Introduction
Breast cancer is the most commonly diagnosed cancer in women and the second‐leading cause of death among women with cancer.1 Whereas outcomes for many breast cancers are favorable, human epidermal growth factor receptor‐2 (HER2)‐positive breast cancers may have an aggressive clinical course and are associated with higher rates of disease recurrence and mortality.2, 3 Such tumors are characterized by overexpression of HER2 and/or amplification of the ERBB2 gene.2, 4 Development of the monoclonal antibody that targets the extracellular domain of HER2, trastuzumab, revolutionized the care of these patients, leading to large improvements in disease‐free and overall survival.5 In addition, development of newer anti‐HER2 therapies has led to further improvements in cancer outcomes for this population.6, 7, 8, 9
HER2 targeted therapies, such as trastuzumab, are generally well tolerated. They do not have significant myelosuppressive side effects nor do they cause typical symptoms associated with chemotherapy, such as emesis and alopecia. However, the safety of therapies directed at HER2, in particular trastuzumab, has been questioned by concerns regarding cardiotoxic effects.10
Clinical Presentation
Cardiac adverse effects from trastuzumab therapy involve decreases in the left ventricular systolic function with or without clinical signs and symptoms of heart failure (HF). Decreases in left ventricular ejection fraction (LVEF) typically manifest during the course of treatment and long‐term follow‐up data up to 10 years do not show evidence of late‐onset cardiac dysfunction associated with HER2 targeted therapy.11, 12, 13 Additional key features that differentiate cardiotoxicity associated with trastuzumab use from that associated with anthracycline therapy, which may occur late and be irreversible, are lack of ultrastructural changes in endomyocardial biopsy specimens, and possible reversibility of cardiac dysfunction.14, 15
Although various groups have proposed a set of criteria to define cardiotoxicity from cancer therapies (Table 1), none have been uniformly accepted.16, 17, 18, 19 The use of various definitions for cardiac adverse events in the trastuzumab trials makes direct comparison of these studies difficult and limits our understanding of the true clinical burden of cardiotoxicity associated with HER2 targeted therapies.
Table 1.
Definitions of Cardiotoxicity Used by Different Organizations
| National Cancer Institute Common Terminology Criteria for Adverse Events (HF) version 418 | ||||
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 |
| Asymptomatic elevation in biomarkers or imaging abnormalities | Symptoms with mild‐to‐moderate exertion | Symptoms with minimal exertion or at rest | Life‐threatening consequences | Death |
| Cardiac Review and Evaluation Committee (CREC)19 | ||||
| LVEF decrease >5%, to less than 55%, that is either global or more severe in the septum, with or without symptoms or HF | ||||
| American Society of Echocardiography (ASE) and European Association of Cardiovascular Imaging (EACVI)17 | ||||
| LVEF decrease >10%, to less than 53%, confirmed on repeat imaging, with or without symptoms of HF | ||||
| European Society of Cardiology (ESC)20 | ||||
| LVEF decrease >10%, to less than 50%, with or without symptoms or HF | ||||
| Trastuzumab labeling | ||||
| LVEF decreased ≥16% from baseline or LVEF decrease ≥10% to institutionally defined normal | ||||
HF indicates heart failure; LVEF, left ventricular ejection fraction.
The Benefits of HER2 Targeted Therapies
The evidence indicating increased rates of cardiotoxicity with the use of HER2 targeted therapies needs to be taken in the context of the significant cancer‐related benefits.
Trastuzumab was the first approved HER2 monoclonal antibody (approved by the US Food and Drug Administration in 1998). Initial trials showed it was well tolerated and produced durable response in patients who had failed first‐ and second‐line chemotherapy.21, 22, 23 Subsequent large, phase III trials showed significant improvements in time to treatment failure and overall mortality in patients with progressive metastatic breast cancer.5 A systematic review published in 2014 concluded that trastuzumab led to 18% and 39% improvements in overall and progression‐free survival in this population (hazard ratio, 0.82; 95% confidence interval [CI], 0.71–0.94; P=0.004; and hazard ratio, 0.61; 95% CI, 0.54–0.70; P<0.00001, respectively).24
In 2005, results of 3 large phase III clinical trials were published showing high efficacy of adjuvant trastuzumab in combination with poly‐chemotherapy for patients with early breast cancer (node positive or high‐risk node negative). In a joint analysis from the NSABP (National Surgical Adjuvant Breast and Bowel Project) trial B‐31 and NCCTG (North Central Cancer Treatment Group) trial N9831, the addition of trastuzumab to standard chemotherapy was associated with 33% and 50% improvements in overall and disease‐free survival,25 which persisted at 10 years of follow‐up.26 Similar results were reported from the HERA (Herceptin Adjuvant) trial.27 The BCIRG (Breast Cancer International Research Group) 006 trial extended these findings by showing benefit from trastuzumab regimens both with and without anthracyclines.28 Most recently, extremely low risk of recurrence was demonstrated with the use of trastuzumab and a single chemotherapy agent (paclitaxel) in patients with small, node‐negative, early‐stage breast cancer.29
Three additional HER2‐directed therapies have been approved for use in patients with breast cancer: lapatinib, pertuzumab, and ado‐trastuzumab emtansine. In the recent CLEOPATRA (A Phase III, Randomized, Double‐blind, Placebo‐controlled Clinical Trial to Evaluate the Efficacy and Safety of Pertuzumab + Trastuzumab + Docetaxel vs. Placebo + Trastuzumab + Docetaxel in Previously Untreated HER2‐positive Metastatic Breast Cancer) trial, the addition of pertuzumab to trastuzumab and chemotherapy led to dramatic improvements in overall and progression‐free survival of patients with metastatic HER2‐positive breast cancer.8 Additionally, pertuzumab is approved for use in the neoadjuvant setting in patients with early breast cancer, and a large trial evaluating its use in the adjuvant setting in combination with trastuzumab has recently reported favorable outcomes.30, 31, 32 The use of lapatinib and ado‐trastuzumab emtansine is restricted to patients with disease progression on trastuzumab in the metastatic setting.33 The addition of lapatinib lead to a 51% decrease in the risk of progression in women with advanced breast cancer that had previously progressed after treatment with trastuzumab.8 In the EMILIA (A Study of Trastuzumab Emtansine Versus Capecitabine + Lapatinib in Participants With HER2‐positive Locally Advanced or Metastatic Breast Cancer) trial, ado‐trastuzumab prolonged overall and progression‐free survival of patients with advanced breast cancer who had been previously treated with trastuzumab.9
The Cardiac Risks of HER2 Targeted Therapies
The first signal for cardiotoxicity associated with trastuzumab did not appear until the first phase III trial of the monoclonal antibody in patients with metastatic breast cancer published in 2001. In this study, risk of symptomatic or asymptomatic cardiac dysfunction was highest among individuals who received trastuzumab in combination with anthracycline and cyclophosphamide, followed by those who received the monoclonal antibody in combination with paclitaxel, as compared with either chemotherapeutic regimen alone (incidence, 27% versus 13% versus 8% versus 1%, respectively).5 Additionally, a significant proportion of these patients developed New York Heart Association class III or IV HF (incidence; 16%, 2%, 3%, and 1%, respectively).5 Such differences in the rates of cardiac dysfunction with the use of different chemotherapeutic regimens raised suspicion for a synergism between trastuzumab and anthracycline. Given these unexpectedly high rates of HF, subsequent trials of trastuzumab adopted stringent criteria for patient enrollment excluding those at increased risk for cardiac adverse events. Exclusion criteria have included: history of uncontrolled hypertension, arrhythmias, valvular disease, coronary artery disease, HF, or asymptomatic left ventricular systolic dysfunction. Enrollment of a lower‐risk population, implementation of strict protocols for monitoring of cardiac function, and changes in chemotherapeutic regimens so that anthracyclines were not given concurrently with trastuzumab or were omitted, likely explain the lower rates of cardiotoxicity observed in the subsequent trials of the monoclonal antibody.
Rates of severe HF, defined as New York Heart Association class III or IV symptoms, were 0.8% and 4.1% in the placebo and trastuzumab groups of the NSABP trial B‐31.34, 35 Similar rates were reported in the NCCTG trial N9831, whether trastuzumab was administered concomitantly or sequentially with adjuvant chemotherapy.13, 36 Although lower rates were reported in the HERA (Herceptin Adjuvant) trial with administration of the monoclonal antibody after completion of chemotherapy,37 these likely reflect differences in timing of randomization of the trials, with the latter only reporting events that occurred following completion of adjuvant chemotherapy. In the HERA and PHARE (Protocol for Herceptin as Adjuvant therapy with Reduced Exposure) trials, longer duration of therapy was associated with higher rates of cardiotoxicity.12, 38, 39 Rates of cardiotoxicity appear to be similar when trastuzumab is added to chemotherapy containing epirubicin as opposed to doxorubicin.40, 41, 42, 43 In a meta‐analysis of adjuvant trastuzumab trials published by the Cochrane group in 2012, there were 135 cases (2.5%) of HF of 5471 patients in the trastuzumab groups compared with 20 cases (0.4%) of 4810 in the control groups, yielding a statistically significant relative risk of 5.1 for development of HF in patients treated with the monoclonal antibody. Additionally, 11.2% of patients in the trastuzumab group had a decline in the LVEF as compared with 5.6% in the control group (relative risk, 1.83; 95% CI, 3.0–8.72).44
Rates of cardiotoxicity appear to be significantly lower when trastuzumab is used with regimens that do not include anthracyclines. In the BCIRG006 trial, docetaxel administered with carboplatin and trastuzumab showed similar efficacy to an anthracycline‐based regimen, while having significantly less cardiac adverse events (0.4% symptomatic HF).45 Similar rates of cardiac adverse events were reported in a recent single‐arm study of paclitaxel plus trastuzumab for small, node‐negative, HER2‐positive breast cancer (0.5% symptomatic HF).46
The combination of pertuzumab and trastuzumab does not appear to increase the risk of cardiotoxicity beyond that expected with trastuzumab alone.8 Similarly, data suggest that lapatinib has a more‐favorable cardiac risk profile than trastuzumab7, 47 and the combination of both does not appear to be more cardiotoxic than the use of trastuzumab alone.6 Trials of ado‐trastuzumab suggest low rates of cardiotoxicity during follow‐up to date.9
A summary of key trastuzumab trials, cardiac monitoring schema used in each trial, and reported rates of cardiotoxicity are provided in Table 2.
