Abstract
The Janus kinase (JAK), signal transducer of activation (STAT) pathway, discovered by investigating interferon gene induction, is now recognized as an evolutionarily conserved signaling pathway employed by diverse cytokines, interferons, growth factors, and related molecules. This pathway provides an elegant, and remarkably straightforward mechanism whereby extracellular factors control gene expression. It thus serves as a fundamental paradigm for how cells sense environmental cues and interpret these signals to regulate cell growth and differentiation. Functionally relevant genetic mutations and polymorphisms are relevant to a variety of human diseases, especially cancer and immune-related conditions. Finally, the clinical relevance of the pathway has been confirmed by the emergence of a new class of therapeutics that target JAKs.
Introduction: A mostly simple membrane-to-nucleus pathway
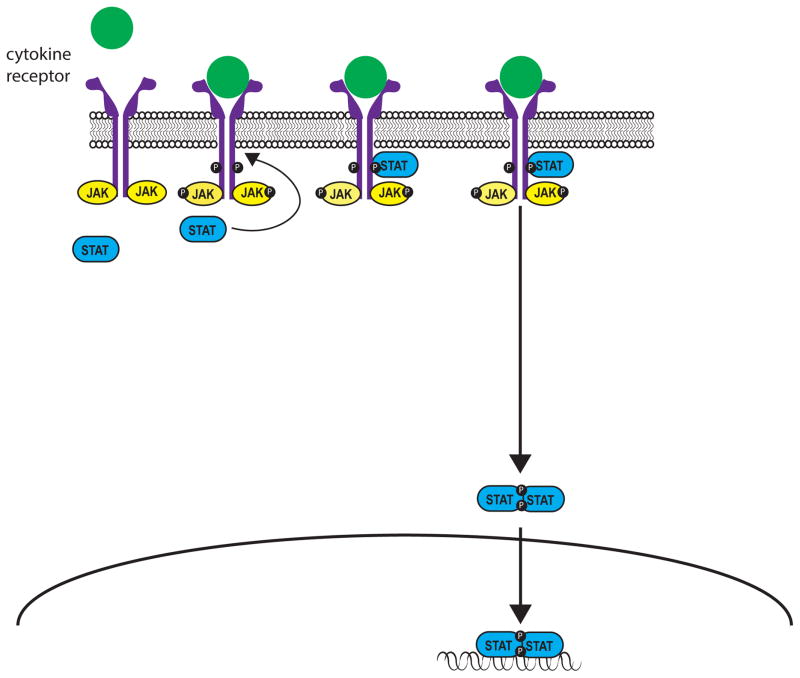
Effective communication between cells is central to development, tissue and organism homeostasis, and host defense. Evolution has provided a number of elegant solutions to this problem, but among these the Jak/STAT pathway is one of the architecturally simplest paradigms, allowing remarkable direct communication from transmembrane receptors to the nucleus (Figure 1). Pioneering work on the control of gene expression by interferons led to the discovery of this pathway; however, it is now recognized that a wide variety of cytokines, colony stimulating factors and hormones employ this mode of signal transduction (Table 1).
Figure 1.
Table 1.
JAKs and STATs with associated cytokines and phenotypes
| JAK/STAT | Important for signaling by | Knockout mouse phenotype | Genetic links to human disease |
|---|---|---|---|
| JAK1 | IFNα/β, IFNγ, IL-2, IL-4, IL-7, IL-9, IL-21, IL-6 family cytokines, IL-10 family cytokines | Perinatally lethal | GOF somatic mutations cause ALL, AML, solid organ malignancies |
| JAK2 | IFNγ, IL-3, IL-5, GM-CSF, EPO, TPO, G-CSF, GH, leptin | Embryonically lethal due to absence of erythropoiesis | GOF mutations cause PCV, MF, ET, hypercoagulable STATe; somatic mutations associated with acute and chronic hematologic malignancies |
| JAK3 | IL-2, IL-4, IL-7, IL-15, IL-21 | Defective T and B cell maturation | Loss of function mutation causes severe combined immunodeficiency (SCID), Jacobsen syndrome |
| TYK2 | IFNα/β, IFNγ, IL-12, IL-23 | Reduced responses to Type I IFN and IL12, and defective Stat3 activation. | Loss-of function mutation causes primary immunodeficiency |
| STAT1 | All IFNs | Impaired responses to Type I and Type II IFN | LOF mutations confer susceptibility to mycobacterial and viral infections; GOF mutations cause chronic mucocutaneous candidiasis |
| STAT2 | Type I IFNs | Impaired response to Type I IFN and susceptibility to viral infections | Deficiency causes increased susceptibility to viral mutations |
| STAT3 | Il-6 and other gp130 cytokines | Embryonically lethal | LOF mutations cause AD-HIES; GOF somatic mutations strongly associated with LGL |
| STAT4 | IL-12, IL-23, type I interferons | Mutations in mouse inhibit Th1 differentiation | Polymorphisms associated with RA, SLE |
| STAT5a/STAT5b | IL-2, EPO, TPO, GM-CSF, GH, IL-7 | Defective hematopoietic cell lines | Deficiency causes autoimmunity, bleeding diathesis, immunodeficiency and dwarfism; somatic mutations associated with LGL |
| STAT6 | IL-4, IL-13 | Mutations in mouse inhibit T helper 2 differentiation | Polymorphisms associated with asthma, atopy, increased levels of IgE |
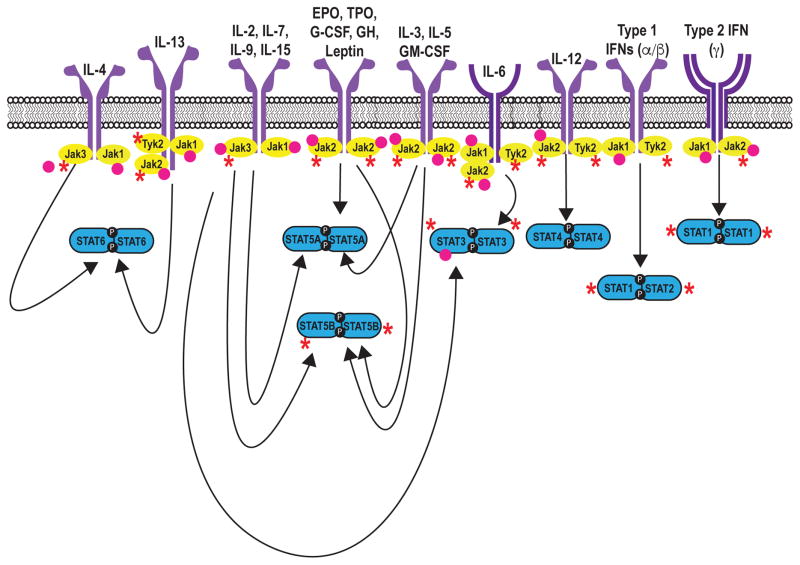
Upon engagement by ligand, receptor-associated Janus Kinases (Jaks) become activated and phosphorylate both each other and the intracellular tail of their receptors, thereby creating docking sites for latent, cytoplasmic transcription factors termed signal transducers and activators of transcription (STATs). Jak-mediated phosphorylation activates STATs, which in turn directly bind DNA and regulate gene expression (1, 2). There are four Jaks, Jak1, Jak2, Jak3, and Tyk2, which selectively bind different receptor chains (Figure 2). The selective usage of Jaks by different receptors explains their distinct in vivo roles (Table 1) and becomes particularly important with the generation of pharmacological inhibitors when specific or relatively discrete functional outcomes are sought.
Figure 2.
Seven mammalian STAT family members have been identified (STAT1, STAT2, STAT3, STAT4, STAT5a, STAT5b, STAT6). Different cytokines have the propensity to activate a particular STAT; however, interactive promiscuity between cytokines and any given STAT operates to varying degrees. The mechanisms by which STATs influence gene transcription have been elucidated using a combination of genetic approaches (e.g. use of knockout mice – Table 1) and new technologies that allow genome -wide assessment of gene expression and transcription factor binding. It is now clear that STATs bind tens of thousands of sites in the genome and regulate transcription of thousands of protein-coding genes, along with microRNAs and long non-coding RNAs. STATs also have important impacts on chromatin structure and the distinctive enhancer landscapes of differentiating cells.
While the aforementioned pathway is critical for the action of many cytokine and hormone receptors, it is important to recognize some inherent complexities. First, pathways other than STATs are activated by cytokines, and other (non-cytokine mediated) pathways can influence the activation of STATs. Growth factors such as epidermal growth factor can induce STAT tyrosine phosphorylation, whereas other pathways induce STAT serine phosphorylation. Additionally, a number of functions have been ascribed to ‘unphosphorylated’ STATs and STATs have non-nuclear functions. STAT3 in particular localizes to mitochondria, where it is thought to promote oxidative phosphorylation and membrane permeability. Conversely, Jaks can also have actions in the nucleus independent of STATs, including phosphorylatinon of histone proteins. In summary, while a straightforward view of the “Jak-Stat” signaling pathway explains a great deal of cytokine biology, it is equally important to recognize the complexities.
