐CABG bleedingb | 4767 (75.8) | 183 (74.7) | 107 (72.7) | 0.60 |
| Post‐CABG transfusionb | 3009 (47.9) | 105 (42.9) | 64 (43.5) | 0.18 |
Unadjusted outcomes in the P2Y12 inhibitor pretreatment group. CABG indicates coronary artery bypass grafting.
Median (25th, 75th percentiles).
Counts with percentages (%).
Discussion
This observational study of current clinical practice in the United States showed that precatheterization P2Y12 inhibitor use occurred in 60% of all NSTEMI patients who underwent catheterization within 24 hours of admission and in 33% of these patients who underwent CABG during the index hospitalization. Patients with precatheterization P2Y12 inhibitor use had longer delays to CABG but remained associated with higher risks of post‐CABG bleeding and transfusion than patients who did not receive precatheterization P2Y12 inhibitor therapy. These associations persisted in sensitivity analyses that excluded patients who were taking home P2Y12 inhibitor immediately before admission. The delay from catheterization to surgery was longer for patients pretreated with prasugrel or ticagrelor than those who received clopidogrel. Postoperative bleeding and transfusion risks did not differ among the three P2Y12 inhibitor agents.
The concept of precatheterization P2Y12 inhibitor use started after an analysis of the percutaneous coronary intervention (PCI) subset of the CURE (Clopidogrel in Unstable Angina to Prevent Recurrent Events) trial showed lower major adverse cardiovascular events in patients pretreated with clopidogrel.8 In that study, the median time from presentation to catheterization was 10 days, considerably longer than current practice. This concept was later challenged when the CREDO (Clopidogrel for the Reduction of Events During Observation) trial showed no benefit from pretreatment with clopidogrel given 3 hours before PCI but suggested potential reduction of events in patients with longer duration from clopidogrel administration to catheterization.10 The ACCOAST (A Comparison of Prasugrel at PCI or Time of Diagnosis of Non‐ST Elevation Myocardial Infarction) trial showed that precatheterization use of prasugrel did not improve cardiovascular outcomes and was associated with an increase in TIMI (Thrombosis in Myocardial Infarction) major bleeding.6 There are no data comparing precatheterization use and nonuse of ticagrelor in NSTEMI. During our study period, guidelines recommended early P2Y12 inhibitor treatment, and we observed precatheterization P2Y12 inhibitor use in 60% of NSTEMI patients undergoing early cardiac catheterization. Ultimately 1 in 3 patients who underwent CABG during the index NSTEMI hospitalization received precatheterization P2Y12 inhibitor. The gradual decline in the proportion of NSTEMI patients who received P2Y12 inhibitors precatheterization over the study period may reflect increasing availability of early catheterization across the United States. Clinicians may have greater clinical equipoise in whether to treat before catheterization or to defer treatment until surgical coronary anatomy is ruled out with catheterization. Additionally, the increased emphasis on reducing hospital length of stay in recent years may also explain the reluctance to use precatheterization P2Y12 inhibitors.
The current study sheds light on the outcomes associated with precatheterization P2Y12 inhibitor use in these patients. Although this study showed precatheterization P2Y12 inhibitor use to be associated with increased duration from catheterization to CABG, the median time from catheterization to CABG (2.9 days in the pretreatment group) is shorter than the washout period recommended by the American College of Cardiology/American Heart Association guidelines between the last dose and planned CABG (5 days for clopidogrel and ticagrelor, 7 days for prasugrel).4, 11 Interestingly, the delay to surgery seems longer for ticagrelor despite its having a shorter half‐life compared with clopidogrel. Importantly, the observed duration from catheterization to CABG is significantly shorter than that seen in the clinical trials studying this topic previously.12, 13 The reluctance of clinicians to wait the recommended period may be possibly explained by a bias toward earlier revascularization5, 14; only a small proportion of NSTEMI patients treated with precatheterization P2Y12 inhibitors deferred CABG to another hospitalization after the index NSTEMI hospitalization. Furthermore, the economic and logistic challenges posed by the increased lengths of stay in the current healthcare environment may encourage earlier surgical intervention.
