Introduction
Cardiovascular characteristics in Marfan syndrome patients are caused by defects in the fibrillin‐1 (FBN1) gene, encoding an extracellular matrix (ECM) protein present in elastic tissues, such as the heart and blood vessels. Of all the Marfan symptoms, the most life threatening is aortic dissection and rupture before the age of 40 years. There is no adequate treatment apart from aortic replacement surgery at an aortic diameter of 4.5 cm. In the meantime, antihypertensive medication, especially β‐blockers and angiotensin receptor blockers, is provided in these normotensive patients. Because of aorta fragility, it is advised that Marfan patients refrain from doing intense sports to prevent a rise in blood pressure during exercise and thereby aortic aneurysm expansion and rupture. This is in sharp contrast to most other vascular patients, who are advised to enhance their sports activities to reduce the burden of their cardiovascular problems. Here, the findings described by Mas‐Stachurska et al1 in this issue of JAHA show in the Marfan Fbn1C1039G/+ mouse model (MF) that exercise is actually beneficial by reducing aneurysm progression, which is counterintuitive but exciting data.
Mas‐Stachurska et al1 showed that MF mice and their wild‐type (WT) littermates already have clear differences at 4 months of age; MF mice exhibit enhanced aortic dilatation, elastic lamina ruptures, aortic stiffness, as well as cardiac hypertrophy, left ventricular fibrosis, and intramyocardial vessel remodeling. At this age, MF and WT mice were split into a sedentary and an exercise group. The treadmill workout protocol was introduced for an additional 5 months in the exercise groups (5 days a week, 1 hour a day). The question was whether ongoing cardiovascular disease in the MF mice would progress. Interestingly, exercise significantly reduced the aortic dilatation rate, as well as cardiac hypertrophy in MF mice, while other pathological characteristics remained equal. So far, this study does not reveal the underlying mechanism, leaving room for multiple hypotheses.
Exercise‐mediated changes relevant to Marfan syndrome are blood pressure alterations, enhanced tissue perfusion, reduced arterial stiffness, ECM remodeling, improved endothelial cell function, and enhanced contractile performance of muscle cells. Tackling these changes 1‐by‐1 may leave some clues.
MF mice in the exercise group showed a slight increase in systolic blood pressure, when compared with the WT mice. This would worry physicians, because of the guidelines indicating to use blood pressure–lowering medication in Marfan patients and provide a negative advice on intense sports. Nowadays, mostly β‐blockers and angiotensin receptor blockers are prescribed to Marfan patients.2 In the study of Mas‐Stachurska et al,1 the blood pressure was increased, while there was a reduced aortic dilatation rate and cardiac hypertrophy. A discrepancy between blood pressure and aortic complications in Marfan has been observed before when the antihypertensive calcium channel blockers were used, resulting in increased aneurysm growth in MF mice and enhanced aortic surgery and dissection risk in Marfan patients.3 These data support the statement that blood pressure lowering per se does not seem to be beneficial in Marfan, but rather that inhibition of specific signaling pathways with β‐blockers and angiotensin receptor blockers is favorable.
Increasing perfusion of tissues in response to exercise may provide better oxygenation and nutrient supply to the heart and aorta to combat cardiovascular damage in Marfan patients. Mas‐Stachurska et al1 did not find a significant expansion of the capillary network in the skeletal muscle of the trained WT mice, while they did show a switch in the oxidative status of these muscular fibers. Unfortunately, these features were not studied in skeletal muscle of MF mice, nor in the heart and aorta. In the heart, smaller‐size intramyocardial vessels with enhanced perivascular fibrosis were found in MF mice compared with WT mice, possibly responsible for part of the cardiac damage (cardiac fibrosis) observed in MF mice. Yet training did not change vessel size or fibrosis, but it did reduce cardiac hypertrophy. Even though improved organ perfusion by enhanced capillary density should still be studied in MF mice, no relief of intramyocardial vessel pathology was observed upon exercise.
