Abstract
Background
Lower muscle mitochondrial energy production may contribute to impaired walking endurance in patients with peripheral arterial disease. A borderline ankle‐brachial index (ABI) of 0.91 to 1.10 is associated with poorer walking endurance compared with higher ABI. We hypothesized that in the absence of peripheral arterial disease, lower ABI is associated with lower mitochondrial energy production.
Methods and Results
We examined 363 men and women participating in the Baltimore Longitudinal Study of Aging with an ABI between 0.90 and 1.40. Muscle mitochondrial energy production was assessed by post‐exercise phosphocreatine recovery rate constant (kPCr) measured by phosphorus magnetic resonance spectroscopy of the left thigh. A lower post‐exercise phosphocreatine recovery rate constant reflects decreased mitochondria energy production.The mean age of the participants was 71±12 years. A total of 18.4% had diabetes mellitus and 4% were current and 40% were former smokers. Compared with participants with an ABI of 1.11 to 1.40, those with an ABI of 0.90 to 1.10 had significantly lower post‐exercise phosphocreatine recovery rate constant (19.3 versus 20.8 ms−1, P=0.015). This difference remained significant after adjusting for age, sex, race, smoking status, diabetes mellitus, body mass index, and cholesterol levels (P=0.028). Similarly, post‐exercise phosphocreatine recovery rate constant was linearly associated with ABI as a continuous variable, both in the ABI ranges of 0.90 to 1.40 (standardized coefficient=0.15, P=0.003) and 1.1 to 1.4 (standardized coefficient=0.12, P=0.0405).
Conclusions
An ABI of 0.90 to 1.10 is associated with lower mitochondrial energy production compared with an ABI of 1.11 to 1.40. These data demonstrate adverse associations of lower ABI values with impaired mitochondrial activity even within the range of a clinically accepted definition of a normal ABI. Further study is needed to determine whether interventions in persons with ABIs of 0.90 to 1.10 can prevent subsequent functional decline.
Keywords: aging, ankle‐brachial index, mitochondrial function
Subject Categories: Peripheral Vascular Disease
Clinical Perspective
What Is New?
Borderline and low normal ankle‐brachial index values are associated with reduced thigh muscle energy production in people free of overt cardiovascular disease
This supports evidence that mildly reduced ankle‐brachial index values are associated with adverse mitochondrial characteristics.
What Are the Clinical Implications?
Future study is needed to determine whether interventions that improve lower extremity perfusion or increase mitochondrial activity can prevent functional decline in persons with an ankle‐brachial index of 0.90 to 1.40.
Introduction
In peripheral arterial disease (PAD), clinically defined as an ankle‐brachial index (ABI) <0.90, chronically reduced blood flow to the lower extremities is associated with reduction in walking endurance.1 Limited walking endurance is associated with lower mitochondrial energy production,2, 3, 4 which is attributed to impaired oxygen delivery5 as well as intrinsic mitochondrial myopathy resulting from the repeated cycles of ischemia/reperfusion injury.6
Growing literature also suggests that compared with those with a normal ABI (1.1–1.4), individuals with borderline ABI (0.90–0.99) and low normal ABI (1.00–1.09) are also more likely to have poor walking endurance, possibly attributable to subclinical atherosclerosis.7, 8, 9 Whether poorer walking performance previously observed in this group is also associated with impaired leg muscle mitochondrial function is unknown.
We hypothesize that as is the case in PAD with ABI <0.90, an ABI of 0.90 to 1.10 is also associated with lower leg muscle mitochondrial energy production compared with an ABI of 1.11 to 1.40. To test this hypothesis, we examined the association between ABI and mitochondrial energy productions, assessed by phosphorus magnetic resonance spectroscopy (31P‐MRS), in participants of the BLSA (Baltimore Longitudinal Study of Aging), free of clinically overt PAD.
Methods
Sample
We analyzed data from 363 men and women participating in the BLSA with ABIs between 0.90 and 1.40, who underwent post‐exercise phosphocreatine recovery rate (kPCr) measurement via 31P‐MRS of the thigh muscle and ABI testing. Participants were divided in 2 groups: those with an ABI of 0.90 to 1.09 and those with an ABI of 1.10 to 1.40. The overall design, study population, and measurement protocols of the BLSA have been previously reported.10 Briefly, the BLSA, established in 1958, is a continuous enrollment cohort study of community‐dwelling adults conducted by the National Institute on Aging Intramural Research Program. Participants with no major chronic conditions or functional impairments are enrolled in the study and follow‐up visits occur at intervals of 1 to 4 years, becoming more frequent for older participants. Trained and certified technicians administered all of the assessments, following standardized protocols. The institutional review board of the National Institute of Environmental Health Sciences approved the study protocol, and all participants gave written informed consent.