Genetic Links Between the Jak-STAT Pathway and Human Disease
Primary immunodeficiency and mutations of Jaks and Stats
Severe combined immunodeficiency
Perhaps the most dramatic evidence for the criticality of JAKs and STATs comes from patients with mutations in genes encoding for these signaling molecules. Severe combined immunodeficiency (SCID) is a devastating primary immunodeficiency in which the combination of nonfunctional T cells and defective immunoglobulin production results in a syndrome of recurrent severe infection, diarrhea, atopic dermatitis, and failure to thrive. An important immunopathological mystery was solved when mutations of a shared or common cytokine receptor subunit termed the common γ chain or γc were found to underlie X-linked SCID (1). In the absence of this receptor subunit, lymphocytes are unable to respond to IL-2, IL-4, IL-7, IL-9, IL-15 and IL-21 (Figure 2), severely impacting T cell and NK cell development, and B cell function. Because Jak3 selectively associates with γc, mutations of JAK3 have the same consequence.
In its classic presentation, the diagnosis of SCID is not difficult. However, not all cases are clinically obvious and the ability to test for a specific genetic mutation can be useful in establishing a diagnosis. Moreover, identification of the disease-causing mutations has major therapeutic implications. SCID is considered a pediatric emergency, necessitating rapid hematopoietic stem cell transplantation (HSCT). However, if a compatible donor is not available, gene therapy might be a reasonable alternative. This approach was effective in addressing primary immune deficiency (2, 3); but clinical trials were complicated by leukemia due to insertional oncogenesis (3, 4). Nonetheless, newer technologies hold future promise for this approach.
Hyperimmunoglobulin E syndrome
Hyperimmunoglobulin E syndrome (HIES, or Job’s syndrome) is a multisystem disorder characterized by recurrent and severe cutaneous and sinopulmonary bacterial infections, chronic dermatitis, elevated serum IgE and connective tissue abnormalities. Underlying many cases of autosomal dominant HIES are dominant negative mutations of STAT3 (5) (6). STAT3 mediates signaling through at least six classes of receptors (7) (Table 1, Figure 2), explaining the range of immunological and somatic abnormalities associated with this disorder. Many of the host defense deficits of this disorder can be explained by the criticality of STAT3 for the production of IL-17 by various lymphocytes. IL-17 acts on broadly expressed receptors to increase the production and recruitment of neutrophils to sites of inflammation and is especially important for host defense against S. aureus and fungal infections (8). It also plays a fundamental role in a range of autoimmune disorders reflecting broader tissue effector function. STAT3 directly binds many of the key genes required for Th17 differentiation, including the Il17 gene itself (9, 10). Increasingly though, it is recognized that Th17 cells are not the only source of IL-17, and that other IL-17- expressing cells can play a key role in controlling bacterial and fungal infections (8).
While failure to produce IL-17 is an important aspect of the immunopathogenesis of Job’s syndrome (6) (5), STAT3 also mediates signaling for another cytokine, IL-22, which is important for epithelial barrier function (11): impaired barrier function in HIES contributes to the atopic dermatitis, staphylococcal skin abscesses, and mucocutaneous candidiasis typical of the disease (12). STAT3 is also important for CD8 T cell memory, so patients with HIES are prone to recurrent Varicella zoster and Epstein-Barr virus infections (13). STAT3 has critical functions in B cells because of its role in signaling by IL-6, IL-21 and IL-10, though the precise mechanism underlying the overproduction of immunoglobulin E is not fully understood. The molecular basis for the craniofacial, skeletal and vascular abnormalities and the role of STAT3 have yet to be thoroughly dissected.
Treatment of HIES is currently directed towards ameliorating disease manifestations, with limited success. In principle, HCST might be a reasonable therapeutic option for HIES; however, STAT3 has important functions in both hematopoietic and non-hematopoietic epithelial cells, which might limit the efficacy of HSCT. Indeed, recent work in a mouse model of HIES would suggest that HSCT only partially rescues host defense deficits in this model, providing a cautionary note (14).
Mucocutaneous candidiasis
Chronic mucocutaneous candidiasis (CMC) comprises a heterogeneous collection of disorders manifested by recurrent or persistent infections of skin, nails and mucosa with Candida organisms, predominantly C. albicans. A recently described genetic lesion underlying CMC is a gain-of-function (GOF) mutation in STAT1. As in HIES, the net effect is poor production of IL-17, but in this case a different mechanism is operative. GOF STAT1 mutations cause exaggerated IFN-γ signaling, which inhibits IL-17 transcription, resulting in susceptibility to fungal infections. Increased IFN-γ signaling may have other relevant functional impacts, and these patients are indeed at risk for autoimmune disease. STAT1 GOF mutations have also been reported in association with autoimmunity, cerebral aneurysms, squamous cell carcinoma, and IPEX-like (immune dysregulation, polyendocrinopathy, enteropathy, X-linked) syndrome with intact regulatory T cells. (15) The exact role of STAT1 and mechanisms underlying these abnormalities are less well defined.
Mycobacterial infection
Contrasting with CMC is the syndrome caused by loss-of-function STAT1 mutations, characterized by recurrent mycobacterial infections and disseminated BCG. This, too, can be ascribed to the role of STAT1 in IFN-γ signaling. LOF STAT1 mutations are dominant negative for type 2 interferon signaling, underlying the patients’ susceptibility to mycobacterial infections, but autosomal recessive for type 1 interferon signaling, resulting in normal responses to viral infections (1).
TYK2 associates with the cytoplasmic domains of receptors for several cytokines including Type I interferon (IFN), IL-6, and IL-12. One patient with TYK2 mutations has been described with a syndrome resembling HIES (16); however, another had a very different phenotype comprising disseminated BCG infection, neurobrucellosis, and cutaneous Herpes zoster infection but no atopy and only mild elevation in IgE levels. (17). The distinctive clinical phenotypes presumably relate to the relative importance of TYK2 for different cytokines and potentially different cells.
STAT2 mutations and susceptibility to viral infection
Only one family with STAT2 deficiency has been described: one sibling developed disseminated vaccine strain measles following a routine immunization, and a second sibling died of severe disseminated viral infection. (18). STAT2 is required for Type 1, but not Type 2, interferon signaling (Figure 2): IFN-γ primarily activates STAT1, creating a STAT1-STAT1 homodimer that localizes to the nucleus to exert downstream effects. Type 1 IFNs, by contrast, activate a complex of transcription factors composed of STAT1, STAT2 and IRF9. Deficiency of STAT2 therefore profoundly affects Type 1 IFN signaling but not Type 2 IFN-γ signaling, explaining the associated clinical phenotype.
Immunodeficiency and growth retardation
Autosomal recessive STAT5B mutations cause a complex syndrome characterized by dwarfism, immunodeficiency, and autoimmunity. These clinical abnormalities can be explained by the role of STAT5 in growth hormone signaling. There are two STAT5 genes, STAT5A and STAT5B, and combined deficiency of both genes in mice profoundly affects all hematopoietic cells (19). Deficiency of STAT5B alone, however, also impacts immune cells, especially T and NK cells (19). STAT5B is important for regulatory T cells and for the key regulatory transcription factor FOXP3. This explains the atopy and autoimmunity seen in some cases of STAT5B deficiency, including early onset juvenile idiopathic arthritis, severe eczema, and immune thrombocytopenic purpura – many of which have been linked with functional deficiencies of regulatory T cells (20, 21). Conversely, STAT5 is also important for T cell memory (22), and thus STAT5B mutation can also be associated with recurrent pneumonia and other infections (1). These observations highlight the dual pro- and anti-inflammatory nature of STAT5: on one hand, it is involved in development and homeostasis of lymphoid cells, while on the other hand, it limits T cell hyperactivity in conditions of immunocompetency.
The JAK-STAT pathway and cancer
Constitutive activation of JAKs and STATs was first recognized as being associated with malignancy in the 1990’s (19). JAK-STAT pathways can be activated by various mechanisms including: autocrine/paracrine cytokine production, activating mutations of JAKs or other upstream oncogenes, that in turn activate STATs, or most rarely activating mutations of STATs themselves.
