Abstract
Background
Arterial stiffness is a well‐known predictor of future cardiovascular events. Search for the underlying mechanism of arterial stiffening is still under way. We investigated the relationship between arterial stiffness and cytomegalovirus infection in terms of T‐cell senescence.
Methods and Results
Arterial stiffness was evaluated using pulse wave velocity measurements in 415 Koreans (age 59±12 years). We also investigated the frequency of CD57+ or CD28null senescent T cells in peripheral blood lymphocytes and analyzed which immune parameters were correlated with pulse wave velocity. Furthermore, cytomegalovirus‐specific T cells were stimulated with overlapping peptides covering pp65 protein, and T‐cell function was evaluated by intracellular cytokine staining of interferon‐γ, tumor necrosis factor‐α, and CD107a. In a multivariate analysis, it was found that the frequency of CD57+ cells in the CD8+ T‐cell subset was independently correlated with pulse wave velocity after adjusting for traditional cardiovascular risk factors such as age, sex, diabetes mellitus history, smoking history, body mass index, blood pressure, serum creatinine, high‐density lipoprotein cholesterol, and high‐sensitivity C‐reactive protein. Cytomegalovirus pp65‐specific T cells were more frequently observed in the CD8+ CD57+ population than in the CD8+ CD57− population, and multivariate analysis revealed that the frequency of cytomegalovirus pp65‐specific interferon‐γ+, tumor necrosis factor‐α+, or CD107a+ cells in the CD8+ T‐cell subset was independently correlated with pulse wave velocity as well.
Conclusions
We demonstrate that arterial stiffness is associated with senescent CD57+ T cells and CMV pp65‐specific T cells in the CD8+ T‐cell subset. The precise role of cytomegalovirus‐specific, senescent T cells in vascular aging needs to be further investigated.
Keywords: arterial stiffness, cytomegalovirus, immunosenescence, pulse wave velocity, T cells
Subject Categories: Inflammation
Clinical Perspective
What Is New?
Previously, immunologic mechanisms for arterial stiffening have not been well addressed.
This is the first study to reveal the relationship between cytomegalovirus‐specific, senescent CD8+ T cells and arterial stiffness in humans.
What Are the Clinical Implications?
Cytomegalovirus‐related T‐cell aging may contribute to age‐associated arterial stiffness and hypertension.
Future studies should address specific mechanisms by which cytomegalovirus‐specific, senescent T cells contribute to arterial stiffening and therapeutic interventions to prevent this process.
Introduction
Growing evidence from recent animal and human studies suggests that T cells contribute to the development of hypertension.1, 2, 3, 4 Previously, we demonstrated that hypertensive patients have an increased frequency of replicative senescent CD8+ T cells in peripheral blood; these cells are characterized by the loss of CD28 and the acquisition of CD57 on their surface.4 CD28 loss in T cells is one of the most prominent changes associated with aging in humans and is caused by the repetitive antigenic stimulation of T cells.5 CD57 expression is known to occur during the late stage of T‐cell differentiation and might be a distinct measure of replicative senescence in T cells.6 Compared with CD28+ or CD57− T cells, CD28null or CD57+ T cells produce more proinflammatory cytokines and exert greater cytotoxicity.7 These senescent T cells are known to be associated with various inflammatory diseases in humans including cardiovascular disease.8, 9, 10
It has been also known that cytomegalovirus infection is involved in the accumulation of CD28null or CD57+ senescent T cells. In humans, cytomegalovirus is known to be one of the most important antigens for repetitive T‐cell stimulation,11 and latent infection with cytomegalovirus has been shown to strongly exert age‐associated changes on peripheral T cell homeostasis.12 The cytomegalovirus‐seropositive population has a higher frequency of CD28null or CD57+, replicative senescent T cells than the cytomegalovirus‐seronegative population.13 Moreover, cytomegalovirus infection is associated with a variety of chronic inflammatory processes in cardiovascular disease14, 15 such as hypertension.16 However, it has not been elucidated how cytomegalovirus infection and senescent T cells contribute to the pathogenesis of cardiovascular disease.
Increased arterial stiffness is one of the major mechanisms underlying the pathogenesis of hypertension.17 Arterial stiffness is increased in the presence of conventional cardiovascular risk factors including aging.18, 19 The degree of arterial stiffness is known to be associated with various markers of inflammation,20, 21 suggesting that immune responses likely play a role in increasing arterial stiffness. Furthermore, a recent study has reported that activated T‐cell markers are associated with arterial stiffness among HIV‐infected patients.22 Therefore, we investigated whether T‐cell senescence is associated with arterial stiffness in the general population, as assessed using pulse wave velocity (PWV) measurements. Next, considering the antigen reactivity of senescent T cells, we examined cytomegalovirus‐specific T‐cell response and analyzed the relationship between these results and the degree of arterial stiffness. Because increased arterial stiffness is characteristic of the vascular aging process, we attempted to elucidate the relationship between vascular aging and T‐cell aging.
Methods
Study Participants
The study population consisted of 415 Koreans who were recruited from subjects initially registered in the Yonsei Cardiovascular Genome cohort. They were recruited from the outpatient clinic of Severance Cardiovascular Hospital irrespective of their disease status. The study population includes those with hypertension, coronary artery disease, diabetes mellitus (DM), and healthy subjects. The average age of the cohort members was 59.4±11.7 years (20–82 years). Hypertension was defined as either a documented systolic blood pressure of >140 mm Hg or a diastolic blood pressure of >90 mm Hg over 3 visits before the use of antihypertensive medication. DM was defined as having fasting plasma glucose levels >126 mg/dL, HbA1c >6.5%, or a history of DM therapy. Coronary artery disease was defined as 1 or more lesions with a >50% diameter reduction either by invasive coronary angiography or CT coronary angiography. At the time of enrollment, patients underwent a complete physical examination and laboratory assessment. Peripheral blood pressure was measured in both arms after 5 minutes of rest in a sitting position using an OMRON HEM 780 device (OMRON Healthcare, Kyoto, Japan),23 and the higher values were used for the analysis. Patients with any of the following conditions were excluded from this study: significant systemic disease, malignant debilitating disease, severe hypertension (>200/140 mm Hg), a history of overt chronic inflammatory disease and/or were receiving anti‐inflammatory medications. The baseline clinical characteristics and laboratory data regarding the study participants are summarized in Table 1. The medications taken by the patients are summarized in Table S1. All the measurements in this study including PWV and the immunologic parameters were obtained while the patients were taking their prescribed medications. This study received prior approval from the Institutional Review Board of the Yonsei University College of Medicine, and the procedures followed were in accordance with institutional guidelines (IRB number: 4‐2001‐0039, 4‐2010‐0500). All participants provided informed consent before enrollment.
Table 1.
