Abstract
Background
Implantable cardioverter‐defibrillators (ICDs) are commonly implanted in older patients, including those with multiple comorbidities. There are few prospective studies assessing the clinical course and end‐of‐life circumstances for these patients.
Methods and Results
We prospectively followed 51 patients with ICDs for up to 18 months to longitudinally assess in terms of (1) advance care planning, (2) health status, (3) healthcare utilization, and (4) end‐of‐life circumstances through quarterly phone interviews and electronic medical record review. The mean age was 71.1±8.3, 74.5% were men, and 19.6% were non‐white. Congestive heart failure was predominant (82.4%), as was chronic kidney disease (92%). At baseline, a total of 12% of subjects met criteria for major depression, and 78.4% met criteria for mild cognitive impairment. From this initial study cohort, 76% survived to 18 months and completed all follow‐up interviews, 18% died, and 19% withdrew or were lost to follow‐up. Though living will completion and healthcare proxy assignment were common (cumulative outcome at 18 months 88% and 98%, respectively), discussions of prognosis were uncommon (baseline, 9.8%; by 18 months, 22.7%), as were conversations regarding ICD deactivation (baseline, 15.7%; by 18 months, 25.5%). Five decedents with available data received shocks in the days immediately prior to death, including 3 of whom ultimately had their ICDs deactivated prior to death.
Conclusions
We demonstrated the feasibility of prospective enrollment and follow‐up of older, vulnerable ICD patients. Early findings suggest a high burden of cognitive and psychological impairment, poor communication with providers, and frequent shocks at the end of life. These findings will inform the design of a larger cohort study designed to further explore the experiences of living and dying with an ICD in this important patient population.
Keywords: end‐of‐life care, health services research, implantable cardioverter‐defibrillator, outcomes research
Subject Categories: Health Services, Quality and Outcomes, Heart Failure, Atrial Fibrillation
Clinical Perspective
What Is New?
This study demonstrates the feasibility of prospective, longitudinal follow‐up of older patients living and dying with ICDs.
Preliminarily results suggest a high burden of cognitive and psychological impairment, poor communication with providers, and frequent shocks at the end of life.
What Are the Clinical Implications?
A larger, multicenter study will build on these findings to better define opportunities to improve the clinical care of patients with ICDs.
Introduction
Implantable cardioverter‐defibrillators (ICDs) are commonly implanted in older patients, including those with multiple comorbidities.1, 2 Over 40% of ICDs are placed in patients aged ≥70 years old, and >10% in patients ≥80.3 Even with an ICD, older patients remain at relatively high risk for short‐term mortality compared to younger patients,4 with nationwide registry data demonstrating that half will either be dead or enrolled in hospice at 5 years of follow‐up.5
Despite these findings and calls for greater research focused on patient‐centered outcomes and healthcare utilization among older cardiac patients,6, 7 the clinical course of older patients following ICD implantation remains largely unexplored. In particular, there have been few prospective studies carefully incorporating granular information on quality of life, functional status, interactions with healthcare providers, and details regarding the dying process which cannot be captured by registry or claims‐based databases.8
To address this gap, we designed the Decisions, Interventions, and Goals in ImplaNtable Cardioverter‐DefIbrillator TherapY (DIGNITY) Study: a prospective study of patients with ICDs to longitudinally assess the experience of vulnerable older patients with ICDs in terms of the following domains: (1) advance care planning, (2) health status, (3) healthcare utilization, and (4) end‐of‐life circumstances. The objective of this report is to present the results of the DIGNITY study pilot to establish the feasibility of the protocol (eg, cohort recruitment, longitudinal collection of outcomes) and generate preliminary findings supportive of a larger study.
Methods
The Beth Israel Deaconess Medical Center (BIDMC) and Hebrew SeniorLife Institute for Aging Research Institutional Review Boards both approved the conduct of this study.
Study Population
The study population included patients followed at the ambulatory cardiac device clinic at BIDMC, an academic tertiary care hospital in Boston. Patient eligibility criteria included: (1) age >18, (2) has an ICD, (3) SHOCKED score >125, (4) English speaking, and (5) sufficient cognitive ability to provide informed consent. SHOCKED is a validated risk score that uses 7 variables (age, creatinine, diabetes mellitus, ejection fraction, atrial fibrillation, chronic obstructive lung disease, and New York Heart Association (NYHA) heart failure class) to estimate mortality among cardiac patients with ICDs.4 We selected a SHOCKED cut‐off score of >125, indicating an estimated 1‐year mortality rate of 10%, because the subject matter of the study was particularly relevant to vulnerable ICD patients with a relatively increased short‐term mortality risk.
To identify eligible patients, the ambulatory device clinic schedule was reviewed monthly. Patients were first screened for age, ability to communicate in English, and presence of an ICD. For these potentially eligible patients, the electronic medical record (EMR) was queried to calculate a SHOCKED score. Patients meeting final eligibility criteria were contacted by phone by a trained research assistant (RA), who obtained verbal informed consent.
Data Collection and Variables
Data were collected at baseline and every 3 months up to 18 months by an RA from two sources: (1) EMR review and (2) telephone interviews with subjects. Subjects who could not be contacted for 2 consecutive interviews were considered lost to follow‐up with regards to interview purposes, but remained eligible for EMR reviews unless they explicitly asked to be withdrawn.