Ankle‐Brachial Index
Simultaneous bilateral brachial and ankle blood pressure (BP) measurements were performed as part of the routine diagnostic procedure to assess bilateral ABI using the oscillometric technique. After a 10‐minute resting period in the supine position, patients underwent 3 repeated BP measurements of both ankles and both arms taken simultaneously using a validated automatic oscillometric device (Colin VP‐2000; Colin Medical Technology). BP cuffs were applied to the arms and ankles of patients and BP was recorded in triplicates. The ABI was calculated as the average of the triplicate readings of resting ankle systolic pressure of the index leg divided by the average of the left and right arm resting brachial systolic pressure readings. We used left ABI for the primary analysis given that muscle spectroscopy was performed on the left thigh muscles; an alternative analysis was performed using the right ABI to examine for the specificity of the association with the ipsilateral readings.
31P‐Magnetic Resonance Spectroscopy
Skeletal muscle mitochondrial energy production was measured using a previously described MRI method.11 In vivo spectra of phosphorous‐containing metabolites were acquired using a 3T Philips Achieva MR scanner (Philips) and a 10‐cm 31P‐tuned surface coil (PulseTeq) fastened over the left thigh vastus lateralis muscle. Participants performed a rapid ballistic knee extension exercise while lying supine in the bore of the magnet; this maneuver was practiced before entering the magnet.11, 12 A series of pulse‐acquire 31P spectra were obtained before, during, and after the knee extension exercise. The pulse sequence consisted of adiabatic radiofrequency excitation pulses with a 90‐degree flip angle, for 300 acquisitions, with a repetition time of 1.5 seconds; signal averaging over 4 successive acquisitions for signal‐to‐noise ratio enhancement, gave effectively 75 spectra obtained with a temporal resolution of 6 seconds. The length of exercise was monitored to achieve between a 33% to 66% reduction in phosphocreatine (PCr) peak height (and never exceeded 42 seconds), with a post‐exercise recovery period of 5.8 to 6.3 minutes. Spectra were processed using jMRUI (version 5.0), and metabolites were quantified using a nonlinear least‐squares algorithm (AMARES).13, 14
Skeletal muscle oxidative ATP resynthesis rate determined by 31P‐MRS
The recovery rate for phosphocreatine was calculated by fitting the post‐exercise time‐dependent change in PCr to a mono‐exponential function of the form:
where PCr0 is the end‐of‐exercise PCr signal area (ie, the PCr signal area at the beginning of the recovery period), ΔPCr is the decrease in signal area from its pre‐exercise baseline value of PCrbaseline to PCr0 resulting from in‐magnet exercise, and τPCr is the PCr exponential recovery time constant. The inverse of τPCr, kPCr (=1/τPCr) is thus the recovery rate constant. Greater kPCr reflects shorter recovery time, and hence higher mitochondrial energy production. This is taken as an index of in vivo muscle oxidative phosphorylation capacity, as there are minimal other energy demands during this resting period. kPCr resynthesis is primarily considered a function of maximum mitochondrial ATP production with no or minimal contribution of anaerobic metabolism.15, 16, 17, 18, 19, 20 The percentage of PCr depletion was calculated as the decrease in the PCr peak area from pre‐exercise PCrbaseline to PCr0.21, 22 Intramuscular pH was also monitored (calculated according to the chemical shift of inorganic phosphate, Pi, relative to PCr) to ensure that intramuscular pH did not drop below 6.80.23
Clinical Variables
Height and weight were objectively measured per standard protocols. Body mass index was determined as kilograms per square meter. Patients were classified as current, former, or never smokers. The use of medications was determined at each study visit. Diabetes mellitus was defined as meeting 2011 American Diabetes Association criteria24 or the use of diabetes mellitus medications. Hypercholesterolemia was defined as a total serum cholesterol level of 200 mg/dL or the use of lipid‐lowering treatment.
