Abstract
IMPORTANCE
Many studies have assessed racial/ethnic and sex disparities in the prevalence of elevated blood pressure (BP) from childhood to adulthood, yet few have examined differences in age-specific transitions between categories of BP over the life course in contemporary, multiracial/multiethnic populations.
OBJECTIVE
To estimate age, racial/ethnic, and sex–specific annual net transition probabilities between categories of BP using Markov modeling of cross-sectional data from the National Health and Nutrition Examination Survey.
DESIGN, SETTING, AND PARTICIPANTS
National probability sample (National Health and Nutrition Examination Survey in 2007–2008, 2009–2010, and 2011–2012) of 17 747 African American, white American, and Mexican American participants aged 8 to 80 years. The data were analyzed from September 2014 to November 2015.
MAIN OUTCOMES AND MEASURES
Age-specific American Heart Association–defined BP categories.
RESULTS
Three National Health and Nutrition Examination Survey cross-sectional samples were used to characterize the ages at which self-reported African American (n = 4973), white American (n = 8886), and Mexican American (n = 3888) populations transitioned between ideal BP, prehypertension, and hypertension across the life course. At age 8 years, disparities in the prevalence of ideal BP were observed, with the prevalence being lower among boys (86.6%–88.8%) compared with girls (93.0%–96.3%). From ages 8 to 30 years, annual net transition probabilities from ideal to prehypertension among male individuals were more than 2 times the net transition probabilities of their female counterparts. The largest net transition probabilities for ages 8 to 30 years occurred in African American young men, among whom a net 2.9% (95% CI, 2.3%–3.4%) of those with ideal BP transitioned to prehypertension 1 year later. Mexican American young women aged 8 to 30 years experienced the lowest ideal to prehypertension net transition probabilities (0.6%; 95% CI, 0.3%–0.8%). After age 40 years, ideal to prehypertension net transition probabilities stabilized or decreased (range, 3.0%–4.5%) for men, whereas net transition probabilities for women increased rapidly (range, 2.6%–13.0%). Mexican American women exhibited the largest ideal to prehypertension net transition probabilities after age 60 years. The largest prehypertension to hypertension net transition probabilities occurred at young ages in boys of white race/ethnicity and African Americans, approximately age 8 years and age 25 years, respectively, while net transition probabilities for white women and Mexican Americans increased over the life course.
CONCLUSIONS AND RELEVANCE
Heterogeneity in net transition probabilities from ideal BP emerge during childhood, with associated rapid declines in ideal BP observed in boys and African Americans, thus introducing disparities. Primordial prevention beginning in childhood and into early adulthood is necessary to preempt the development of prehypertension and hypertension, as well as associated racial/ethnic and sex disparities.
High blood pressure (BP), the most common primary care diagnosis in the United States, affects more than 31% of adults1 and is a major risk factor for the development of cardiovascular and renal diseases.2–4 Even elevated levels of BP below clinical treatment thresholds are associated with increased subclinical atherosclerosis, target-organ damage, and cardiovascular morbidity and mortality compared with ideal BP.5,6 Increases in BP have been documented in childhood, with racial/ethnic minorities and young men disproportionately contributing to the total US burden of high BP at younger ages.1,7–11 Considering that half of the US population develops high BP by age 60 years, characterizing the ages at which populations transition between BP categories is needed to inform prevention and management efforts.12
Although the benefits of maintaining ideal levels of BP13–15 are well known, much of the literature characterizing changes in BP across the life course relies on prevalence estimates owing to the lack of contemporary multiracial/multiethnic longitudinal studies spanning childhood to adulthood. While cross-sectional data enable the estimation of the prevalence of BP levels at consecutive ages, prevalence estimates do not separate the counterbalancing probabilities of net transitions between BP categories. A method that estimates net transition probabilities between BP categories from widely available cross-sectional data can overcome this deficiency and inform health policy for age-appropriate intervention strategies. Herein, we used Markov-type net transition models specifically designed to estimate 1-year net transition probabilities from cross-sectional data16 for the estimation of age, racial/ethnic, and sex–specific net transition probabilities between levels of BP over the life course.
Methods
Study Populations
Three National Health and Nutrition Examination Survey (NHANES)17 cross-sectional samples (2007–2008, 2009–2010, and 2011–2012) were used to characterize the ages (range, 8–80 years) at which self-reported African American (AA) (n = 4973), white American (WA) (n = 8886), and Mexican American (MA) (n = 3888) populations transitioned between ideal BP, prehypertension, and hypertension across the life course. This analysis began in September 2014 and was completed in November 2015. Participants who had a physical limitation on both arms (eg, rashes, casts, or edema) that prevented measurement of BP were excluded. We also excluded non-Hispanic Asian, other Hispanic, and other race/ethnicity (including multiracial/multiethnic) because of small sample sizes. Demographic and health information collected in the study17 followed appropriate procedures for written informed consent, and the study was approved by local institutional review boards.