Polycythemia vera and JAK2 mutations
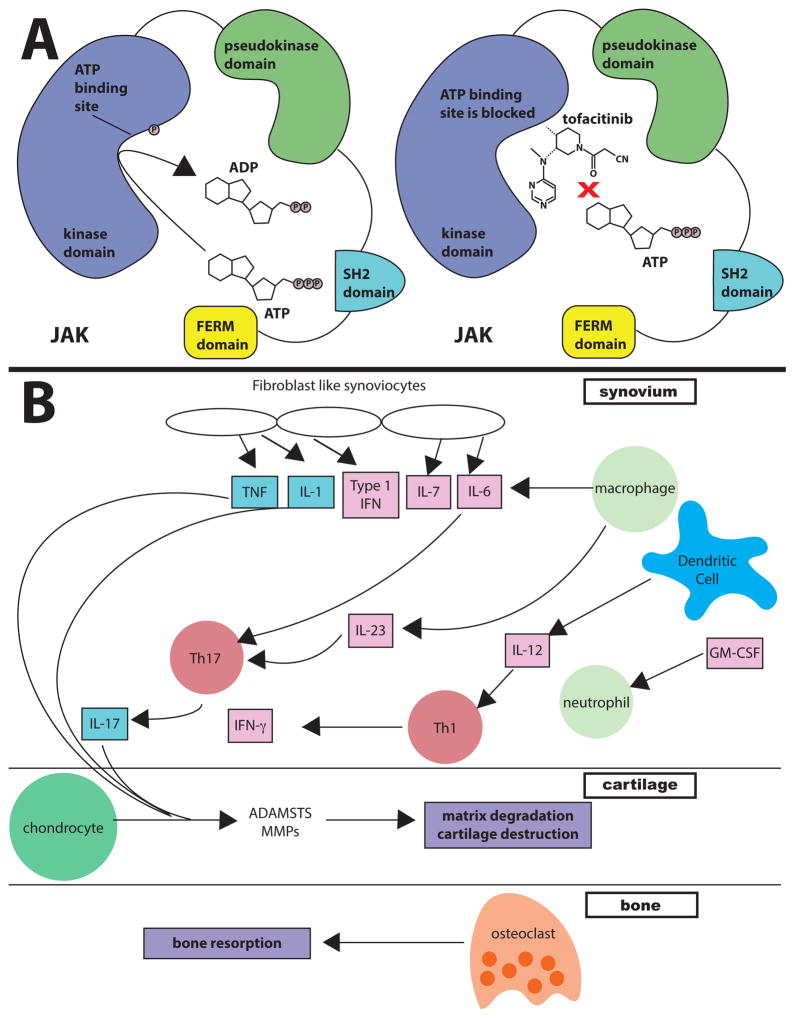
Polycythemia vera (PV), essential thrombocytosis (ET) and primary myelofibrosis (PMF) are closely related myeloproliferative diseases (MPD), all characterized by elevated bone marrow production of erythrocytes and megakaryocytes. Early reports classified each syndrome according to a distinct clinical phenotype, but in the 1950s it was recognized that the three could overlap significantly (23). The genetic basis for this relationship was identified with the discovery of activating mutations of JAK2, most commonly V617F, in almost all patients with PV and in many with ET and PMF (24). JAK2 is crucial for signal transduction downstream of the erythropoietin, thrombopoetin, and related receptors that control erythrocyte and megakaryocyte expansion (1). The V617F mutation lies within the pseudokinase domain, which historically was presumed to be a catalytically inactive negative regulator of JAK2 (25). More recent work, however, has suggested that that this domain has catalytic activity responsible for its inhibitory function (25). Elaboration of the pseudokinase domain’s crystal structure has created opportunities to develop targeted JAK2 inhibitors for the treatment of MPDs. (25, 26) JAK2 can also be targeted for activation: eltrombopag, a small molecule JAK2 activator, is used to treat refractory aplastic anemia and ITP (27).
Gain-of-function somatic mutations of Jaks in cancer
Predating the discovery of JAK2 mutations and MPD, somatic activating mutations in JAKs have been described in various malignancies (28), although their contribution to disease pathogenesis is incompletely defined. Somatic JAK2 mutations have been linked to a number of hematologic malignancies (28, 29), and a cohort study indicated that the V617F mutation was associated with increased mortality in the general population (30). Mutations in JAK1 have been associated with the development of AML, although there is debate surrounding this point (31). JAK3 mutations have been associated with leukemia and lymphoma (28). Secondary mutations in JAK3 have been described in juvenile myelomonocytic leukemia, and are thought to be associated with disease progression (32).
STATs and oncogenesis
Aberrant activation of STATs has been found in many tumors; STAT3 is constitutively activated in solid and hematological cancers (33, 34), and cytokines produced by T cells can activate STAT3 in cancer cells to impact stemness and tumorigenicity (35). STAT3 has also been implicated in the pathogenesis of diffuse large B cell lymphoma (36) and solid organ malignancies such as breast and nasopharyngeal carcinomas (34). Mutations of the SH2 domain of STAT3 are present in 40% of large granular leukemias (37). Similar mutations have been identified in approximately 30% of chronic natural killer cell lymphoproliferative disorders (38), as well as in the context of aplastic anemia and myelodysplastic syndromes (39, 40). Curiously, some of the mutations seen in large granular leukemia affect the same residues that are altered in HIES – a disease associated with STAT3 loss of function and an increase in the incidence of both T and B cell lymphomas (1). This is notable because STAT3 mutations typically result in a hypofunctional protein and it is unclear why patients with HIES have an increased lymphoma risk.
Aberrant STAT5 signaling has also been implicated in the pathogenesis of hematologic and solid organ malignancies (33, 41). Chronic myeloid leukemia (CML) is characterized by the presence of the BCR-Abl oncogene resulting in a constitutively active tyrosine kinase (42). CML can be mimicked in mice by forced expression of BCR-Abl. Mice with bone marrow deficient in STAT5, however, are resistant to developing CML, suggesting that STAT5 is essential for the development of CML (42).
Despite their role in promoting oncogenesis, there is substantial literature that also points to a protective role of STATs in cancer. IFNs are critical for the elimination phase of cancer immunoediting, in which the immune system recognizes and destroys transformed malignant cells. (43) A subset of human tumors lack the ability to signal through the IFN receptors, including some types of lung cancer, prostate cancer, melanoma, and breast cancer. (43, 44). Much of the effect of IFNs is mediated by STAT1. Differential STAT1 signaling may influence the clinical phenotype caused by the V617F mutation in JAK2 (45).
JAKs and STATs and common, multigenic human diseases
Beyond the identification of monogenic loss- and gain- of function alleles, an explosion of genome-wide association studies (GWAS) has implicated the Jak-STAT pathway in common human diseases. Polymorphisms of STAT1 have been associated with an increased risk of malignancy. (46) and polymorphisms of STAT3 are associated with Crohn’s disease and psoriasis (47). STAT4 SNPs are associated with rheumatoid arthritis and systemic lupus erythematosus (SLE) (38); STAT6 SNPs are associated with asthma and allergy (48). As is the case for most GWAS studies, the functional implications of these polymorphisms remain obscure. Nonetheless, a large body of evidence points to fundamental roles for the JAK-STAT pathway in human health and disease, from rare monogenic disorders to more common complex diseases.
Therapeutic targeting of Janus kinases
Given the breadth of data implicating Jak/STAT signaling in autoimmune disease and malignancy, it is not surprising that this pathway has become an attractive therapeutic target. While this is an area of intense research, we will attempt to summarize recent and impending breakthroughs. We will first briefly review the FDA-approved JAK inhibitors or Jakinibs and then discuss the implications for future work in targeting JAKs and possibly STATs.
Tofacitinib
Tofacitinib was the first selective Jakinib to be tested and later approved in humans. The initial rationale underlying its development was JAK3’s essential, non-redundant function in lymphocytes. JAK3 was a particularly attractive therapeutic target because deficiency does not affect non-immunologic organs or tissues, indicating that the adverse effect profile of a selective inhibitor could be favorable, at least in terms of non-immunologic or hematologic toxicity (49–50). Subsequently tofacitinib was found to inhibit JAK1 and to a lesser degree JAK2 (51–52). This broader spectrum targets cytokines and hormones that are important to host defense, development, and homeostasis of hematopoietic and other cells. However, clinical study data thus far available have established that the drug appears to have an acceptable safety profile, sufficient as to allow utilization and exploration across a range of immune-mediated diseases, and the ability of tofacitinib to inhibit JAK1 and JAK2 may have improved tofacitinib’s efficacy in autoimmunity.
Thus, tofacitinib was effective in preclinical models of several immune-mediated diseases ranging from inflammatory arthritis and transplantation rejection to allergic models (53). A subsequent comprehensive phase II/III clinical trial program demonstrated the acceptable safety and efficacy of tofacitinib in RA (Table S1 – online). Tofacitinib is effective used as monotherapy or in conjunction with methotrexate, and is effective in distinct patient populations, including those refractory to standard therapy. (54–56) Tofacitinib was found to be noninferior to the TNF inhibitor adalimumab, representing normal first choice biologic in the RA paradigm (57). Patients with disease refractory to both conventional and biologic DMARDs were responsive to tofacitinib (58), and tofacitinib halted radiographic progression of RA (59). Treatment-naïve patients exhibited particularly impressive response rates and in these studies outperformed methotrexate, a hurdle not previously achieved in such study designs by other modes of action.
Clinical trials evaluating tofacitinib have shown promising results in treating several other autoimmune disorders including psoriasis, psoriatic arthritis, juvenile idiopathic arthritis (JIA), keratoconjunctivitis sicca, and transplant rejection (60–66). Results were similarly encouraging in ulcerative colitis (67), but not in Crohn’s disease (68). (http://clinicaltrials.gov/ct2/show/NCT01786668?term=tofacitinib&rank=9; http://clinicaltrials.gov/ct2/show/NCT01500551?term=tofacitinib&rank=28).
The efficacy of tofacitinib in RA led to FDA approval in 2012 for patients unresponsive to or intolerant of methotrexate. However, the approval required a “black box” warning and a postmarketing study. In RA and other trials Infection rates with tofacitinib have generally been higher than those seen with placebo. (54–59, 69–70). Most of these have been mild upper respiratory infections, urinary tract infections, and episodes of viral gastroenteritis. However, serious and opportunistic infections have also been reported including Mycobacterium tuberculosis, Herpes zoster, Cytomegalovirus, Pneumocystis jirovecii pneumonia, and bacterial pneumonias (69, 71–73). There may be a relationship to significant lymphopenia, and SAEs occurred with higher frequency in older patients, those with diabetes and those on higher doses of glucocorticoids. This AE profile is not unexpected, as the efficacy of tofacitinib relates directly to the fundamental role of JAKs in immune responses, including response to infection – they will need to be carefully evaluated going forward to ensure appropriate patient selection and observation over time. Moreover new agents targeting Jaks should also be carefully evaluated with this in mind.