Laboratory Studies
Fasting plasma glucose, triglyceride, total cholesterol, and high‐density lipoprotein cholesterol levels were measured. Low‐density lipoprotein cholesterol concentrations were estimated using the Friedewald formula.25
Statistical Analyses
Descriptive characteristics and kPCr values were compared between participants with an ABI of 0.90 to 1.10 and those with an ABI of 1.11 to 1.40 using Student t test and chi‐square statistics. Bivariate correlation between kPCr and left and right ABIs was performed to examine the specificity of the association between left thigh kPCr with ipsilateral versus contralateral ABI. To test whether the difference in kPCr between the 2 groups was independent of other covariates, we used general linear models with ABI as a categorical variable (ie, ABI 0.90–1.10 versus ABI 1.11–1.40) to compute and compare the least‐square means (marginal means) of kPCr for both categories adjusting for age, sex, race, smoking status, diabetes mellitus, hypertension, body mass index, and cholesterol levels. To examine whether the association between ABI and kPCr displays a threshold effect or whether it persists in an ABI range of 1.11 to 1.40, we fit linear regression models, using kPCr and ABI as continuous variables, in the larger ABI range of 0.90 to 1.40 and limited to the group with an ABI of 1.11 to 1.40. ABI was entered as a quadratic term to test for nonlinearity in the association and removed from the model when nonsignificant. All analyses were performed with SAS for Windows (version 9.3; SAS Institute Inc).
Results
Descriptive characteristics are shown on Table 1. The sample included 363 participants with a mean age of 71±12 years; 18% of the participants had diabetes mellitus and 3.2% were current and 36.9% were former smokers. A total of 37% depletion of PCr was observed with exercise with a recovery rate constant (kPCr) of 20.6 ms−1. These findings are consistent with reports from similar populations.16, 26 The average ABI was 1.16±0.08, with 82 participants (22%) having ABI of 0.90 to 1.10. Compared with participants with an ABI of 1.11 to 1.40, those with an ABI of 0.90 to 1.11 included a higher prevalence of women (73% versus 54.1%, P=0.001) and had slower PCr recovery (19.3 versus 20.8 ms−1, P=0.015), with no difference in percentage of PCr depletion. Interestingly, while left and right ABI were strongly correlated (r=0.75, P<0.001), kPCr was significantly correlated with the ipsilateral, left ABI (r=0.12, P=0.003); however, the association was weaker and marginally significant with the contralateral ABI (r=0.075, P=0.073) (Table 2). The rest of the analysis was performed using left ABI, referred to simply as ABI. Figure 1 shows that the difference in kPCr between the 2 groups remained significant using least‐square means adjusted for age, sex, race, diabetes mellitus, smoking status, body mass index, mean brachial BP, and cholesterol levels. There was no significant interaction between sex and ABI in the association with kPCr (data not shown).
Table 1.
Descriptive Characteristics
| Variable | Total (N=363) | ABI 0.9 to 1.01 (n=82) | ABI 1.1 to 1.4 (n=281) | P Value |
|---|---|---|---|---|
| Mean±SD, No. (%) | ||||
| Age | 71.0±12.7 | 71.9±14.8 | 71.3±12.1 | 0.7027 |
| Women | 212 (58.4) | 60 (73.2) | 152 (54.1) | 0.0012 |
| White race | 247 (68.0) | 56 (68.3) | 191 (67.9) | 0.6864 |
| Smoking | 0.7967 | |||
| Never | 206 (59.9) | 48 (61.5) | 161 (59.1) | |
| Former | 127 (36.9) | 28 (35.9) | 101 (37.6) | |
| Current | 11 (3.2) | 2 (2.6) | 10 (3.3) | |
| Left ABI | 1.16±0.08 | 1.05±0.04 | 1.19±0.06 | <0.0001 |
| kPCr, ms−1 | 20.6±0.005 | 19.3±5.6 | 20.8±4.7 | 0.0146 |
| PCr depletion, % | 37.7±11.1 | 37.5±11.0 | 37.5±11.1 | 0.9640 |
| Brachial SBP | 119.3±14.4 | 120.3±16.2 | 119.5±14.3 | 0.6809 |
| Hypertension | 135 (38.9) | 30 (39.5) | 105 (38.8) | 0.7059 |
| Hypertension treatment | 122 (90.0) | 28 (93.0) | 94 (89.5) | 0.4564 |
| Diabetes mellitus | 64 (18.4) | 11 (14.5) | 53 (19.6) | 0.6117 |
| Glucose, mg/dL | 90.44±16.4 | 91.4±25.2 | 90.1±12.8 | 0.5055 |
| LDL cholesterol, mg/dL | 98.7±31.7 | 103.6±32.9 | 97.0±31.4 | 0.1006 |
| HDL cholesterol, mg/dL | 62.7±17.8 | 63.1±18.1 | 62.6±17.6 | 0.8578 |
| Total cholesterol, mg/dL | 180.2±36.8 | 185.6±37.1 | 178.4±36.6 | 0.1235 |
| BMI, kg/m2 | 29.7±4.1 | 26.4±4.2 | 26.8±4.1 | 0.4083 |
ABI indicates ankle‐brachial index; BMI, body mass index; HDL, high‐density lipoprotein; kPCr, post‐exercise phosphocreatine recovery rate constant of the thigh muscle; LDL, low‐density lipoprotein; PCr, phosphocreatine recovery rate constant; SBP, systolic blood pressure.