Table 2.
Summary of Key Trastuzumab Trials Including Treatment Protocols, Strategies for Monitoring for Cardiac Dysfunction, and Reported Rates of Cardiac Adverse Events
| Study | Study Arms | Monitoring Protocol | Rates of Cardiac Adverse Events | |
|---|---|---|---|---|
| Modality | Frequency | |||
| Slamon et al, NEJM, 20015 |
1. AC±Tras 2. Pac±Tras |
Not specified | Not specified |
NYHA class III or IV, or death from HF: 1. 3% vs 16% (with Tras) 2. 2% vs 1% (with Tras) Any cardiac dysfunction: 1. 8% vs 27% 2. 1% vs 13% |
| NSABP trial B‐3125, 26, 34, 35, 36 | AC+Pac±Tras (concurrent with paclitaxel) for 1 y | MUGA | Study entry, after completion of doxorubicin and cyclophosphamide, and at 6, 9, and 12 mo after randomization |
19% discontinued the medication for cardiac adverse events NYHA class III or IV, or death from HF: 0.8% vs 4.1% (with Tras) |
| NCCTG trial N983113, 25, 34, 36, 48 |
AC+Pac±Tras: 1. Sequential, for 1 y 2. Concurrent with Pac, for 1 y |
MUGA or echocardiography | Study entry, after completion of doxorubicin and cyclophosphamide and at 6, 9, and 12 mo after randomization |
NYHA class III or IV, or death from HF: 0.3% vs 2.8% (sequential Tras) vs 3.3% (concurrent Tras) |
| HERA trial12, 27, 37, 49 |
Surgery+adjuvant and/or neoadjuvant chemotherapy (94% anthracycline; 26% taxane)±radiation±sequential Tras for: 1. 2 y 2. 1 y |
MUGA or echocardiography | At baseline, 3, 6, 12, 18, 24, 30, 36, and 60 mo after randomization |
Asymptomatic decrease in LVEF ≥10%, or to <50%: 2.2% vs 7.2 (Tras for 2 y) vs 4.1% (Tras for 1 y) Severe HF (NYHA class III or IV, or cardiac death): 0.1% vs 1.0% vs 0.8% |
| Neoadjuvant trastuzumab41, 42 | Pac+FEC±concurrent Tras for 24 wk before surgery | Echocardiography |
0 symptomatic HF Asymptomatic decreases in LVEF ≥10%: 26% vs 16% (with Tras) |
|
| FinHER28 |
1. Docetaxel+FEC±concurrent Tras for 9 wk 2. Vinorelbine+FEC±concurrent Tras for 9 wk |
Echocardiography or isotope cardiography | At baseline, after last FEC cycle, at 12 and 36 mo after chemotherapy |
0% symptomatic HF in trastuzumab group >15% decrease in LVEF at any point: 6% vs 3.5% |
| PACS‐0440 |
1. FEC±sequential Tras for 1 y 2. ET±sequential Tras for 1 y |
MUGA or echocardiography | At mo 1, 2, 5, 8, and 12 during trastuzumab administration, and at 6 mo and 5 y after completion of trastuzumab |
Asymptomatic declined in LVEF >15% to <50%: 1. 14.1% vs 3.5% (with Tras) 2. 8% vs 1.6% (with Tras) Symptomatic HF: 1.5% vs 0.37% (with Tras) |
| NOAH43 | Neoadjuvant AC+Pac followed by methotrexate+fluorouracil±concurrent Tras followed by adjuvant Tras for 1 y | MUGA or echocardiography | At baseline, completion of doxorubicin+paclitaxel, completion of paclitaxel, before surgery, and end of trastuzumab treatment or 1 y from first dose of chemotherapy |
Asymptomatic decrease in LVEF >10%: 17% vs 24.5% (with Tras) Symptomatic HF: 0% vs 1.7% (with Tras) |
| BCIRG00645 |
1. ACT±concurrent Tras for 1 y 2. TCH |
MUGA or echocardiography | LVEF assessment after doxorubicin+cyclophosphamide, after the second dose of docetaxel, at the end of chemotherapy, and 3, 12 and 36 mo after randomization |
Asymptomatic decrease in LVEF >10%: 1. 11.2% vs 18.6% (with Tras) 2. 9.4% Grade 3 or 4 HF (According to NCI criteria): 1. 0.7% vs 2% (with Tras) 2. 0.4% |
| PHARE38, 39 | Standard chemotherapy (89% received anthracycline and 84% taxane)+6 mo of Tras±6 additional mo of Tras | MUGA or echocardiography | Every 3 mo during the first 2 y and then every 6 mo afterwards | Symptomatic or asymptomatic decrease in LVEF: 1.9% vs 5.7% |
| Tolaney et al, NEJM, 201529, 46 | Pac+Tras for 1 y | MUGA or echocardiography | At baseline, 12 wk, 6 mo, and 1 y | Symptomatic, grade 3 or 4 HF: 0.5% Aymptomatic decline in LVEF that lead to discontinuation of therapy: 3.2% |
AC indicates doxorubicin+cyclophosphamide; ACT, doxorubicin+cyclophosphamide+docetaxel; HF, heart failure; FEC, fluorouracil, epirubicin, cyclophosphamide; LVEF, left ventricular ejection fraction; MUGA, Multigated Acquisition Scan; NCI, National Cancer Institute; NYHA, New York Heart Association; Pac, paclitaxel; TCH, docetaxel+carboplatin+trastuzumab; Tras, trastuzumab.
Risk of Cardiotoxicity Outside of Clinical Trials
Experience outside of clinical trials suggests higher risks of cardiac toxicity associated with trastuzumab compared with that reported in clinical trials.50 In a retrospective analysis of older women with early‐stage breast cancer, compared with patients who did not receive either adjuvant chemotherapy or trastuzumab, use of trastuzumab alone or the combination of trastuzumab and anthracycline were associated with absolute increases in the adjusted incidence rate of HF or cardiomyopathy of 14% and 23.8%.51 Similarly, in a retrospective analysis of women treated for metastatic breast cancer at the MD Anderson where 5% had a history of cardiovascular disease (CVD), 26.5% of those who received HER2 targeted therapies had symptomatic HF, which was reversible in the majority of cases.52 Data from the health maintenance organization Cancer Research Network reported the cumulative incidence of HF at 1 and 5 years was 6.2% and 20.1% for women who received a combination of anthracycline and trastuzumab and 3.6% and 12.1% for women who received trastuzumab alone.50 Such cumulative incidence increased significantly, with increasing age at cancer diagnosis being as high as 40.7% among women who were aged ≥75 years and received a combination of anthracycline and trastuzumab.49 Importantly, there were significant differences in the number of comorbidities of each treatment group, which may reflect treatment selection biases by providers. The increased rates of cardiotoxicity in such observational studies likely reflect the use of trastuzumab in older populations with more‐adverse cardiac risk profiles and reduced cardiac reserve.
Risk Factors for Cardiotoxicity Associated With HER2 Targeted Therapy
Characteristics associated with increased risk of cardiotoxicity from trastuzumab use are summarized in Table 3. Past use of anthracyclines, especially at high cumulative doses (>250 mg/m2 of doxorubicin or >600 mg/m2 of epirubicin),53 appears to be the most important risk factor for subsequent cardiac dysfunction.49 In 1 retrospective analysis, past anthracycline use was the only significant predictor of trastuzumab‐induced cardiotoxicity.54 Additionally, concomitant use of anthracycline or short period (3 weeks versus 3 months) between anthracycline use and administration of trastuzumab appear to increase the risk of cardiac adverse events in some studies.14, 55 Given these observations, many cancer centers are now favoring chemotherapeutic regimens that do not contain anthracyclines when HER2 targeted therapies are used.56
Table 3.
Risk Factors for Cardiotoxicity From HER2 Targeted Therapies
| High‐Risk Characteristics |
|---|
| Anthracycline use |
| Heart failure |
| Asymptomatic systolic dysfunction at baseline (LVEF ≤50%) |
| Coronary artery disease |
| Atrial fibrillation/flutter |
| Hypertension |
| Diabetes mellitus |
| Obesity (BMI ≥30 kg/m2) |
| Dyslipidemia |
| Renal failure |
| Age ≥60 y |
BMI indicates body mass index; HER2, human epidermal growth factor receptor‐2; LVEF, left ventricular ejection fraction.
In the N9831 trial, older age (≥60 years), lower baseline LVEF, and use of antihypertensive medications were associated with increased risk of cardiotoxicity.48 In a late follow‐up of the NSABP B‐31 trial, older age and lower baseline LVEF (50–54%) were associated with trastuzumab‐induced cardiotoxicity.11 Risk of cardiotoxicity appears to increase progressively with increasing age in several studies.11, 48, 50, 57 Higher body mass index has also been shown to significantly increase the odds of cardiac dysfunction associated with anthracycline or sequential treatment with anthracycline and trastuzumab. In a meta‐analysis of 15 studies, a body mass index >25 or >30 kg/m2 was associated with 1.32 (95% CI, 1.06–1.80) and 1.47 (95% CI, 0.95–2.28) times the odds of cardiotoxicity compared with a normal body mass index.58 Cardiac risk scores have been developed to predict the risk of cardiotoxicity associated with trastuzumab, including several risk factors such as age, hypertension, diabetes mellitus, coronary artery disease, atrial fibrillation or flutter, renal dysfunction, and use of adjuvant chemotherapy.11, 57 However, lack of prospective independent validation limits the use of such tools. Importantly, the risk associated with trastuzumab use in patients with pre‐existing HF and systolic dysfunction is largely unknown.