Three consecutive seated BP measurements were obtained after a 5-minute rest by a trained examiner following a standardized protocol; the mean of the second and third readings was used for analysis.18 For participants 20 years or older, BP was categorized using American Heart Association19 cut points as ideal (systolic BP [SBP]<120 mm Hg and diastolic BP [DBP]<80 mm Hg, untreated), prehypertension (SBP 120–139 mm Hg or DBP 80–89 mm Hg, treated or untreated), or hypertension (SBP≥140 mm Hg or DBP≥90 mm Hg, treated or un-treated) (eAppendix in the Supplement). For participants aged 8 to 19 years, BP was classified as ideal (<90th percentile by age and sex), prehypertension (90th–95th percentiles or SBP≥120 mm Hg or DBP≥80 mm Hg), or hypertension (>95th percentile) using the American Heart Association guidelines.20 Given these category definitions, populations treated with antihypertensive agents were ineligible for classification as ideal, regardless of their BP level. Antihypertensive medication use was determined by participant self-report.17
Statistical Analysis
We leveraged Markov-type transition models and cross-sectional data to estimate 1-year net transition probabilities between BP categories. As an example of net transition probabilities, consider a hypothetical cohort of 70 participants aged 35 years, 50 with ideal BP and 20 with prehypertension. If by age 36 years, 8 participants with ideal BP transitioned to prehypertension and 3 participants with prehypertension transitioned to ideal BP, the ideal to prehypertension net transition would be 5 participants (ie, 8 minus 3); dividing by the number of participants with ideal BP at age 35 years (n = 50) would yield a 1-year net transition probability of 10%. The 1-year net transition from prehypertension to ideal BP is 0 because fewer participants transitioned from prehypertension to ideal BP than from ideal to prehypertension (a 3-level example is provided in the eAppendix in the Supplement). Estimation of the individual transition probabilities instead of net transition probabilities would require longitudinal data spanning adolescence to late adulthood, which are generally unavailable for multiracial/multiethnic populations, are not widely generalizable, or are outdated.
Using Markov-type models to estimate net transition probabilities required 3 steps. First, we smoothed the data using multinomial P-spline models to estimate the age, racial/ethnic, and sex–specific smoothed prevalence of ideal BP, pre-hypertension, and hypertension.21–23 Although BP was categorized using percentiles for participants aged 8 to 19 years and standard BP thresholds for participants 20 years or older, no evidence of discontinuities by race/ethnicity or sex across the 2 age groups was observed; therefore, we present smoothed results across ages 8 to 80 years. Second, we used the smoothed prevalence estimates to calculate the age, racial/ethnic, and sex–specific net transition probabilities using a series of simplex algorithms from linear programming theory (eAppendix in the Supplement).16,24 Third, 95% CIs were calculated using bootstrapping. Although all net transition probabilities presented herein are age, racial/ethnic, and sex–specific 1-year net transition probabilities, the term net transition probability was adopted to improve readability.
Calibration of Net Transition Probabilities
To represent the cost of moving from one BP category to another, estimation of net transition probabilities required specification of a cost constant.16 Conceptually, the cost constant reflected our belief that movement from ideal BP to hypertension (a 2-step movement) in 1 year was unlikely; instead, staying in the same BP category or a 1-step movement (ie, from ideal BP to prehypertension or from prehypertension to ideal BP) was more plausible. Results of our calibration investigation using longitudinal Coronary Artery Risk Development in Young Adults (CARDIA) Study25 data suggested that the cost constants of 4, 8, and 17 were optimal for describing 0-step, 1-step, and 2-step movements between BP categories (eAppendix in the Supplement).
To place net transition probabilities in a population context, estimates were extrapolated to the US population to evaluate the net number of noninstitutionalized AAs, WAs, and MAs transitioning between BP levels at each age. The net number of individuals was calculated by multiplying the appropriate net transition probabilities (relative scale) by the prevalence of the BP category and the age, racial/ethnic, and sex–specific 2010 noninstitutionalized population size. Statistical analyses were performed using the following software programs: SAS version 9.3 (SAS Institute Inc), Stata 12 (StataCorp LP), and R (The R Foundation).
Results
We analyzed 17 747 NHANES participants (28.0% AA, 50.1% WA, and 21.9% MA) for whom BP measurements were available (Table). The median age ranged from 33 to 44 years, with AAs and MAs more likely to be younger and female compared with WAs. Antihypertensive medication use varied by race/ethnicity, with 22.8% to 31.1% of AAs reporting antihypertensive medication use compared with 9.8% to 12.7% among MAs. The median SBP and DBP were also higher among AAs and WAs than among MAs.
Table.
Racial/Ethnic and Sex–Specific Demographics Used to Characterize Age-Specific Net Probability of Transitioning Between Ideal Blood Pressure, Prehypertension, and Hypertensiona
| African Americans | White Americans | Mexican Americans | ||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Variable | Male | Female | Male | Female | Male | Female |
| Unweighted No. | 2445 | 2528 | 4498 | 4388 | 1963 | 1925 |
|
| ||||||
| Age, median (IQR), y | 37 (20–51) | 39 (22–53) | 43 (26–58) | 44 (27–60) | 33 (19–43) | 33 (18–45) |
|
| ||||||
| Blood pressure category among adults aged 20–80 y, % | ||||||
|
| ||||||
| Ideal | 42.0 | 49.0 | 42.2 | 53.3 | 55.7 | 71.3 |
|
| ||||||
| Prehypertension | 40.0 | 34.3 | 44.0 | 34.3 | 34.4 | 20.4 |
|
| ||||||
| Hypertension | 18.0 | 16.7 | 13.7 | 12.4 | 9.8 | 8.2 |
|
| ||||||
| Antihypertensive medication use, % | 22.8 | 31.3 | 22.1 | 24.6 | 9.8 | 12.7 |
|
| ||||||
| Blood pressure among adults aged 20–80 y, median (IQR), mm Hg | ||||||
|
| ||||||
| Systolic | 123 (114–135) | 119 (109–133) | 121 (113–130) | 117 (107–129) | 119 (112–128) | 112 (105–123) |
|
| ||||||
| Diastolic | 73 (65–81) | 71 (62–78) | 73 (65–79) | 69 (63–76) | 71 (64–78) | 67 (80–74) |
|
| ||||||
| BMI, median (IQR) | 27 (24–32) | 31 (26–36) | 28 (24–31) | 27 (23–32) | 28 (25–32) | 29 (25–34) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); IQR, interquartile range.