Baseline Characteristics of the Study Population (n=415)
| Age, y | 59.4±11.7 |
| Male sex | 273 (65.8%) |
| CAD | 281 (67.7%) |
| Hypertension | 247 (59.5%) |
| DM | 148 (35.7%) |
| Hyperlipidemia | 179 (43.1%) |
| Current smoker | 51 (12.3%) |
| BMI, kg/m2 | 25.2±3.1 |
| SBP, mm Hg | 131.3±17.1 |
| DBP, mm Hg | 80.8±11.0 |
| Creatinine, mg/dL | 1.0±0.7 |
| Total cholesterol, mg/dL | 163.1±41.1 |
| HDL‐cholesterol, mg/dL | 48.2±11.9 |
| LDL‐cholesterol, mg/dL | 88.1±34.7 |
| Triglyceride, mg/dL | 135.8±122.2 |
| hsCRP, mg/L | 1.5±3.1 |
| hfPWV, cm/s | 1.016.6±234.6 |
| Frequency of T‐cell subset, % | |
| CD57+ cells in CD4+ T cells | 6.1±6.0 |
| CD28null cells in CD4+ T cells | 4.4±5.7 |
| CD57+ cells in CD8+ T cells | 43.0±17.1 |
| CD28null cells in CD8+ T cells | 41.9±17.6 |
Data are presented as the means±SD or n (%). BMI indicates body mass index; CAD, coronary artery disease; DBP, diastolic blood pressure; DM, diabetes mellitus; HDL, high‐density lipoprotein; hfPWV, heart‐femoral pulse wave velocity; hsCRP, high‐sensitivity C‐reactive protein; LDL, low‐density lipoprotein; SBP, systolic blood pressure.
PWV Measurements
PWV was measured as heart‐femoral PWV using a VP‐2000 pulse wave unit (Nippon Colin Ltd, Komaki City, Japan) as previously described.24 The method was validated in 948 patients with hypertension or coronary artery disease by showing high correlation and good agreement with the conventional SphygmoCor system.25 Briefly, carotid and femoral artery pressure waveforms were recorded in multielement tonometry sensors at the left carotid and left femoral arteries with patients in a supine position. The ECG was monitored from electrodes placed on both wrists. Heart sounds S1 and S2 were detected by placing a microphone on the left edge of the sternum at the third intercostal space. The waveform analyzer measured the time intervals between S2 and the notch of the carotid pulse wave (Thc) and between the carotid and femoral artery pulse waves (Tcf). The sum of Thc and Tcf represents the time required for pulse waves to travel from the heart to the femoral artery (Thf). The heart‐femoral PWV, a marker of central aortic stiffness,26 was calculated as Lhf/(Thc+Tcf), where Lhf is the distance from the heart to the femoral artery. Lhf and the distance between the sampling points were calculated based on patient height and then divided by the time interval for the waveform from each measuring point.
Immunophenotype Analysis of Peripheral Blood Mononuclear Cells
Peripheral blood mononuclear cells from anticoagulated blood were isolated using Ficoll‐Hypaque (GE Healthcare, Uppsala, Sweden) density gradient centrifugation. For surface staining, peripheral blood mononuclear cells were incubated with directly conjugated monoclonal antibodies for 20 minutes at 4°C. The antibodies used were anti‐CD3 (horizon V500), anti‐CD4 (PE‐Cy7), anti‐CD8 (APC‐H7), anti‐CD19 (PerCP‐Cy5.5), anti‐CD28 (APC) (all from BD Biosciences, San Jose, CA), and anti‐CD57 (eFluor 450) (Biolegend, San Diego, CA). Multicolor flow cytometry was performed using an LSR II instrument (BD Biosciences), and FlowJo software (Treestar, San Carlos, CA) was used to analyze the data.
In Vitro Stimulation of T Cells and Intracellular Cytokine Staining
To analyze the specific antigen reactivity of T cells, peripheral blood mononuclear cells were stimulated with overlapping peptides from cytomegalovirus pp65 (0.6 nmol of each peptide/mL; Miltenyi Biotec), which could directly bind to MHC class I or II molecules on antigen‐presenting cells and be presented to CD8+ or CD4+ T cells in peripheral blood mononuclear cell cultures, for 6 hours in the presence of phycoerythrin (PE)‐conjugated anti‐CD107a (BD Biosciences). The CD107a molecule is exposed to the cell surface during degranulation of cytotoxic proteins such as perforin and granzymes, and therefore PE‐conjugated anti‐CD107a was added to the cultures for the detection of T cells with cytotoxic activity.27, 28 After 1 hour of incubation, brefeldin A (GolgiPlug, BD Biosciences) and monensin (GolgiStop, BD Biosciences) were added to accumulate cytokine protein intracellularly. Following surface staining with anti‐CD3 (horizon V500), anti‐CD4 (PerCP‐Cy5.5), anti‐CD8 (APC‐H7), anti‐CD28 (horizon V450), and anti‐CD57 (APC), the cells were fixed and permeabilized using a Fixation/Permeabilization Buffer Kit and further stained for intracellular cytokines with anti‐interferon‐γ (IFN‐γ) (fluorescein isothiocyanate conjugated) and anti‐tumor necrosis factor‐α (TNF‐α) (PE‐Cy7) (both from BD Biosciences). All samples were assessed using an LSR II Flow Cytometer (BD Biosciences), and the data were analyzed using FlowJo software (Treestar, San Carlos, CA). This assay enables enumeration of cytomegalovirus pp65‐specific T cells in both T‐cell compartments, CD8+ and CD4+ T cells.28, 29
Measurement of Serum Anti‐Cytomegalovirus IgG Antibody Titers
Serum anti‐cytomegalovirus immunoglobulin G (IgG) titers were determined using a commercially available chemiluminescent microparticle immunoassay (Abbott Laboratories, Chicago, IL) in accordance with the manufacturer's instructions. A titer of 6.0 antibody units/mL of IgG or greater was considered cytomegalovirus IgG‐seropositive.
Statistical Analysis
Continuous variables are summarized as the mean±SD. Categorical variables are summarized as a percentage of the group total. Continuous variables were compared using Student t test and ANOVA. Intragroup comparisons were summarized using the paired t test, and the Wilcoxon signed‐rank test was used to verify the results. Pearson's correlation analysis was used for the simple correlation between continuous variables. To examine the association of senescent T cells and cytomegalovirus‐specific T‐cell responses with arterial stiffness, multiple linear regression models were used, using heart‐femoral PWV as the dependent variables and the frequencies of senescent or cytomegalovirus‐specific T cells as the main independent variables of interest. All P values were 2‐sided and considered significant at the 0.05 level. All statistical analyses were performed using SPSS 13.0 (SPSS Inc, Chicago, IL).