Reports on the use of trastuzumab outside of clinical trials, in the “real‐world” setting, have suggested similar risk factors for cardiotoxicity. Among older women exposed to trastuzumab, factors associated with a higher risk of HF included black race, history of CVD, diabetes mellitus, hypertension, and renal failure.51 In the MD Anderson study, lower baseline LVEF and older age (≥60 years) were associated with increased risk of cardiotoxicity.52
Pathophysiology
HER2 belongs to a family of tyrosine kinase transmembrane receptors (ErbB1‐4) that regulate growth, differentiation, and survival of cells. After ligand binding, ErbB receptors form homodimers or heterodimers, which activate tyrosine kinase function and recruit downstream effectors. Amplification or overexpression of the ErbB2 gene occurs in ≈20% of breast cancer cases and is oncogenic.59 In tumor cells, trastuzumab binds to the subdomain IV of the extracellular domain of HER2, which blocks HER2 cleavage, stimulating antibody‐dependent cellular cytotoxicity and inhibiting HER2‐mediated mitogenic signaling.60
The mechanisms underlying cardiotoxicity from use of trastuzumab are incompletely understood. It has been long known that ErbB2 is expressed in embryonic hearts and has a critical role in cardiac development61 with relatively low expression in adult cardiomyocytes. Subsequent to the clinical trials showing cardiotoxicity from trastuzumab use, emerging research found that HER2 receptors expressed in the membranes of adult cardiomyocytes have an important role in transmitting growth and survival signals.62 In response to the ligand, neuregulin‐1, ErbB2 forms heterodimers that activate cell hypertrophy and survival pathways through activation of the phosphoinositide 3‐kinase and protein kinase A pathways as well as the mitogen‐activated protein kinase cascade.60, 63 In murine models, deletion of ErbB2 leads to development of spontaneous dilated cardiomyopathy and makes these mice more sensitive to triggers of cardiomyopathy such as pressure overload and anthracyclines.64, 65 Taken together, this evidence suggests an important role of ErbB2 in the maintenance of normal cardiac structure and function, especially under stress conditions.
A major risk factor for cardiotoxicity associated with trastuzumab is use of anthracycline‐containing chemotherapy. Given the important protective role of ErbB2 in stress conditions, a “2‐hit” model has been postulated as being responsible for the synergism between anthracycline and trastuzumab in causing cardiac dysfunction. In this model, anthracyclines activate cardiac stress pathways through several mechanisms that include generation of reactive oxygen species and oxidative damage of cardiomyocytes,66 and inhibition of topoisomerase 2β leading to double‐stranded breaks in DNA.67 Concomitant ErbB2 inhibition disrupts cardioprotective and prosurvival signaling, diminishing the heart's ability to tolerate noxious stimuli and recover.68, 69, 70, 71 Indeed, preclinical studies showed that activation of ErbB2 by recombinant neuregulin‐1 protected cardiomyocytes from the myofibrillar disarray caused by anthracyclines,72 whereas administration of an ErbB2 antibody increased susceptibility of myofilaments to doxorubicin.73
HER2 targeted therapies currently available have different mechanisms of action that might underlie the variable risks of cardiotoxicity. Ado‐trastuzumab emtansine is a combination of trastuzumab with a cytotoxic agent that allows intracellular drug delivery that is specific to HER2‐overexpressing cells and is therefore associated with low rates of cardiotoxicity.9 Pertuzumab is a similar antibody to trastuzumab, but binds to a different HER2 epitope, subdomain II. After binding, it prevents its dimerization with HER3, which causes similar activation of antibody‐dependent cellular cytotoxicity and prevention of HER2 downstream signaling.74 Because trastuzumab and pertuzumab bind to different receptor subdomains, they have complementary mechanisms of action and clinical synergism without increased cardiotoxicity.8, 75 Lapatinib is an oral small molecule that inhibits the tyrosine kinases of HER2 and epidermal growth factor receptor type 1 (HER1). Clinical studies have not demonstrated significant cardiotoxicity associated with use of lapatinib.7, 47
Monitoring for Cardiac Dysfunction During HER2‐Targeted Therapy
The ideal modality, frequency, and duration of monitoring for cardiac dysfunction during HER2 targeted therapy are unknown. Table 4 summarizes the published recommendations by major societies.17, 20, 53, 76, 77 Clinical trials have used various monitoring protocols, including echocardiography or multigated acquisition (MUGA) scan, none of which has been prospectively validated. Despite that, routine cardiac monitoring with either echocardiogram or MUGA is recommended in the labeling of trastuzumab.
Table 4.
Recommendations for Surveillance for Cardiac Dysfunction According to Major Societies
| Society | Modality of Choice | Frequency of Monitoring |
|---|---|---|
| American Society of Clinical Oncology (ASCO)53 |
|
Frequency of surveillance should be determined by the provider based on patient's clinical characteristics. |
| American Society of Echocardiography (ASE) and European Association of Cardiovascular Imaging (EACVI)17 |
|
Every 3 mo during therapy. |
| European Society for Medical Oncology (ESMO)77 |
|
Baseline, 3, 6, 9, 12, and 18 months after initiation of treatment. For patients with metastatic disease, obtain baseline measurement and only repeat if patient develops symptoms of HF. |
| European Society of Cardiology (ESC)20 |
|
Baseline, every 3 mo during therapy, and once after completion. |
| Canadian cardiovascular Society (CCS)76 |
|
No specific recommendation. |
| Trastuzumab Labeling |
|
Baseline (immediately preceding initiation of trastuzumab), every 3 mo during and upon completion of therapy, and at every 6 mo for at least 2 y following completion of therapy. |
BNP indicates brain natriuretic peptide; LVEF, left ventricular ejection fraction; MRI, magnetic resonance imaging; MUGA, multigated acquisition.
Echocardiography is currently recommended as the modality of choice for routine monitoring and detection of cardiac dysfunction.17, 53 Volumetric assessment of LVEF using 3‐dimentional echocardiography provides a more‐accurate and reproducible measurement of LVEF than 2‐dimentional echocardiography; however, this method is not available at all centers. The biplane method of disks (modified Simpson's rule) is recommended for calculation of LVEF when 2‐dimensional echocardiography is the modality of choice.17 Assessment of LVEF by MUGA has higher accuracy and reproducibility than 2‐dimensional echocardiography.78 Limitations to the routine use of MUGA include patient exposure to radiation, lack of detailed information on right ventricular function, atrial size, valvular, or pericardial disease. Cardiac magnetic resonance is the references standard in the evaluation of cardiac volumes, mass, and function, with high inter‐ and intraobserver reproducibility.79, 80 However, its use is limited because of lesser availability and elevated operational costs. This method can be particularly helpful when echocardiographic images are suboptimal, or when LVEF assessed by other methods is borderline and discontinuation of cancer therapy is being entertained.17, 81
A growing body of research is investigating methods for early detection of subclinical cardiotoxicity that would allow for early implementation of therapies to prevent overt cardiac dysfunction and HF. Assessment of LVEF alone by either method appears insensitive to detect subclinical changes and predict subsequent cardiotoxicity. Decreases in myocardial deformation (strain) precede changes in LVEF and have been consistently predictive of cardiac dysfunction from trastuzumab.82, 83 The American Society of Echocardiography recommends global longitudinal strain measured by speckle tracking echocardiography as the modality of choice for detection of subclinical myocardial changes and risk prediction.17 A decrease of <8% from baseline is likely insignificant, whereas a relative drop of more than 15% is likely pathological.82 Importantly, there have been no studies assessing whether interventions based on changes in strain alter outcomes.
The prognostic value of several cardiac biomarkers has also been evaluated, yielding conflicting results. Cardiac troponins are well‐established markers of myocardial injury and appear to correlate best with incident cardiac dysfunction following chemotherapy. Three studies have reported that elevated and increasing troponin I following chemotherapy, particularly if such increase persists at 1 month after treatment,84 is associated with subsequent cardiac dysfunction as well as with lower likelihood of cardiac recovery in patients receiving trastuzumab.85, 86, 87 During treatment, negative cardiac troponin has a high negative predictive value; however, minute elevations can be commonly detected in patients following chemotherapy and have low positive predictive value.88 Additionally, optimal timing and frequency of measurement as well as the ideal cutoff have not yet been determined. An integrated approach using strain imaging and high‐sensitivity troponin may provide incremental value in predicting subsequent cardiac dysfunction, but needs further investigation.83 The recently published guidelines from the American Society of Clinical Oncology gives a moderate strength of recommendation for routine use of biomarkers and echocardiographic‐derived strain imaging for surveillance of cardiotoxicity, citing intermediate quality of evidence.53 Baseline N‐terminal pro–B‐type natriuretic peptide, a marker of hemodynamic stress, high‐sensitivity C‐reactive protein and growth differentiation factor‐15, markers of inflammation and oxidative stress, placental growth factor, a marker of angiogenesis, galectin‐3, a marker of fibrosis, soluble fms‐like tyrosine kinase receptor‐1, and others, have not shown significant association with future cardiac dysfunction.83, 85 Increases in N‐terminal pro–B‐type natriuretic peptide over time appear to correlate with changes in cardiac function; however, no thresholds have been established.87
Prevention and Treatment of Cardiotoxicity Associated With HER2 Targeted Therapy
Observational studies and small randomized clinical trials suggest a benefit in early initiation of angiotensin‐converting enzyme inhibitors and beta‐blockers for the prevention of cardiotoxicity.15, 89, 90, 91, 92, 93 The PRADA (Prevention of Cardiac Dysfunction During Adjuvant Breast Cancer Therapy) trial tested whether angiotensin receptor blockers and/or beta‐blockers would be effective in preventing cardiotoxicity in patients diagnosed with breast cancer receiving anthracycline‐based chemotherapy with and without trastuzumab. The study showed modest benefit of candesartan, but not metoprolol, in preventing LVEF reduction assessed by magnetic resonance imaging94; however, neither medication was effective in preventing increases in high‐sensitivity troponin, a marker of subclinical myocardinal injury. A more‐recent randomized controlled trial failed to reproduce such beneficial effects of candesartan in patients treated with trastuzumab.95 Another small trial reported modest benefit of bisoprolol and perindopril in preventing cardiac dysfunction, but not remodeling, associated with trastuzumab use.96 The ongoing SAFEHEART (Spanish Familial Hypercholesterolaemia Cohort Study) study is testing whether administration of various HER2 targeted therapies is safe among patients with mild systolic dysfunction (LVEF 40–50%) who are on appropriate HF medical therapy.97 At this time, there are insufficient data to recommend routine preventive use of angiotensin‐converting enzyme inhibitors, angiotensin receptor blockers, or beta‐blockers for patients receiving HER2 targeted therapies. Screening for and optimizing management of CVD risk factors (eg, smoking, hypertension, obesity, etc) is currently recommended as part of efforts to prevent cardiotoxicity; however, the impact of such measures on cardiotoxicity has not been evaluated.53
Management of cardiac adverse events associated with HER2 targeted treatment varied across clinical trials and different strategies have not been compared to one another. In most studies, following development of cardiac dysfunction, HER2 targeted therapy was temporarily discontinued for 4 weeks and initiation of guideline‐directed medical therapy was left at the discretion of managing providers. Partial or complete recovery was observed in the majority of cases and following recovery of systolic function most patients tolerated restarting the HER2 targeted medication. Whether discontinuation of the HER2 targeted therapy is at all necessary is not known, but could have important implications on cancer related outcomes. In one study, interruption of trastuzumab treatment was common after diagnosis of asymptomatic cardiac dysfunction and resulted in lower cumulative doses of the medication.98 Tripathy et al reported that in the trastuzumab pivotal trial published in 2001,5 33 patients continued to receive trastuzumab therapy for a median of 26 weeks after development of cardiac dysfunction, and the cardiac status of 28 (85%) either improved or remained stable.99 However, not all cases of cardiotoxicity associated with the use of the trastuzumab are reversible. In the BCIRG006, of 194 patients randomized to doxorubicin+cyclophosphamide+docetaxel+trastuzumab who had a decline of at least 10% in LVEF, the decrease persisted for at least 4 years in 33%.45 Even in the absence of anthracycline use, persistent declines in LVEF following trastuzumab administration have been reported.46 Further research is needed to help identify patients at high risk for persistence of left ventricular dysfunction for whom discontinuation of the antibody and more‐aggressive therapeutic interventions may be advised.