The baseline EMR review ascertained information about patient characteristics and recent healthcare utilization. Demographics variables included age, sex, race (white, black, or other), and religion (Christian, Jewish, or other). Clinical characteristics included cardiac conditions, non‐cardiac conditions, diagnostic studies, heart failure, and ICD details. Cardiac conditions included coronary artery disease, congestive heart failure, New York Heart Association (NYHA) class (I–IV), atrial fibrillation, and history of sustained ventricular tachycardia or ventricular fibrillation. Non‐cardiac conditions included diabetes mellitus, chronic obstructive lung disease, history of malignancy, and chronic kidney disease. Diagnostic studies included most recent glomerular filtration rate (mL/min) and left ventricular ejection fraction (%). ICD details included date of implant, original device type (single‐chamber, dual‐chamber, or biventricular), prior generator replacement, and history of either appropriate or inappropriate ICD therapies (anti‐tachycardia pacing or shocks).
Finally, healthcare utilization variables collected at the baseline EMR review included whether patients had experienced any of the following within the 3 months prior to enrollment: BIDMC emergency room visits, BIDMC hospitalizations, short or long‐stay nursing home admission, hospice enrollment, and office visits with a primary care physician, cardiologist, or any other physician specialist.
At each 3‐month EMR review, data were collected on new interval diagnoses of the cardiac and non‐cardiac conditions, and documented device‐related events such as generator deactivation, replacement, or new anti‐tachycardia pacing or shocks. The follow‐up EMR reviews also ascertained the aforementioned healthcare utilization variables experienced by the patient in the intervening period.
Patient deaths were identified through monthly review of the EMR. In these cases, EMR review was performed to identify the cause and location of death, code status at time of death, ICD shocks within 90 days of death, and any surgeries or interventions in the 90 days prior to death. In addition, we attempted to contact the healthcare proxy or next of kin for each decedent in order to administer a post‐death interview assessing the quality of care at the end of life and impact of the ICD on end‐of‐life experiences.
The baseline and follow‐up interviews ascertained information about the patients’ experience in the following domains: (1) advance care planning, (2) functional status, (3) depression, (4) cognitive function, and (5) protocol acceptability. Advance care planning was evaluated by assessing the presence of a living will and formally appointed healthcare proxy. Patients were also asked the extent to which they agreed or disagreed with the following two statements (asked independently, eg, not mutually exclusive): “The most important goal of my health care at this time is to extend my life as long as possible even if that requires potentially life‐prolonging medical interventions that may cause pain or discomfort”, and “The most important goal of my health care at this time is to remain as comfortable as possible even if that means avoiding potentially life‐prolonging medical interventions that may cause pain or discomfort.”
In addition, patients were asked the following questions: (1) “At the time you were deciding whether or not to get the device, did your cardiologist who placed your ICD ask you about your overall goals of care?”; (2) “Since you received your device, has your cardiologist asked you about your overall goals of care?”; (3) “Has your cardiologist talked to you about how long you are expected to live?”; (4) “At the time you received your ICD, did any healthcare professional discuss with you the possibility of turning off your defibrillator?”; (5) “Since you received your ICD, has any healthcare professional ever discussed the possibility of turning off your defibrillator with you?”, and (6) “To the best of your knowledge, can an ICD be turned off?” Patients' perceptions of the quality of the decision‐making process related to ICD implantation was further evaluated using the decision satisfaction inventory,9 (range, 0–100, with higher scores indicating greater satisfaction with decision‐making).
Functional status was assessed by asking patients to self‐rate their health as excellent, very good, good, fair, or poor. In addition, patients were asked “Do you feel full of energy?”, a component of the validated Study of Osteoporotic Frailty measure, a negative response to which indicates pre‐frailty,10, 11 and “Which of the following would you say best characterizes the impact of the ICD on your quality of life?: greatly improved, somewhat improved, no impact, somewhat worsened, or greatly worsened.” Functional status was further characterized using the Katz Activity of Daily Living (ADL)12 scale (range, 0–6, higher scores indicating greater impairment), which assesses in a binary fashion whether patients experience any impairment across a range of basic daily activities. We additionally used the Lawton Instrumental Activities of Daily Living (IADL) scale13 (range, 0–16, higher scores indicating greater impairment), which assesses more complex daily activities (such management of medication).
Depression was assessed using the Patient Health Questionnaire—8 instrument, which is a well‐validated depression screening tool widely used in cardiovascular cohorts.14, 15, 16 Questions cover mental health domains including mood, physical symptoms, and self‐care, evaluating the prevalence and frequency of specific symptoms to yield a score ranging from 0 to 24, with higher scores indicating worse symptoms and a score of >10 indicating “major depression.”17 Cognitive function was assessed using the Telephone Interview of Cognitive Status (TICS) instrument (score range 0–39, with higher scores indicating greater preservation of cognitive function), which has been well‐validated particularly to detect mild cognitive impairment.18 We used scores ≤25 to indicate mild cognitive impairment and <15 to indicate dementia.18, 19, 20 Lastly, protocol acceptability was noted by asking patients to rate their comfort with the interview questions and process.
At each 3 month interval, all interview questions were repeated except for those focusing on the initial implantation decision and discussions.
Statistical Analysis
All baseline demographic data, clinical information, and procedural variables were described using frequencies for categorical variables and means/medians with standard deviations/interquartile ranges for continuous variables.