Results
The Frequency of CD8+CD57+ T Cells Is Independently Correlated With Arterial Stiffness
In the present study, we investigated the significance of T‐cell senescence in arterial stiffness. The study population consisted of 415 Koreans who were registered in the Yonsei Cardiovascular Genome cohort. The baseline characteristics and laboratory data of the study participants are summarized in Table 1. The mean frequencies of CD57+ and CD28null T cells among the peripheral blood CD8+ T‐cell population were 43.0±17.1% and 41.9±17.6%, respectively. In Figure 1A, representative flow cytometry plots are presented for CD57 and CD28 expression in the CD8+ T‐cell subset from young and old subjects. As expected, the frequency of CD57+ (r=0.410, P<0.001) or CD28null (r=0.391, P<0.001) cells among the CD8+ T‐cell subset was positively correlated with age (Figure 1B). The study population showed a significant, positive relationship between age and systolic blood pressure (Figure 1C). We analyzed the relationship between senescent CD8+ T cells and blood pressure (Figure 1D) and found that the frequency of CD8+CD57+ T cells was positively correlated with systolic blood pressure (r=0.103, P=0.036). Next, we analyzed the relationship between the frequency of senescent T cells and PWV. The frequencies of CD8+CD57+ (r=0.322, P<0.001) and CD8+CD28null (r=0.300, P<0.001) T cells were both positively correlated with PWV (Figure 1E and 1F). Because PWV is strongly affected by age and blood pressure, we performed a multiple linear regression analysis of the association between the frequency of senescent CD8+ T cells and PWV after adjusting for traditional cardiovascular risk factors including age, sex, systolic blood pressure, medical history, and laboratory findings (Table 2). Based on this analysis, the frequency of CD8+CD57+ T cells was independently correlated with PWV (B=0.037, 95% confidence interval [CI], 0.001–0.073, P=0.047).
Figure 1.
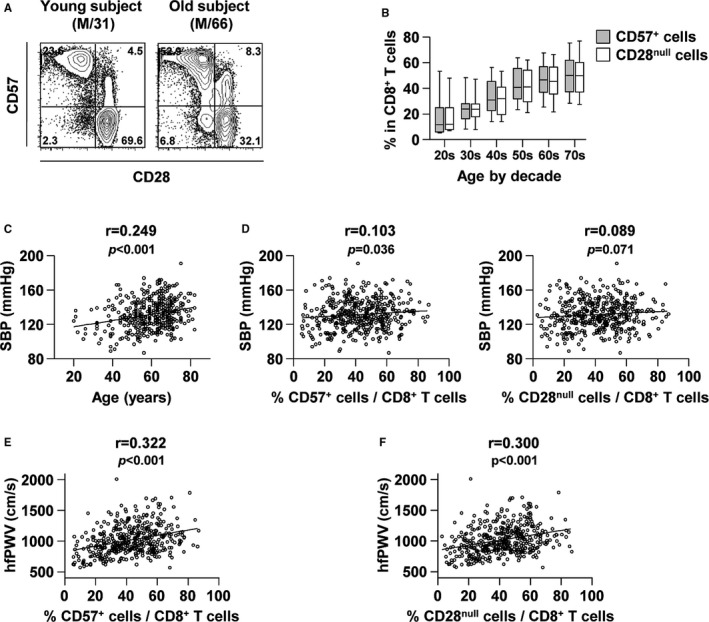
The significance of the frequency of senescent CD8+ T cells in blood pressure and PWV. Immunophenotyping of peripheral blood mononuclear cells obtained from 415 individuals was performed. A, Representative flow cytometry plots are presented for CD57 and CD28 expression in CD8+ T cells in a young (left) and an old subject (right). B, The frequency of CD57+ (gray) or CD28null (white) cells in CD8+ T cells is plotted against age. C, Pearson's correlation scatterplot of SBP and the age of all participants. D, Pearson's correlation scatterplot of SBP and the frequency of CD57+ (left) or CD28null (right) cells in CD8+ T cells. E and F, Pearson's correlation presented by plotting hfPWV against the frequency of CD57+ (E) or CD28null (F) cells in CD8+ T cells of the overall population. hfPWV indicates heart‐femoral pulse wave velocity; PWV, pulse wave velocity; SBP, systolic blood pressure.
Table 2.
Multiple Linear Regression Analysis for Determinants of PWV
| Regression Coefficient | 95% CI | P Value | |
|---|---|---|---|
| CD8+CD57+ T cells (R 2=0.513) | |||
| Age, y | 0.004 | 0.003 to 0.005 | <0.001 |
| Female sex | −0.020 | −0.039 to −0.001 | 0.041 |
| DM history | 0.025 | 0.009 to 0.041 | 0.002 |
| Smoking history | 0.007 | −0.005 to 0.019 | 0.264 |
| BMI, kg/m2 | −0.002 | −0.004 to 0.001 | 0.150 |
| SBP, mm Hg | 0.002 | 0.001 to 0.002 | <0.001 |
| Creatinine, mg/dL | 0.004 | −0.006 to 0.015 | 0.409 |
| HDL‐cholesterol, mg/dL | 0.000 | −0.001 to 0.000 | 0.576 |
| hsCRP, mg/L | 0.001 | −0.001 to 0.003 | 0.314 |
| Percent CD57+ in CD8+ T cellsa | 0.037 | 0.001 to 0.073 | 0.047 |
| CD8+CD28null T cells (R 2=0.512) | |||
| Age, y | 0.004 | 0.003 to 0.005 | <0.001 |
| Female sex | −0.021 | −0.040 to −0.002 | 0.031 |
| DM history | 0.025 | 0.009 to 0.040 | 0.003 |
| Smoking history | 0.007 | −0.005 to 0.020 | 0.249 |
| BMI, kg/m2 | −0.002 | −0.004 to 0.001 | 0.149 |
| SBP, mm Hg | 0.002 | 0.001 to 0.002 | <0.001 |
| Creatinine, mg/dL | 0.005 | −0.006 to 0.015 | 0.404 |
| HDL‐cholesterol, mg/dL | 0.000 | −0.001 to 0.000 | 0.576 |
| hsCRP, mg/L | 0.001 | −0.001 to 0.003 | 0.323 |
| Percent CD28null in CD8+ T cellsa | 0.029 | −0.004 to 0.062 | 0.086 |
BMI indicates body mass index; CI, confidence interval; DM, diabetes mellitus; HDL, high‐density lipoprotein; hsCRP, high‐sensitivity C‐reactive protein; PWV, pulse wave velocity; SBP, systolic blood pressure.
Log‐transformed.
Cytomegalovirus pp65‐Specific T Cells Are More Frequently Observed in the CD57+ T‐Cell Population
Because a major population within CD8+CD57+ T cells is known to be reactive to cytomegalovirus,30 we analyzed cytomegalovirus‐specific immune response parameters. First, cytomegalovirus serostatus was evaluated in a subgroup of 123 individuals, and all of these individuals were seropositive for cytomegalovirus (data not shown). This result confirms previous reports showing high (96–98%) cytomegalovirus seroprevalence in Korean adults.31, 32 Next, we investigated cytomegalovirus‐specific T‐cell responses by stimulating peripheral blood mononuclear cells with overlapping peptides covering cytomegalovirus pp65 protein in all 415 individuals. T‐cell function was evaluated using intracellular cytokine staining of IFN‐γ and TNF‐α. In addition, cytotoxic function of T cells was simultaneously evaluated by CD107a staining, which measures the degranulation of cytotoxic proteins such as perforin and granzymes.27 Figure 2A shows representative flow cytometry plots for cytomegalovirus pp65‐specific IFN‐γ and TNF‐α secretion and CD107a staining in the CD8+ T‐cell population against CD57 expression. Figure S1 shows the specificity of this assay by representative flow cytometry plots with or without stimulation with cytomegalovirus pp65 overlapping peptides. As previously found, cytomegalovirus pp65‐specific T cells were more frequent in the CD8+CD57+ population than in the CD8+CD57− population (Figure 2B), and the degree of cytomegalovirus pp65‐specific CD8+ T‐cell responses was positively correlated with increasing age (Figure 2C). Interestingly, cytomegalovirus pp65‐specifically IFN‐γ‐producing cells represented >10% of the CD8+CD57+ T‐cell population in certain subjects (Figure 2B). We demonstrated that a major population of CD8+CD57+ T cells was derived from cytomegalovirus infection and that cytomegalovirus‐specific CD8+ T cells are preferentially present in the senescent CD57+ population.