In 17 747 National Health and Nutrition Examination Survey17 (2007–2012) participants adjusted for sampling weights (except for N) to represent noninstitutionalized, civilian, African American, White American, and Mexican American US residents.
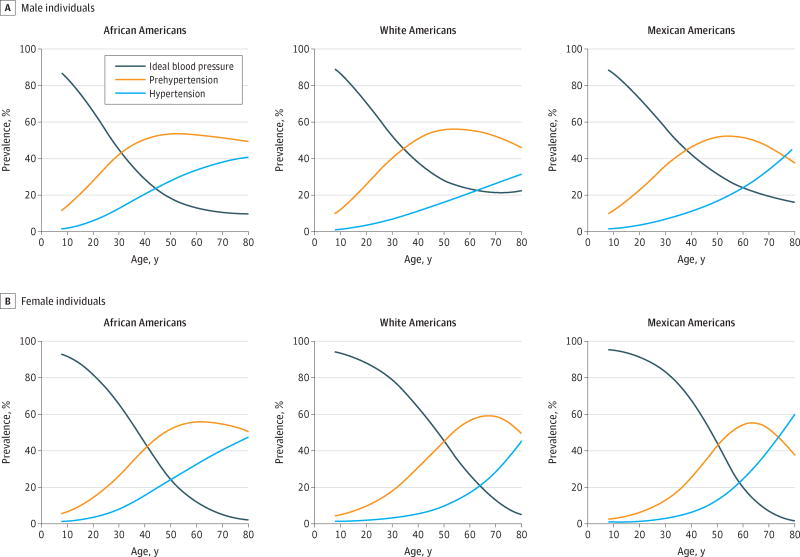
At age 8 years, the prevalence of hypertension ranged from 1.0% to 1.6%, showing little difference by race/ethnicity or sex (Figure 1). In contrast, disparities in the prevalence of ideal BP and prehypertension were observed at this age, with a lower prevalence of ideal BP among boys (86.6%–88.8%) compared with girls (93.0%–96.3%) and, conversely, a higher prevalence of prehypertension among boys (10.0%–11.8%) compared with girls (2.7%–5.9%). By age 19 years, disparities in the prevalence of ideal BP (range, 67.9%–92.6%) and prehypertension (range, 5.9%–26.6%) had widened, with AAs and boys having the highest prevalence of prehypertension.
Figure 1. Smoothed Age (Range, 8–80 Years), Race/Ethnic, and Sex–Specific Prevalence Proportions of Ideal Blood Pressure, Prehypertension, and Hypertension.
Shown are estimates in 17 747 National Health and Nutrition Examination Survey17 (2007–2012) participants.
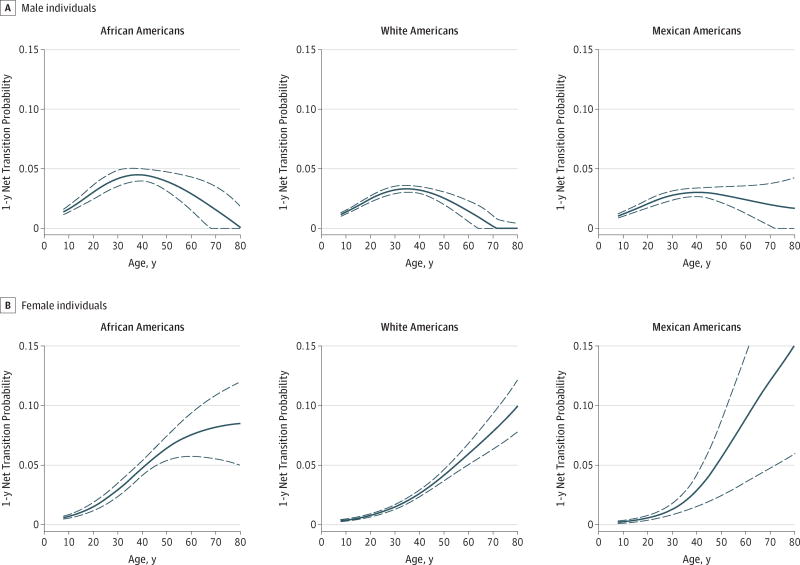
Estimated net transition probabilities across the life course consistently demonstrated a transition from ideal BP to prehypertension, with net transition probabilities being generally more comparable by sex than race/ethnicity, particularly in early life (Figure 2). From ages 8 to 30 years, annual net transition probabilities from ideal BP to prehypertension became higher at each consecutive age, with net transition probabilities among male individuals being more than 2 times the net transition probabilities of their female counterparts. At age 8 years, a net 1.4% (95% CI, 1.2%–1.6%) of AA boys with ideal BP transitioned to prehypertension by age 9 years compared with a net 0.6% (95% CI, 0.5%–0.7%) of AA girls. The largest net transition probabilities over this age range (8–30 years) were observed in AA young men, for whom a net of 2.9% (95% CI, 2.3%–3.4%) of those with ideal BP transitioned to prehypertension 1 year later. The lowest ideal to prehypertension net transition probabilities (0.6%, 95% CI, 0.3%–0.8%) were seen in MA young women aged 8 to 30 years. Differences in net transition probabilities from ideal BP to prehypertension among men by race/ethnicity were particularly large between ages 35 and 40 years, with the largest net transition probabilities in this age range estimated among AA men aged 38 years (4.5%; 95% CI, 4.0%–5.0%) and the smallest estimated among MA men aged 35 years (3.3%; 95% CI, 3.0%–3.6%). At age 40 years, ideal BP to prehypertension net transition probabilities for men and women were approximately equal within racial/ethnic groups. After age 40 years, net transition probabilities for men decreased or remained constant, while net transition probabilities for women increased rapidly (range, 2.6%–13.0%), with MA women exhibiting the largest net transition probabilities from ideal BP after age 60 years.