For the Katz ADL and Lawton IADL scores, the mean and standard deviations were calculated for baseline scores, and the distribution of scores (0–6 and 0–16, respectively) across the available patient sample at each interview timepoint were calculated. Scores on the decision satisfaction inventory, PHQ‐8, and TICS‐M were summarized as medians with interquartile ranges, with proportions presented for relevant cut‐off values.
Responses to baseline and follow‐up EMR‐derived variables and interview questions were summarized as proportions. In addition, we calculated cumulative outcome rates for longitudinal assessments for measures related to advance care planning and communication. Thus, at each time period, the percentages derived included positive responses to each question of those who lived to that timepoint as well as the last response of those who had previously died or were lost to follow‐up.
Results
Study Cohort
From March 2013 to February 2015, 370 patients with ICDs were screened for eligibility, of whom 244 (65.9%) were excluded due to SHOCKED scores <125 (see Figure 1). Of the remaining eligible patients, 51 (46%) agreed to participate. Demographic and clinical features of eligible patients who declined were similar to those who enrolled.
Figure 1.
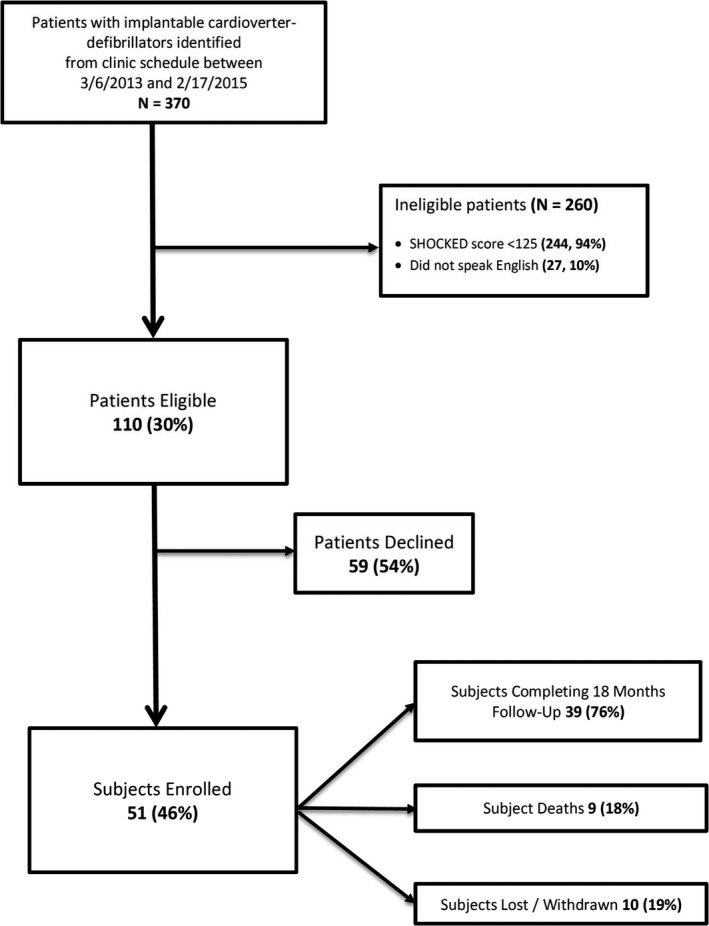
Study flow from derivation of the subject cohort through study completion. Reasons for ineligibility not were not mutually exclusive. All subjects who withdrew or were lost to follow‐up for interviews were still followed by electronic medical record review.
Table 1 presents the baseline demographic and clinical characteristics of enrolled patients ascertained from the EMR. The mean age was 71.1±8.3, 74.5% were men, and 19.6% were non‐white. Congestive heart failure was predominant (82.4%), including more than half in NYHA functional class II–III, with large proportions of patients reporting prior histories of atrial fibrillation (43.1%) and ventricular tachycardia or fibrillation (41.2%). Non‐cardiac comorbidities were also common, particularly chronic kidney disease (92%) and diabetes mellitus (45.1%). At the time of enrollment, patients were on average 5.5±4.4 years post‐implantation of their first ICD, and accordingly, 45.1% had already received at least one ICD generator replacement. The majority of ICDs (72.5%) were placed for primary prevention originally, though 29.4% of patients had received appropriate ICD therapies (eg, shocks or anti‐tachycardia pacing for ventricular tachyarrhythmia) at the time of enrollment.
Table 1.