Table 2.
Correlation Matrix
| Age | kPCr | Left ABI | Right ABI | |
|---|---|---|---|---|
| Age | 1 | −0.30731a | 0.05338 | 0.0092 |
| kPCr | 1 | 0.12282b | 0.07587 | |
| Left ABI | 1 | 0.75336a | ||
| Right ABI | 1 |
ABI indicates ankle‐brachial index; kPCr, post‐exercise phosphocreatine recovery rate constant of the thigh muscle.
P<0.0001.
P<0.001.
Figure 1.
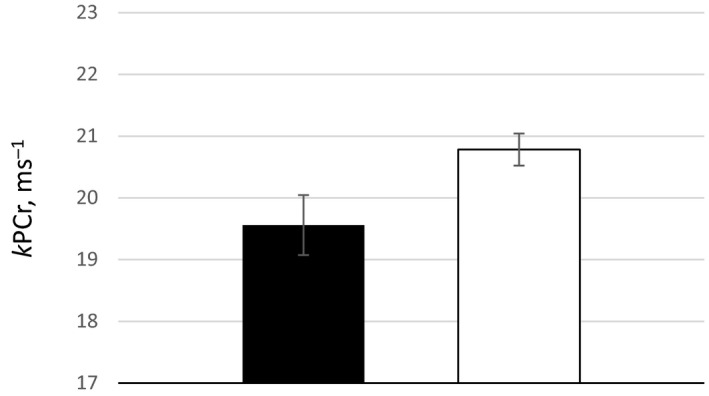
Least‐square means and standard errors of post‐exercise phosphocreatine recovery rate constant (kPCr) in patients with an ankle‐brachial index (ABI) 0.9 to 1.1 (black bar) and patients with an ABI>1.1 (white bar), adjusting for age, sex, race, diabetes mellitus, smoking, and body mass index.
To examine whether the association between ABI and kPCr has a threshold effect, we performed bivariate and multivariate linear regression analysis predicting kPCr among participants with an ABI of 0.90 to 1.40 and among those with an ABI of 1.10 to 1.40. ABI was linearly associated with kPCr in a bivariate analysis (Figure 2). Similarly, multivariate analysis showed that ABI was significantly associated with kPCr (standardized coefficient [B]=0.15, P=0.0027) adjusting for the covariates mentioned above (Table 3). The association was linear across the ABI range of 0.90 to 1.40, evidenced by the nonsignificant quadratic term for ABI in the model (data not shown). The association between ABI and kPCr remained significant when the analysis was restricted to the ABI range of 1.11 to 1.40 (Table 3).
Figure 2.
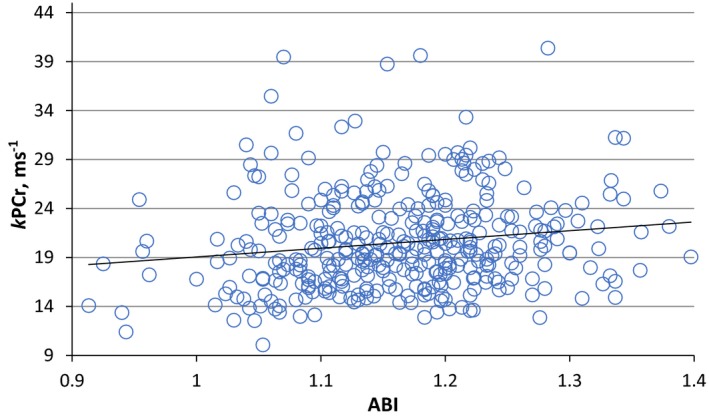
Linear regression analysis showing the association between post‐exercise phosphocreatine recovery rate constant (kPCr) of the thigh muscle with ankle‐brachial index.