Cardiovascular events in patients on tofacitinib have been reported. (59, 69, 70) Concern regarding cardiovascular disease has largely stemmed from the modest but statistically significant increases in HDL and LDL noted in clinical trials. However, the clinical significance of these laboratory abnormalities is not yet clear. Metabolic and immune regulator networks are intertwined and as such these are not entirely unexpected observations. Moreover, RA patients treated with tocilizumab, which also causes hypercholesterolemia mediated through its blockade of IL-6R, do not have increased rates of cardiovascular disease observed thus far, although ongoing outcomes studies are evaluating this risk (74).
Another adverse effect of tofacitinib is anemia and neutropenia, usually mild, and presumably related to JAK2 inhibition. Elevations in serum creatinine and transaminases have been noted (73) but consistent effects on renal function are not emerging although isolated significant problems have been reported.
Malignancies have been associated with tofacitinib, but as with other RA drugs, it has been difficult to establish whether this represents a significant risk (73). In principle, tofacitinib may adversely affect malignancy risk through its effects on JAK1 and JAK2, which are important for IFN signaling and therefore for cancer immunoediting. (43) Given the relatively small number of malignancies reported, further studies will be needed to clarify risk and relationship to duration of therapy.
Ruxolitinib
A potent inhibitor of JAK1 and JAK2, Ruxolitinib was first FDA approved Jakinib. It was approved for the treatment of intermediate and high risk PMF after the COMFORT-I and COMFORT-II Trials demonstrated marked responses to therapy. Efficacy was irrespective of the presence of JAK2V617F mutations (52). Ruxolitinib has also been studied in psoriasis and in RA, where preliminary results have been encouraging (52). Further studies are ongoing for the treatment of PV, ET, a variety of malignancies, and alopecia areata. (http://clinicaltrials.gov/ct2/show/NCT01751425?term=ruxolitinib&rank=1; http://clinicaltrials.gov/ct2/show/NCT02119676?term=ruxolitinib&rank=6; http://clinicaltrials.gov/ct2/show/NCT00726232?term=ruxolitinib&rank=11; http://clinicaltrials.gov/ct2/show/NCT01950780?term=ruxolitinib+alopecia&rank=1)
Oclacitinib
Oclacitinib is a pan-JAK inhibitor recently approved for the treatment of atopic dermatitis in canines (75). While it is not currently being studied in humans, its effectiveness in treating allergic skin disease illustrates the breadth of anti-inflammatory effects that can be seen with Jakinibs, and provides rationale for the evaluation of this class of drugs in immune-mediated dermatological conditions. (http://clinicaltrials.gov/ct2/show/NCT02001181?term=tofacitinib+dermatitis&rank=1).
Second generation Jakinibs and the future of targeting Jaks
At present, 25 Jakinibs are currently being tested in various conditions from asthma, to malignancies and myeloproliferative diseases, to a host of autoimmune conditions (Table 3). The first, FDA-approved Jakinibs inhibit multiple JAKs and consequently inhibit a relatively broad spectrum of cytokines. This is also true for other Jakinibs in clinical trials. Baricitinib, a Jak1/Jak2 inhibitor, has shown promising results in the treatment of RA (50); phase II trials are also ongoing for RA and psoriasis, and in the treatment of autoinflammatory diseases. (http://clinicaltrials.gov/ct2/show/NCT01710358?term=baricitinib&rank=12; http://clinicaltrials.gov/ct2/show/NCT01724580?term=baricitinib&rank=16; http://clinicaltrials.gov/ct2/show/NCT01490632?term=LY3009104+psoriasis&rank=1) The JAK1/2 inhibitor momelotinib has shown encouraging results in the treatment of myelofibrosis; (76) a Phase III trial is ongoing. (http://clinicaltrials.gov/ct2/show/NCT01969838?term=momelotinib+myelofibrosis&rank=2). The ability to inhibit multiple cytokines has implications for efficacy and adverse effect profiles that can be traced directly to the mechanism of action. Via JAK1 and JAK2 targeting, they inhibit all cytokine receptors containing the γc chain, βc common and gp130, along with interferons, IL-12, IL-23 and IL-27 and the hormone-like cytokines (Figure 2). Many of these cytokines are inhibited to various degrees, due to relative selectivity and pharmacokinetics, giving these medications an acceptable therapeutic index. However, the goal of more selectively targeting a single JAK remains, especially in the setting of long-term use for autoimmune disease.
Table 3.
Jakinibs and Statinibs
| Drug | Target | Status | Diseases |
|---|---|---|---|
| Ruxolitinib (INC424) | JAK1, JAK2 |
FDA approved Phase II Phase 2b |
Polycythemia, Myelofibrosis, Various cancers Psoriasis (topical) |
| Tofacitinib | JAK3>JAK1≫ (JAK2) |
FDA approved Phase III Phase II |
RA Psoriasis, Ulcerative colitis spondyloarthropathy, JIA Transplant rejection |
| Oclacitinib | JAK1 | FDA approved | Canine allergic dermatitis |
| ABT494 | JAK1 | Phase II | RA, Crohn’s |
| Baricitinib | JAK1, JAK2 | Phase II | RA, Psoriasis, Diabetic nephropathy, autoinflammatory disease |
| Momelitinib | JAK1, JAK2 | Phase III | Myelofibrosis |
| GLPG0634(filgotinib) | JAK1 | Phase II | RA, Crohn’s |
| INCB047986 | JAK inhibitor | Phase I | Lymphoma, solid tumors |
| INCB039110 | JAK1, JAK2 | Phase II | Psoriasis, RA |
| CYT387 | JAK1, JAK2 | Phase II | Myelofibrosis |
| ASP015K | JAK 3/JAK1≫ JAK2/TYK2 |
Phase II | Psoriasis, RA |
| R333 | JAK/SYK | Phase II | Discoid lupus (topical) |
| PF-04965842 | JAK1 | Phase I | healthy adults |
| GLG0778 | JAK1 | Phase II | SLE |
| GSK2586184 | JAK1 | Phase II | SLE, Psoriasis |
| VX-509 (decernotinib) | JAK3 | Phase IIb | RA |
| Lestaurtinib | FLT3, JAK2, TRKs | Phase II | AML, PCV/ET, myelofibrosis |
| Pacritinib | JAK2 | Phase II | Myelofibrosis, myeloid leukemias, MDS |
| LY2784544 | JAK2 | Phase II Phase I |
myelofibrosis various cancers |
| AZD1480 | JAK1, JAK2 | Phase I | myeloproliferative diseases, various cancers |
| XL019 | JAK2 | Phase I, terminated | Myelofibrosis, PCV |
| BMS-911543 | JAK2 | Phase II | Myelofibrosis |
| NS-108 | JAK2, SRC | Phase II | Myelofibrosis |
| PF-06263276 | pan-JAK | Phase I | healthy (topical) |
| SV1578 | JAK2, Flt3 | Phase I | healthy adults |
| ISIS-STAT3Rx (AZD9150) | STAT3 | Phase II | various cancers |
| OPB-51602 | STAT3 | Phase I | nasopharyngeal carcinoma |
| OPB-31121 | STAT3 | Phase I | various cancers |
Phase II trials have demonstrated efficacy of the JAK3 inhibitor VX-509 (77) and the JAK1 inhibitor GLPG0634 (78, 79) in treating RA. It must be acknowledged that even many of these second generation inhibitors are not entirely specific to one JAK, and that larger confirmatory studies will be required as this class of medicines continues to expand.
Another crucial question for clinical use relates to the optimal dosing regimens and utility in various phases of different diseases. Thus, although tofacitinib has been evaluated in comparison with DMARDs, it may be more effective as an induction regimen in acute immune-mediated disease, in place of steroids or even cyclophosphamide. As with steroids, flexible dosing regimens with dose tapering may have utility. Moreover, the development of topical formulations has considerable implications for dermatological and pulmonary disease. Thus, it may take time to fully appreciate the optimal ways in which these drugs can be used to treat the broad range of clinical scenarios for which they are being evaluated. Moreover could they offer utility for tissue repair, establishing immune tolerance and facilitating stratification by virtue of accessible biomarker profiles.
The Prospect of STAT inhibitors
Because STATs are also key nodes in signal transduction and are frequently activated in the setting of malignancy, considerable effort has been expended to develop STAT inhibitors, for over two decades. This has met with limited success due to issues with bioavailability, in vivo efficacy, and selectivity. Conceptually, rational targeting of STATs may be achieved by: (1) blocking phosphorylation (2) disrupting the SH2 domains that mediate binding to phosphorylated receptors and dimerization or (3) interfering with DNA binding. It is the last of these methods that led to the development of the first STAT inhibitors appropriate for clinical use. Oligonucleotide-based STAT inhibitors are currently being tested in the treatment of various malignancies (Table 3). Clinical trials for advanced malignancies are also underway for an even newer group of small molecule inhibitors targeting STAT3, including OPB-51062 (http://clinicaltrials.gov/ct2/show/NCT02058017?term=stat3&rank=4) and OPB-31121 (http://clinicaltrials.gov/ct2/show/NCT01406574?term=OPB-31121&rank=4). Preclinical results indicate that STAT inhibitors are effective in animal models of autoimmune disease (80) Intrabodies, which bind with great specificity to phosphorylated STAT3 (81), represent a possible novel avenue for the development of STAT inhibitors.