Figure 2.
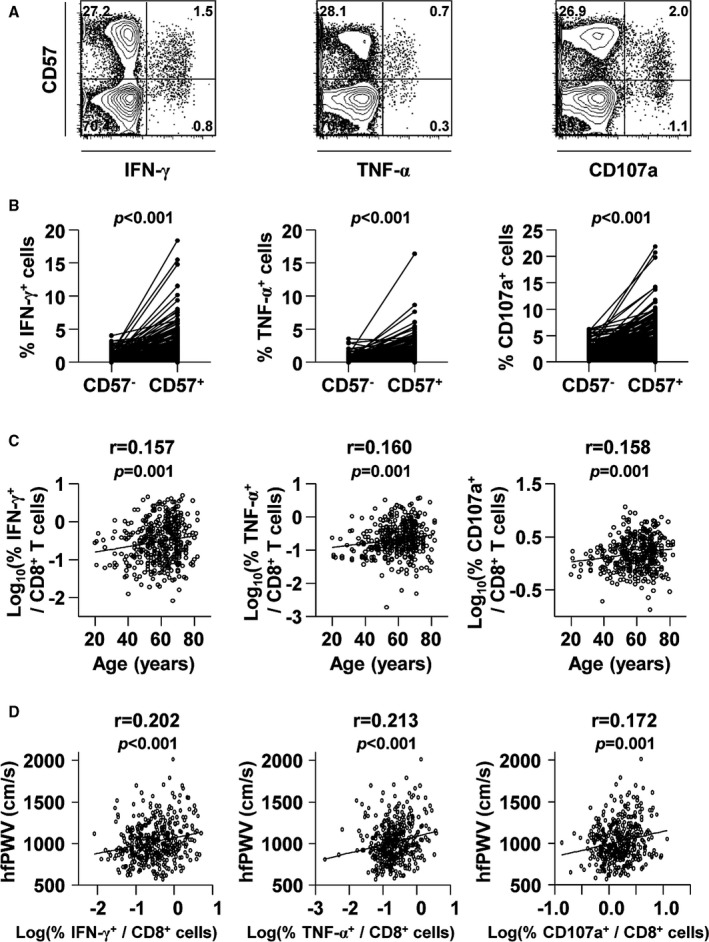
The significance of cytomegalovirus pp65‐specific CD8+ T‐cell responses in the CD57+ population and PWV. Peripheral blood mononuclear cells were stimulated with cytomegalovirus pp65 overlapping peptides, and intracellular staining for IFN‐γ, TNF‐α, and CD107a was performed. A, Representative flow cytometry plots are presented for IFN‐γ and TNF‐α secretion and for CD107a expression in the CD8+ T‐cell populations. B, The frequencies of cytomegalovirus pp65‐specific IFN‐γ‐, TNF‐α‐, and CD107a‐expressing cells were compared between CD8+CD57+ and CD8+CD57− T‐cell populations. Cytomegalovirus pp65‐specific responses were more frequently observed in CD8+CD57+ T cells than in the CD8+CD57− population. The P value was calculated using the paired t test. C, Pearson's correlation presented as a scatterplot of age and the frequency of cytomegalovirus pp65‐specific IFN‐γ‐, TNF‐α‐, and CD107a‐expressing cells (log‐transformed) in CD8+ T cells. D, Pearson's correlation presented as a plot of hfPWV against the frequency of cytomegalovirus pp65‐specific IFN‐γ‐, TNF‐α‐, and CD107a‐expressing cells (log‐transformed) in CD8+ T cells of the overall population. hfPWV indicates heart‐femoral pulse wave velocity; IFN‐γ, interferon‐ γ; TNF‐α, tumor necrosis factor‐ α.
Cytomegalovirus‐Specific CD8+ T Cells Are Independently Correlated With Arterial Stiffness
The relationship between cytomegalovirus pp65‐specific CD8+ T‐cell responses and the degree of arterial stiffness was assessed as well. The frequencies of cytomegalovirus pp65‐specific IFN‐γ and TNF‐α secretion and CD107a staining in CD8+ T cells were positively correlated with PWV (r=0.202, P<0.001 for IFN‐γ; r=0.213, P<0.001 for TNF‐α; and r=0.172, P=0.001 for CD107a) (Figure 2D). We also performed multiple linear regression analysis of the association between cytomegalovirus pp65‐specific CD8+ T‐cell responses and PWV after adjusting for traditional cardiovascular risk factors (Table 3). The frequency of cytomegalovirus pp65‐specific IFN‐γ and TNF‐α secretion and the cytotoxic function of CD8+ T cells were independently correlated with PWV (B=0.020, 95% CI, 0.007–0.033, P=0.003 for IFN‐γ; B=0.021, 95% CI, 0.006–0.036, P=0.006 for TNF‐α; and B=0.033, 95% CI, 0.010–0.056, P=0.006 for CD107a).
Table 3.
Multiple Linear Regression Analysis of the Association Between Cytomegalovirus pp65‐Specific CD8+ T Cells and PWV
| Regression Coefficient | 95% CI | P Value | |
|---|---|---|---|
| Pp65‐specific IFN‐γ secretion (R 2=0.519) | |||
| Age, y | 0.004 | 0.004 to 0.005 | <0.001 |
| Female sex | −0.021 | −0.039 to −0.002 | 0.031 |
| DM history | 0.026 | 0.010 to 0.042 | 0.001 |
| Smoking history | 0.009 | −0.003 to 0.021 | 0.161 |
| BMI, kg/m2 | −0.002 | −0.005 to 0.000 | 0.067 |
| SBP, mm Hg | 0.002 | 0.001 to 0.002 | <0.001 |
| Creatinine, mg/dL | 0.003 | −0.008 to 0.014 | 0.579 |
| HDL‐cholesterol, mg/dL | 0.000 | −0.001 to 0.000 | 0.237 |
| hsCRP, mg/L | 0.001 | −0.001 to 0.003 | 0.361 |
| Percent IFN‐γ+ in CD8+ T cellsa | 0.020 | 0.007 to 0.033 | 0.003 |
| pp65‐specific TNF‐α secretion (R 2=0.517) | |||
| Age, y | 0.004 | 0.004 to 0.005 | <0.001 |
| Female sex | −0.021 | −0.040 to −0.002 | 0.030 |
| DM history | 0.025 | 0.010 to 0.041 | 0.002 |
| Smoking history | 0.008 | −0.004 to 0.021 | 0.181 |
| BMI, kg/m2 | −0.002 | −0.005 to 0.000 | 0.084 |
| SBP, mm Hg | 0.002 | 0.001 to 0.002 | <0.001 |
| Creatinine, mg/dL | 0.004 | −0.007 to 0.015 | 0.453 |
| HDL‐cholesterol, mg/dL | 0.000 | −0.001 to 0.000 | 0.256 |
| hsCRP, mg/L | 0.001 | −0.001 to 0.003 | 0.351 |
| Percent TNF‐α+ in CD8+ T cellsa | 0.021 | 0.006 to 0.036 | 0.006 |
| Pp65‐specific CD107a expression (R 2=0.517) | |||
| Age, y | 0.004 | 0.003 to 0.005 | <0.001 |
| Female sex | −0.021 | −0.040 to −0.002 | 0.027 |
| DM history | 0.027 | 0.011 to 0.043 | 0.001 |
| Smoking history | 0.009 | −0.004 to 0.021 | 0.174 |
| BMI, kg/m2 | −0.002 | −0.004 to 0.000 | 0.116 |
| SBP, mm Hg | 0.002 | 0.001 to 0.002 | <0.001 |
| Creatinine, mg/dL | 0.004 | −0.007 to 0.015 | 0.466 |
| HDL‐cholesterol, mg/dL | 0.000 | −0.001 to 0.000 | 0.330 |
| hsCRP, mg/L | 0.001 | −0.001 to 0.003 | 0.314 |
| Percent CD107a+ in CD8+ T cellsa | 0.033 | 0.010 to 0.056 | 0.006 |
BMI indicates body mass index; CI, confidence interval; DM, diabetes mellitus; HDL, high‐density lipoprotein; hsCRP, high‐sensitivity C‐reactive protein; IFN‐γ, interferon‐γ; PWV, pulse wave velocity; SBP, systolic blood pressure; TNF‐α, tumor necrosis factor‐α.