Demographic and Clinical Characteristics of Enrolled Patients Ascertained From Electronic Medical Records at Baseline
| Characteristic | Baseline (N=51)N (%) |
|---|---|
| Demographics | |
| Age (y), mean±SD | 71.1±8.3 |
| Men | 38 (74.5%) |
| White Race | 41 (80.4%) |
| Religion | |
| Christian | 33 (64.7%) |
| Jewish | 6 (11.8%) |
| Other | 12 (23.5%) |
| Cardiac conditions | |
| Coronary artery disease | 32 (62.7%) |
| Congestive heart failure | 42 (82.4%) |
| New York Heart Association Class | |
| I | 23 (45.1%) |
| II | 14 (27.5%) |
| III | 13 (25.5%) |
| IV | 1 (2.0%) |
| Atrial fibrillation | 22 (43.1%) |
| History of sustained ventricular tachycardia/fibrillation | 21 (41.2%) |
| Noncardiac conditions | |
| Diabetes mellitus | 23 (45.1%) |
| Chronic obstructive lung disease | 8 (15.7%) |
| History of malignancy | 1 (2.0%) |
| Chronic kidney disease | 47 (92.2%) |
| Diagnostic studies | |
| Left ventricular ejection fraction (%), mean±SD | 30.0±13.1 |
| Glomerular filtration rate (mL/min), mean±SD | 44.4±17.2 |
| Device history | |
| Duration of implant (y), mean±SD | 5.5±4.4 |
| Cardiac resynchronization therapy | 12 (23.5%) |
| Primary prevention implant | 37 (72.5%) |
| Appropriate implantable cardioverter‐defibrillator therapies prior to enrollment | 15 (29.4%) |
| Implantable cardioverter‐defibrillator generator replaced prior to enrollment | 23 (45.1%) |
From this initial study cohort, 76% survived to 18 months and completed all follow‐up interviews, while 19% withdrew or were lost to follow‐up. Nine (18%) patients died during the course of follow‐up, 5 of whom had completed all follow‐up interviews for which they were eligible. A total of 319 EMR extractions (51 baseline, 268 follow‐up) and 271 phone interviews (51 baseline, 220 follow‐up) were performed.
Advance Care Planning and Goals of Care
At baseline 62.7% of patients had completed a living will, and 84% had identified a healthcare proxy. Over the course of the 18 month follow‐up period, these cumulative outcomes had increased to 88% and 98%, respectively (Table 2). At the first interview, while 56.9% of patients agreed or strongly agreed that life extension was their primary goal of care, 62.7% agreed or strongly agreed that comfort was their primary goal of care. Notably, 37% of patients indicated that both life extension and comfort were their primary goals of care. Over time, both life extension and comfort as goals of care increased (83.3% and 94.0%, respectively), though in decedents comfort was more common (88.9% versus 66.7% for life extension) at their last recorded interview.
Table 2.
Selected Responses to Telephone Interviews of Patients With ICDs Regarding Advance Care Planning and Communication at Enrollment and During Longitudinal Follow‐Up
| Interview Questions | Baseline Responses (N=51)N (%) | 3 Months (N=51) | 6 Months (N=47) | 9 Months (N=46) | 12 Month (N=45) | 15 Months (N=44) | 18 Months (N=42) | Decedents (N=09) |
|---|---|---|---|---|---|---|---|---|
| Living Will | 32 (62.7%) | 36 (70.6%) | 40 (80.0%) | 41 (82.0%) | 42 (84.0%) | 42 (84.0%) | 43 (87.8%) | 7 (77.8%) |
| Healthcare Proxy | 43 (84.3%) | 47 (92.2%) | 48 (94.1%) | 48 (94.1%) | 48 (94.1%) | 49 (96.1%) | 50 (98.0%) | 9 (100.0%) |
| Primary Goal is Life Extension | ||||||||
| Strongly agree or agree | 29 (56.9%) | 34 (66.7%) | 35 (68.6%) | 39 (78.0%) | 39 (79.6%) | 40 (81.6%) | 40 (83.3%) | 6 (66.7%) |
| Primary Goal is Comfort | ||||||||
| Strongly agree or agree | 32 (62.7%) | 40 (78.4%) | 43 (86.0%) | 46 (92.0%) | 46 (92.0%) | 46 (92.0%) | 47 (94.0%) | 8 (88.9%) |
| Cardiologist elicited goals of care | ||||||||
| At time of implantation | 28 (54.9%) | |||||||
| Since implantation | 28 (54.9%) | 36 (70.6%) | 37 (75.5%) | 39 (81.3%) | 40 (83.3%) | 40 (85.1%) | 40 (85.1%) | 5 (55.6%) |
| Cardiologist discussed prognosis | 5 (9.8%) | 6 (11.8%) | 7 (14.9%) | 9 (19.6%) | 9 (20.0%) | 9 (20.5%) | 10 (22.7%) | 2 (22.2%) |
| Discussion of ICD deactivation | 9 (17.6%) | 10 (19.6%) | 10 (19.6%) | 10 (19.6%) | 12 (23.5%) | 13 (25.5%) | 13 (25.5%) | 2 (22.2%) |
Percentages represent cumulative acquisition of a positive response to the interview question. Thus, at each time period, the data presented include the responses of those who lived to that timepoint as well as the last response of those who had previously died or were lost to follow‐up. For decedents (last column), the responses at their last interview performed are shown. ICD indicates implantable cardioverter‐defibrillators.
Only 54% of patients reported that their cardiologist had asked about these goals either at the time of ICD implantation or since, and only 9.8% reported that their cardiologist had ever discussed their prognosis with them. A minority of patients stated that their cardiologist discussed the possibility of ICD deactivation either prior to ICD implantation (5.9%) or ever (15.7%), and 53% of patients were not aware that the device could in fact be turned off. In the follow‐up period, the cumulative proportion of discussion of goals of care rose to 85.1%, whereas discussion of prognosis remained low (22.7%). Discussion of ICD deactivation rose only to 25.5% over the follow‐up period.
With regard to the decision to initially have the ICD implanted, the mean decision satisfaction inventory score was 77.7±12.3, a value corresponding to generally high satisfaction with decision‐making.9
Health Status
In terms of functional status, at baseline 59% of patients noted that they did not feel “full of energy”. Most (70.6%) patients indicated that their ICD had greatly or somewhat improved their quality of life at the initial interview, though this declined to 58.6% by 18 months of follow‐up. Patients predominantly (70.6%) described their own health status as excellent, very good, or good at baseline, with only slight variation (60–80%) recorded with repeated assessments to 18 months.