A challenge with respect to the development of STAT inhibitors relates to specificity. The homology of STAT3 with other STATs, especially STAT1, is a factor that presents a singular challenge in the design of STAT inhibitors. STAT1 mediates IFN signaling and is critical for apoptosis, cell death, and defense against pathogens; a safe and effective STAT3 inhibitor would presumably have minimal activity against STAT1. In this regard, it is important to keep in mind that STAT3 also has important diverse roles in barrier function and host defense, as well as inhibiting tumorigenesis; these factors will need to considered in clinical trials of STAT inhibitors.
Empiric targeting of STATs is another strategy that has been employed and a variety of drugs have been “repurposed” as STAT inhibitors. These include drugs such as lysofilline, fludarabine, pimozide, sulforaphane, pyrimethamine and the neutraceutical curcumin. The precise molecular and structural basis through which they interfere with STAT action is incompletely understood.
Conclusions
The discovery of the JAK/STAT pathway and its role in health and disease represents one of the most exciting developments in modern medicine that now serves as a paradigm for cell signaling and translational science. Basic molecular strategies together with genetic and phenotypic analysis have led to better immunopathogenic insights, diagnostic advances, and new therapeutic options for both rare and common diseases. Clearly there are many challenges that remain in elucidating how this evolutionarily conserved pathway regulates chromatin biology and cellular differentiation. Furthermore, much work remains in dissecting the precise mechanisms by which Jakinibs exert their effects vis-à-vis the various cytokines that are inhibited in different clinical scenarios, The second generation selective jakinibs also need to be investigated further, to determine whether they represent an advance over existing drugs. It will undoubtedly be exciting to see how the story unfolds over the next few years, as we learn precisely how to use these and other inhibitors of the JAK-STAT pathway.
Figure 3.
Table 2.
| Trial | Notes | Phase | Patient Population | Rx regimen | ACR20 TOFA | Other measures |
AEs | Serious AEs | Infectionn TOFA (serious infection TOFA) |
|---|---|---|---|---|---|---|---|---|---|
| Kremer A&R 2009 | 2a | RA with inadequate responses to MTX or ETA, ADA, INF (most were MTX failures) | PBO vs TOFA 5mg BID vs 15mg BID vs 30mg BID × 6 weeks à DC for 6 weeks with followup | 5mg: 70.5% 15mg: 80.2% 30mg: 76.8% PBO: 29.2% |
ACR50/70, HAQ-DI, DAS28-ESR, DAS28-CRP | 5mg: 59% 15mg: 75% 30mg: 77% PBO: 59% |
5mg: none 15mg: 1 patient 30mg: 1 patient PBO: 1 patient |
5mg: 24.6% 15mg: 30.4% 30mg: 30.4% PBO: 26.2% |
|
| Tanaka 2011 | 2b | MTX failures (Note low dose of background MTX avg 8mg weekly) | Background MTX vs MTX + TOFA 1mg BID vs 3mg BID vs 5mg BID vs 10 mg BID × 12 weeks | 1mg = 64.3% 3mg =77.8% 5mg = 96.3 10mg = 80.8% PBO 14.3% |
ACR50/70, HAQ-DI, DAS28-CRP | 1mg: 53.6% 3mg: 48.1% 5mg: 77.7% 10mg: 73.4% PBO: 35.8% |
1mg: 1 patient 3mg: 1 patient 5mg: 1 patient 10mg: 2 patients PBO: none |
1mg = 10.7% 3mg =29.6% 5mg = 11.1% 10mg = 42.3% PBO 21.4% |
|
| Kremer 2011 ACR | ORAL Sync, Abstract only | 3 | Failed nonbiologic DMARDs | Monotherapy with TOFA 5mg BID × 12mo vs 10mg BID × 12 mo vs PBO → TOFA 5mg or 10mg after 3mo for nonresponders and 6mo for all patients. Abstract reprsents 6mo interim analysis | 5mg = 52.7% 10mg =58.3% PBO: 31.2% |
ACR50/70, HAQ-DI, DAS28-ESR |
Months 0–3 5mg: 52.7% 10mg: 54.4% PBO: 61% Months 3–6 5mg: 38.4% 10mg: 39% PBO: 25.9% |
Months 0–3 5mg: 2.9% 10mg: 2.5% PBO: 3.8% Months 3–6 5mg: 1.6% 10mg: 2.2% PBO: 0% |
Four opportunistic infections. Serious infectious events in 3 (5mg BID) and 5 (10mg BID) patients from months 0–6. No data in PBO-treated patients. |
| Fleischmann 2012 A&R | 2b | Failed DMARDs (mainly MTX failures at mod/approp doses 10–15mg weekly) | Monotherapy with TOFA 1mg BID vs 3mg BID vs 5mg BID vs 10mg BID vs 15mg BID vs ADA 40mg every 14d × 12 weeks followed by TOFA 5mg BID for 12 weeks | 1mg: 31.5% 3mg: 39.2% 5mg: 59.2% 10mg: 70.5% 15mg: 71.9% PBO: 22% ADA (wk 12): 35.9% |
ACR50/70, HAQ-DI, SF-36, FACIT-F, DAS28-ESR, DAS28-CRP | 1mg: 51.4% 3mg: 52.9% 5mg: 55.1% 10mg: 59% 15mg: 61.4% PBO: 47.1% ADA (wk12): 50.9% ADA→TOFA (wk24): 63.6% |
1mg: 5.4% 3mg: 2.9% 5mg: 0% 10mg: 1.6% 15mg: 7% PBO: 5.9% ADA (wk12): 1.9% ADA→TOFA (wk24): 9.1% |
1mg: 29.7% (5.9%) 3mg: 20.6% (0%) 5mg: 34.7% (0%) 10mg: 34.3% (0%) 15mg: 33.3% (1.8%) PBO: 17.6% (2.9%) ADA (wk12): 18.9% (0%) ADA→TOFA (wk24): 25% (2.3%) |
|
| Kremer 2012 A&R | 2b | MTX failures | MTX background + TOFA 20mg daily vs 1mg BID vs 3mg BID vs 5mg BID vs 10mg BID vs 15mg BID × 24 weeks | 1mg: ???% 3mg: 52.9% 5mg: 50.7% 10mg: 58.1% 15mg: 56.0% 20mg: 53.8% PBO: 33.3% |
ACR50/70, HAQ-DI, DAS28-CRP | 1mg: 59.2% 3mg: 69.1% 5mg: 66.2% 10mg: 67.6% 15mg: 76% 20mg: 61.2% PBO: 56.9% |
1mg: 2% 3mg: 7.3% 5mg: 5.6% 10mg: 1.4% 15mg: 8% 20mg: 6% PBO: 0% |
1mg: 14.3% (0%) 3mg: 20% (0%) 5mg: 22.5% (1.4%) 10mg: 17.6% (1.4%) 15mg: 18.7% (0%) 20mg: 19.4% (1.5%) PBO: 5.9% (0%) |
|
| Van Vollenhoven 2012 NEJM | ORAL Standard | 3 | MTX failures | MTX background in all groups + TOFA 5mg BID × 12 mo vs TOFA 10mg BID × 12 mo vs ADA 40mg q2weeks × 12 mo vs PBO → nonresponders switched to TOFA 5mg or 10mg BID at mo3 and all patients switched to TOFA 5mg or 10mg BID at mo6 | 5mg: 51.5% 10mg: 52.6% PBO: 28.3% ADA: 47.2% |
ACR50/70, HAQ-DI, DAS28-ESR, DAS28-CRP |
Months 0–3 5mg: 52% 10mg: 46.8% PBO: 47.2% ADA: 51.5% Months 3–6 5mg: 32.8% 10mg: 30.8% PBO: 27.1% PBO→5mg: 25% PBO→10mg: 42.9% ADA: 33.3% Months 6–12 5mg: 43.6% 10mg: 41.8% PBO→5mg: 32.1% PBO→10mg: 40.4% ADA: 40.7% |
Months 0–3 5mg: 5.9% 10mg: 5% PBO: 1.9% ADA: 2.5% Months 3–6 5mg: 4.9% 10mg: 3.5% PBO: 3.4% PBO→5mg: 0% PBO→10mg: 0% ADA: 2.9% Months 6–12 5mg: 4.9% 10mg: 3% PBO→5mg: 1.8% PBO→10mg: 7.7% ADA: 3.4% |
(Serious only) Months 0–3 5mg: 1.5% 10mg: 2% PBO: 0.9% ADA: 0% Months 3–6 5mg: 1% 10mg: 0.5% PBO: 0% PBO→5mg: 0% PBO→10mg: 0% ADA: 1% Months 6–12 5mg: 1% 10mg: 1.5% PBO→5mg: 0% PBO→10mg: 1.9% ADA: 0.5% |
| Fleischmann 2012 NEJM | ORAL Solo | 3 | DMARD or biologic nonresponders, about 80% methotrexate failures | Monotherapy with TOFA 5mg BID × 6 mo vs TOFA 10mg BID × 6 mo vs PBO × 3mo → TOFA 5mg BID × 3 mo vs PBO × 3mo → TOFA 10mg BID × 3 mo | 5mg: 59.8% 10mg: 65.7% PBO: 26.7% |
ACR50/70, HAQ-DI, DAS28-ESR, FACIT-F |
Months 0–3 5mg: 51% 10mg: 56.7% PBO: 54.9% Months 3–6 5mg: 39.9% 10mg: 41.2% PBO→5mg: 36.1% PBO→10mg: 39.3% |
Months 0–3 5mg: 0.4% 10mg: 2% PBO: 4.9% Months 3–6 5mg: 2.1% 10mg: 2.4% PBO→5mg: 1.6% PBO→10mg: 0% |
(Serious only) Months 0–3 5mg: 0% 10mg: 0.4% PBO: 0% Months 3–6 5mg: 0.4% 10mg: 1.2% PBO→5mg: 1.6% PBO→10mg: 0% |
| Burmester 2012 Lancet | ORAL Step | 3 | TNFi nonresponders only, 30% had failed 2 + TNFis | MTX background in all groups + TOFA 5mg BID × 6 mo vs TOFA 10mg BID × 6 mo vs PBO × 3mo → TOFA 5mg BID × 3 mo vs PBO × 3mo → TOFA 10mg BID × 3 mo | 5mg: 41.7% 10mg: 48.1% PBO: 24.4% |
ACR50/70, HAQ-DI, DAS28-ESR, DAS28-CRP, FACIT-F |
Months 0–3 5mg: 53.4% 10mg: 56.7% PBO: 56.8% Months 3–6 5mg: 42.9% 10mg: 43.3% PBO→5mg: 36.4% PBO→10mg: 42.4% |