Figure 2. Age (Range, 8–80 Years), Race/Ethnic, and Sex–Specific Ideal Blood Pressure to Prehypertension Net Transition Probabilities.
Shown are estimates in 17 747 National Health and Nutrition Examination Survey17 (2007–2012) participants. Dashed lines indicate 95%CIs.
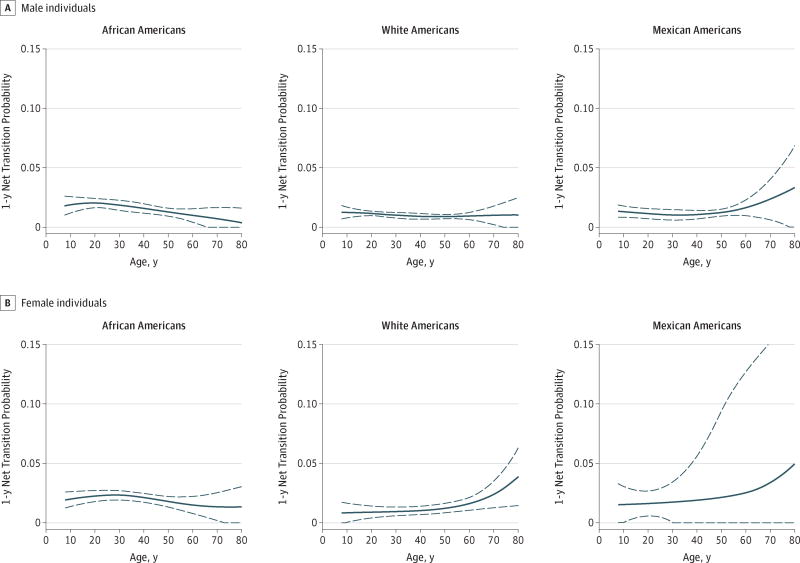
Similar to net transition probabilities from ideal BP to prehypertension, net transition probabilities between prehypertension and hypertension consistently exhibited a net flow toward higher BP categories compared with lower categories and heterogeneity by race/ethnicity and sex (Figure 3). In WA boys, estimated net transition probabilities from prehypertension to hypertension were highest at age 8 years, the earliest age under investigation (net transition probability, 1.2%; 95% CI, 0.7%–1.8%) and slightly decreased over the life course until age 60 years. Prehypertension to hypertension net transition probabilities for AA boys at 8 years (net transition probability, 1.8%; 95% CI, 1.0%–2.6%) were higher than those for WA boys and continued to become higher until approximately a decade later at age 20 years (net transition probability, 2.0%; 95% CI, 1.6%–2.4%); elevated prehypertension to hypertension net transition probabilities for AA women also accelerated into early adulthood at age 28 years (net transition probability, 2.3%; 95% CI, 1.9%–2.7%). In contrast to the net transition probabilities for AAs that decreased after early adulthood, prehypertension to hypertension net transition probabilities for WA women and MAs generally increased throughout adulthood, with increases accelerating at approximately age 55 years.
Figure 3. Age (Range, 8–80 Years), Race/Ethnic, and Sex–Specific Prehypertension to Hypertension Net Transition Probabilities.
Shown are estimates in 17 747 National Health and Nutrition Examination Survey17 (2002–2012) participants. Dashed lines indicate 95%CIs.
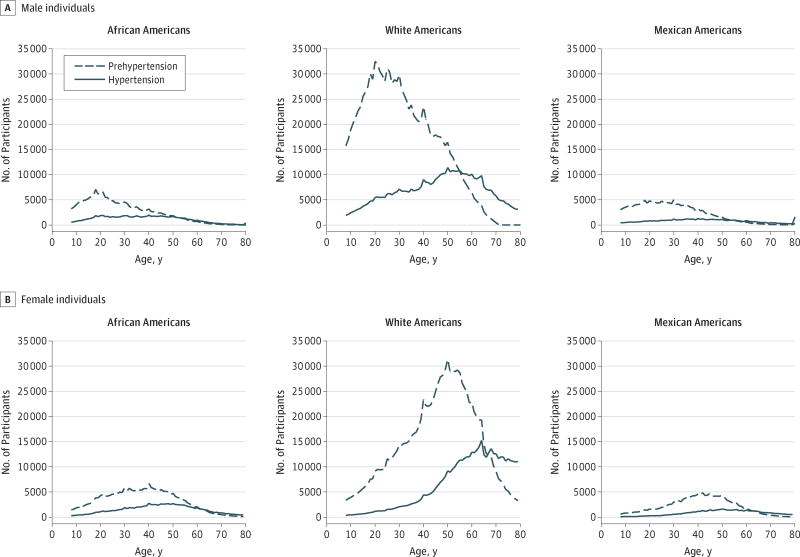
When net transition probabilities were extrapolated to the 2010 US population of noninstitutionalized AAs, WAs, and MAs aged 8 to 80 years, substantial differences between estimated net transition probabilities and net increases in the population were observed (Figure 4). These differences reflect both the relative magnitude of estimated net transition probabilities and the size of the population eligible to transition into the adjacent BP category. For example, ideal BP to prehypertension net transition probabilities among men peaked at approximately age 40 years. Yet, when extrapolated to the 2010 US noninstitutionalized population, the largest age-specific increases in the net number of men transitioning from ideal BP to prehypertension occurred approximately 2 decades earlier at age 20 years, with estimated 1-year net increases of 6187 in AA men, 22 340 in WA men, and 4830 in MA men. Although net transition probabilities from ideal BP to prehypertension showed increases in net transition probabilities into late life for AA, WA, and MA women, few participants with ideal BP remained; therefore, net increases in the population with prehypertension peaked around age 45 years for all racial/ethnic groups.