At baseline, the mean Katz ADL score was 0.53±0.83, and the mean Lawton IADL score was 1.31±2.57. At least 1 impairment (a Katz ADL score ≥1) was noted in 35% of patients, and similarly 43% had a Lawton IADL score ≥1, indicating at least some difficulty with independent daily activity. The overall proportions of patients reporting these and higher degrees of functional impairment remained similar over time (Figure 2). Regarding depression, the cohort's mean score on the PHQ‐8 score was 4.3±4.5, with 6 patients (12%) meeting the cut‐off of >10 (indicating major depression) at baseline. During longitudinal follow‐up, 5 (10%) additional people crossed this pre‐specified threshold for major depression.
Figure 2.
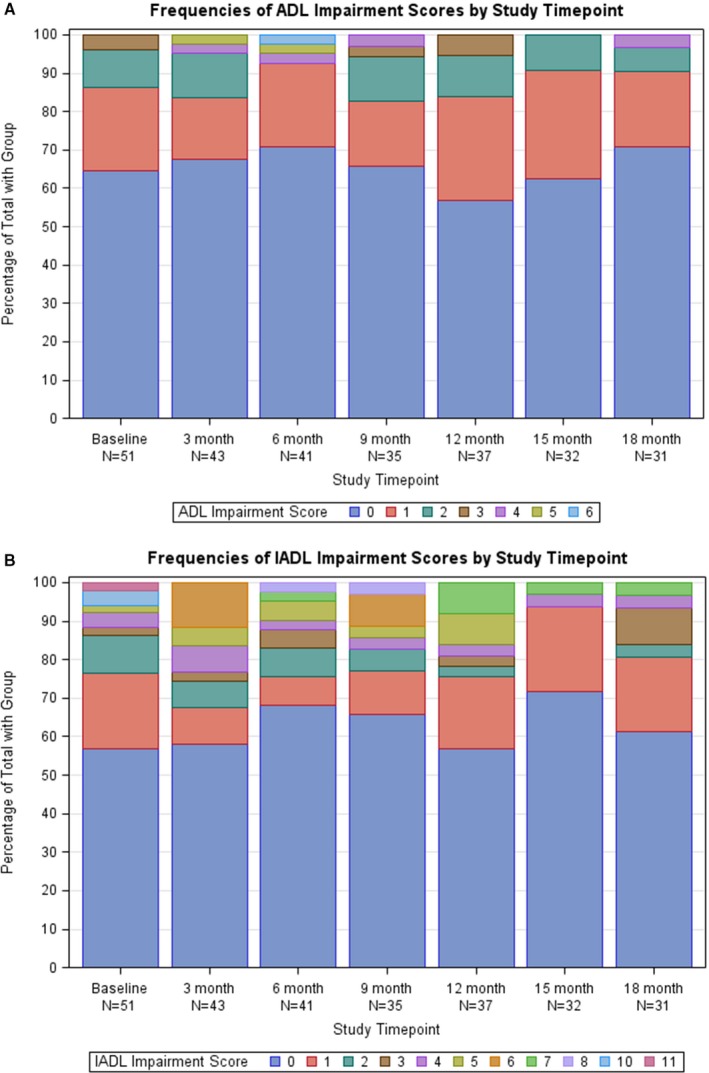
Distribution of scores according to the Katz Activity of Daily Living (A) and Lawton Instrumental Activities of Daily Living (B) scales among patients with implantable cardioverter‐defibrillators. For both scales, higher scores indicate a greater number of activities for which the patients reported impairment or required assistance. ADL indicates activity of daily living; IADL, instrumental activities of daily living.
Cognitive testing demonstrated a mean score on the TICS‐M of 20.3±4.8, with 78.4% meeting criteria for mild cognitive impairment (score <25) and 11.8% meeting the cut‐off for dementia (<15) at baseline (Table 3).
Table 3.
TICS Questionnaire Among Patients With Implantable Cardioverter‐Defibrillators, (Range 0–39, With Higher Scores Indicating Greater Preservation of Cognitive Function, <25 Indicating Mild Cognitive Impairment and <15 Indicating Dementia)
| Baseline Responses (N=51)N (%) | 3 Months (N=43) | 6 Months (N=40) | 9 Months (N=35) | 12 Months (N=37) | 15 Months (N=32) | 18 Months (N=31) | |
|---|---|---|---|---|---|---|---|
| TICS Questionnaire (mean±SD) | 21.3±4.8 | 22.9±3.6 | 25.1±4.3 | 24.7±4.3 | 26.0±4.5 | 25.9±5.3 | 25.6±5.1 |
| ≤30 | 49 (96.1%) | 42 (97.7%) | 34 (85.0%) | 33 (94.3%) | 33 (89.2%) | 27 (84.4%) | 26 (83.9%) |
| ≤25 | 40 (78.4%) | 30 (69.8%) | 20 (50.0%) | 21 (60.0%) | 14 (37.8%) | 14 (43.8%) | 13 (41.9%) |
| ≤20 | 22 (43.1%) | 13 (30.2%) | 6 (15.0%) | 5 (14.3%) | 4 (10.8%) | 4 (12.5%) | 6 (19.4%) |
| ≤15 | 6 (11.8%) | 2 (4.7%) | 0 (0.0%) | 1 (2.9%) | 1 (2.7%) | 2 (6.3%) | 1 (3.2%) |
TICS indicates telephone interview of cognitive status.