In our study, the associations between precatheterization P2Y12 inhibitor use and higher post‐CABG bleeding and transfusion risks echo those reported in prior smaller studies showing increased postoperative bleeding when clopidogrel is discontinued less than 5 to 7 days pre‐CABG.14, 15, 16 In a subgroup analysis of CABG patients in the CURE trial,12 pretreatment with clopidogrel was found to have similar clinical benefits without incurring increased risk of bleeding when CABG was performed more than 5 days after drug cessation but was associated with increased life‐threatening bleeding when clopidogrel was discontinued less than 5 days before CABG. Our study again showed that bleeding and transfusion rates were higher when CABG occurred within 5 days of precatheterization P2Y12 inhibitor exposure, compared with CABG that occurred >5 days afterwards.
The higher risks of post‐CABG bleeding associated with precatheterization P2Y12 inhibitor use have important implications on the overall management of this patient group. Our study showed that precatheterization P2Y12 inhibitor use was associated with longer index hospital length of stay and higher likelihood of requiring surgical intervention to control bleeding. Prior studies have shown post‐CABG bleeding to be associated with higher rates of reoperation, hospital length of stay, and treatment costs.17 A post hoc analysis of the ACUITY (Acute Catheterization and Urgent Intervention Triage Strategy) trial showed correlation between post‐CABG bleeding and decreased survival.18 In our study, we avoided mortality and ischemic events as end points due to the high risk of error in attributing perioperative death or infarction to bleeding caused by P2Y12 inhibitor exposure, particularly in the absence of adjudicated cause of death and clinical details that influence such perioperative events in this registry. Additionally, because the preoperative duration of antiplatelet and anticoagulant therapies and the timing of therapy resumption post‐CABG were not captured, off‐treatment risk of ischemic events could not be ascertained. The role of shorter‐acting P2Y12 inhibitor agents should be examined in this clinical context, as these agents confer early ischemic protection of early P2Y12 inhibitor use with the advantage of rapid offset of antiplatelet effect when surgical revascularization is required.
Although our study provides insight into contemporary practice in one of the largest cohorts of patients undergoing in‐hospital CABG for NSTEMI, several limitations need to be considered. First, this is a retrospective observational study; therefore, causal relationships cannot be inferred, and the potential for unmeasured confounding remains despite adjustment for a wide range of covariates. Patients treated with precatheterization P2Y12 inhibitors appear to have more significant comorbid conditions and therefore may be more likely to have significant adverse outcomes even after adjustment for multiple measured confounders. The timing of CABG and postoperative bleeding and transfusion may be influenced by clinical factors (eg, postcatheterization hemodynamic instability) that the registry does not collect and are therefore unaccounted for in this study. Second, this study focused on NSTEMI patients who underwent early catheterization (within 24 hours of admission), as this is a population that may have more equipoise for precatheterization P2Y12 inhibitor use or deferral. These findings cannot be generalized to NSTEMI patients who do not undergo early catheterization. Third, the contemporary nature of the data permitted assessment of practice patterns in the setting of the novel antiplatelet agents prasugrel and ticagrelor being available for clinical use. However, because of the modest number of patients pretreated with these newer P2Y12 inhibitor agents, our results have wide confidence intervals and should be validated in future studies. Fourth, ACTION Registry captures P2Y12 inhibitor use only during the first 24 hours of admission and does not capture when the P2Y12 inhibitor was discontinued relative to the time of CABG. Our analysis assumes that, for most patients found to have surgical disease on angiography, no further P2Y12 inhibitor doses are administered postcatheterization. We also included patients who were on P2Y12 inhibitor therapy before admission because the antiplatelet effect should be present precatheterization. Sensitivity analyses excluding patients without home P2Y12 inhibitor use showed similar results. Fifth, among our study population, 287 patients received PCI with stenting before in‐hospital CABG. This small number precludes insight into the optimal antiplatelet strategy for these patients. Finally, the association of precatheterization P2Y12 inhibitor use with long‐term bleeding and transfusion outcomes cannot be ascertained.
Conclusions
In contemporary practice, 60% of NSTEMI patients who underwent early catheterization received precatheterization P2Y12 inhibitor therapy. Among patients who then underwent CABG, precatheterization P2Y12 inhibitor use was associated with longer delays to CABG, although the majority of patients proceeded to CABG before guideline‐recommended drug washout. Precatheterization P2Y12 inhibitor use was associated with higher risks of post‐CABG bleeding and transfusion. These results suggest the need for randomized prospective studies to define the benefit of P2Y12 inhibitor pretreatment in NSTEMI patients who undergo early coronary angiography.