Log‐transformed.
Anti‐cytomegalovirus Humoral Immune Response Is Not Correlated With Arterial Stiffness
Finally, we analyzed the relationship between the anti‐cytomegalovirus humoral immune response and arterial stiffness. As described above, all individuals were positive for anti‐cytomegalovirus IgG antibody in a subgroup of 123 individuals. When we divided the individuals into 3 groups according to anti‐cytomegalovirus IgG antibody titer, no significant relationship was found between anti‐cytomegalovirus IgG antibody titer and age (Figure 3A) or PWV (Figure 3B). In addition, anti‐cytomegalovirus IgG antibody titer was not associated with the intensity of cytomegalovirus‐specific T‐cell responses (Figure 3C). In summary, in view of the immune response to cytomegalovirus, we found that arterial stiffness is related only to T‐cell‐mediated immunity and not to the humoral immune response against cytomegalovirus. Taken together, our data suggest that cytomegalovirus‐specific, replicative senescent T cells, but not cytomegalovirus‐specific antibody, might contribute to increased arterial stiffness in the general population.
Figure 3.
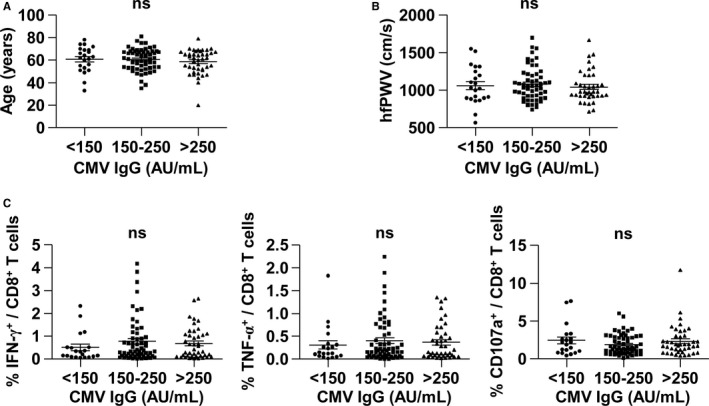
Anti‐cytomegalovirus IgG titer is not correlated with age, PWV, or cytomegalovirus pp65‐specific CD8+ T‐cell responses. Serum anti‐cytomegalovirus IgG antibody titer was assessed in a subgroup of 123 individuals. The population was divided into 3 groups according to antibody titer (<150, 150–250, and >250 AU/mL). Age (A), hfPWV (B), and cytomegalovirus pp65‐specific CD8+ T‐cell responses (C) were comparable among the 3 groups. A P<0.05 was considered significant (ANOVA). AU indicates antibody units; CMV, cytomegalovirus; hfPWV, heart‐femoral pulse wave velocity; IFN‐γ, interferon‐ γ; IgG, immunoglobulin G; ns, not significant; TNF‐α, tumor necrosis factor‐α.
Discussion
Our findings demonstrate that the frequency of senescent CD8+CD57+ T cells in peripheral blood is independently correlated with arterial stiffness. Cytomegalovirus‐specific T‐cell responses were analyzed because cytomegalovirus is a major driving antigen for replicative senescence in T cells.11 We found that cytomegalovirus‐specific CD8+ T cells were more frequently observed in the CD57+ population, and cytomegalovirus‐specific IFN‐γ and TNF‐α‐secretion and the cytotoxic degranulation of CD8+ T cells were independently associated with PWV. In the present study, we evaluated cytomegalovirus pp65‐specific T‐cell activity not only by IFN‐γ and TNF‐α staining but also by CD107a staining. CD107a molecule is exposed to the cell surface during degranulation of cytotoxic proteins such as perforin and granzymes, and therefore we can detect T cells with cytotoxic activity by adding fluorophore‐conjugated anti‐CD107a to the culture of T cells during antigen stimulation.27, 28 Our data showed that the frequency of cytomegalovirus pp65‐specific CD107a‐stained cells among CD8+ T cells was independently correlated with PWV. These data suggest that immune aging, especially T‐cell senescence that is linked to cytomegalovirus infection, might play a role in the progression of vascular aging.