Healthcare Utilization
Contact with healthcare providers was common throughout the study. Among 325 total assessments (baseline and quarterly), 19% indicated the patient had experienced a BIDMC hospitalization or emergency room visit in the prior 3 months, 42% had at least one office visit with a primary care physician, 67% had seen their cardiologist, and 49% had appointments with other physician specialists.
Nursing home admission and hospice utilization were relatively rare. Only 6 patients were admitted temporarily to a nursing home for sub‐acute care, 2 patients were admitted to a nursing home for long‐term care, and 3 patients enrolled in hospice.
Circumstances at Death
During study follow‐up, 9 patients died. The causes and circumstances of death are noted in Table 4. Two patients’ circumstances were entirely unknown. Of the remainder, 5 of 7 patients received ICD shocks in the days immediately prior to death. Four patients died of progressive heart failure and all had their ICDs deactivated, but only after having received multiple shocks. Two patients died of sudden cardiac arrest and were shocked multiple times either by their ICDs alone or in concert with attempted resuscitation.
Table 4.
Causes and Circumstances of Death Among Patients With ICDs
| Patient | Cause of Death | Place of Death | Hospice Services at Time of Death | Code Status at Time of Death | Shocks Proximate to Dying Process | ICD Deactivated? |
|---|---|---|---|---|---|---|
| 1 | Progressive heart failure | Home | Yes | Comfort measures only | Yes—VT storm with multiple shocks in setting of progressive heart failure | Yes |
| 2 | Pulseless electrical activity arrest | Emergency Room | No | Full | Yes—Multiple shocks during dying process/ED code | No |
| 3 | Progressive heart failure | Hospital | No | Comfort measures only | Yes—Multiple shocks in last several days of life in hospital prior to ICD deactivation | Yes |
| 4 | Progressive heart failure, sepsis | Hospital | No | Comfort measures only | Yes—Multiple shocks in last several days of life in hospital prior to ICD deactivation | Yes |
| 5 | Cardiac arrest | Home | No | Full | Yes—Multiple shocks at home in setting of cardiac arrest | No |
| 6 | Progressive heart failure | Home | Yes | Comfort measures only | No | Yes |
| 7 | Liver cancer | Home | Yes | Comfort measures only | No | Yes |
| 8 | Unknown | Unknown | Unknown | Unknown | Unknown | Unknown |
| 9 | Unknown | Unknown | Unknown | Unknown | Unknown | Unknown |
ICD indicates implantable cardioverter‐defibrillators.
Protocol Acceptability
Among the 267 phone interviews performed, 56% were “very comfortable” and 39% “comfortable” with the process and questions. Only 2 (0.7%) experiences (from different patients) were characterized as “very uncomfortable”.
Discussion
This pilot prospective study demonstrates the feasibility of recruiting and longitudinally following vulnerable ICD recipients through repeated EMR review and telephone interviews. Our study protocol was well‐tolerated by participants, and our preliminary findings indicate shortcomings in advance care planning and communication between providers and patients, particularly in terms of infrequent discussions of goals of care, prognosis, and the possibility of ICD deactivation, despite very frequent contact with providers. Our prospective recording of patients’ end of life experiences demonstrates that ICD shocks immediately prior to death are common, further supporting the need for additional prospective study on the ways in which management of ICDs at the end of patients’ lives can be tailored towards their goals and quality of life.
While the granular end‐of‐life experience of patients with ICDs has received growing attention,21 prospective studies in this area have been limited. One retrospective survey of family members of decedents with ICDs (N=100) revealed that 27% of patients were shocked within the last month of life, including 8 in the final minutes of life.22 Another retrospective study leveraging ICDs explanted from decedents showed that 30% of patients were shocked within the final hours of life. In that same series, most (65%) patients with a do‐not‐resuscitate order still had active ICD therapy at the time of death. Our prospective findings build upon these worrisome data. Five of the 7 patients for whom circumstances could be described had received shocks in their final days, though 3 of these patients (and 5 overall) had their ICDs deactivated prior to death. Thus, our study reinforces the importance of prospectively characterizing the incidence and timing of ICD shocks in the dying process, evaluating the availability and use of ICD deactivation, and the broader influence of an ICD on the quality of life at the end of life and the experience of the dying process of patients and loved ones. In addition, though discussions with providers around healthcare goals became increasingly common over time in our study, discussion around prognosis and ICD deactivation were not, suggesting an important disconnect between the information presented to patients and their actual clinical needs.