Recommendations
Although several guideline groups, including the American Society of Echocardiography, the American Society of Clinical Oncology, the Heart Failure Association of the European Society of Cardiology (ESC), the European Society of Cardiology, and the European Society of Medical Oncology, have published recommendations and consensus statements for the diagnosis and management of cardiotoxicity from cancer therapies17, 20, 53, 68, 77 no widely accepted international guidelines are available. In the United States, neither the American Heart Association nor the American College of Cardiology have published specific recommendations.
The authors of this review suggest that all patients diagnosed with HER2‐positive breast cancer who will be receiving HER2 targeted therapies have a baseline assessment of the risk of cardiotoxicity (Figure). Such evaluation should include a thorough history and physical exam focused on identification of existing CVD risk factors and established CVD, as well as predictors of trastuzumab cardiotoxicity such as age and anthracycline use. Use of risk scores has not been prospectively validated, but could facilitate risk/benefit assessment and discussions between patients and providers.
Figure 1.
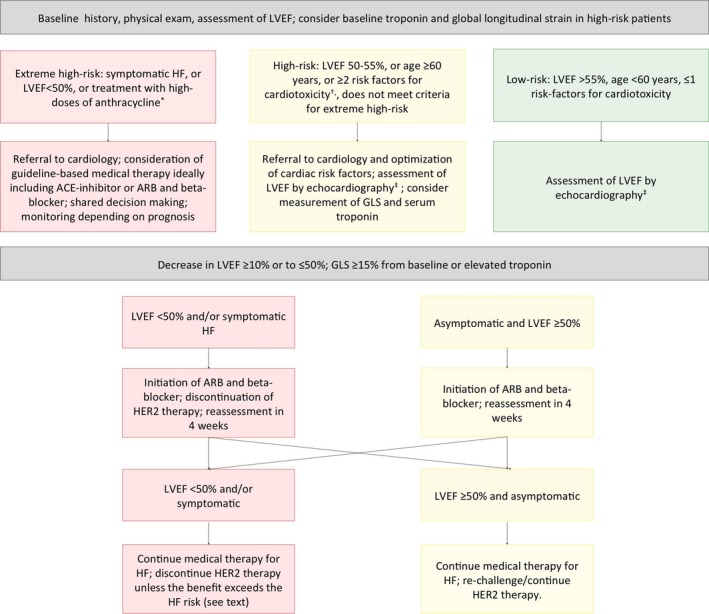
Proposed algorithm for risk stratification, monitoring, and management of cardiotoxicity during therapy with trastuzumab. *>250 mg/m2 of doxorubicin or >600 mg/m2 of epirubicin. †Refer to Table 3. ‡Assessment of LVEF at baseline, every 3 months during the first year of therapy, every 6 months during the second year of therapy, and once after completion. ACE indicates angiotensin‐converting enzyme; ARB, angiotensin receptor blocker; GLS, global longitudinal strain; HER2, human epidermal growth factor receptor‐2; HF, heart failure; LVEF, left ventricular ejection fraction.
We recommend that all patients have their LVEF assessed by echocardiography at baseline. Assessment of LVEF should be done with 3‐dimensional echocardiography, or using the 2‐dimensional biplane Simpson's method when the first is not available. MUGA is an acceptable alternative when echocardiography is not available, but providers should be mindful of the potential harms of repeated exposure to radiation. At this time there are insufficient data to suggest additional benefit from routine use of magnetic resonance imaging for assessment of cardiac function; however, this method should be considered when echocardiographic images are suboptimal, or if the LVEF as assessed by echocardiography is borderline and discontinuation of therapy is being considered. Repeat assessment of LVEF should be carried every 3 months during the first year of therapy, every 6 months during the second year of therapy, once after completion of therapy, and when clinically indicated thereafter. Measurement of global longitudinal strain by speckle tracking echocardiography and serum troponin may be considered for high‐risk patients in combination with imaging at baseline and during follow‐up.17
We recommend lifestyle modification to all patients as well as optimization of medical management of CVD risk factors. Weight gain is common following the diagnosis of breast cancer and associated with poor outcomes.100 Counseling on weight loss, avoidance of weight gain, and routine physical activity may have a particularly important role in this population. If patients with pre‐existing CVD or baseline low LVEF (<50%) are not already on an angiotensin‐converting enzyme inhibitor or angiotensin receptor blocker, and a beta‐blocker, initiation should be considered.
We suggest initiation of an angiotensin‐converting enzyme inhibitor or an angiotensin receptor blocker followed by a beta‐blocker for any evidence of significant cardiac injury, including: asymptomatic decrease in LVEF ≥10% or to ≤50%, or a relative change in global longitudinal strain ≥15%. The treating oncologist and cardiologist should collaborate to determine the course of care, but in most cases it is appropriate to temporarily discontinue HER2 targeted therapy if LVEF decreases to <50% or if the patient develops symptoms of HF. Rechallenge may be considered if there is partial or complete recovery on repeat assessment of cardiac function at 4 weeks.
There are no data on the ideal strategy for management of patients with pre‐existing cardiac conditions or with persistent left ventricular dysfunction despite discontinuation of therapy. Patients and providers should engage in a careful review of the possible risks and benefits of starting/continuing trastuzumab versus progressive cardiac dysfunction in a shared decision‐making process. The balance might favor continued therapy with the monoclonal antibody among those with advanced breast cancer, whereas the decision might be more difficult for patients with early stages of the disease. There are several factors to consider in this decision, for example: (1) the degree of cardiac dysfunction; (2) whether there is further reduction in ejection fraction with continued therapy after the initiation of HF medical therapy; (3) the degree of HF symptoms; and (4) the risk of cancer progression. The results of the ongoing SAFEHEART trial are expected to help inform this decision.
Close collaboration between oncologists and cardiologists is key for successful prevention and management of cardiotoxicity from cancer therapies. We suggest that high‐risk patients, especially those with pre‐existing CVD, and patients who develop cardiac dysfunction be referred for consultation with a cardiologist, ideally someone with cardio‐oncology expertise. Clinical decisions about discontinuation of therapy ought to be informed by both providers and shared with patients, in a collaborative process.
Conclusions
Trastuzumab has contributed to significant improvements in outcomes of patients with HER2‐positive breast cancer over the past 15 years. The addition of the monoclonal antibody, trastuzumab, is associated with 20% and 34% improvements in overall survival of patients with metastatic and locally advanced disease, and recent evidence suggests improved outcomes in patients with early, node‐negative malignancies. Trastuzumab is associated with increased rates of symptomatic and asymptomatic cardiac dysfunction, particularly if administered in older patients, those with pre‐existing CVD risk factors or CVD, and those receiving anthracycline‐based chemotherapy. Such risk can be significantly mitigated by the use of less‐cardiotoxic chemotherapeutic regimens, preferentially without concomitant anthracyclines. Novel HER2 targeted therapies appear to have a more‐favorable cardiac risk profile. Overall, the large benefits of HER2 targeted therapies in patients with HER2‐positive breast cancer justify its use in most patients with cautious monitoring for cardiotoxicity. Research is needed to guide management of patients with pre‐existing CVD or other risk factors.
Disclosures
None.