Recently, several research groups have reported the existence and relevance of senescent T cells in various cardiovascular diseases. In particular, the role of senescent T cells in atherosclerotic disease has been extensively studied. Accelerated telomere shortening in T cells and other leukocyte subpopulations has been reported in patients with coronary heart disease.33 It is not yet clear whether T cells with shorter telomeres lead to increased cardiovascular risk or telomere shortening in T cells is caused by the chronic inflammation associated with cardiovascular disease. The former explanation is more likely because proinflammatory cytokines (such as interleukin‐6 and TNF‐α, which are secreted by senescent T cells) are also found in atherosclerotic plaque lesions.34 Other studies reported that increased senescent memory CD4+ T cells are associated with subclinical atherosclerosis, as assessed based on carotid artery intimal media thickness and coronary artery calcification,35 particularly in patients with rheumatoid arthritis.36 In addition, a higher frequency of CD8+CD28nullCD57+ T cells was found to be correlated with an increased prevalence of carotid artery lesions in a study of HIV‐infected patients.37 Liuzzo et al38 reported that the number of CD4+CD28null T cells increased significantly in the peripheral blood of patients with unstable angina. Furthermore, it was shown that increased frequency of CD4+CD28null T cells was associated with the recurrence of acute coronary events.39 Another study found that the presence of CD4+CD28null T cells is correlated with the occurrence of a first cardiovascular event and with a worse outcome after an acute coronary syndrome in diabetic patients.40 More recently, a direct role of T cells in hypertension has been demonstrated in angiotensin II‐infused humanized mice.41
Recent studies have suggested that cytomegalovirus infection is associated with a variety of chronic inflammatory processes in cardiovascular disease.14, 15 An increase in the number of peripheral CD8+CD57+ and CD8+CD28null T cells has been reported in patients with coronary artery disease in relation to cytomegalovirus infection.33, 42 Cytomegalovirus‐specific T‐cell responses have independently been associated with carotid atherosclerosis in HIV‐infected patients.43 In terms of prognostic implications, the occurrence of cytomegalovirus DNAemia is strongly associated with the death or readmission of patients with acute heart failure.44 Furthermore, cytomegalovirus‐seropositive subjects exhibiting high C‐reactive protein levels were at significantly higher risk for all‐cause and cardiovascular disease–related mortality among the general population.45 Cytomegalovirus infection has effects beyond atherosclerotic disease; cytomegalovirus caused a significant increase in blood pressure that was independent of atherosclerotic plaque formation in the aorta in a mouse model.16 Moreover, recent research has shown that cytomegalovirus seropositivity is independently associated with increased arterial stiffness in patients with chronic kidney disease.46
Most studies on cytomegalovirus infection or on the immune response to cytomegalovirus in humans have focused on cytomegalovirus seroprevalence according to anti‐cytomegalovirus IgG antibody titers. However, the cytomegalovirus seropositivity of nonwhites tends to be 20% to 30% higher than that of whites, and some nonwhites, especially black and Asian populations, exhibit cytomegalovirus seroprevalences approaching 100%.47 This is also the case in Koreans.31, 32 Among our study participants, the subgroup of 123 individuals exhibited 100% cytomegalovirus seropositivity; therefore, we were unable to analyze differences in the degree of arterial stiffness according to cytomegalovirus seroprevalence. Instead, we focused on cytomegalovirus‐specific T cells and found that the PWV is strongly correlated with the frequency of cytomegalovirus‐specific CD8+ T cells but is not correlated with anti‐cytomegalovirus IgG antibody titer. To the best of our knowledge, this is the first study to demonstrate an independent correlation between cytomegalovirus‐specific CD8+ T‐cell response and arterial stiffness. Considering the correlation between cytomegalovirus‐specific T cells and arterial stiffness, it is hypothesized that cytomegalovirus‐specific T cells are more important than cytomegalovirus itself during vascular pathogenesis in cytomegalovirus‐seropositive subjects.
The mechanism by which senescent T cells or cytomegalovirus‐specific T cells contribute to vascular aging has not yet been clearly identified. Considering the fact that senescent T cells produce more proinflammatory cytokines than nonsenescent T cells,48 proinflammatory cytokines such as IFN‐γ and TNF‐α might injure vessel walls directly or activate other immune cells. Indeed, senescent T cells have been shown to produce large amounts of IFN‐γ that induce macrophage activation, thereby releasing metalloproteinases that degrade the extracellular matrix.49, 50 Moreover, senescent T cells exert higher cytotoxicity than nonsenescent T cells.51 In the current study, arterial stiffness was independently correlated with frequency of CD8+CD57+ T cells, but not with CD4+CD57+ T cells. This result suggests that CD8+ T‐cell cytotoxicity might be a major effector function in increasing arterial stiffness. Homing properties of T cells need to be considered in T‐cell‐mediated vascular injury. Bolovan‐Fritts et al52 revealed that cytomegalovirus‐reactive T cells induce endothelial damage via fractalkine‐CX3C motif chemokine receptor 1 (CX3CR1) interaction. We previously confirmed that CD8+CD57+ T cells express higher levels of CX3CR1,53 suggesting that these senescent T cells might participate in endothelial dysfunction progression.
The present study has limitations. First, this is a human observational study, rather than an interventional study. Therefore, it is difficult to identify a direct cause‐and‐effect relationship. Even though the statistical methods of multivariate analysis in the current study are clear and sound, the principal correlations are relatively weak with limited explanatory power. However, the results of multivariate analysis are not a final outcome in itself, but rather serve as a bridgehead for the pathophysiologic evaluation of arterial stiffening in terms of cytomegalovirus infection. Future studies are required to address specific mechanisms by which cytomegalovirus‐specific, senescent T cells contribute to arterial stiffening. Second, we could not use live/dead cell staining in the flow cytometry analysis because of a limitation in the multicolor staining panel. However, we have confirmed that autofluorescence from dead cells in our analysis was minimal and almost negligible based on our previous experiments.
Conclusion
Arterial stiffness is associated with CD8+CD57+ T cells and cytomegalovirus‐specific IFN‐γ and TNF‐α‐secretion and with the cytotoxic degranulation of CD8+ T cells. These findings might provide a new perspective on the immune‐related mechanism of arterial stiffness and might lead to a new therapeutic strategy for arresting the progression of human hypertension. The precise role of these CD8+CD57+ T cells and cytomegalovirus‐specific CD8+ T‐cell responses warrants further investigation.
Sources of Funding
This work was supported by a grant of the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (HI13C0715), by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (NRF‐2015R1A2A2A01007346, 2015R1C1A1A02036645, 2017R1C1B1008292), by the KAIST Future Systems Healthcare Project from the Ministry of Science, ICT & Future Planning of Korea, and by Hallym University Research Fund 2017 (HURF‐2017‐24).
Disclosures
None.
Supporting information
Table S1. Summary of the Medications Taken by the Study Population (n=415)
Figure S1. Representative flow cytometry plots are presented for IFN‐γ and TNF‐α secretion and for CD107a staining in the CD8+ T‐cell populations with or without stimulation with cytomegalovirus pp65 overlapping peptides.
(J Am Heart Assoc. 2017;6:e006535 DOI: 10.1161/JAHA.117.006535.)28847915
Contributor Information
Sungha Park, Email: shpark0530@yuhs.ac.
Eui‐Cheol Shin, Email: ecshin@kaist.ac.kr.