Sources of Funding
This study was supported by the American College of Cardiology Foundation's National Cardiovascular Data Registry.
Disclosures
Dr Wang received research grants to the Duke Clinical Research Institute from AstraZeneca, Boston Scientific, Bristol Myers Squibb, Daiichi Sankyo, Eli Lilly, Gilead Sciences, Glaxo Smith Kline, Pfizer, and Regeneron Pharmaceuticals. Dr Wang also consulted/received honoraria from AstraZeneca, Eli Lilly, Merck, and Pfizer, Inc. All other authors have no financial relationships to disclose.
Supporting information
Figure S1. Balance of categorical variables before (red symbols) and after inverse probability‐weighting adjustment (black symbols).
Figure S2. Balance of continuous variables before (red symbols) and after inverse probability‐weighting adjustment (black symbols).
(J Am Heart Assoc. 2017;6:e006508 DOI: 10.1161/JAHA.117.006508.)28939715
References
- 1. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST‐segment elevation. N Engl J Med. 2001;345:494–502. [DOI] [PubMed] [Google Scholar]
- 2. Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, Neumann F‐J, Ardissino D, De Servi S, Murphy SA, Riesmeyer J, Weerakkody G, Gibson CM, Antman EM. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001–2015. [DOI] [PubMed] [Google Scholar]
- 3. Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, Horrow J, Husted S, James S, Katus H, Mahaffey KW, Scirica BM, Skene A, Steg PG, Storey RF, Harrington RA. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045–1057. [DOI] [PubMed] [Google Scholar]
- 4. Jneid H, Anderson JL, Wright RS, Adams CD, Bridges CR, Casey DE, Ettinger SM, Fesmire FM, Ganiats TG, Lincoff AM, Peterson ED, Philippides GJ, Theroux P, Wenger NK, Zidar JP. 2012 ACCF/AHA focused update of the guideline for the management of patients with unstable angina/non‐ST‐elevation myocardial infarction (updating the 2007 guideline and replacing the 2011 focused update): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2012;60:645–681. [DOI] [PubMed] [Google Scholar]
- 5. Sherwood MW, Wiviott SD, Peng SA, Roe MT, DeLemos J, Peterson ED, Wang TY. Early clopidogrel versus prasugrel use among contemporary STEMI and NSTEMI patients in the US: insights from the National Cardiovascular Data Registry. J Am Heart Assoc. 2014;3:e000849 DOI: 10.1161/JAHA.114.000849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Montalescot G, Bolognese L, Dudek D, Goldstein P, Hamm C, Tanguay J‐F, ten Berg JM, Miller DL, Costigan TM, Goedicke J, Silvain J, Angioli P, Legutko J, Niethammer M, Motovska Z, Jakubowski JA, Cayla G, Visconti LO, Vicaut E, Widimsky P; ACCOAST Investigators . Pretreatment with prasugrel in non‐ST‐segment elevation acute coronary syndromes. N Engl J Med. 2013;369:999–1010. [DOI] [PubMed] [Google Scholar]
- 7. Khera S, Kolte D, Aronow WS, Palaniswamy C, Subramanian KS, Hashim T, Mujib M, Jain D, Paudel R, Ahmed A, Frishman WH, Bhatt DL, Panza JA, Fonarow GC. Non‐ST‐elevation myocardial infarction in the United States: contemporary trends in incidence, utilization of the early invasive strategy, and in‐hospital outcomes. J Am Heart Assoc. 2014;3:e000995 DOI: 10.1161/JAHA.114.000995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mehta SR, Yusuf S, Peters RJ, Bertrand ME, Lewis BS, Natarajan MK, Malmberg K, Rupprecht H, Zhao F, Chrolavicius S, Copland I, Fox KA; Clopidogrel in Unstable angina to prevent Recurrent Events trial (CURE) Investigators . Effects of pretreatment with clopidogrel and aspirin followed by long‐term therapy in patients undergoing percutaneous coronary intervention: the PCI‐CURE study. Lancet. 2001;358:527–533. [DOI] [PubMed] [Google Scholar]