Figure 4. One-Year Age-Specific Population Extrapolations of the Net Number of Participants Transitioning to Prehypertension and Hypertension.
Shown are noninstitutionalized African American, white American, and Mexican American male individuals and female individuals.
Discussion
Higher net transition probabilities away from ideal BP and prehypertension observed among AAs and male individuals in early life widened the racial/ethnic and sex disparities observed in the burden of ideal BP and prehypertension at age 8 years. After midlife, higher 1-year net transition probabilities from ideal BP and prehypertension for women and MAs contributed to a higher burden of hypertension in late life. However, when net transition probabilities were extrapolated to the US population, earlier peaks were observed during young adulthood and midlife. Together, these findings emphasize the importance of primordial prevention early in life, particularly for AAs and men.6
Large-scale efforts to reduce the burden of elevated BP and its sequelae have principally focused on the clinical management of BP in adults at levels that exceed actionable thresholds.26 However, the high net transition probabilities to prehypertension and hypertension at young ages reported herein suggest that the biggest gains in primordial prevention will occur from preventing transitions away from ideal BP in children and young adults more resolutely, thereby necessitating greater clinical and public health efforts to prevent or delay the loss of ideal BP, sustained BP elevation, and disparities in the subpopulations most affected. A growing evidence base supports efforts to decrease BP in early life (eg, through increased physical activity and improved dietary intake), regardless of changes in adiposity or starting BP level.27 In addition to school, family, and community–based programs,27,28 the high use of pediatric care services29 also offers an under-used opportunity for physicians to promote healthy behaviors known to assist with the maintenance of ideal BP in childhood and connect families with community resources.30
Heterogeneity in racial/ethnic and sex–specific net transition probabilities to prehypertension and hypertension levels also documents important differences in the natural history of BP elevation31,32 and again invites a reassessment of an archetype that considers unfavorable BP levels an actionable attribute at middle age or late life. Of particular importance is the burden of undetected elevations of BP in early adulthood, especially among AA young men. For illustration, while the largest net transition probabilities to prehypertension and hypertension among AAs and men occurred between the second and fourth decades of life, 23% of the population aged 18 to 44 years reported no health care visit within the past year.33 Limited health care use during ages critical to BP transitions poses challenges that disproportionately affect population subgroups and suggest the need for increased public health programs aimed at the prevention of BP elevation in young adulthood. Public awareness of these large net transition probabilities could motivate individuals toward engagement with primary care during a period of life when such care is often a low priority. Research to test innovative prevention and detection programs that reach populations known to be vulnerable to early elevations in BP in culturally and sex–appropriate settings are few but much needed, particularly for AAs and young men, whose increased salt sensitivity, early-life covariate patterns, or hormonal predisposition may place them at early risk for sustained loss of ideal BP.34–37 Consistent with the literature on protective hormonal effects before menopause,31 large net transition probabilities to prehypertension and hypertension were generally observed in older women compared with younger women.38,39 On the other hand, extrapolations to the US population showed large net increases in the population with prehypertension and hypertension in midlife, further emphasizing the importance of primordial prevention in early life before loss of ideal BP.
Reflecting perhaps a focus on clinically defined hypertension, much of the literature to date has concentrated on the prevention of hypertension, with less emphasis placed on the timing of onset and duration of prehypertension and its influence on the risk of downstream chronic disease.5,6,40 Although the prevalence of hypertension has been stable for almost 2 decades,12 increases in prehypertension in early life extend the period at risk for BP-associated cardiovascular and renal complications.41,42 Therefore, the evaluation of age, racial/ethnic, and sex–specific net transition probabilities between BP categories can identify epochs of increased vulnerability to developing prehypertension annually and suggest target groups, particularly in early life, for health policy priorities and the allocation resources. Studies that compare contemporary net transition probabilities (estimated in earlier NHANES population cross-sections) with net transition probabilities estimated in earlier waves of the NHANES (eg, NHANES 1999–2000) can also help identify changes in the ages at which large net transition probabilities to prehypertension have occurred between these periods to gauge vulnerabilities and trends.