Aortic stiffness is a typical characteristic in Marfan patients and mice,4, 5 which is in part caused by reduced elastin fiber integrity and ECM remodeling. Mas‐Stachurska et al1 show increased aortic stiffness in MF mice, which was not reversed by exercise. This may be explained by similar elastic lamina disruption and collagen deposition in trained and sedentary MF mice. Since fibrillin‐1, the most commonly mutated protein in Marfan, is a core component of elastic laminae, accumulation of elastic lamina ruptures usually precedes aortic dilatation. Going against this dogma here, aneurysm progression in MF mice is reduced by exercise despite continuous accumulation of elastic lamina ruptures. While elastin and collagen have been examined in this study, there are many other ECM proteins to investigate in the aneurysmal aorta as shown by proteomics approaches,6, 7 which may have been beneficially altered by exercise to facilitate aortic repair. It is not merely the abundance of these ECM proteins that determines the stability of the aorta, but the quality of the fibers and network they form together that is important. This also determines the (dys)function of the endothelial cells and smooth muscle cells living in it.
Endothelial cells are essential in flow sensing and communication with the underlying smooth muscle cells to maintain vascular integrity.8 Endothelial cell dysfunction has been reported in Marfan patients,9, 10 so improving endothelial function by exercise may influence aneurysm formation. Interestingly, an exercise‐induced cytokine produced and secreted by skeletal and heart muscle cells, the so‐called “myokine” irisin, reverses endothelial cell dysfunction, thereby reducing atherosclerosis in hyperlipidemic mice.11 Communication via training‐induced myokines may be a pathway involved in improvement of cardiovascular function and requires additional research.
Smooth muscle cells in the aorta vessel wall are mechanosensing cells, indicating that mechanical changes induce signaling and thereby determine cellular behavior. An essential role for smooth muscle cell contractile force in vascular integrity should not come as a surprise, illustrated by a number of other aneurysmal diseases with defects in smooth muscle contractile genes, such as ACTA2, MYLK, and MYH11. Moreover, in Marfan aorta and cultured smooth muscle cells there is a clear dysregulation of these contractile proteins.4 Exercise induces enhanced mechanical force in muscle cells. Where Mas‐Stachurska et al1 show a metabolic switch in response to exercise in skeletal muscle cells, they did not investigate changes in the oxidative state of aortic smooth muscle cells, potentially promoting cell survival in the vessel wall.
In Marfan, a prominent detrimental signaling cascade is the extracellular signal–regulated kinase (ERK)‐1/2,12 and it would be interesting to know whether exercise affects smooth muscle cell ERK1/2 signaling. Mas‐Stachurska et al1 propose, however, that the enhanced nitric oxide synthase‐2 in MF mouse aorta13 may be downregulated by exercise,14, 15 explaining normalization of the aortic dilatation rate to WT level. Continuous nitric oxide generation by nitric oxide synthase‐2 induces vascular relaxation and hampers smooth muscle cell contractility.
In summary, heart and aorta perfusion, ECM remodeling in the aorta, endothelial dysfunction, and smooth muscle cell contractility are processes altered by exercise in Marfan syndrome, which may deserve further investigation in preclinical and clinical studies (Figure). Very recently, Gibson and colleagues found similar results on exercise in Marfan mice16, showing that the data are very robust. Ultimately, these studies may lead to a paradigm shift in the management of Marfan syndrome, so that physicians may advise Marfan patients to initiate a moderate exercise program.
Figure 1.