References
- 1. Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med. 2007;204:2449–2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vinh A, Chen W, Blinder Y, Weiss D, Taylor WR, Goronzy JJ, Weyand CM, Harrison DG, Guzik TJ. Inhibition and genetic ablation of the B7/CD28 T‐cell costimulation axis prevents experimental hypertension. Circulation. 2010;122:2529–2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Trott DW, Thabet SR, Kirabo A, Saleh MA, Itani H, Norlander AE, Wu J, Goldstein A, Arendshorst WJ, Madhur MS, Chen W, Li CI, Shyr Y, Harrison DG. Oligoclonal CD8+ T cells play a critical role in the development of hypertension. Hypertension. 2014;64:1108–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Youn JC, Yu HT, Lim BJ, Koh MJ, Lee J, Chang DY, Choi YS, Lee SH, Kang SM, Jang Y, Yoo OJ, Shin EC, Park S. Immunosenescent CD8+ T cells and C‐X‐C chemokine receptor type 3 chemokines are increased in human hypertension. Hypertension. 2013;62:126–133. [DOI] [PubMed] [Google Scholar]
- 5. Weng NP, Akbar AN, Goronzy J. CD28(‐) T cells: their role in the age‐associated decline of immune function. Trends Immunol. 2009;30:306–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Strioga M, Pasukoniene V, Characiejus D. CD8+ CD28‐ and CD8+ CD57+ T cells and their role in health and disease. Immunology. 2011;134:17–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dumitriu IE, Araguas ET, Baboonian C, Kaski JC. CD4+ CD28 null T cells in coronary artery disease: when helpers become killers. Cardiovasc Res. 2009;81:11–19. [DOI] [PubMed] [Google Scholar]
- 8. Maeda T, Yamada H, Nagamine R, Shuto T, Nakashima Y, Hirata G, Iwamoto Y. Involvement of CD4+, CD57+ T cells in the disease activity of rheumatoid arthritis. Arthritis Rheum. 2002;46:379–384. [DOI] [PubMed] [Google Scholar]
- 9. Palmer BE, Blyveis N, Fontenot AP, Wilson CC. Functional and phenotypic characterization of CD57+CD4+ T cells and their association with HIV‐1‐induced T cell dysfunction. J Immunol. 2005;175:8415–8423. [DOI] [PubMed] [Google Scholar]
- 10. Palmer BE, Mack DG, Martin AK, Maier LA, Fontenot AP. CD57 expression correlates with alveolitis severity in subjects with beryllium‐induced disease. J Allergy Clin Immunol. 2007;120:184–191. [DOI] [PubMed] [Google Scholar]
- 11. Pawelec G, Derhovanessian E, Larbi A, Strindhall J, Wikby A. Cytomegalovirus and human immunosenescence. Rev Med Virol. 2009;19:47–56. [DOI] [PubMed] [Google Scholar]
- 12. Wang EC, Taylor‐Wiedeman J, Perera P, Fisher J, Borysiewicz LK. Subsets of CD8+, CD57+ cells in normal, healthy individuals: correlations with human cytomegalovirus (HCMV) carrier status, phenotypic and functional analyses. Clin Exp Immunol. 1993;94:297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Derhovanessian E, Larbi A, Pawelec G. Biomarkers of human immunosenescence: impact of cytomegalovirus infection. Curr Opin Immunol. 2009;21:440–445. [DOI] [PubMed] [Google Scholar]
- 14. Nieto FJ, Adam E, Sorlie P, Farzadegan H, Melnick JL, Comstock GW, Szklo M. Cohort study of cytomegalovirus infection as a risk factor for carotid intimal‐medial thickening, a measure of subclinical atherosclerosis. Circulation. 1996;94:922–927. [DOI] [PubMed] [Google Scholar]
- 15. Melnick JL, Adam E, DeBakey ME. Possible role of cytomegalovirus in atherogenesis. JAMA. 1990;263:2204–2207. [PubMed] [Google Scholar]
- 16. Cheng J, Ke Q, Jin Z, Wang H, Kocher O, Morgan JP, Zhang J, Crumpacker CS. Cytomegalovirus infection causes an increase of arterial blood pressure. PLoS Pathog. 2009;5:e1000427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kannel WB, Wolf PA, McGee DL, Dawber TR, McNamara P, Castelli WP. Systolic blood pressure, arterial rigidity, and risk of stroke. The Framingham study. JAMA. 1981;245:1225–1229. [PubMed] [Google Scholar]
- 18. Vaitkevicius PV, Fleg JL, Engel JH, O'Connor FC, Wright JG, Lakatta LE, Yin FC, Lakatta EG. Effects of age and aerobic capacity on arterial stiffness in healthy adults. Circulation. 1993;88:1456–1462. [DOI] [PubMed] [Google Scholar]
- 19. Laurent S, Boutouyrie P, Asmar R, Gautier I, Laloux B, Guize L, Ducimetiere P, Benetos A. Aortic stiffness is an independent predictor of all‐cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37:1236–1241. [DOI] [PubMed] [Google Scholar]
- 20. Mahmud A, Feely J. Arterial stiffness is related to systemic inflammation in essential hypertension. Hypertension. 2005;46:1118–1122. [DOI] [PubMed] [Google Scholar]
- 21. Tuttolomondo A, Di Raimondo D, Pecoraro R, Serio A, D'Aguanno G, Pinto A, Licata G. Immune‐inflammatory markers and arterial stiffness indexes in subjects with acute ischemic stroke. Atherosclerosis. 2010;213:311–318. [DOI] [PubMed] [Google Scholar]
- 22. Kaplan RC, Sinclair E, Landay AL, Lurain N, Sharrett AR, Gange SJ, Xue X, Parrinello CM, Hunt P, Deeks SG, Hodis HN. T cell activation predicts carotid artery stiffness among HIV‐infected women. Atherosclerosis. 2011;217:207–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. El Feghali RN, Topouchian JA, Pannier BM, El Assaad HA, Asmar RG; European Society of H . Validation of the OMRON M7 (HEM‐780‐E) blood pressure measuring device in a population requiring large cuff use according to the International Protocol of the European Society of Hypertension. Blood Press Monit. 2007;12:173–178. [DOI] [PubMed] [Google Scholar]
- 24. Youn JC, Kim C, Park S, Lee SH, Kang SM, Choi D, Son NH, Shin DJ, Jang Y. Adiponectin and progression of arterial stiffness in hypertensive patients. Int J Cardiol. 2013;163:316–319. [DOI] [PubMed] [Google Scholar]
- 25. Youn JC, Kim JY, Park S, Kwon J, Lee HS, Shin DH, Lee SH, Kang SM, Hoon Son N, Jang Y. Comparison of arterial stiffness indices measured by the Colins and SphygmoCor systems. Hypertens Res. 2012;35:1180–1184. [DOI] [PubMed] [Google Scholar]
- 26. Kimoto E, Shoji T, Shinohara K, Inaba M, Okuno Y, Miki T, Koyama H, Emoto M, Nishizawa Y. Preferential stiffening of central over peripheral arteries in type 2 diabetes. Diabetes. 2003;52:448–452. [DOI] [PubMed] [Google Scholar]
- 27. Alter G, Malenfant JM, Altfeld M. CD107a as a functional marker for the identification of natural killer cell activity. J Immunol Methods. 2004;294:15–22. [DOI] [PubMed] [Google Scholar]
- 28. Betts MR, Nason MC, West SM, De Rosa SC, Migueles SA, Abraham J, Lederman MM, Benito JM, Goepfert PA, Connors M, Roederer M, Koup RA. HIV nonprogressors preferentially maintain highly functional HIV‐specific CD8+ T cells. Blood. 2006;107:4781–4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Karlsson AC, Martin JN, Younger SR, Bredt BM, Epling L, Ronquillo R, Varma A, Deeks SG, McCune JM, Nixon DF, Sinclair E. Comparison of the ELISPOT and cytokine flow cytometry assays for the enumeration of antigen‐specific T cells. J Immunol Methods. 2003;283:141–153. [DOI] [PubMed] [Google Scholar]