The high prevalence of cognitive impairment in this cohort underscores an important aspect of their medical vulnerability, as well as a potential barrier in their ability to meaningfully participate in shared decision‐making regarding treatments and preferences. Mild cognitive impairment may be seen in >30% of heart failure patients,23 but has not been studied in ICD populations specifically. Even under ideal circumstances, decisions around ICD implantation and management remain challenged by biases and misinformation,24 and cognitive barriers will make shared decision‐making even less attainable. Cognitive impairment further challenges attempts to improve communication around ICD management, while also reinforcing the necessity of comprehensive advance care planning. Our findings that both comfort and life extension were common (and not mutually exclusive) goals of care may reflect difficulty with the presentation of the question, ambivalence about goals, or real strong preferences for both. Elucidating this under ideal circumstances may be difficult, and likely more so with cognitive deficits. Similarly, while qualitative work suggests that patients with advanced cardiac disease are willing to have discussions about prognosis and goals,25 either real or perceived cognitive impairment may make such conversations less fruitful. Thus, confirming the prevalence and spectrum of cognitive impairment among ICD recipients, and its potential influence on defining goals, decision‐making, and communication, may be useful in designing interventions such as decision aids for ICD replacement or other advanced therapies such as ventricular assist devices.26
These findings should be viewed in the context of several potential limitations. As a single‐center pilot study, our results should be considered the basis for a larger cohort, and may not generalize to ICD patients in general. Though the burden on patients was minimized, approximately half of eligible patients approached regarding our study declined. Thus, the study population may reflect subtle biases regarding their preferences for communication and engagement with their providers. Some patients withdrew or were lost to interview contact, though our use of EMR allowed for continued tracking of their clinical course. The overall prevalence of cognitive impairment was higher than we had anticipated, and this may have influenced patients’ ability to answer certain questions requiring recollection of conversations in particular. Queries regarding initial implantation discussions may be limited by recall, given the time lag between the procedure and our initial interview. While our interview instruments were designed to be as clear as possible, it is possible that some patients (particularly those with cognitive impairment) did not clearly understand specific questions, such as those regarding goals of care. We excluded patients who did not speak English, and extension of our protocol towards non‐native speakers would require significant additional effort. Longitudinal responses to some questions, such as cognitive testing, may have been affected by growing familiarity with the instrument. Our use of the EMR was limited to data from our hospital network, and thus information about events occurring elsewhere (including end‐of‐life experiences for 2 of 9 decedents) were not captured.
In summary, we demonstrated the feasibility of prospective enrollment and follow‐up of older, vulnerable ICD patients. Early findings suggest a high burden of cognitive, functional, and psychological impairment, poor communication with providers, and frequent shocks at the end of life. These findings will inform the design of a larger cohort study designed to further explore the experiences of living and dying with an ICD in this important patient population.
Sources of Funding
Dr Kramer is supported by a Paul B. Beeson Career Development Award in Aging Research (K23AG045963) and the Greenwall Faculty Scholars Program. Dr Mitchell is supported by NIH‐NIA K24AG033640. There are no other financial or commercial financial conflicts of interest related to the study topic to report.
Disclosures
Kramer serves as a consultant to the Circulatory Systems Advisory Panel of the Food and Drug Administration as well as the Baim Institute for Clinical Research (on studies unrelated to the current topic). Reynolds reports a consulting contract with Medtronic. The remaining authors have no disclosures to report.
(J Am Heart Assoc. 2017;6:e006881 DOI: 10.1161/JAHA.117.006881.)28939708
References
- 1. Kramer DB, Matlock DD, Buxton AE, Goldstein NE, Goodwin C, Green AR, Kirkpatrick JN, Knoepke C, Lampert R, Mueller PS, Reynolds MR, Spertus JA, Stevenson LW, Mitchell SL. Implantable cardioverter‐defibrillator use in older adults: proceedings of a Hartford Change AGEnts Symposium. Circ Cardiovasc Qual Outcomes. 2015;8:437–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Green AR, Leff B, Wang Y, Spatz ES, Masoudi FA, Peterson PN, Daugherty SL, Matlock DD. Geriatric conditions in patients undergoing defibrillator implantation for prevention of sudden cardiac death: prevalence and impact on mortality. Circ Cardiovasc Qual Outcomes. 2016;9:23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Epstein AE, Kay GN, Plumb VJ, McElderry HT, Doppalapudi H, Yamada T, Shafiroff J, Syed ZA, Shkurovich S. Implantable cardioverter‐defibrillator prescription in the elderly. Heart Rhythm. 2009;6:1136–1143. [DOI] [PubMed] [Google Scholar]