- 9. Mathews R, Peterson ED, Chen AY, Wang TY, Chin CT, Fonarow GC, Cannon CP, Rumsfeld JS, Roe MT, Alexander KP. In‐hospital major bleeding during ST‐elevation and non‐ST‐elevation myocardial infarction care: derivation and validation of a model from the ACTION Registry®‐GWTGTM . Am J Cardiol. 2011;107:1136–1143. [DOI] [PubMed] [Google Scholar]
- 10. Steinhubl SR, Berger PB, Mann JT, Fry ETA, DeLago A, Wilmer C, Topol EJ; CREDO Investigators. Clopidogrel for the Reduction of Events During Observation . Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial. JAMA. 2002;288:2411–2420. [DOI] [PubMed] [Google Scholar]
- 11. Amsterdam EA, Wenger NK, Brindis RG, Casey DE, Ganiats TG, Holmes DR, Jaffe AS, Jneid H, Kelly RF, Kontos MC, Levine GN, Liebson PR, Mukherjee D, Peterson ED, Sabatine MS, Smalling RW, Zieman SJ. 2014 AHA/ACC guideline for the management of patients with non–ST‐elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;64:e139–e228. [DOI] [PubMed] [Google Scholar]
- 12. Fox KAA, Mehta SR, Peters R, Zhao F, Lakkis N, Gersh BJ, Yusuf S; Clopidogrel in Unstable angina to prevent Recurrent ischemic Events Trial . Benefits and risks of the combination of clopidogrel and aspirin in patients undergoing surgical revascularization for non‐ST‐elevation acute coronary syndrome: the Clopidogrel in Unstable angina to prevent Recurrent ischemic Events (CURE) Trial. Circulation. 2004;110:1202–1208. [DOI] [PubMed] [Google Scholar]
- 13. Ebrahimi R, Dyke C, Mehran R, Manoukian SV, Feit F, Cox DA, Gersh BJ, Ohman EM, White HD, Moses JW, Ware JH, Lincoff AM, Stone GW. Outcomes following pre‐operative clopidogrel administration in patients with acute coronary syndromes undergoing coronary artery bypass surgery: the ACUITY (Acute Catheterization and Urgent Intervention Triage strategY) trial. J Am Coll Cardiol. 2009;53:1965–1972. [DOI] [PubMed] [Google Scholar]
- 14. Mehta RH, Roe MT, Mulgund J, Ohman EM, Cannon CP, Gibler WB, Pollack CV, Smith SC, Ferguson TB, Peterson ED. Acute clopidogrel use and outcomes in patients with non‐ST‐segment elevation acute coronary syndromes undergoing coronary artery bypass surgery. J Am Coll Cardiol. 2006;48:281–286. [DOI] [PubMed] [Google Scholar]
- 15. Pickard AS, Becker RC, Schumock GT, Frye CB. Clopidogrel‐associated bleeding and related complications in patients undergoing coronary artery bypass grafting. Pharmacotherapy. 2008;28:376–392. [DOI] [PubMed] [Google Scholar]
- 16. Berger JS, Frye CB, Harshaw Q, Edwards FH, Steinhubl SR, Becker RC. Impact of clopidogrel in patients with acute coronary syndromes requiring coronary artery bypass surgery: a multicenter analysis. J Am Coll Cardiol. 2008;52:1693–1701. [DOI] [PubMed] [Google Scholar]
- 17. Reeves BC, Murphy GJ. Increased mortality, morbidity, and cost associated with red blood cell transfusion after cardiac surgery. Curr Opin Cardiol. 2008;23:607–612. [DOI] [PubMed] [Google Scholar]
- 18. Stone GW, Clayton TC, Mehran R, Dangas G, Parise H, Fahy M, Pocock SJ. Impact of major bleeding and blood transfusions after cardiac surgery: analysis from the Acute Catheterization and Urgent Intervention Triage strategY (ACUITY) trial. Am Heart J. 2012;163:522–529. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Balance of categorical variables before (red symbols) and after inverse probability‐weighting adjustment (black symbols).
Figure S2. Balance of continuous variables before (red symbols) and after inverse probability‐weighting adjustment (black symbols).