Months 0–3 5mg: 1.5% 10mg: 1.5% PBO: 4.5% Months 3–6 5mg: 3.8% 10mg: 4.5% PBO→5mg: 4.5% PBO→10mg: 3% |
(Serious only) Months 0–3 5mg: 0% 10mg: 0% PBO: 0% Months 3–6 5mg: 1.5% 10mg: 1.5% PBO→5mg: 1.5% PBO→10mg: 0% |
| Lee 2012 ACR | ORAL Start. Abstract only (interim analysis) | 3 (ongoing) | MTX naïve patients | TOFA 5mg BID vs. 10mg BID vs MTX 10mg/week titrated up to 20mg/week; patients treated × 24 mo (data from 12 mo interim analysis) | 5mg: 71% 10mg: 75.8% MTX: 50.5% |
ACR50/70, HAQ-DI, Sharp-van der Hejide Score (SHS) | 5mg: 70.1% 10mg: 74.4% MTX: 69.9% |
5mg: 6.5% 10mg: 6.1% MTX: 7% |
5mg: 31.8% 10mg: 38.7% MTX: 27.4% |
| Van der Hejide 2013 A&R | ORAL Scan | 3 | Active erosive RA with erosive disease, MTX failures (10–20% failed biologics) | MTX background in all groups + TOFA 5mg BID × 24 mo vs TOFA 10mg BID × 24 mo vs PBO → nonresponders switched to TOFA 5mg or 10mg BID at mo3 and all patients switched to TOFA 5mg or 10mg BID at mo6 |
At month 6 5mg: 51.5% 10mg: 61.8% PBO: 25.3% At month 12 5mg: 48.5% 10mg: 57% |
ACR50/70, HAQ-DI, DAS28-ESR, Sharp-van der Hejide Score (SHS), FACIT-F |
Months 0–3 5mg: 48.9% 10mg: 54.1% PBO: 45.6% Months 3–6 5mg: 45.2% 10mg: 35.1% PBO→5mg: 42.9% PBO→10mg: 40.5% Months 6–12 5mg: 51.7% 10mg: 55.1% PBO→5mg: 42% PBO→10mg: 44.3% |
Months 0–3 5mg: 3.7% 10mg: 3.2% PBO: 3.1% Months 3–6 5mg: 5.3% 10mg: 2.2% PBO→5mg: 2.4% PBO→10mg: 2.7% Months 6–12 5mg: 4% 10mg: 2.8% PBO→5mg: 1.2% PBO→10mg: 5.1% |
Months 0–3 5mg: 0.6% 10mg: 0.6% PBO: 0% Months 3–6 5mg: 2.5% 10mg: 0.6% PBO→5mg: 1.2% PBO→10mg: 1.3% Months 6–12 5mg: 0.3% 10mg: 0.3% PBO→5mg: 0% PBO→10mg: 1.3% |
References
- 1.O’Shea JJ, Holland SM, Staudt LM. JAKs and STATs in immunity, immunodeficiency, and cancer. N Engl J Med. 2013;368:161–70. doi: 10.1056/NEJMra1202117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hacein-Bey-Abina S, Le Deist F, Carlier F, et al. Sustained correction of X-linked severe combined immunodeficiency by ex vivo gene therapy. N Engl J Med. 2002;346:1185–93. doi: 10.1056/NEJMoa012616. [DOI] [PubMed] [Google Scholar]
- 3.Rivat C, Santilli G, Gaspar HB, Thrasher AJ. Gene therapy for primary immunodeficiencies. Hum Gene Ther. 2012;23:668–75. doi: 10.1089/hum.2012.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hacein-Bey-Abina S, von Kalle C, Schmidt M, et al. A serious adverse event after successful gene therapy for X-linked severe combined immunodeficiency. N Engl J Med. 2003;348:255–6. doi: 10.1056/NEJM200301163480314. [DOI] [PubMed] [Google Scholar]
- 5.Minegishi Y, Saito M, Tsuchiya S, et al. Dominant-negative mutations in the DNA-binding domain of STAT3 cause hyper-IgE syndrome. Nature. 2007;448:1058–62. doi: 10.1038/nature06096. [DOI] [PubMed] [Google Scholar]
- 6.Holland SM, DeLeo FR, Elloumi HZ, et al. STAT3 mutations in the hyper-IgE syndrome. N Engl J Med. 2007;357:1608–19. doi: 10.1056/NEJMoa073687. [DOI] [PubMed] [Google Scholar]
- 7.Casanova JL, Holland SM, Notarangelo LD. Inborn errors of human JAKs and STATs. Immunity. 2012;36:515–28. doi: 10.1016/j.immuni.2012.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miossec P, Kolls JK. Targeting IL-17 and TH17 cells in chronic inflammation. Nat Rev Drug Discov. 2012;11:763–76. doi: 10.1038/nrd3794. [DOI] [PubMed] [Google Scholar]
- 9.Durant L, Watford WT, Ramos HL, et al. Diverse targets of the transcription factor STAT3 contribute to T cell pathogenicity and homeostasis. Immunity. 2010;32:605–15. doi: 10.1016/j.immuni.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ciofani M, Madar A, Galan C, et al. A validated regulatory network for Th17 cell specification. Cell. 2012;151:289–303. doi: 10.1016/j.cell.2012.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sonnenberg GF, Fouser LA, Artis D. Border patrol: regulation of immunity, inflammation and tissue homeostasis at barrier surfaces by IL-22. Nat Immunol. 2011;12:383–90. [Google Scholar]
- 12.Mogensen TH. STAT3 and the Hyper-IgE syndrome: Clinical presentation, genetic origin, pathogenesis, novel findings and remaining uncertainties. JAKSTAT. 2013;2:e23435. doi: 10.4161/jkst.23435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siegel AM, Heimall J, Freeman AF, et al. A critical role for STAT3 transcription factor signaling in the development and maintenance of human T cell memory. Immunity. 2011;35:806–18. doi: 10.1016/j.immuni.2011.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steward-Tharp SM, Laurence A, Kanno Y, et al. A mouse model of HIES reveals pro and anti-inflammatory functions of STAT3. Blood. 2014 doi: 10.1182/blood-2013-09-523167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Uzel G, Sampaio EP, Lawrence MG, et al. Dominant gain-of-function STAT1 mutations in FOXP3 wild-type immune dysregulation-polyendocrinopathy-enteropathy-X-linked-like syndrome. J Allergy Clin Immunol. 2013;131:1611–23. doi: 10.1016/j.jaci.2012.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Minegishi Y, Saito M, Morio T, et al. Human tyrosine kinase 2 deficiency reveals its requisite roles in multiple cytokine signals involved in innate and acquired immunity. Immunity. 2006;25:745–55. doi: 10.1016/j.immuni.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 17.Kilic SS, Hacimustafaoglu M, Boisson-Dupuis S, et al. A patient with tyrosine kinase 2 deficiency without hyper-IgE syndrome. J Pediatr. 2012;160:1055–7. doi: 10.1016/j.jpeds.2012.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hambleton S, Goodbourn S, Young DF, et al. STAT2 deficiency and susceptibility to viral illness in humans. Proc Natl Acad Sci U S A. 2013;110:3053–8. doi: 10.1073/pnas.1220098110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leonard WJ, O’Shea JJ. Jaks and STATs: biological implications. Annu Rev Immunol. 1998;16:293–322. doi: 10.1146/annurev.immunol.16.1.293. [DOI] [PubMed] [Google Scholar]
- 20.Cohen AC, Nadeau KC, Tu W, et al. Cutting Edge: Decreased Accumulation and Regulatory Function of CD4+CD25high T Cells in Human STAT5b Deficiency. The Journal of Immunology. 2006;177:2770–74. doi: 10.4049/jimmunol.177.5.2770. [DOI] [PubMed] [Google Scholar]
- 21.Semple JW. ITP three R’s: regulation, routing, rituximab. Blood. 2008;112:927–8. doi: 10.1182/blood-2008-05-155770. [DOI] [PubMed] [Google Scholar]
- 22.Hand TW, Cui W, Jung YW, et al. Differential effects of STAT5 and PI3K/AKT signaling on effector and memory CD8 T-cell survival. Proc Natl Acad Sci U S A. 2010;107:16601–6. doi: 10.1073/pnas.1003457107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levine RL, Gilliland DG. Myeloproliferative disorders. Blood. 2008;112:2190–8. doi: 10.1182/blood-2008-03-077966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spivak JL. Narrative review: Thrombocytosis, polycythemia vera, and JAK2 mutations: The phenotypic mimicry of chronic myeloproliferation. Ann Intern Med. 2010;152:300–6. doi: 10.7326/0003-4819-152-5-201003020-00008. [DOI] [PubMed] [Google Scholar]