Table 3.
Linear Regression Model Predicting kPCr
| ABI 0.9 to 1.4 (N=363) | ABI 1.1 to 1.4 (N=281) | |||||
|---|---|---|---|---|---|---|
| β | Ba | P Value | β | Bb | P Value | |
| Age | −0.13 | −0.28 | <0.0001 | −0.14 | −0.37 | <0.0001 |
| Men | 0.46 | 0.04 | 0.3484 | 0.93 | 0.09 | 0.1334 |
| White race | 1.91 | 0.16 | 0.0011 | 1.19 | 0.12 | 0.0492 |
| PCr depletion, % | −0.13 | −0.29 | <0.0001 | −0.12 | −0.29 | <0.0001 |
| ABI | 8.4 | 0.15 | 0.0027 | 9.0 | 0.12 | 0.0405 |
| Smoking, ever | −0.11 | −0.01 | 0.8146 | 0.14 | 0.018 | 0.7565 |
| Diabetes mellitus | 0.01 | 0.001 | 0.9982 | 0.36 | 0.03 | 0.6069 |
| BMI, kg/m2 | −0.03 | −0.03 | 0.5622 | −0.04 | −0.04 | 0.5711 |
| HR, beats per min | −0.06 | −0.11 | 0.0202 | −0.06 | −0.10 | 0.0705 |
| MAP, mm Hg | 0.03 | 0.08 | 0.085 | 0.04 | 0.08 | 0.1453 |
| LDL cholesterol, mg/dL | 0.01 | 0.05 | 0.2847 | 0.01 | 0.08 | 0.1563 |
| HDL cholesterol, mg/dL | 0.01 | 0.04 | 0.4700 | 0.01 | 0.04 | 0.5549 |
| Triglycerides, mg/dL | 0.006 | 0.05 | 0.3141 | 0.006 | 0.06 | 0.3194 |
ABI indicates ankle brachial index; BMI, body mass index; HDL, high‐density lipoprotein; HR, heart rate; kPCr, post‐exercise phosphocreatine recovery rate constant of the thigh muscle; LDL, low‐density lipoprotein; MAP, mean arterial pressure; PCr, phosphocreatine recovery rate constant.
Β, nonstandardized coefficients.
B, standardized β coefficients.
Discussion
Main Findings
This analysis shows a statically significantly lower muscle mitochondrial energy production in persons with a modestly reduced ABI of 0.90 to 1.09 compared with those with an ABI of 1.10 to 1.40. These findings suggest the possibility that a modestly lower ABI in the absence of overt PAD may have a negative association with mitochondria function. Interestingly, in this study, the positive association between ABI and lower muscle energy production was linear along the entire ABI range of 1.10 to 1.40.
Lower ABI is Linearly Associated With Reduced Mitochondrial Energy Production
These findings expand on previous reports showing a significant reduction in mitochondrial energy production in patients with PAD2, 3, 4 and suggest that even modest alterations in lower extremity perfusion may cause reduced muscle mitochondrial energy production.
The mechanism that links ABI with muscle mitochondrial energetics is not clear. There is a debate on whether the reduced mitochondrial energy production in PAD is a result of decreased perfusion and oxygen delivery during exercise or results from intrinsic mitochondrial impairment caused by repetitive cycles of ischemia‐reperfusion injury.2 Decreased perfusion via cuff‐induced stenosis causes a reduction in post‐exercise mitochondrial energy production in healthy men and women, supporting the notion that reduced oxygen delivery during exercise affects mitochondrial function.5 Further studies with assessment of leg arterial anatomy and flow are needed to examine whether a modest reduction in ABI is a result of subclinical flow limiting lesion in arteries of the lower extremities.