J Am Heart Assoc. 2017;6:e006915 DOI: 10.1161/JAHA.117.006915.28939718
References
- 1. American Cancer Society . Breast Cancer Facts & Figures 2015–2016. Atlanta, GA: American Cancer Society; 2015. [Google Scholar]
- 2. Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER‐2/neu oncogene. Science. 1987;235:177–182. [DOI] [PubMed] [Google Scholar]
- 3. Gonzalez‐Angulo AM, Litton JK, Broglio KR, Meric‐Bernstam F, Rakkhit R, Cardoso F, Peintinger F, Hanrahan EO, Sahin A, Guray M, Larsimont D, Feoli F, Stranzl H, Buchholz TA, Valero V, Theriault R, Piccart‐Gebhart M, Ravdin PM, Berry DA, Hortobagyi GN. High risk of recurrence for patients with breast cancer who have human epidermal growth factor receptor 2‐positive, node‐negative tumors 1 cm or smaller. J Clin Oncol. 2009;27:5700–5706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, Levin WJ, Stuart SG, Udove J, Ullrich A, Press MF. Studies of the HER‐2/neu proto‐oncogene in human breast and ovarian cancer. Science. 1989;244:707–712. [DOI] [PubMed] [Google Scholar]
- 5. Slamon DJ, Leyland‐Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J, Norton L. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. [DOI] [PubMed] [Google Scholar]
- 6. Blackwell KL, Burstein HJ, Storniolo AM, Rugo H, Sledge G, Koehler M, Ellis C, Casey M, Vukelja S, Bischoff J, Baselga J, O'Shaughnessy J. Randomized study of lapatinib alone or in combination with trastuzumab in women with ErbB2‐positive, trastuzumab‐refractory metastatic breast cancer. J Clin Oncol. 2010;28:1124–1130. [DOI] [PubMed] [Google Scholar]
- 7. Geyer CE, Forster J, Lindquist D, Chan S, Romieu CG, Pienkowski T, Jagiello‐Gruszfeld A, Crown J, Chan A, Kaufman B, Skarlos D, Campone M, Davidson N, Berger M, Oliva C, Rubin SD, Stein S, Cameron D. Lapatinib plus capecitabine for HER2‐positive advanced breast cancer. N Engl J Med. 2006;355:2733–2743. [DOI] [PubMed] [Google Scholar]
- 8. Baselga J, Cortés J, Kim SB, Im SA, Hegg R, Im YH, Roman L, Pedrini JL, Pienkowski T, Knott A, Clark E, Benyunes MC, Ross G, Swain SM; CLEOPATRA Study Group . Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366:109–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Verma S, Miles D, Gianni L, Krop IE, Welslau M, Baselga J, Pegram M, Oh DY, Diéras V, Guardino E, Fang L, Lu MW, Olsen S, Blackwell K; EMILIA Study Group . Trastuzumab emtansine for HER2‐positive advanced breast cancer. N Engl J Med. 2012;367:1783–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Morris PG, Hudis CA. Trastuzumab‐related cardiotoxicity following anthracycline‐based adjuvant chemotherapy: how worried should we be? J Clin Oncol. 2010;28:3407–3410. [DOI] [PubMed] [Google Scholar]
- 11. Romond EH, Jeong JH, Rastogi P, Swain SM, Geyer CE Jr, Ewer MS, Rathi V, Fehrenbacher L, Brufsky A, Azar CA, Flynn PJ, Zapas JL, Polikoff J, Gross HM, Biggs DD, Atkins JN, Tan‐Chiu E, Zheng P, Yothers G, Mamounas EP, Wolmark N. Seven‐year follow‐up assessment of cardiac function in NSABP B‐31, a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel (ACP) with ACP plus trastuzumab as adjuvant therapy for patients with node‐positive, human epidermal growth factor receptor 2‐positive breast cancer. J Clin Oncol. 2012;30:3792–3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Goldhirsch A, Gelber RD, Piccart‐Gebhart MJ, de Azambuja E, Procter M, Suter TM, Jackisch C, Cameron D, Weber HA, Heinzmann D, Dal Lago L, McFadden E, Dowsett M, Untch M, Gianni L, Bell R, Kohne CH, Vindevoghel A, Andersson M, Brunt AM, Otero‐Reyes D, Song S, Smith I, Leyland‐Jones B, Baselga J; Herceptin Adjuvant (HERA) Trial Study Team . 2 years versus 1 year of adjuvant trastuzumab for HER2‐positive breast cancer (HERA): an open‐label, randomised controlled trial. Lancet. 2013;382:1021–1028. [DOI] [PubMed] [Google Scholar]
- 13. Advani PP, Ballman KV, Dockter TJ, Colon‐Otero G, Perez EA. Long‐term cardiac safety analysis of NCCTG N9831 (Alliance) adjuvant trastuzumab trial. J Clin Oncol. 2016;34:581–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ewer MS, Lippman SM. Type II chemotherapy‐related cardiac dysfunction: time to recognize a new entity. J Clin Oncol. 2005;23:2900–2902. [DOI] [PubMed] [Google Scholar]
- 15. Ewer MS, Vooletich MT, Durand JB, Woods ML, Davis JR, Valero V, Lenihan DJ. Reversibility of trastuzumab‐related cardiotoxicity: new insights based on clinical course and response to medical treatment. J Clin Oncol. 2005;23:7820–7826. [DOI] [PubMed] [Google Scholar]
- 16. Zamorano JL, Lancellotti P, Rodriguez Munoz D, Aboyans V, Asteggiano R, Galderisi M, Habib G, Lenihan DJ, Lip GY, Lyon AR, Lopez Fernandez T, Mohty D, Piepoli MF, Tamargo J, Torbicki A, Suter TM; Authors/Task Force Members; ESC Committee for Practice Guidelines (CPG) . 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: the Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur Heart J. 2016;37:2768–2801. [DOI] [PubMed] [Google Scholar]
- 17. Plana JC, Galderisi M, Barac A, Ewer MS, Ky B, Scherrer‐Crosbie M, Ganame J, Sebag IA, Agler DA, Badano LP, Banchs J, Cardinale D, Carver J, Cerqueira M, DeCara JM, Edvardsen T, Flamm SD, Force T, Griffin BP, Jerusalem G, Liu JE, Magalhaes A, Marwick T, Sanchez LY, Sicari R, Villarraga HR, Lancellotti P. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2014;15:1063–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. NCI . Common Terminology Criteria for Adverse Events (CTCAE). 2009.
- 19. Seidman A, Hudis C, Pierri MK, Shak S, Paton V, Ashby M, Murphy M, Stewart SJ, Keefe D. Cardiac dysfunction in the trastuzumab clinical trials experience. J Clin Oncol. 2002;20:1215–1221. [DOI] [PubMed] [Google Scholar]
- 20. Zamorano JL, Lancellotti P, Rodriguez Muñoz D, Aboyans V, Asteggiano R, Galderisi M, Habib G, Lenihan DJ, Lip GY, Lyon AR, Lopez Fernandez T, Mohty D, Piepoli MF, Tamargo J, Torbicki A, Suter TM, Zamorano JL, Aboyans V, Achenbach S, Agewall S, Badimon L, Baron‐Esquivias G, Baumgartner H, Bax JJ, Bueno H, Carerj S, Dean V, Erol C, Fitzsimons D, Gaemperli O, Kirchhof P, Kolh P, Lancellotti P, Lip GY, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Roffi M, Torbicki A, Vaz Carneiro A, Windecker S; Authors/Task Force Members; ESC Committee for Practice Guidelines (CPG); Document Reviewers . 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: the Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur J Heart Fail. 2017;19:9–42. [DOI] [PubMed] [Google Scholar]
- 21. Cobleigh MA, Vogel CL, Tripathy D, Robert NJ, Scholl S, Fehrenbacher L, Wolter JM, Paton V, Shak S, Lieberman G, Slamon DJ. Multinational study of the efficacy and safety of humanized anti‐HER2 monoclonal antibody in women who have HER2‐overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol. 1999;17:2639–2648. [DOI] [PubMed] [Google Scholar]
- 22. Baselga J, Tripathy D, Mendelsohn J, Baughman S, Benz CC, Dantis L, Sklarin NT, Seidman AD, Hudis CA, Moore J, Rosen PP, Twaddell T, Henderson IC, Norton L. Phase II study of weekly intravenous recombinant humanized anti‐p185HER2 monoclonal antibody in patients with HER2/neu‐overexpressing metastatic breast cancer. J Clin Oncol. 1996;14:737–744. [DOI] [PubMed] [Google Scholar]
- 23. Pegram MD, Lipton A, Hayes DF, Weber BL, Baselga JM, Tripathy D, Baly D, Baughman SA, Twaddell T, Glaspy JA, Slamon DJ. Phase II study of receptor‐enhanced chemosensitivity using recombinant humanized anti‐p185HER2/neu monoclonal antibody plus cisplatin in patients with HER2/neu‐overexpressing metastatic breast cancer refractory to chemotherapy treatment. J Clin Oncol. 1998;16:2659–2671. [DOI] [PubMed] [Google Scholar]
- 24. Balduzzi S, Mantarro S, Guarneri V, Tagliabue L, Pistotti V, Moja L, D'Amico R. Trastuzumab‐containing regimens for metastatic breast cancer. Cochrane Database Syst Rev. 2014:CD006242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE Jr, Davidson NE, Tan‐Chiu E, Martino S, Paik S, Kaufman PA, Swain SM, Pisansky TM, Fehrenbacher L, Kutteh LA, Vogel VG, Visscher DW, Yothers G, Jenkins RB, Brown AM, Dakhil SR, Mamounas EP, Lingle WL, Klein PM, Ingle JN, Wolmark N. Trastuzumab plus adjuvant chemotherapy for operable HER2‐positive breast cancer. N Engl J Med. 2005;353:1673–1684. [DOI] [PubMed] [Google Scholar]