Strengths and Limitations
Strengths of this study include the use of a novel approach to estimate annual net transition probabilities between BP categories over the life course using contemporary cross-sectional data. These results differentiate movement into a BP category from movement out of it and uncover differences by subpopulations that are potentially unseen using prevalence estimates. For example, while the prevalence of prehypertension at ages 37 and 38 years was approximately 48.7% and 49.5%, respectively, for AA and WA men, examination of the net transition probabilities revealed that a net 4.5% (95% CI, 0.4%–0.5%) of AA men with ideal BP at age 37 years transitioned to prehypertension by age 38 years compared with a net 3.3% (95% CI, 3.0%–3.6%) of WA men. Unlike trajectories that often require longitudinal follow-up,3,16,43–45 our use of net transition probabilities allows for the use of data with current trends in BP in multiracial/multiethnic populations to explore disparities over the life course and increase generalizability of results, making this approach well suited for future application when contemporary longitudinal data are unattainable or when risk factor profiles in the population rapidly change. Further strengths are contributed by the use of NHANES data, a large, nationally representative multiracial/multiethnic population inclusive of ages from childhood to late adulthood and generalizable to approximately 86% of the US racial/ethnic populations, with no exclusions for secondary causes of hypertension.46
Several limitations of our data source and methods should be noted. First, BP measurements were not collected in NHANES on children younger than age 8 years, by which time sizable proportions of the population had already transitioned from ideal BP to prehypertension. As a result, net transition probabilities in children younger than age 8 years are not described. Second, the timing of BP measurements and the timing of antihypertensive medication use were not available, as is the case for other plausible correlates of BP that could have introduced some degree of error in our estimates. Third, we were unable to evaluate the influence of other factors that may affect net transition probabilities (eg, obesity47 or marital status48) because this examination would require further stratification of our data, likely beyond the ability of the current sample sizes. Fourth, individuals with elevated BP that has been controlled to below the treatment threshold of 140/90 mm Hg were classified as having prehypertension; therefore, net transitions from prehypertension to hypertension may reflect differences in BP treatment levels between racial/ethnic and sex groups, particularly in middle to late adulthood, when antihypertensive treatment was more common.
Conclusions
Heterogeneity in net transition probabilities from ideal BP emerge during childhood and early adolescence, with rapid declines in ideal BP observed in boys or young men and AAs, thus introducing disparities in desirable BP levels. This contemporary and multiracial/multiethnic study of net transition probabilities in AA, WA, and MA populations identified critical ages for which primordial prevention efforts could target those with increased susceptibility to transitioning to prehypertension and hypertension across the life course. Our results suggest the need for innovative clinical and public health partnerships that develop and implement primordial prevention efforts in childhood and early adulthood to preempt racial/ethnic and sex disparities in undesirable levels of BP.49
Supplementary Material
Key Points.
Question
Does age at transition from ideal blood pressure and prehypertension vary by race/ethnicity and sex?
Findings
A cross-sectional study using Markov modeling indicates that higher net transition probabilities away from ideal blood pressure and prehypertension are observed among African Americans and young men in early life, widening the racial/ethnic and sex disparities observed in the burden of ideal and prehypertension at age 8 years.
Meaning
A focus on primordial prevention in childhood and early adulthood is needed to preempt the development of prehypertension and hypertension, as well as racial/ethnic and sex disparities.
Acknowledgments
Funding/Support: The Coronary Artery Risk Development in Young Adults (CARDIA) Study is conducted and supported by the National Heart, Lung, and Blood Institute (NHLBI) in collaboration with The University of Alabama at Birmingham (HHSN268201300025C and HHSN268201300026C), Northwestern University (HHSN268201300027C), University of Minnesota (HHSN268201300028C), Kaiser Foundation Research Institute (HHSN268201300029C), and The Johns Hopkins University School of Medicine (HHSN268200900041C). The CARDIA Study is also partially supported by the Intramural Research Program of the National Institute on Aging (NIA) and an intra-agency agreement between the NIA and the NHLBI (grant AG0005). This research was supported in part by a National Institute of Diabetes and Digestive and Kidney Diseases training grant to the UNC Kidney Center at The University of North Carolina at Chapel Hill (grant T32-DK007750) (Ms Hardy).
Role of the Funder/Sponsor:The manuscript was reviewed by the CARDIA Study investigators for scientific content. Otherwise, the funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Author Contributions: Ms Hardy and Dr Avery had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Hardy, Holliday, Chakladar, Shay, Avery.
Acquisition, analysis, or interpretation of data:All authors.
Drafting of the manuscript: Hardy, Chakladar, Engeda, Avery.
Critical revision of the manuscript for important intellectual content: Hardy, Holliday, Allen, Heiss, Lloyd-Jones, Schreiner, Shay, Lin, Zeng, Avery.
Statistical analysis: Hardy, Holliday, Chakladar, Shay, Lin, Zeng, Avery.
Obtained funding: Heiss, Avery.
Administrative, technical, or material support: Hardy, Holliday.
Study supervision: Hardy, Holliday, Avery.
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest, and none were reported.