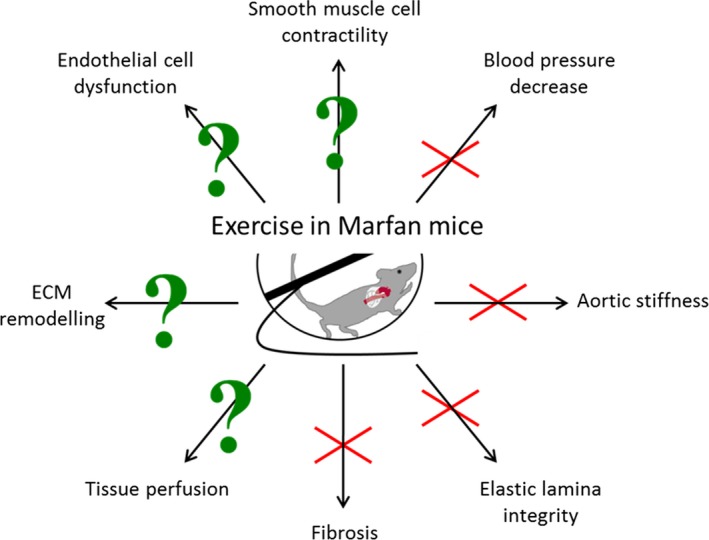
A schematic overview of cardiovascular processes influenced by exercise. Fbn1 C1039G/+ Marfan mice were challenged for 5 months with daily (1 hour) treadmill exercise, which decreased aortic aneurysm progression. The red cross marks pathways studied by Mas‐Stachurska et al, which were not altered upon exercise, thus ruling them out for further investigation. The pathways with a green question mark were not (extensively) studied yet upon exercise and deserve further attention. ECM indicates extracellular matrix.
Disclosures
None.
J Am Heart Assoc. 2017;6:e007465 DOI: 10.1161/JAHA.117.007465.28947564
The opinions expressed in this article are not necessarily those of the editors or of the American Heart Association.
References
- 1. Mas‐Stachurska A, Siegert AM, Batlle M, Gorbenko del Blanco D, Meirelles T, Rubies C, Bonorino F, Serra‐Peinado C, Bijnens B, Baudin J, Sitges M, Mont L, Guasch E, Egea G. Cardiovascular benefits of moderate exercise training in Marfan syndrome: insights from an animal model. J Am Heart Assoc. 2017;6:e006438 DOI: 10.1161/JAHA.117.006438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Franken R, Mulder BJ. Aortic disease: Losartan versus atenolol in the Marfan aorta‐how to treat? Nat Rev Cardiol. 2015;12:447–448. [DOI] [PubMed] [Google Scholar]
- 3. Doyle JJ, Doyle AJ, Wilson NK, Habashi JP, Bedja D, Whitworth RE, Lindsay ME, Schoenhoff F, Myers L, Huso N, Bachir S, Squires O, Rusholme B, Ehsan H, Huso D, Thomas CJ, Caulfield MJ, Van Eyk JE, Judge DP, Dietz HC; GenTAC Registry Consortium; MIBAVA Leducq Consortium . A deleterious gene‐by‐environment interaction imposed by calcium channel blockers in Marfan syndrome. Elife. 2015;4:e08648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Crosas‐Molist E, Meirelles T, López‐Luque J, Serra‐Peinado C, Selva J, Caja L, Gorbenko Del Blanco D, Uriarte JJ, Bertran E, Mendizábal Y, Hernández V, García‐Calero C, Busnadiego O, Condom E, Toral D, Castellà M, Forteza A, Navajas D, Sarri E, Rodríguez‐Pascual F, Dietz HC, Fabregat I, Egea G. Vascular smooth muscle cell phenotypic changes in patients with Marfan syndrome. Arterioscler Thromb Vasc Biol. 2015;35:960–972. [DOI] [PubMed] [Google Scholar]
- 5. Wanga SA, Hibender S, Ridwan Y, van Roomen C, Vos M, van der Made I, van Vliet N, Franken R, van Riel LA, Groenink M, Zwinderman AH, Mulder BJ, de Vries CJ, Essers J, de Waard V. Aortic microcalcification associates with elastin fragmentation in Marfan syndrome. J Pathol. 2017. DOI: 10.1002/path.4949. Available at: http://onlinelibrary.wiley.com/doi/10.1002/path.4949/full. Accessed September 22, 2017. [DOI] [PubMed] [Google Scholar]
- 6. Abdulkareem N, Skroblin P, Jahangiri M, Mayr M. Proteomics in aortic aneurysm—what have we learnt so far? Proteomics Clin Appl. 2013;7:504–515. [DOI] [PubMed] [Google Scholar]
- 7. Pilop C, Aregger F, Gorman RC, Brunisholz R, Gerrits B, Schaffner T, Gorman JH III, Matyas G, Carrel T, Frey BM. Proteomic analysis in aortic media of patients with Marfan syndrome reveals increased activity of calpain 2 in aortic aneurysms. Circulation. 2009;120:983–991. [DOI] [PubMed] [Google Scholar]