- 26. Perez EA, Romond EH, Suman VJ, Jeong JH, Sledge G, Geyer CE Jr, Martino S, Rastogi P, Gralow J, Swain SM, Winer EP, Colon‐Otero G, Davidson NE, Mamounas E, Zujewski JA, Wolmark N. Trastuzumab plus adjuvant chemotherapy for human epidermal growth factor receptor 2‐positive breast cancer: planned joint analysis of overall survival from NSABP B‐31 and NCCTG N9831. J Clin Oncol. 2014;32:3744–3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Piccart‐Gebhart MJ, Procter M, Leyland‐Jones B, Goldhirsch A, Untch M, Smith I, Gianni L, Baselga J, Bell R, Jackisch C, Cameron D, Dowsett M, Barrios CH, Steger G, Huang CS, Andersson M, Inbar M, Lichinitser M, Láng I, Nitz U, Iwata H, Thomssen C, Lohrisch C, Suter TM, Rüschoff J, Suto T, Greatorex V, Ward C, Straehle C, McFadden E, Dolci MS, Gelber RD; Herceptin Adjuvant (HERA) Trial Study Team . Trastuzumab after adjuvant chemotherapy in HER2‐positive breast cancer. N Engl J Med. 2005;353:1659–1672. [DOI] [PubMed] [Google Scholar]
- 28. Joensuu H, Kellokumpu‐Lehtinen PL, Bono P, Alanko T, Kataja V, Asola R, Utriainen T, Kokko R, Hemminki A, Tarkkanen M, Turpeenniemi‐Hujanen T, Jyrkkiö S, Flander M, Helle L, Ingalsuo S, Johansson K, Jaaskelainen AS, Pajunen M, Rauhala M, Kaleva‐Kerola J, Salminen T, Leinonen M, Elomaa I, Isola J; FinHer Study Investigators . Adjuvant docetaxel or vinorelbine with or without trastuzumab for breast cancer. N Engl J Med. 2006;354:809–820. [DOI] [PubMed] [Google Scholar]
- 29. Tolaney SM, Barry WT, Dang CT, Yardley DA, Moy B, Marcom PK, Albain KS, Rugo HS, Ellis M, Shapira I, Wolff AC, Carey LA, Overmoyer BA, Partridge AH, Guo H, Hudis CA, Krop IE, Burstein HJ, Winer EP. Adjuvant paclitaxel and trastuzumab for node‐negative, HER2‐positive breast cancer. N Engl J Med. 2015;372:134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gianni L, Pienkowski T, Im YH, Tseng LM, Liu MC, Lluch A, Staroslawska E, de la Haba‐Rodriguez J, Im SA, Pedrini JL, Poirier B, Morandi P, Semiglazov V, Srimuninnimit V, Bianchi GV, Magazzu D, McNally V, Douthwaite H, Ross G, Valagussa P. 5‐year analysis of neoadjuvant pertuzumab and trastuzumab in patients with locally advanced, inflammatory, or early‐stage HER2‐positive breast cancer (NeoSphere): a multicentre, open‐label, phase 2 randomised trial. Lancet Oncol. 2016;17:791–800. [DOI] [PubMed] [Google Scholar]
- 31. Schneeweiss A, Chia S, Hickish T, Harvey V, Eniu A, Hegg R, Tausch C, Seo JH, Tsai YF, Ratnayake J, McNally V, Ross G, Cortes J. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline‐containing and anthracycline‐free chemotherapy regimens in patients with HER2‐positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA). Ann Oncol. 2013;24:2278–2284. [DOI] [PubMed] [Google Scholar]
- 32. von Minckwitz G, Procter M, de Azambuja E, Zardavas D, Benyunes M, Viale G, Suter T, Arahmani A, Rouchet N, Clark E, Knott A, Lang I, Levy C, Yardley DA, Bines J, Gelber RD, Piccart M, Baselga J; APHINITY Steering Committee and Investigators . Adjuvant pertuzumab and trastuzumab in early HER2‐positive breast cancer. N Engl J Med. 2017;377:122–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Figueroa‐Magalhaes MC, Jelovac D, Connolly RM, Wolff AC. Treatment of HER2‐positive breast cancer. Breast. 2014;23:128–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Perez EA, Romond EH, Suman VJ, Jeong JH, Davidson NE, Geyer CE Jr, Martino S, Mamounas EP, Kaufman PA, Wolmark N. Four‐year follow‐up of trastuzumab plus adjuvant chemotherapy for operable human epidermal growth factor receptor 2‐positive breast cancer: joint analysis of data from NCCTG N9831 and NSABP B‐31. J Clin Oncol. 2011;29:3366–3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tan‐Chiu E, Yothers G, Romond E, Geyer CE Jr, Ewer M, Keefe D, Shannon RP, Swain SM, Brown A, Fehrenbacher L, Vogel VG, Seay TE, Rastogi P, Mamounas EP, Wolmark N, Bryant J. Assessment of cardiac dysfunction in a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel, with or without trastuzumab as adjuvant therapy in node‐positive, human epidermal growth factor receptor 2‐overexpressing breast cancer: NSABP B‐31. J Clin Oncol. 2005;23:7811–7819. [DOI] [PubMed] [Google Scholar]
- 36. Russell SD, Blackwell KL, Lawrence J, Pippen JE Jr, Roe MT, Wood F, Paton V, Holmgren E, Mahaffey KW. Independent adjudication of symptomatic heart failure with the use of doxorubicin and cyclophosphamide followed by trastuzumab adjuvant therapy: a combined review of cardiac data from the National Surgical Adjuvant breast and Bowel Project B‐31 and the North Central Cancer Treatment Group N9831 clinical trials. J Clin Oncol. 2010;28:3416–3421. [DOI] [PubMed] [Google Scholar]
- 37. Smith I, Procter M, Gelber RD, Guillaume S, Feyereislova A, Dowsett M, Goldhirsch A, Untch M, Mariani G, Baselga J, Kaufmann M, Cameron D, Bell R, Bergh J, Coleman R, Wardley A, Harbeck N, Lopez RI, Mallmann P, Gelmon K, Wilcken N, Wist E, Sánchez Rovira P, Piccart‐Gebhart MJ; HERA study team . 2‐year follow‐up of trastuzumab after adjuvant chemotherapy in HER2‐positive breast cancer: a randomised controlled trial. Lancet. 2007;369:29–36. [DOI] [PubMed] [Google Scholar]
- 38. Pivot X, Romieu G, Debled M, Pierga JY, Kerbrat P, Bachelot T, Lortholary A, Espié M, Fumoleau P, Serin D, Jacquin JP, Jouannaud C, Rios M, Abadie‐Lacourtoisie S, Tubiana‐Mathieu N, Cany L, Catala S, Khayat D, Pauporté I, Kramar A; PHARE trial investogators . 6 months versus 12 months of adjuvant trastuzumab for patients with HER2‐positive early breast cancer (PHARE): a randomised phase 3 trial. Lancet Oncol. 2013;14:741–748. [DOI] [PubMed] [Google Scholar]
- 39. Pivot X, Suter T, Nabholtz JM, Pierga JY, Espie M, Lortholary A, Khayat D, Pauporte I, Romieu G, Kramar A, Fumoleau P. Cardiac toxicity events in the PHARE trial, an adjuvant trastuzumab randomised phase III study. Eur J Cancer. 2015;51:1660–1666. [DOI] [PubMed] [Google Scholar]
- 40. Spielmann M, Roche H, Delozier T, Canon JL, Romieu G, Bourgeois H, Extra JM, Serin D, Kerbrat P, Machiels JP, Lortholary A, Orfeuvre H, Campone M, Hardy‐Bessard AC, Coudert B, Maerevoet M, Piot G, Kramar A, Martin AL, Penault‐Llorca F. Trastuzumab for patients with axillary‐node‐positive breast cancer: results of the FNCLCC‐PACS 04 trial. J Clin Oncol. 2009;27:6129–6134. [DOI] [PubMed] [Google Scholar]
- 41. Buzdar AU, Ibrahim NK, Francis D, Booser DJ, Thomas ES, Theriault RL, Pusztai L, Green MC, Arun BK, Giordano SH, Cristofanilli M, Frye DK, Smith TL, Hunt KK, Singletary SE, Sahin AA, Ewer MS, Buchholz TA, Berry D, Hortobagyi GN. Significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: results of a randomized trial in human epidermal growth factor receptor 2‐positive operable breast cancer. J Clin Oncol. 2005;23:3676–3685. [DOI] [PubMed] [Google Scholar]
- 42. Buzdar AU, Valero V, Ibrahim NK, Francis D, Broglio KR, Theriault RL, Pusztai L, Green MC, Singletary SE, Hunt KK, Sahin AA, Esteva F, Symmans WF, Ewer MS, Buchholz TA, Hortobagyi GN. Neoadjuvant therapy with paclitaxel followed by 5‐fluorouracil, epirubicin, and cyclophosphamide chemotherapy and concurrent trastuzumab in human epidermal growth factor receptor 2‐positive operable breast cancer: an update of the initial randomized study population and data of additional patients treated with the same regimen. Clin Cancer Res. 2007;13:228–233. [DOI] [PubMed] [Google Scholar]
- 43. Gianni L, Eiermann W, Semiglazov V, Manikhas A, Lluch A, Tjulandin S, Zambetti M, Vazquez F, Byakhow M, Lichinitser M, Climent MA, Ciruelos E, Ojeda B, Mansutti M, Bozhok A, Baronio R, Feyereislova A, Barton C, Valagussa P, Baselga J. Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2‐positive locally advanced breast cancer (the NOAH trial): a randomised controlled superiority trial with a parallel HER2‐negative cohort. Lancet. 2010;375:377–384. [DOI] [PubMed] [Google Scholar]
- 44. Moja L, Tagliabue L, Balduzzi S, Parmelli E, Pistotti V, Guarneri V and D'Amico R. Trastuzumab containing regimens for early breast cancer. Cochrane Database Syst Rev. 2012:CD006243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Slamon D, Eiermann W, Robert N, Pienkowski T, Martin M, Press M, Mackey J, Glaspy J, Chan A, Pawlicki M, Pinter T, Valero V, Liu MC, Sauter G, von Minckwitz G, Visco F, Bee V, Buyse M, Bendahmane B, Tabah‐Fisch I, Lindsay MA, Riva A, Crown J; Breast Cancer International Research Group . Adjuvant trastuzumab in HER2‐positive breast cancer. N Engl J Med. 2011;365:1273–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dang C, Guo H, Najita J, Yardley D, Marcom K, Albain K, Rugo H, Miller K, Ellis M, Shapira I, Wolff AC, Carey LA, Moy B, Groarke J, Moslehi J, Krop I, Burstein HJ, Hudis C, Winer EP, Tolaney SM. Cardiac outcomes of patients receiving adjuvant weekly paclitaxel and trastuzumab for node‐negative, ERBB2‐positive breast cancer. JAMA Oncol. 2016;2:29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Perez EA, Koehler M, Byrne J, Preston AJ, Rappold E, Ewer MS. Cardiac safety of lapatinib: pooled analysis of 3689 patients enrolled in clinical trials. Mayo Clin Proc. 2008;83:679–686. [DOI] [PubMed] [Google Scholar]