- 4. Bilchick KC, Stukenborg GJ, Kamath S, Cheng A. Prediction of mortality in clinical practice for Medicare patients undergoing defibrillator implantation for primary prevention of sudden cardiac death. J Am Coll Cardiol. 2012;60:1647–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kramer DB, Reynolds MR, Normand SL, Parzynski CS, Spertus JA, Mor V, Mitchell SL. Hospice use following implantable cardioverter‐defibrillator implantation in older patients: results from the National Cardiovascular Data Registry. Circulation. 2016;133:2030–2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Forman DE, Rich MW, Alexander KP, Zieman S, Maurer MS, Najjar SS, Cleveland JC Jr, Krumholz HM, Wenger NK. Cardiac care for older adults. Time for a new paradigm. J Am Coll Cardiol. 2011;57:1801–1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tinetti ME, Naik AD, Dodson JA. Moving from disease‐centered to patient goals‐directed care for patients with multiple chronic conditions: patient value‐based care. JAMA Cardiol. 2016;1:9–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Allen LA, Stevenson LW, Grady KL, Goldstein NE, Matlock DD, Arnold RM, Cook NR, Felker GM, Francis GS, Hauptman PJ, Havranek EP, Krumholz HM, Mancini D, Riegel B, Spertus JA; American Heart A, Council on Quality of C, Outcomes R, Council on Cardiovascular N, Council on Clinical C, Council on Cardiovascular R, Intervention, Council on Cardiovascular S, Anesthesia . Decision making in advanced heart failure: a scientific statement from the American Heart Association. Circulation. 2012;125:1928–1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Givens JL, Kiely DK, Carey K, Mitchell SL. Healthcare proxies of nursing home residents with advanced dementia: decisions they confront and their satisfaction with decision‐making. J Am Geriatr Soc. 2009;57:1149–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ensrud KE, Ewing SK, Taylor BC, Fink HA, Cawthon PM, Stone KL, Hillier TA, Cauley JA, Hochberg MC, Rodondi N, Tracy JK, Cummings SR. Comparison of 2 frailty indexes for prediction of falls, disability, fractures, and death in older women. Arch Intern Med. 2008;168:382–389. [DOI] [PubMed] [Google Scholar]
- 11. Ensrud KE, Ewing SK, Cawthon PM, Fink HA, Taylor BC, Cauley JA, Dam TT, Marshall LM, Orwoll ES, Cummings SR; Osteoporotic Fractures in Men Research G . A comparison of frailty indexes for the prediction of falls, disability, fractures, and mortality in older men. J Am Geriatr Soc. 2009;57:492–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. The index of ADL: a standardized measure of biological and psychosocial function. JAMA. 1963;185:914–919. [DOI] [PubMed] [Google Scholar]
- 13. Lawton MP, Brody EM. Assessment of older people: self‐maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–186. [PubMed] [Google Scholar]
- 14. Razykov I, Ziegelstein RC, Whooley MA, Thombs BD. The PHQ‐9 versus the PHQ‐8—is item 9 useful for assessing suicide risk in coronary artery disease patients? Data from the Heart and Soul Study. J Psychosom Res. 2012;73:163–168. [DOI] [PubMed] [Google Scholar]
- 15. Stafford L, Berk M, Jackson HJ. Validity of the Hospital Anxiety and Depression Scale and Patient Health Questionnaire‐9 to screen for depression in patients with coronary artery disease. Gen Hosp Psychiatry. 2007;29:417–424. [DOI] [PubMed] [Google Scholar]
- 16. Smolderen KG, Buchanan DM, Amin AA, Gosch K, Nugent K, Riggs L, Seavey G, Spertus JA. Real‐world lessons from the implementation of a depression screening protocol in acute myocardial infarction patients: implications for the American Heart Association depression screening advisory. Circ Cardiovasc Qual Outcomes. 2011;4:283–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kroenke K, Strine TW, Spitzer RL, Williams JB, Berry JT, Mokdad AH. The PHQ‐8 as a measure of current depression in the general population. J Affect Disord. 2009;114:163–173. [DOI] [PubMed] [Google Scholar]
- 18. de Jager CA, Budge MM, Clarke R. Utility of TICS‐M for the assessment of cognitive function in older adults. Int J Geriatr Psychiatry. 2003;18:318–324. [DOI] [PubMed] [Google Scholar]
- 19. Manly JJ, Schupf N, Stern Y, Brickman AM, Tang MX, Mayeux R. Telephone‐based identification of mild cognitive impairment and dementia in a multicultural cohort. Arch Neurol. 2011;68:607–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Knopman DS, Roberts RO, Geda YE, Pankratz VS, Christianson TJ, Petersen RC, Rocca WA. Validation of the telephone interview for cognitive status‐modified in subjects with normal cognition, mild cognitive impairment, or dementia. Neuroepidemiology. 2010;34:34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Russo JE. Original research: deactivation of ICDs at the end of life: a systematic review of clinical practices and provider and patient attitudes. Am J Nurs. 2011;111:26–35. [DOI] [PubMed] [Google Scholar]
- 22. Goldstein NE, Lampert R, Bradley E, Lynn J, Krumholz HM. Management of implantable cardioverter defibrillators in end‐of‐life care. Ann Intern Med. 2004;141:835–838. [DOI] [PubMed] [Google Scholar]
- 23. Yohannes AM, Chen W, Moga AM, Leroi I, Connolly MJ. Cognitive impairment in chronic obstructive pulmonary disease and chronic heart failure: a systematic review and meta‐analysis of observational studies. J Am Med Dir Assoc. 2017;18:451.e1–451.e11. [DOI] [PubMed] [Google Scholar]
- 24. Matlock DD, Jones J, Nowels CT, Jenkins A, Allen LA, Kutner JS. Evidence of cognitive bias in decision making around implantable‐cardioverter defibrillators: a qualitative framework analysis. J Card Fail. 2017; doi: 10.1016/j.cardfail.2017.03.008 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Narayan M, Jones J, Portalupi LB, McIlvennan CK, Matlock DD, Allen LA. Patient perspectives on communication of individualized survival estimates in heart failure. J Card Fail. 2017;23:272–277. [DOI] [PubMed] [Google Scholar]
- 26. McIlvennan CK, Thompson JS, Matlock DD, Cleveland JC Jr, Dunlay SM, LaRue SJ, Lewis EF, Patel CB, Walsh MN, Allen LA. A multicenter trial of a shared decision support intervention for patients and their caregivers offered destination therapy for advanced heart failure: DECIDE‐LVAD: rationale, design, and pilot data. J Cardiovasc Nurs. 2016;31:E8–E20. [DOI] [PubMed] [Google Scholar]