An additional explanation of the linear association between ABI and mitochondrial function within the “normal” range is that a modest reduction in ABI is a marker of arterial stiffness, which has been associated with universal organ dysfunction with aging, and not restricted to the legs. A normal ABI (1.10–1.40) reflects physiologic pulse pressure amplification, ie, higher systolic BP, in distal versus proximal arterial sites.8, 27 This phenomena is caused by the stiffer peripheral versus central arteries and by wave reflection hemodynamics that amplify pressure to a greater extent in distal versus proximal arterial sites. Given the profound central arterial stiffening and alterations to wave reflection dynamics with aging,28 peripheral amplification and subsequently ABI are reduced. Hence, a modest reduction in ABI within the range of 1.1 to 1.4 could also be a marker of central arterial stiffness, and not necessarily a reflection of limb‐specific flow impairment. Further studies with a larger sample size and repeated measures are needed to examine whether measures of arterial stiffness and pulse pressure amplification explain the association between ABI and mitochondrial energy production and functional capacity.
Reduced Mitochondrial Energy Production as a Potential Link Between Low ABI and Impaired Walk Endurance
McDermott et al29 have shown that an ABI between 0.90 and 1.10 was associated with reduced functional capacity measured by 6‐meter walking speed. Furthermore, an ABI of 0.90 to 1.10 was prospectively associated with a greater decline in 6‐minute walk performance as well as a greater risk for developing mobility loss over 5 years, when compared with an ABI of 1.11 to 1.40.9 These findings suggest that a gradual decline in ABI may be a marker of subclinical atherosclerotic disease in the lower extremities as well as in other organs that cumulatively affect lower extremity performance.8
Our findings suggest that a possible modest restriction of blood flow with lower ABI could potentially compromise muscle mitochondrial function and directly contribute to poorer walking function in this group. We have previously reported, using data from the same cohort, that reduced mitochondrial energy production was associated with less exercise endurance, assessed by 400‐meter walking speed.30 Future studies are needed to demonstrate whether modest reduction of ABI when associated with impaired mitochondrial function predicts the risk of developing overt PAD. In addition, our findings raise the question of whether persons with ABIs of 0.90 to 1.10 would benefit from interventions aimed at preventing further mitochondrial damage and, therefore, its functional consequences and perhaps even full development of PAD.
Strengths and Limitations
To our knowledge, this is the first study to examine the relationship between skeletal muscle mitochondrial energy production assessed by 31P‐MRS of thigh muscle and ABI over the range of ABI of 0.90 to 1.40. However, it is important to consider the study limitations. This is a cross‐sectional analysis; hence, inferences regarding causality cannot be made. The design of the BLSA prevented the inclusion of participants with PAD, which would have provided a wider range of ABI and kPCr. However, we believe that this topic has been studied in a PAD population, making the findings of this report complementary to those previously reported.
In addition, the magnitude of difference between the 2 groups is smaller compared with that reported between normal and PAD. While this is expected, it raises questions about the clinical relevance of these findings. We have previously reported, using data from this cohort, that kPCR was strongly associated with 400‐meter walking speed,30 which suggests that even such variations in this normal range might underlie subclinical alterations in function. We are in the process of collecting longitudinal data of ABI, mitochondrial function, and walking endurance from a larger sample, which will allow further elaboration on these findings.
Another limitation of our study is that mitochondrial testing was performed on the thigh muscle. This is likely to be affected only by proximal flow limitation compared with calf muscles, which are more frequently involved. Last, we measured ABI using the oscillometric rather than the auscultatory technique. This technique does not provide separate tibial and peroneal measures. However, the oscillometric technique has the clear advantage of being less operator dependent.
Perspectives
An ABI of 0.91 to 1.10 is associated with reduced mitochondrial energy production. The association between lower ABI and reduced mitochondrial energy production is also evident in the normal ABI range of 1.11 to 1.40. Further studies are needed to examine whether this subclinical decline in mitochondrial energy production is associated with future decline in walking endurance and the development of PAD symptomatology.
Sources of Funding
This research was supported by the Intramural Research Program of the National Institutes of Health, National Institute on Aging (USA).
Disclosures
None.