- 48. Perez EA, Suman VJ, Davidson NE, Sledge GW, Kaufman PA, Hudis CA, Martino S, Gralow JR, Dakhil SR, Ingle JN, Winer EP, Gelmon KA, Gersh BJ, Jaffe AS, Rodeheffer RJ. Cardiac safety analysis of doxorubicin and cyclophosphamide followed by paclitaxel with or without trastuzumab in the North Central Cancer Treatment Group N9831 adjuvant breast cancer trial. J Clin Oncol. 2008;26:1231–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Suter TM, Procter M, van Veldhuisen DJ, Muscholl M, Bergh J, Carlomagno C, Perren T, Passalacqua R, Bighin C, Klijn JG, Ageev FT, Hitre E, Groetz J, Iwata H, Knap M, Gnant M, Muehlbauer S, Spence A, Gelber RD, Piccart‐Gebhart MJ. Trastuzumab‐associated cardiac adverse effects in the herceptin adjuvant trial. J Clin Oncol. 2007;25:3859–3865. [DOI] [PubMed] [Google Scholar]
- 50. Bowles EJ, Wellman R, Feigelson HS, Onitilo AA, Freedman AN, Delate T, Allen LA, Nekhlyudov L, Goddard KA, Davis RL, Habel LA, Yood MU, McCarty C, Magid DJ, Wagner EH; Pharmacovigilance Study T eam. Risk of heart failure in breast cancer patients after anthracycline and trastuzumab treatment: a retrospective cohort study. J Natl Cancer Inst. 2012;104:1293–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chen J, Long JB, Hurria A, Owusu C, Steingart RM, Gross CP. Incidence of heart failure or cardiomyopathy after adjuvant trastuzumab therapy for breast cancer. J Am Coll Cardiol. 2012;60:2504–2512. [DOI] [PubMed] [Google Scholar]
- 52. Guarneri V, Lenihan DJ, Valero V, Durand JB, Broglio K, Hess KR, Michaud LB, Gonzalez‐Angulo AM, Hortobagyi GN, Esteva FJ. Long‐term cardiac tolerability of trastuzumab in metastatic breast cancer: the M.D. Anderson Cancer Center experience. J Clin Oncol. 2006;24:4107–4115. [DOI] [PubMed] [Google Scholar]
- 53. Armenian SH, Lacchetti C, Barac A, Carver J, Constine LS, Denduluri N, Dent S, Douglas PS, Durand JB, Ewer M, Fabian C, Hudson M, Jessup M, Jones LW, Ky B, Mayer EL, Moslehi J, Oeffinger K, Ray K, Ruddy K, Lenihan D. Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2017;35:893–911. [DOI] [PubMed] [Google Scholar]
- 54. Farolfi A, Melegari E, Aquilina M, Scarpi E, Ibrahim T, Maltoni R, Sarti S, Cecconetto L, Pietri E, Ferrario C, Fedeli A, Faedi M, Nanni O, Frassineti GL, Amadori D, Rocca A. Trastuzumab‐induced cardiotoxicity in early breast cancer patients: a retrospective study of possible risk and protective factors. Heart. 2013;99:634–639. [DOI] [PubMed] [Google Scholar]
- 55. Ewer MS, Ewer SM. Cardiotoxicity of anticancer treatments. Nat Rev Cardiol. 2015;12:547–558. [DOI] [PubMed] [Google Scholar]
- 56. Giordano SH, Lin YL, Kuo YF, Hortobagyi GN, Goodwin JS. Decline in the use of anthracyclines for breast cancer. J Clin Oncol. 2012;30:2232–2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ezaz G, Long JB, Gross CP, Chen J. Risk prediction model for heart failure and cardiomyopathy after adjuvant trastuzumab therapy for breast cancer. J Am Heart Assoc. 2014;3:e000472. doi: 10.1161/JAHA.113.000472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Guenancia C, Lefebvre A, Cardinale D, Yu AF, Ladoire S, Ghiringhelli F, Zeller M, Rochette L, Cottin Y, Vergely C. Obesity as a risk factor for anthracyclines and trastuzumab cardiotoxicity in breast cancer: a systematic review and meta‐analysis. J Clin Oncol. 2016;34:3157–3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Arteaga CL, Engelman JA. ERBB receptors: from oncogene discovery to basic science to mechanism‐based cancer therapeutics. Cancer Cell. 2014;25:282–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hahn VS, Lenihan DJ, Ky B. Cancer therapy‐induced cardiotoxicity: basic mechanisms and potential cardioprotective therapies. J Am Heart Assoc. 2014;3:e000665 DOI: 10.1161/JAHA.113.000665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lee KF, Simon H, Chen H, Bates B, Hung MC, Hauser C. Requirement for neuregulin receptor erbB2 in neural and cardiac development. Nature. 1995;378:394–398. [DOI] [PubMed] [Google Scholar]
- 62. De Keulenaer GW, Doggen K, Lemmens K. The vulnerability of the heart as a pluricellular paracrine organ: lessons from unexpected triggers of heart failure in targeted ErbB2 anticancer therapy. Circ Res. 2010;106:35–46. [DOI] [PubMed] [Google Scholar]
- 63. Zhao YY, Sawyer DR, Baliga RR, Opel DJ, Han X, Marchionni MA, Kelly RA. Neuregulins promote survival and growth of cardiac myocytes. Persistence of ErbB2 and ErbB4 expression in neonatal and adult ventricular myocytes. J Biol Chem. 1998;273:10261–10269. [DOI] [PubMed] [Google Scholar]
- 64. Ozcelik C, Erdmann B, Pilz B, Wettschureck N, Britsch S, Hubner N, Chien KR, Birchmeier C, Garratt AN. Conditional mutation of the ErbB2 (HER2) receptor in cardiomyocytes leads to dilated cardiomyopathy. Proc Natl Acad Sci USA. 2002;99:8880–8885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Crone SA, Zhao YY, Fan L, Gu Y, Minamisawa S, Liu Y, Peterson KL, Chen J, Kahn R, Condorelli G, Ross J Jr, Chien KR, Lee KF. ErbB2 is essential in the prevention of dilated cardiomyopathy. Nat Med. 2002;8:459–465. [DOI] [PubMed] [Google Scholar]
- 66. Lyu YL, Kerrigan JE, Lin CP, Azarova AM, Tsai YC, Ban Y, Liu LF. Topoisomerase IIbeta mediated DNA double‐strand breaks: implications in doxorubicin cardiotoxicity and prevention by dexrazoxane. Cancer Res. 2007;67:8839–8846. [DOI] [PubMed] [Google Scholar]
- 67. Zhang S, Liu X, Bawa‐Khalfe T, Lu LS, Lyu YL, Liu LF, Yeh ET. Identification of the molecular basis of doxorubicin‐induced cardiotoxicity. Nat Med. 2012;18:1639–1642. [DOI] [PubMed] [Google Scholar]
- 68. Eschenhagen T, Force T, Ewer MS, de Keulenaer GW, Suter TM, Anker SD, Avkiran M, de Azambuja E, Balligand JL, Brutsaert DL, Condorelli G, Hansen A, Heymans S, Hill JA, Hirsch E, Hilfiker‐Kleiner D, Janssens S, de Jong S, Neubauer G, Pieske B, Ponikowski P, Pirmohamed M, Rauchhaus M, Sawyer D, Sugden PH, Wojta J, Zannad F, Shah AM. Cardiovascular side effects of cancer therapies: a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2011;13:1–10. [DOI] [PubMed] [Google Scholar]
- 69. Sawyer DB, Peng X, Chen B, Pentassuglia L, Lim CC. Mechanisms of anthracycline cardiac injury: can we identify strategies for cardioprotection? Prog Cardiovasc Dis. 2010;53:105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Chien KR. Herceptin and the heart—a molecular modifier of cardiac failure. N Engl J Med. 2006;354:789–790. [DOI] [PubMed] [Google Scholar]
- 71. Cote GM, Sawyer DB, Chabner BA. ERBB2 inhibition and heart failure. N Engl J Med. 2012;367:2150–2153. [DOI] [PubMed] [Google Scholar]
- 72. Jay SM, Murthy AC, Hawkins JF, Wortzel JR, Steinhauser ML, Alvarez LM, Gannon J, Macrae CA, Griffith LG, Lee RT. An engineered bivalent neuregulin protects against doxorubicin‐induced cardiotoxicity with reduced proneoplastic potential. Circulation. 2013;128:152–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Sawyer DB, Zuppinger C, Miller TA, Eppenberger HM, Suter TM. Modulation of anthracycline‐induced myofibrillar disarray in rat ventricular myocytes by neuregulin‐1beta and anti‐erbB2: potential mechanism for trastuzumab‐induced cardiotoxicity. Circulation. 2002;105:1551–1554. [DOI] [PubMed] [Google Scholar]
- 74. Scheuer W, Friess T, Burtscher H, Bossenmaier B, Endl J, Hasmann M. Strongly enhanced antitumor activity of trastuzumab and pertuzumab combination treatment on HER2‐positive human xenograft tumor models. Cancer Res. 2009;69:9330–9336. [DOI] [PubMed] [Google Scholar]
- 75. Swain SM, Ewer MS, Cortes J, Amadori D, Miles D, Knott A, Clark E, Benyunes MC, Ross G, Baselga J. Cardiac tolerability of pertuzumab plus trastuzumab plus docetaxel in patients with HER2‐positive metastatic breast cancer in CLEOPATRA: a randomized, double‐blind, placebo‐controlled phase III study. Oncologist. 2013;18:257–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Virani SA, Dent S, Brezden‐Masley C, Clarke B, Davis MK, Jassal DS, Johnson C, Lemieux J, Paterson I, Sebag IA, Simmons C, Sulpher J, Thain K, Thavendiranathan P, Wentzell JR, Wurtele N, Cote MA, Fine NM, Haddad H, Hayley BD, Hopkins S, Joy AA, Rayson D, Stadnick E, Straatman L. Canadian Cardiovascular Society guidelines for evaluation and management of cardiovascular complications of cancer therapy. Can J Cardiol. 2016;32:831–841. [DOI] [PubMed] [Google Scholar]
- 77. Curigliano G, Cardinale D, Suter T, Plataniotis G, de Azambuja E, Sandri MT, Criscitiello C, Goldhirsch A, Cipolla C, Roila F; ESMO Guidelines Working Group . Cardiovascular toxicity induced by chemotherapy, targeted agents and radiotherapy: ESMO Clinical Practice Guidelines. Ann Oncol. 2012;23(Suppl 7):vii155–vii166. [DOI] [PubMed] [Google Scholar]