- 25.Ungureanu D, Wu J, Pekkala T, et al. The pseudokinase domain of JAK2 is a dual-specificity protein kinase that negatively regulates cytokine signaling. Nat Struct Mol Biol. 2011;18:971–6. doi: 10.1038/nsmb.2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bandaranayake RM, Ungureanu D, Shan Y, et al. Crystal structures of the JAK2 pseudokinase domain and the pathogenic mutant V617F. Nat Struct Mol Biol. 2012;19:754–9. doi: 10.1038/nsmb.2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Y, Kolesar JM. Eltrombopag: an oral thrombopoietin receptor agonist for the treatment of idiopathic thrombocytopenic purpura. Clin Ther. 2011;33:1560–76. doi: 10.1016/j.clinthera.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 28.Tefferi A. JAK and MPL mutations in myeloid malignancies. Leuk Lymphoma. 2008;49:388–97. doi: 10.1080/10428190801895360. [DOI] [PubMed] [Google Scholar]
- 29.Scott LM. The JAK2 exon 12 mutations: a comprehensive review. Am J Hematol. 2011;86:668–76. doi: 10.1002/ajh.22063. [DOI] [PubMed] [Google Scholar]
- 30.Nielsen C, Birgens HS, Nordestgaard BG, et al. The JAK2 V617F somatic mutation, mortality and cancer risk in the general population. Haematologica. 2011;96:450–3. doi: 10.3324/haematol.2010.033191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xiang Z, Zhao Y, Mitaksov V, et al. Identification of somatic JAK1 mutations in patients with acute myeloid leukemia. Blood. 2008;111:4809–12. doi: 10.1182/blood-2007-05-090308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sakaguchi H, Okuno Y, Muramatsu H, et al. Exome sequencing identifies secondary mutations of SETBP1 and JAK3 in juvenile myelomonocytic leukemia. Nat Genet. 2013;45:937–41. doi: 10.1038/ng.2698. [DOI] [PubMed] [Google Scholar]
- 33.Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer. 2009;9:798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Song JI, Grandis JR. STAT signaling in head and neck cancer. Oncogene. 2000;19:2489–95. doi: 10.1038/sj.onc.1203483. [DOI] [PubMed] [Google Scholar]
- 35.Kryczek I, Lin Y, Nagarsheth N, et al. IL-22+CD4+ T Cells Promote Colorectal Cancer Stemness via STAT3 Transcription Factor Activation and Induction of the Methyltransferase DOT1L. Immunity. 2014 doi: 10.1016/j.immuni.2014.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lam LT, Wright G, Davis RE, et al. Cooperative signaling through the signal transducer and activator of transcription 3 and nuclear factor-{kappa}B pathways in subtypes of diffuse large B-cell lymphoma. Blood. 2008;111:3701–13. doi: 10.1182/blood-2007-09-111948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koskela HL, Eldfors S, Ellonen P, et al. Somatic STAT3 mutations in large granular lymphocytic leukemia. N Engl J Med. 2012;366:1905–13. doi: 10.1056/NEJMoa1114885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jerez A, Clemente MJ, Makishima H, et al. STAT3 mutations unify the pathogenesis of chronic lymphoproliferative disorders of NK cells and T-cell large granular lymphocyte leukemia. Blood. 2012;120:3048–57. doi: 10.1182/blood-2012-06-435297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jerez A, Clemente MJ, Makishima H, et al. STAT3 mutations indicate the presence of subclinical T-cell clones in a subset of aplastic anemia and myelodysplastic syndrome patients. Blood. 2013;122:2453–9. doi: 10.1182/blood-2013-04-494930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ishida F, Matsuda K, Sekiguchi N, et al. STAT3 gene mutations and their association with pure red cell aplasia in large granular lymphocyte leukemia. Cancer Sci. 2014;105:342–6. doi: 10.1111/cas.12341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rajala HL, Eldfors S, Kuusanmaki H, et al. Discovery of somatic STAT5b mutations in large granular lymphocytic leukemia. Blood. 2013;121:4541–50. doi: 10.1182/blood-2012-12-474577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nelson EA, Walker SR, Weisberg E, et al. The STAT5 inhibitor pimozide decreases survival of chronic myelogenous leukemia cells resistant to kinase inhibitors. Blood. 2011;117:3421–9. doi: 10.1182/blood-2009-11-255232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dunn GP, Koebel CM, Schreiber RD. Interferons, immunity and cancer immunoediting. Nat Rev Immunol. 2006;6:836–48. doi: 10.1038/nri1961. [DOI] [PubMed] [Google Scholar]
- 44.Chan SR, Rickert CG, Vermi W, et al. Dysregulated STAT1-SOCS1 control of JAK2 promotes mammary luminal progenitor cell survival and drives ERalpha(+) tumorigenesis. Cell Death Differ. 2014;21:234–46. doi: 10.1038/cdd.2013.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen E, Beer PA, Godfrey AL, et al. Distinct clinical phenotypes associated with JAK2V617F reflect differential STAT1 signaling. Cancer Cell. 2010;18:524–35. doi: 10.1016/j.ccr.2010.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Butterbach K, Beckmann L, de Sanjose S, et al. Association of JAK-STAT pathway related genes with lymphoma risk: results of a European case-control study (EpiLymph) Br J Haematol. 2011;153:318–33. doi: 10.1111/j.1365-2141.2011.08632.x. [DOI] [PubMed] [Google Scholar]
- 47.Ellinghaus D, Ellinghaus E, Nair RP, et al. Combined analysis of genome-wide association studies for Crohn disease and psoriasis identifies seven shared susceptibility loci. Am J Hum Genet. 2012;90:636–47. doi: 10.1016/j.ajhg.2012.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Duetsch G, Illig T, Loesgen S, et al. STAT6 as an asthma candidate gene: polymorphism-screening, association and haplotype analysis in a Caucasian sib-pair study. Hum Mol Genet. 2002;11:613–21. doi: 10.1093/hmg/11.6.613. [DOI] [PubMed] [Google Scholar]
- 49.Macchi P, Villa A, Giliani S, et al. Mutations of Jak-3 gene in patients with autosomal severe combined immune deficiency (SCID) Nature. 1995;377:65–8. doi: 10.1038/377065a0. [DOI] [PubMed] [Google Scholar]
- 50.Russell SM, Tayebi N, Nakajima H, et al. Mutation of Jak3 in a patient with SCID: essential role of Jak3 in lymphoid development. Science. 1995;270:797–800. doi: 10.1126/science.270.5237.797. [DOI] [PubMed] [Google Scholar]
- 51.Riese RJ, Krishnaswami S, Kremer J. Inhibition of JAK kinases in patients with rheumatoid arthritis: scientific rationale and clinical outcomes. Best Pract Res Clin Rheumatol. 2010;24:513–26. doi: 10.1016/j.berh.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 52.Clark JD, Flanagan ME, Telliez JB. Discovery and Development of Janus Kinase (JAK) Inhibitors for Inflammatory Diseases. J Med Chem. 2014 doi: 10.1021/jm401490p. [DOI] [PubMed] [Google Scholar]
- 53.Kudlacz E, Perry B, Sawyer P, et al. The Novel JAK-3 Inhibitor CP-690550 Is a Potent Immunosuppressive Agent in Various Murine Models. American Journal of Transplantation. 2004;4:51–57. doi: 10.1046/j.1600-6143.2003.00281.x. [DOI] [PubMed] [Google Scholar]
- 54.Kremer JM, Bloom BJ, Breedveld FC, et al. The safety and efficacy of a JAK inhibitor in patients with active rheumatoid arthritis: Results of a double-blind, placebo-controlled phase IIa trial of three dosage levels of CP-690,550 versus placebo. Arthritis Rheum. 2009;60:1895–905. doi: 10.1002/art.24567. [DOI] [PubMed] [Google Scholar]
- 55.Tanaka Y, Suzuki M, Nakamura H, et al. Phase II study of tofacitinib (CP-690,550) combined with methotrexate in patients with rheumatoid arthritis and an inadequate response to methotrexate. Arthritis Care Res (Hoboken) 2011;63:1150–8. doi: 10.1002/acr.20494. [DOI] [PubMed] [Google Scholar]