- 8. Hergenreider E, Heydt S, Tréguer K, Boettger T, Horrevoets AJ, Zeiher AM, Scheffer MP, Frangakis AS, Yin X, Mayr M, Braun T, Urbich C, Boon RA, Dimmeler S. Atheroprotective communication between endothelial cells and smooth muscle cells through miRNAs. Nat Cell Biol. 2012;14:249–256. [DOI] [PubMed] [Google Scholar]
- 9. Wilson DG, Bellamy MF, Ramsey MW, Goodfellow J, Brownlee M, Davies S, Wilson JF, Lewis MJ, Stuart AG. Endothelial function in Marfan syndrome: selective impairment of flow‐mediated vasodilation. Circulation. 1999;99:909–915. [DOI] [PubMed] [Google Scholar]
- 10. Takata M, Amiya E, Watanabe M, Omori K, Imai Y, Fujita D, Nishimura H, Kato M, Morota T, Nawata K, Ozeki A, Watanabe A, Kawarasaki S, Hosoya Y, Nakao T, Maemura K, Nagai R, Hirata Y, Komuro I. Impairment of flow‐mediated dilation correlates with aortic dilation in patients with Marfan syndrome. Heart Vessels. 2014;29:478–485. [DOI] [PubMed] [Google Scholar]
- 11. Zhang Y, Mu Q, Zhou Z, Song H, Zhang Y, Wu F, Jiang M, Wang F, Zhang W, Li L, Shao L, Wang X, Li S, Yang L, Wu Q, Zhang M, Tang D. Protective effect of irisin on atherosclerosis via suppressing oxidized low density lipoprotein induced vascular inflammation and endothelial dysfunction. PLoS One. 2016;11:e0158038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Holm TM, Habashi JP, Doyle JJ, Bedja D, Chen Y, van Erp C, Lindsay ME, Kim D, Schoenhoff F, Cohn RD, Loeys BL, Thomas CJ, Patnaik S, Marugan JJ, Judge DP, Dietz HC. Noncanonical TGFβ signaling contributes to aortic aneurysm progression in Marfan syndrome mice. Science. 2011;332:358–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Oller J, Méndez‐Barbero N, Ruiz EJ, Villahoz S, Renard M, Canelas LI, Briones AM, Alberca R, Lozano‐Vidal N, Hurlé MA, Milewicz D, Evangelista A, Salaices M, Nistal JF, Jiménez‐Borreguero LJ, De Backer J, Campanero MR, Redondo JM. Nitric oxide mediates aortic disease in mice deficient in the metalloprotease Adamts1 and in a mouse model of Marfan syndrome. Nat Med. 2017;23:200–212. [DOI] [PubMed] [Google Scholar]
- 14. Silva JF, Correa IC, Diniz TF, Lima PM, Santos RL, Cortes SF, Coimbra CC, Lemos VS. Obesity, inflammation, and exercise training: relative contribution of iNOS and eNOS in the modulation of vascular function in the mouse aorta. Front Physiol. 2016;7:386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kleindienst A, Battault S, Belaidi E, Tanguy S, Rosselin M, Boulghobra D, Meyer G, Gayrard S, Walther G, Geny B, Durand G, Cazorla O, Reboul C. Exercise does not activate the β3 adrenergic receptor–eNOS pathway, but reduces inducible NOS expression to protect the heart of obese diabetic mice. Basic Res Cardiol. 2016;111:40. [DOI] [PubMed] [Google Scholar]
- 16. Gibson C, Nielsen C, Alex R, Cooper K, Farney M, Gaufin D, Cui JZ, van Breemen C, Broderick TL, Vallejo‐Elias J, Esfandiarei M. Mild aerobic exercise blocks elastin fiber fragmentation and aortic dilatation in a mouse model of Marfan syndrome associated aortic aneurysm. J Appl Physiol. 2017;123:147–160. [DOI] [PubMed] [Google Scholar]