References
- 1.Fields LE, Burt VL, Cutler JA, Hughes J, Roccella EJ, Sorlie P. The burden of adult hypertension in the United States 1999 to 2000: a rising tide. Hypertension. 2004;44(4):398–404. doi: 10.1161/01.HYP.0000142248.54761.56. [DOI] [PubMed] [Google Scholar]
- 2.Roger VL, Go AS, Lloyd-Jones DM, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics: 2011 update: a report from the American Heart Association. Circulation. 2011;123(4):e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kannel WB, Wolf PA, Verter J, McNamara PM. Epidemiologic assessment of the role of blood pressure in stroke: the Framingham Study: 1970. JAMA. 1996;276(15):1269–1278. [PubMed] [Google Scholar]
- 4.Klag MJ, Whelton PK, Randall BL, et al. Blood pressure and end-stage renal disease in men. N Engl J Med. 1996;334(1):13–18. doi: 10.1056/NEJM199601043340103. [DOI] [PubMed] [Google Scholar]
- 5.Pletcher MJ, Bibbins-Domingo K, Lewis CE, et al. Prehypertension during young adulthood and coronary calcium later in life. Ann Intern Med. 2008;149(2):91–99. doi: 10.7326/0003-4819-149-2-200807150-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hsia J, Margolis KL, Eaton CB, et al. Women’s Health Initiative Investigators. Prehypertension and cardiovascular disease risk in the Women’s Health Initiative. Circulation. 2007;115(7):855–860. doi: 10.1161/CIRCULATIONAHA.106.656850. [DOI] [PubMed] [Google Scholar]
- 7.Hertz RP, Unger AN, Cornell JA, Saunders E. Racial disparities in hypertension prevalence, awareness, and management. Arch Intern Med. 2005;165(18):2098–2104. doi: 10.1001/archinte.165.18.2098. [DOI] [PubMed] [Google Scholar]
- 8.Chobanian AV, Bakris GL, Black HR, et al. Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42(6):1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 9.Brummett BH, Babyak MA, Siegler IC, et al. Systolic blood pressure, socioeconomic status, and biobehavioral risk factors in a nationally representative US young adult sample. Hypertension. 2011;58(2):161–166. doi: 10.1161/HYPERTENSIONAHA.111.171272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nguyen QC, Tabor JW, Entzel PP, et al. Discordance in national estimates of hypertension among young adults. Epidemiology. 2011;22(4):532–541. doi: 10.1097/EDE.0b013e31821c79d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lauer RM, Clarke WR. Childhood risk factors for high adult blood pressure: the Muscatine Study. Pediatrics. 1989;84(4):633–641. [PubMed] [Google Scholar]
- 12.Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988–2008. JAMA. 2010;303(20):2043–2050. doi: 10.1001/jama.2010.650. [DOI] [PubMed] [Google Scholar]
- 13.Stamler J, Stamler R, Neaton JD, et al. Low risk-factor profile and long-term cardiovascular and noncardiovascular mortality and life expectancy: findings for 5 large cohorts of young adult and middle-aged men and women. JAMA. 1999;282(21):2012–2018. doi: 10.1001/jama.282.21.2012. [DOI] [PubMed] [Google Scholar]
- 14.Xanthakis V, Enserro DM, Murabito JM, et al. Ideal cardiovascular health: associations with biomarkers and subclinical disease and impact on incidence of cardiovascular disease in the Framingham Offspring Study. Circulation. 2014;130(19):1676–1683. doi: 10.1161/CIRCULATIONAHA.114.009273. [DOI] [PubMed] [Google Scholar]
- 15.Laitinen TT, Pahkala K, Magnussen CG, et al. Ideal cardiovascular health in childhood and cardiometabolic outcomes in adulthood: the Cardiovascular Risk in Young Finns Study. Circulation. 2012;125(16):1971–1978. doi: 10.1161/CIRCULATIONAHA.111.073585. [DOI] [PubMed] [Google Scholar]
- 16.Kassteele Jv, Hoogenveen RT, Engelfriet PM, Baal PH, Boshuizen HC. Estimating net transition probabilities from cross-sectional data with application to risk factors in chronic disease modeling. Stat Med. 2012;31(6):533–543. doi: 10.1002/sim.4423. [DOI] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey. [Accessed October 29, 2012]; http://www.cdc.gov/nchs/nhanes.htm. Published 2012.
- 18.Hajjar I, Kotchen TA. Trends in prevalence, awareness, treatment, and control of hypertension in the United States, 1988–2000. JAMA. 2003;290(2):199–206. doi: 10.1001/jama.290.2.199. [DOI] [PubMed] [Google Scholar]
- 19.Lloyd-Jones DM, Hong Y, Labarthe D, et al. American Heart Association Strategic Planning Task Force and Statistics Committee. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation. 2010;121(4):586–613. doi: 10.1161/CIRCULATIONAHA.109.192703. [DOI] [PubMed] [Google Scholar]
- 20.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114((2)(suppl 4th report)):555–576. [PubMed] [Google Scholar]
- 21.Hosmer DW, Lemeshow S. Applied Logistic Regression. 2. New York, NY: Wiley; 2000. [Google Scholar]
- 22.McCullagh P, Nelder J. Generalized Linear Models. 2. London, England: Chapman & Hall/CRC; 1989. [Google Scholar]
- 23.Eilers PHC, Marx BD. Flexible smoothing with B-splines and penalties (with discussion) Stat Sci. 1996;11(2):89–121. [Google Scholar]
- 24.Golub GH, Welsch JH. Calculation of Gauss quadrature rules. Math Comput. 1969;23(106):221–230. [Google Scholar]
- 25.Friedman GD, Cutter GR, Donahue RP, et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41(11):1105–1116. doi: 10.1016/0895-4356(88)90080-7. [DOI] [PubMed] [Google Scholar]
- 26.James PA, Oparil S, Carter BL, et al. 2014 Evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8) JAMA. 2014;311(5):520. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 27.Cai L, Wu Y, Wilson RF, Segal JB, Kim MT, Wang Y. Effect of childhood obesity prevention programs on blood pressure: a systematic review and meta-analysis. Circulation. 2014;129(18):1832–1839. doi: 10.1161/CIRCULATIONAHA.113.005666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gittelsohn J, Kumar MB. Preventing childhood obesity and diabetes: is it time to move out of the school? Pediatr Diabetes. 2007;8(suppl 9):55–69. doi: 10.1111/j.1399-5448.2007.00333.x. [DOI] [PubMed] [Google Scholar]