(J Am Heart Assoc. 2017;6:e006604 DOI: 10.1161/JAHA.117.006604.)28855165
References
- 1. McDermott MM, Liu K, Greenland P, Guralnik JM, Criqui MH, Chan C, Pearce WH, Schneider JR, Ferrucci L, Celic L, Taylor LM, Vonesh E, Martin GJ, Clark E. Functional decline in peripheral arterial disease: associations with the ankle brachial index and leg symptoms. JAMA. 2004;292:453–461. [DOI] [PubMed] [Google Scholar]
- 2. Pipinos II, Judge AR, Selsby JT, Zhu Z, Swanson SA, Nella AA, Dodd SL. The myopathy of peripheral arterial occlusive disease: part 1. Functional and histomorphological changes and evidence for mitochondrial dysfunction. Vasc Endovascular Surg. 2008;41:481–489. [DOI] [PubMed] [Google Scholar]
- 3. Esterhammer R, Schocke M, Gorny O, Posch L, Messner H, Jaschke W, Fraedrich G, Greiner A. Phosphocreatine kinetics in the calf muscle of patients with bilateral symptomatic peripheral arterial disease during exhaustive incremental exercise. Mol Imaging Biol. 10:30–39. [DOI] [PubMed] [Google Scholar]
- 4. Pipinos II, Sharov VG, Shepard AD, Anagnostopoulos PV, Katsamouris A, Todor A, Filis KA, Sabbah HN. Abnormal mitochondrial respiration in skeletal muscle in patients with peripheral arterial disease. J Vasc Surg. 2003;38:827–832. [DOI] [PubMed] [Google Scholar]
- 5. Greiner A, Esterhammer R, Bammer D, Messner H, Kremser C, Jaschke WR, Fraedrich G, Schocke MFH. High‐energy phosphate metabolism in the calf muscle of healthy humans during incremental calf exercise with and without moderate cuff stenosis. Eur J Appl Physiol. 2007;99:519–531. [DOI] [PubMed] [Google Scholar]
- 6. Bauer TA, Brass EP, Hiatt WR. Impaired muscle oxygen use at onset of exercise in peripheral arterial disease. J Vasc Surg. 2004;40:488–493. [DOI] [PubMed] [Google Scholar]
- 7. Wang JC, Criqui MH, Denenberg JO, McDermott MM, Golomb BA, Fronek A. Exertional leg pain in patients with and without peripheral arterial disease. Circulation. 2005;112:3501–3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McDermott MM, Liu K, Criqui MH, Ruth K, Goff D, Saad MF, Wu C, Homma S, Sharrett AR. Ankle‐brachial index and subclinical cardiac and carotid disease: the multi‐ethnic study of atherosclerosis. Am J Epidemiol. 2005;162:33–41. [DOI] [PubMed] [Google Scholar]
- 9. McDermott MM, Guralnik JM, Tian L, Liu K, Ferrucci L, Liao Y, Sharma L, Criqui MH. Associations of borderline and low normal ankle‐brachial index values with functional decline at 5‐year follow‐up: the WALCS (Walking and Leg Circulation Study). J Am Coll Cardiol. 2009;53:1056–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shock NW, Greulich RC, Andres R, Arenberg D, Costa PT, Lakatta EG, Tobin JD. Normal Human Aging: The Baltimore Longitudinal Study on Aging. Washington, DC: US Gov Print Off; 1984. [Google Scholar]
- 11. Choi S, Reiter DA, Shardell M, Simonsick EM, Studenski S, Spencer RG, Fishbein KW, Ferrucci L. 31P magnetic resonance spectroscopy assessment of muscle bioenergetics as a predictor of gait speed in the Baltimore Longitudinal Study of Aging. J Gerontol A Biol Sci Med Sci. 2016;71:1638–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Coen PM, Jubrias SA, Distefano G, Amati F, Mackey DC, Glynn NW, Manini TM, Wohlgemuth SE, Leeuwenburgh C, Cummings SR, Newman AB, Ferrucci L, Toledo FGS, Shankland E, Conley KE, Goodpaster BH. Skeletal muscle mitochondrial energetics are associated with maximal aerobic capacity and walking speed in older adults. J Gerontol A Biol Sci Med Sci. 2013;68:447–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vanhamme L, Van Huffel S, Van Hecke P, van Ormondt D. Time‐domain quantification of series of biomedical magnetic resonance spectroscopy signals. J Magn Reson. 1999;140:120–130. [DOI] [PubMed] [Google Scholar]
- 14. Naressi A, Couturier C, Castang I, de Beer R, Graveron‐Demilly D. Java‐based graphical user interface for MRUI, a software package for quantitation of in vivo/medical magnetic resonance spectroscopy signals. Comput Biol Med. 2001;31:269–286. [DOI] [PubMed] [Google Scholar]