- 30. Kern F, Khatamzas E, Surel I, Frommel C, Reinke P, Waldrop SL, Picker LJ, Volk HD. Distribution of human CMV‐specific memory T cells among the CD8pos. subsets defined by CD57, CD27, and CD45 isoforms. Eur J Immunol. 1999;29:2908–2915. [DOI] [PubMed] [Google Scholar]
- 31. Sohn YM, Park KI, Lee C, Han DG, Lee WY. Congenital cytomegalovirus infection in Korean population with very high prevalence of maternal immunity. J Korean Med Sci. 1992;7:47–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Seo S, Cho Y, Park J. Serologic screening of pregnant Korean women for primary human cytomegalovirus infection using IgG avidity test. Korean J Lab Med. 2009;29:557–562. [DOI] [PubMed] [Google Scholar]
- 33. Spyridopoulos I, Hoffmann J, Aicher A, Brummendorf TH, Doerr HW, Zeiher AM, Dimmeler S. Accelerated telomere shortening in leukocyte subpopulations of patients with coronary heart disease: role of cytomegalovirus seropositivity. Circulation. 2009;120:1364–1372. [DOI] [PubMed] [Google Scholar]
- 34. Galkina E, Ley K. Immune and inflammatory mechanisms of atherosclerosis (*). Annu Rev Immunol. 2009;27:165–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Olson NC, Doyle MF, Jenny NS, Huber SA, Psaty BM, Kronmal RA, Tracy RP. Decreased naive and increased memory CD4(+) T cells are associated with subclinical atherosclerosis: the Multi‐Ethnic Study of Atherosclerosis. PLoS One. 2013;8:e71498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Winchester R, Giles JT, Nativ S, Downer K, Zhang HZ, Bag‐Ozbek A, Zartoshti A, Bokhari S, Bathon JM. Association of elevations of specific T cell and monocyte subpopulations in rheumatoid arthritis with subclinical coronary artery atherosclerosis. Arthritis Rheumatol. 2016;68:92–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kaplan RC, Sinclair E, Landay AL, Lurain N, Sharrett AR, Gange SJ, Xue X, Hunt P, Karim R, Kern DM, Hodis HN, Deeks SG. T cell activation and senescence predict subclinical carotid artery disease in HIV‐infected women. J Infect Dis. 2011;203:452–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Liuzzo G, Kopecky SL, Frye RL, O'Fallon WM, Maseri A, Goronzy JJ, Weyand CM. Perturbation of the T‐cell repertoire in patients with unstable angina. Circulation. 1999;100:2135–2139. [DOI] [PubMed] [Google Scholar]
- 39. Liuzzo G, Biasucci LM, Trotta G, Brugaletta S, Pinnelli M, Digianuario G, Rizzello V, Rebuzzi AG, Rumi C, Maseri A, Crea F. Unusual CD4+CD28null T lymphocytes and recurrence of acute coronary events. J Am Coll Cardiol. 2007;50:1450–1458. [DOI] [PubMed] [Google Scholar]
- 40. Giubilato S, Liuzzo G, Brugaletta S, Pitocco D, Graziani F, Smaldone C, Montone RA, Pazzano V, Pedicino D, Biasucci LM, Ghirlanda G, Crea F. Expansion of CD4+CD28null T‐lymphocytes in diabetic patients: exploring new pathogenetic mechanisms of increased cardiovascular risk in diabetes mellitus. Eur Heart J. 2011;32:1214–1226. [DOI] [PubMed] [Google Scholar]
- 41. Itani HA, McMaster WG Jr, Saleh MA, Nazarewicz RR, Mikolajczyk TP, Kaszuba AM, Konior A, Prejbisz A, Januszewicz A, Norlander AE, Chen W, Bonami RH, Marshall AF, Poffenberger G, Weyand CM, Madhur MS, Moore DJ, Harrison DG, Guzik TJ. Activation of human T cells in hypertension: studies of humanized mice and hypertensive humans. Hypertension. 2016;68:123–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jonasson L, Tompa A, Wikby A. Expansion of peripheral CD8+ T cells in patients with coronary artery disease: relation to cytomegalovirus infection. J Intern Med. 2003;254:472–478. [DOI] [PubMed] [Google Scholar]
- 43. Hsue PY, Hunt PW, Sinclair E, Bredt B, Franklin A, Killian M, Hoh R, Martin JN, McCune JM, Waters DD, Deeks SG. Increased carotid intima‐media thickness in HIV patients is associated with increased cytomegalovirus‐specific T‐cell responses. AIDS. 2006;20:2275–2283. [DOI] [PubMed] [Google Scholar]
- 44. Nunez J, Chilet M, Sanchis J, Bodi V, Nunez E, Minana G, Tormo N, Clari MA, Pellicer M, Chorro FJ, Llacer A, Navarro D. Prevalence and prognostic implications of active cytomegalovirus infection in patients with acute heart failure. Clin Sci (Lond). 2010;119:443–452. [DOI] [PubMed] [Google Scholar]
- 45. Simanek AM, Dowd JB, Pawelec G, Melzer D, Dutta A, Aiello AE. Seropositivity to cytomegalovirus, inflammation, all‐cause and cardiovascular disease‐related mortality in the United States. PLoS One. 2011;6:e16103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wall NA, Chue CD, Edwards NC, Pankhurst T, Harper L, Steeds RP, Lauder S, Townend JN, Moss P, Ferro CJ. Cytomegalovirus seropositivity is associated with increased arterial stiffness in patients with chronic kidney disease. PLoS One. 2013;8:e55686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cannon MJ, Schmid DS, Hyde TB. Review of cytomegalovirus seroprevalence and demographic characteristics associated with infection. Rev Med Virol. 2010;20:202–213. [DOI] [PubMed] [Google Scholar]
- 48. Weyand CM, Brandes JC, Schmidt D, Fulbright JW, Goronzy JJ. Functional properties of CD4+ CD28‐ T cells in the aging immune system. Mech Ageing Dev. 1998;102:131–147. [DOI] [PubMed] [Google Scholar]
- 49. Leon ML, Zuckerman SH. Gamma interferon: a central mediator in atherosclerosis. Inflamm Res. 2005;54:395–411. [DOI] [PubMed] [Google Scholar]
- 50. Johnson JL. Matrix metalloproteinases: influence on smooth muscle cells and atherosclerotic plaque stability. Expert Rev Cardiovasc Ther. 2007;5:265–282. [DOI] [PubMed] [Google Scholar]
- 51. Nakajima T, Schulte S, Warrington KJ, Kopecky SL, Frye RL, Goronzy JJ, Weyand CM. T‐cell‐mediated lysis of endothelial cells in acute coronary syndromes. Circulation. 2002;105:570–575. [DOI] [PubMed] [Google Scholar]
- 52. Bolovan‐Fritts CA, Spector SA. Endothelial damage from cytomegalovirus‐specific host immune response can be prevented by targeted disruption of fractalkine‐CX3CR1 interaction. Blood. 2008;111:175–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Yu HT, Youn JC, Lee J, Park S, Chi HS, Lee J, Choi C, Park S, Choi D, Ha JW, Shin EC. Characterization of CD8(+)CD57(+) T cells in patients with acute myocardial infarction. Cell Mol Immunol. 2015;12:466–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Summary of the Medications Taken by the Study Population (n=415)
Figure S1. Representative flow cytometry plots are presented for IFN‐γ and TNF‐α secretion and for CD107a staining in the CD8+ T‐cell populations with or without stimulation with cytomegalovirus pp65 overlapping peptides.