- 29.Larson K, Cull WL, Racine AD, Olson LM. Trends in access to health care services for US children: 2000–2014. Pediatrics. 2016;138(6):e20162176. doi: 10.1542/peds.2016-2176. [DOI] [PubMed] [Google Scholar]
- 30.Abramson S, Stein J, Schaufele M, Frates E, Rogan S. Personal exercise habits and counseling practices of primary care physicians: a national survey. Clin J Sport Med. 2000;10(1):40–48. doi: 10.1097/00042752-200001000-00008. [DOI] [PubMed] [Google Scholar]
- 31.Reckelhoff JF. Gender differences in the regulation of blood pressure. Hypertension. 2001;37(5):1199–1208. doi: 10.1161/01.hyp.37.5.1199. [DOI] [PubMed] [Google Scholar]
- 32.Harshfield GA, Alpert BS, Willey ES, Somes GW, Murphy JK, Dupaul LM. Race and gender influence ambulatory blood pressure patterns of adolescents. Hypertension. 1989;14(6):598–603. doi: 10.1161/01.hyp.14.6.598. [DOI] [PubMed] [Google Scholar]
- 33.National Center for Health Statistics. Health, United States, 2015: With Special Feature on Racial and Ethnic Health Disparities. Hyattsville, MD: National Center for Health Statistics; 2016. Report 2016-1232. [PubMed] [Google Scholar]
- 34.Hess PL, Reingold JS, Jones J, et al. Barbershops as hypertension detection, referral, and follow-up centers for black men. Hypertension. 2007;49(5):1040–1046. doi: 10.1161/HYPERTENSIONAHA.106.080432. [DOI] [PubMed] [Google Scholar]
- 35.Victor RG, Ravenell JE, Freeman A, et al. Effectiveness of a barber-based intervention for improving hypertension control in black men: the BARBER-1 study: a cluster randomized trial. Arch Intern Med. 2011;171(4):342–350. doi: 10.1001/archinternmed.2010.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rader F, Elashoff RM, Niknezhad S, Victor RG. Differential treatment of hypertension by primary care providers and hypertension specialists in a barber-based intervention trial to control hypertension in black men. Am J Cardiol. 2013;112(9):1421–1426. doi: 10.1016/j.amjcard.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fleming S, Atherton H, McCartney D, et al. Self-screening and non-physician screening for hypertension in communities: a systematic review. Am J Hypertens. 2015;28(11):1316–1324. doi: 10.1093/ajh/hpv029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wassertheil-Smoller S, Anderson G, Psaty BM, et al. Hypertension and its treatment in postmenopausal women: baseline data from the Women’s Health Initiative. Hypertension. 2000;36(5):780–789. doi: 10.1161/01.hyp.36.5.780. [DOI] [PubMed] [Google Scholar]
- 39.Wright JT, Jr, Williamson JD, Whelton PK, et al. SPRINT Research Group. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373(22):2103–2116. doi: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kurioka S, Horie S, Inoue A, Mafune K, Tsuda Y, Otsuji Y. Risk of progression to hypertension in nonhypertensive Japanese workers aged 20–64 years. J Hypertens. 2014;32(2):236–244. doi: 10.1097/HJH.0000000000000031. [DOI] [PubMed] [Google Scholar]
- 41.Redwine KM, Falkner B. Progression of prehypertension to hypertension in adolescents. Curr Hypertens Rep. 2012;14(6):619–625. doi: 10.1007/s11906-012-0299-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kit BK, Kuklina E, Carroll MD, Ostchega Y, Freedman DS, Ogden CL. Prevalence of and trends in dyslipidemia and blood pressure among US children and adolescents, 1999–2012. JAMA Pediatr. 2015;169(3):272–279. doi: 10.1001/jamapediatrics.2014.3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kannel WB, Schwartz MJ, McNamara PM. Blood pressure and risk of coronary heart disease: the Framingham Study. Dis Chest. 1969;56(1):43–52. doi: 10.1378/chest.56.1.43. [DOI] [PubMed] [Google Scholar]
- 44.Rodriguez CJ, Swett K, Agarwal SK, et al. Systolic blood pressure levels among adults with hypertension and incident cardiovascular events: the Atherosclerosis Risk in Communities Study. JAMA Intern Med. 2014;174(8):1252–1261. doi: 10.1001/jamainternmed.2014.2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Allen NB, Siddique J, Wilkins JT, et al. Blood pressure trajectories in early adulthood and subclinical atherosclerosis in middle age [published correction appears in JAMA. 2014; 311(15):1568] JAMA. 2014;311(5):490–497. doi: 10.1001/jama.2013.285122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Passel JS, Cohn D. US population projections: 2005–2050. [Accessed April 28, 2016]; http://www.pewhispanic.org/2008/02/11/us-population-projections-2005-2050/. Published February 11, 2008.
- 47.Falkner B, Gidding S. Childhood obesity and blood pressure: back to the future? Hypertension. 2011;58(5):754–755. doi: 10.1161/HYPERTENSIONAHA.111.180430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Causland FRM, Sacks FM, Forman JP. Marital status, dipping and nocturnal blood pressure: results from the Dietary Approaches to Stop Hypertension trial. J Hypertens. 2014;32(4):756–761. doi: 10.1097/HJH.0000000000000107. [DOI] [PubMed] [Google Scholar]
- 49.Kavey RE, Daniels SR, Lauer RM, Atkins DL, Hayman LL, Taubert K American Heart Association. American Heart Association guidelines for primary prevention of atherosclerotic cardiovascular disease beginning in childhood. Circulation. 2003;107(11):1562–1566. doi: 10.1161/01.cir.0000061521.15730.6e. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.