- 78. van Royen N, Jaffe CC, Krumholz HM, Johnson KM, Lynch PJ, Natale D, Atkinson P, Deman P, Wackers FJ. Comparison and reproducibility of visual echocardiographic and quantitative radionuclide left ventricular ejection fractions. Am J Cardiol. 1996;77:843–850. [DOI] [PubMed] [Google Scholar]
- 79. Bellenger NG, Burgess MI, Ray SG, Lahiri A, Coats AJ, Cleland JG, Pennell DJ. Comparison of left ventricular ejection fraction and volumes in heart failure by echocardiography, radionuclide ventriculography and cardiovascular magnetic resonance; are they interchangeable? Eur Heart J. 2000;21:1387–1396. [DOI] [PubMed] [Google Scholar]
- 80. Grothues F, Smith GC, Moon JC, Bellenger NG, Collins P, Klein HU, Pennell DJ. Comparison of interstudy reproducibility of cardiovascular magnetic resonance with two‐dimensional echocardiography in normal subjects and in patients with heart failure or left ventricular hypertrophy. Am J Cardiol. 2002;90:29–34. [DOI] [PubMed] [Google Scholar]
- 81. Armstrong GT, Plana JC, Zhang N, Srivastava D, Green DM, Ness KK, Daniel Donovan F, Metzger ML, Arevalo A, Durand JB, Joshi V, Hudson MM, Robison LL, Flamm SD. Screening adult survivors of childhood cancer for cardiomyopathy: comparison of echocardiography and cardiac magnetic resonance imaging. J Clin Oncol. 2012;30:2876–2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Negishi K, Negishi T, Hare JL, Haluska BA, Plana JC, Marwick TH. Independent and incremental value of deformation indices for prediction of trastuzumab‐induced cardiotoxicity. J Am Soc Echocardiogr. 2013;26:493–498. [DOI] [PubMed] [Google Scholar]
- 83. Sawaya H, Sebag IA, Plana JC, Januzzi JL, Ky B, Tan TC, Cohen V, Banchs J, Carver JR, Wiegers SE, Martin RP, Picard MH, Gerszten RE, Halpern EF, Passeri J, Kuter I, Scherrer‐Crosbie M. Assessment of echocardiography and biomarkers for the extended prediction of cardiotoxicity in patients treated with anthracyclines, taxanes, and trastuzumab. Circ Cardiovasc Imaging. 2012;5:596–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Cardinale D, Sandri MT, Colombo A, Colombo N, Boeri M, Lamantia G, Civelli M, Peccatori F, Martinelli G, Fiorentini C, Cipolla CM. Prognostic value of troponin I in cardiac risk stratification of cancer patients undergoing high‐dose chemotherapy. Circulation. 2004;109:2749–2754. [DOI] [PubMed] [Google Scholar]
- 85. Ky B, Putt M, Sawaya H, French B, Januzzi JL Jr, Sebag IA, Plana JC, Cohen V, Banchs J, Carver JR, Wiegers SE, Martin RP, Picard MH, Gerszten RE, Halpern EF, Passeri J, Kuter I, Scherrer‐Crosbie M. Early increases in multiple biomarkers predict subsequent cardiotoxicity in patients with breast cancer treated with doxorubicin, taxanes, and trastuzumab. J Am Coll Cardiol. 2014;63:809–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Cardinale D, Colombo A, Torrisi R, Sandri MT, Civelli M, Salvatici M, Lamantia G, Colombo N, Cortinovis S, Dessanai MA, Nole F, Veglia F, Cipolla CM. Trastuzumab‐induced cardiotoxicity: clinical and prognostic implications of troponin I evaluation. J Clin Oncol. 2010;28:3910–3916. [DOI] [PubMed] [Google Scholar]
- 87. Zardavas D, Suter TM, Van Veldhuisen DJ, Steinseifer J, Noe J, Lauer S, Al‐Sakaff N, Piccart‐Gebhart MJ, de Azambuja E. Role of troponins I and T and N‐terminal prohormone of brain natriuretic peptide in monitoring cardiac safety of patients with early‐stage human epidermal growth factor receptor 2‐positive breast cancer receiving trastuzumab: a herceptin adjuvant study cardiac marker substudy. J Clin Oncol. 2017;35:878–884. [DOI] [PubMed] [Google Scholar]
- 88. Morris PG, Chen C, Steingart R, Fleisher M, Lin N, Moy B, Come S, Sugarman S, Abbruzzi A, Lehman R, Patil S, Dickler M, McArthur HL, Winer E, Norton L, Hudis CA, Dang CT. Troponin I and C‐reactive protein are commonly detected in patients with breast cancer treated with dose‐dense chemotherapy incorporating trastuzumab and lapatinib. Clin Cancer Res. 2011;17:3490–3499. [DOI] [PubMed] [Google Scholar]
- 89. Seicean S, Seicean A, Alan N, Plana JC, Budd GT, Marwick TH. Cardioprotective effect of beta‐adrenoceptor blockade in patients with breast cancer undergoing chemotherapy: follow‐up study of heart failure. Circ Heart Fail. 2013;6:420–426. [DOI] [PubMed] [Google Scholar]
- 90. Cardinale D, Colombo A, Sandri MT, Lamantia G, Colombo N, Civelli M, Martinelli G, Veglia F, Fiorentini C, Cipolla CM. Prevention of high‐dose chemotherapy‐induced cardiotoxicity in high‐risk patients by angiotensin‐converting enzyme inhibition. Circulation. 2006;114:2474–2481. [DOI] [PubMed] [Google Scholar]
- 91. Bosch X, Rovira M, Sitges M, Domenech A, Ortiz‐Perez JT, de Caralt TM, Morales‐Ruiz M, Perea RJ, Monzo M, Esteve J. Enalapril and carvedilol for preventing chemotherapy‐induced left ventricular systolic dysfunction in patients with malignant hemopathies: the OVERCOME trial (preventiOn of left Ventricular dysfunction with Enalapril and caRvedilol in patients submitted to intensive ChemOtherapy for the treatment of Malignant hEmopathies). J Am Coll Cardiol. 2013;61:2355–2362. [DOI] [PubMed] [Google Scholar]
- 92. Kalay N, Basar E, Ozdogru I, Er O, Cetinkaya Y, Dogan A, Inanc T, Oguzhan A, Eryol NK, Topsakal R, Ergin A. Protective effects of carvedilol against anthracycline‐induced cardiomyopathy. J Am Coll Cardiol. 2006;48:2258–2262. [DOI] [PubMed] [Google Scholar]
- 93. Oliva S, Cioffi G, Frattini S, Simoncini EL, Faggiano P, Boccardi L, Pulignano G, Fioretti AM, Giotta F, Lestuzzi C, Maurea N, Sabatini S, Tarantini L; Italian Cardio‐Oncological Network . Administration of angiotensin‐converting enzyme inhibitors and beta‐blockers during adjuvant trastuzumab chemotherapy for nonmetastatic breast cancer: marker of risk or cardioprotection in the real world? Oncologist. 2012;17:917–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Gulati G, Heck SL, Ree AH, Hoffmann P, Schulz‐Menger J, Fagerland MW, Gravdehaug B, von Knobelsdorff‐Brenkenhoff F, Bratland A, Storas TH, Hagve TA, Rosjo H, Steine K, Geisler J, Omland T. Prevention of cardiac dysfunction during adjuvant breast cancer therapy (PRADA): a 2 × 2 factorial, randomized, placebo‐controlled, double‐blind clinical trial of candesartan and metoprolol. Eur Heart J. 2016;37:1671–1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Boekhout AH, Gietema JA, Milojkovic Kerklaan B, van Werkhoven ED, Altena R, Honkoop A, Los M, Smit WM, Nieboer P, Smorenburg CH, Mandigers CM, van der Wouw AJ, Kessels L, van der Velden AW, Ottevanger PB, Smilde T, de Boer J, van Veldhuisen DJ, Kema IP, de Vries EG, Schellens JH. Angiotensin II‐receptor inhibition with candesartan to prevent trastuzumab‐related cardiotoxic effects in patients with early breast cancer: a randomized clinical trial. JAMA Oncol. 2016;2:1030–1037. [DOI] [PubMed] [Google Scholar]
- 96. Pituskin E, Mackey JR, Koshman S, Jassal D, Pitz M, Haykowsky MJ, Pagano JJ, Chow K, Thompson RB, Vos LJ, Ghosh S, Oudit GY, Ezekowitz JA, Paterson DI. Multidisciplinary Approach to Novel Therapies in Cardio‐Oncology Research (MANTICORE 101‐Breast): a randomized trial for the prevention of trastuzumab‐associated cardiotoxicity. J Clin Oncol. 2017;35:870–877. [DOI] [PubMed] [Google Scholar]
- 97. Lynce F, Barac A, Tan MT, Asch FM, Smith KL, Dang C, Isaacs C, Swain SM. SAFE‐HEaRt: rationale and design of a pilot study investigating cardiac safety of HER2 targeted therapy in patients with HER2‐positive breast cancer and reduced left ventricular function. Oncologist. 2017;22:518–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Yu AF, Yadav NU, Lung BY, Eaton AA, Thaler HT, Hudis CA, Dang CT, Steingart RM. Trastuzumab interruption and treatment‐induced cardiotoxicity in early HER2‐positive breast cancer. Breast Cancer Res Treat. 2015;149:489–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Tripathy D, Seidman A, Keefe D, Hudis C, Paton V, Lieberman G. Effect of cardiac dysfunction on treatment outcomes in women receiving trastuzumab for HER2‐overexpressing metastatic breast cancer. Clin Breast Cancer. 2004;5:293–298. [DOI] [PubMed] [Google Scholar]
- 100. Ligibel JA, Alfano CM, Courneya KS, Demark‐Wahnefried W, Burger RA, Chlebowski RT, Fabian CJ, Gucalp A, Hershman DL, Hudson MM, Jones LW, Kakarala M, Ness KK, Merrill JK, Wollins DS, Hudis CA. American Society of Clinical Oncology position statement on obesity and cancer. J Clin Oncol. 2014;32:3568–3574. [DOI] [PMC free article] [PubMed] [Google Scholar]