- 56.Kremer JM, Cohen S, Wilkinson BE, et al. A phase IIb dose-ranging study of the oral JAK inhibitor tofacitinib (CP-690,550) versus placebo in combination with background methotrexate in patients with active rheumatoid arthritis and an inadequate response to methotrexate alone. Arthritis Rheum. 2012;64:970–81. doi: 10.1002/art.33419. [DOI] [PubMed] [Google Scholar]
- 57.Fleischmann R, Cutolo M, Genovese MC, et al. Phase IIb dose-ranging study of the oral JAK inhibitor tofacitinib (CP-690,550) or adalimumab monotherapy versus placebo in patients with active rheumatoid arthritis with an inadequate response to disease-modifying antirheumatic drugs. Arthritis Rheum. 2012;64:617–29. doi: 10.1002/art.33383. [DOI] [PubMed] [Google Scholar]
- 58.Burmester GR, Blanco R, Charles-Schoeman C, et al. Tofacitinib (CP-690,550) in combination with methotrexate in patients with active rheumatoid arthritis with an inadequate response to tumour necrosis factor inhibitors: a randomised phase 3 trial. The Lancet. 2013;381:451–60. doi: 10.1016/S0140-6736(12)61424-X. [DOI] [PubMed] [Google Scholar]
- 59.van der Heijde D, Tanaka Y, Fleischmann R, et al. Tofacitinib (CP-690,550) in patients with rheumatoid arthritis receiving methotrexate: twelve-month data from a twenty-four-month phase III randomized radiographic study. Arthritis Rheum. 2013;65:559–70. doi: 10.1002/art.37816. [DOI] [PubMed] [Google Scholar]
- 60.Vincenti F, Tedesco Silva H, Busque S, et al. Randomized phase 2b trial of tofacitinib (CP-690,550) in de novo kidney transplant patients: efficacy, renal function and safety at 1 year. Am J Transplant. 2012;12:2446–56. doi: 10.1111/j.1600-6143.2012.04127.x. [DOI] [PubMed] [Google Scholar]
- 61.Liew SH, Nichols KK, Klamerus KJ, et al. Tofacitinib (CP-690,550), a Janus kinase inhibitor for dry eye disease: results from a phase 1/2 trial. Ophthalmology. 2012;119:1328–35. doi: 10.1016/j.ophtha.2012.01.028. [DOI] [PubMed] [Google Scholar]
- 62.Papp KA, Menter A, Strober B, et al. Efficacy and safety of tofacitinib, an oral Janus kinase inhibitor, in the treatment of psoriasis: a Phase 2b randomized placebo-controlled dose-ranging study. Br J Dermatol. 2012;167:668–77. doi: 10.1111/j.1365-2133.2012.11168.x. [DOI] [PubMed] [Google Scholar]
- 63.Strober B, Buonanno M, Clark JD, et al. Effect of tofacitinib, a Janus kinase inhibitor, on haematological parameters during 12 weeks of psoriasis treatment. Br J Dermatol. 2013;169:992–9. doi: 10.1111/bjd.12517. [DOI] [PubMed] [Google Scholar]
- 64.Ports WC, Khan S, Lan S, et al. A randomized phase 2a efficacy and safety trial of the topical Janus kinase inhibitor tofacitinib in the treatment of chronic plaque psoriasis. Br J Dermatol. 2013;169:137–45. doi: 10.1111/bjd.12266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mamolo C, Harness J, Tan H, Menter A. Tofacitinib (CP-690,550), an oral Janus kinase inhibitor, improves patient-reported outcomes in a phase 2b, randomized, double-blind, placebo-controlled study in patients with moderate-to-severe psoriasis. J Eur Acad Dermatol Venereol. 2013 doi: 10.1111/jdv.12081. [DOI] [PubMed] [Google Scholar]
- 66.Menter A, Papp KA, Tan H, et al. Efficacy of tofacitinib, an oral janus kinase inhibitor, on clinical signs of moderate-to-severe plaque psoriasis in different body regions. J Drugs Dermatol. 2014;13:252–6. [PubMed] [Google Scholar]
- 67.Sandborn WJ, Ghosh S, Panes J, et al. Tofacitinib, an oral Janus kinase inhibitor, in active ulcerative colitis. N Engl J Med. 2012;367:616–24. doi: 10.1056/NEJMoa1112168. [DOI] [PubMed] [Google Scholar]
- 68.Sandborn WJ, Ghosh S, Panes J, et al. A Phase 2 Study of Tofacitinib, an Oral Janus Kinase Inhibitor, in Patients With Crohn’s Disease. Clin Gastroenterol Hepatol. 2014 doi: 10.1016/j.cgh.2014.01.029. [DOI] [PubMed] [Google Scholar]
- 69.Fleischmann R, Kremer J, Cush J, et al. Placebo-controlled trial of tofacitinib monotherapy in rheumatoid arthritis. N Engl J Med. 2012;367:495–507. doi: 10.1056/NEJMoa1109071. [DOI] [PubMed] [Google Scholar]
- 70.Kremer JM, Li Z-G, Hall S, et al. Tofacitinib (cp-690,550), an oral JAK inhibitor, in combination with traditional DMARDs: phase 3 study in patients with active rheumatoid arthritis with inadequate response to DMARDs. Ann Rheum Dis. 2011;70:170. [Google Scholar]
- 71.van Vollenhoven RF, Fleischmann R, Cohen S, et al. Tofacitinib or adalimumab versus placebo in rheumatoid arthritis. N Engl J Med. 2012;367:508–19. doi: 10.1056/NEJMoa1112072. [DOI] [PubMed] [Google Scholar]
- 72.Lee EB, Fleischmann R, Hall S. Radiographic, clinical and functional comparison of tofacitinib monotherapy versus methotrexate in methotrexate-naïve patients with rheumatoid arthritis. Arthritis Rheum. 2012;64:S1049. [Google Scholar]
- 73.He Y, Wong AY, Chan EW, et al. Efficacy and safety of tofacitinib in the treatment of rheumatoid arthritis: a systematic review and meta-analysis. BMC Musculoskelet Disord. 2013;14:298. doi: 10.1186/1471-2474-14-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rao VU, Pavlov A, Klearman M, et al. Risk Factors for Major Adverse Cardiovascular Events in Rheumatoid Arthritis Patients Treated with the Interleukin-6 Receptor Inhibitor Tocilizumab. Journal of the American College of Cardiology. 2012;59:E1648. [Google Scholar]
- 75.Cosgrove SB, Wren JA, Cleaver DM, et al. A blinded, randomized, placebo-controlled trial of the efficacy and safety of the Janus kinase inhibitor oclacitinib (Apoquel(R)) in client-owned dogs with atopic dermatitis. Vet Dermatol. 2013;24:587–97. e141–2. doi: 10.1111/vde.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pardanani A, Gotlib K, Gupta V, et al. Update On The Long-Term Efficacy and Safety Of Momelotinib, a JAK1 and JAK2 Inhibitor, For The Treatment Of Myelofibrosis. Blood. 2013;122:108. doi: 10.1038/leu.2017.330. [DOI] [PubMed] [Google Scholar]
- 77.Strand V, Suthoff E, Fleischmann R, et al. Effects Of VX-509, An Investigational Oral Selective Janus Kinase 3 (JAK3) Inhibitor, On Patient-Reported Outcomes In a Phase 2A Study Of Patients With Active Rheumatoid Arthritis. Arthritis Rheum. 2013;65:S1004–S05. [Google Scholar]
- 78.Tasset C, Harrison P, Van der Aa A, et al. The JAK1-Selective Inhibitor GLPG0634 Is Safe and Rapidly Reduces Disease Activity in Patients With Moderate To Severe Rheumatoid Arthritis; Results Of a 4-Week Dose Renging STudy. Arthritis Rheum. 2013;65:S1018. [Google Scholar]
- 79.Vanhoutte F, Mazur M, Van der Aa A, et al. Selective JAK1 Inhibition in the Treatment of Rheumatoid Arthritis: Proof of Concept with GLPG0634. Arthritis Rheum. 2012;64:S1051. [Google Scholar]
- 80.Park J-S, Kwok S-K, Lim M-A, et al. STA-21, a Promising STAT-3 Inhibitor That Reciprocally Regulates Th17 and Treg Cells, Inhibits Osteoclastogenesis in Mice and Humans and Alleviates Autoimmune Inflammation in an Experimental Model of Rheumatoid Arthritis. Arthritis & Rheumatology. 2014;66:918–29. doi: 10.1002/art.38305. [DOI] [PubMed] [Google Scholar]
- 81.Koo MY, Park J, Lim JM, et al. Selective inhibition of the function of tyrosine-phosphorylated STAT3 with a phosphorylation site-specific intrabody. Proc Natl Acad Sci U S A. 2014 doi: 10.1073/pnas.1316815111. [DOI] [PMC free article] [PubMed] [Google Scholar]