- 15. Arnold DL, Matthews PM, Radda GK. Metabolic recovery after exercise and the assessment of mitochondrial function in vivo in human skeletal muscle by means of 31P NMR. Magn Reson Med. 1984;1:307–315. [DOI] [PubMed] [Google Scholar]
- 16.Edwards LM1, Tyler DJ, Kemp GJ, Dwyer RM, Johnson A, Holloway CJ, Nevill AM, Clarke K. The reproducibility of 31‐phosphorus MRS measures of muscle energetics at 3 Tesla in trained men. PLoS One. 2012;7:e37237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McCully KK, Fielding RA, Evans WJ, Leigh JS, Posner JD. Relationships between in vivo and in vitro measurements of metabolism in young and old human calf muscles. J Appl Physiol. 1993;75:813–819. [DOI] [PubMed] [Google Scholar]
- 18. Conley KE, Jubrias SA, Esselman PC. Oxidative capacity and ageing in human muscle. J Physiol. 2000;526(pt 1):203–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McCully KK, Turner TN, Langley J, Zhao Q. The reproducibility of measurements of intramuscular magnesium concentrations and muscle oxidative capacity using 31P MRS. Dyn Med. 2009;8:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Broskey NT, Greggio C, Boss A, Boutant M, Dwyer A, Schlueter L, Hans D, Gremion G, Kreis R, Boesch C, Canto C, Amati F. Skeletal muscle mitochondria in the elderly: effects of physical fitness and exercise training. J Clin Endocrinol Metab. 2014;99:1852–1861. [DOI] [PubMed] [Google Scholar]
- 21. Walsh B, Tonkonogi M, Söderlund K, Hultman E, Saks V, Sahlin K. The role of phosphorylcreatine and creatine in the regulation of mitochondrial respiration in human skeletal muscle. J Physiol. 2001;537:971–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Smith SA, Montain SJ, Zientara GP, Fielding RA. Use of phosphocreatine kinetics to determine the influence of creatine on muscle mitochondrial respiration: an in vivo 31P‐MRS study of oral creatine ingestion. J Appl Physiol. 2004;96:2288–2292. [DOI] [PubMed] [Google Scholar]
- 23. Taylor DJ, Styles P, Matthews PM, Arnold DA, Gadian DG, Bore P, Radda GK. Energetics of human muscle: exercise‐induced ATP depletion. Magn Reson Med. 1986;3:44–54. [DOI] [PubMed] [Google Scholar]
- 24. American Diabetes Association . Standards of medical care in diabetes––2011. Diabetes Care. 2011;34(suppl 1):S11–S61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low‐density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 26. Isbell DC, Berr SS, Toledano AY, Epstein FH, Meyer CH, Rogers WJ, Harthun NL, Hagspiel KD, Weltman A, Kramer CM. Delayed calf muscle phosphocreatine recovery after exercise identifies peripheral arterial disease. J Am Coll Cardiol. 2006;47:2289–2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Aboyans V, Criqui MH, Abraham P, Allison MA, Creager MA, Diehm C, Fowkes FGR, Hiatt WR, Jönsson B, Lacroix P, Marin B, McDermott MM, Norgren L, Pande RL, Preux P‐M, Stoffers HEJ, Treat‐Jacobson D. Measurement and interpretation of the ankle‐brachial index: a scientific statement from the American Heart Association. Circulation. 2012;126:2890–2909. [DOI] [PubMed] [Google Scholar]
- 28. AlGhatrif M, Lakatta EG. The Reality of Aging Viewed from the Arterial Wall In: Blood Pressure and Arterial Wall Mechanics in Cardiovascular Diseases. London, England: Springer London; 2014:137–153. [Google Scholar]
- 29. McDermott MM, Greenland P, Liu K, Guralnik JM, Celic L, Criqui MH, Chan C, Martin GJ, Schneider J, Pearce WH, Taylor LM, Clark E. The ankle brachial index is associated with leg function and physical activity: the Walking and Leg Circulation Study. Ann Intern Med. 2002;136:873–883. [DOI] [PubMed] [Google Scholar]
- 30. Zane AC, Reiter DA, Shardell M, Cameron D, Simonsick EM, Fishbein KW, Studenski SA, Spencer RG, Ferrucci L. Muscle strength mediates the relationship between mitochondrial energetics and walking performance. Aging Cell. 2017;16:461–468. [DOI] [PMC free article] [PubMed] [Google Scholar]


