Abstract
The hippocampus has been the target of stem cell transplantations in preclinical studies focused on Alzheimer's disease, with results showing improvements in histological and behavioral outcomes. The corpus callosum is another structure that is affected early in Alzheimer's disease. Therefore, we hypothesize that this structure is a novel target for human neural stem cell transplantation in transgenic Alzheimer's disease mouse models. This study demonstrates the feasibility of targeting the corpus callosum and identifies an effective immunosuppression regimen for transplanted neural stem cell survival. These results support further preclinical development of the corpus callosum as a therapeutic target in Alzheimer's disease.
Keywords: Alzheimer's disease, corpus callosum, immunosuppression, FK‐506, tacrolimus, stem cell transplantation
Introduction
Cellular therapies represent a potential disease‐modifying treatment for Alzheimer's disease (AD), with multifaceted therapeutic benefits including tissue replacement, secretion of neuroprotective trophic factors, and/or modulation of inflammation.1, 2, 3, 4, 5, 6 As such, transplantation of stem cells from various sources into the hippocampus improves cognitive impairment in murine AD models.1, 7, 8 With the success of hippocampal targeting, interest has grown in identifying other intracranial targets suitable for stem cell transplantation. One of the proposed targets is the corpus callosum (CC), a large white matter tract that includes the neural pathway starting at the nucleus basalis of Meynert, and connects the majority of frontal, parietal, and occipital cortical areas to corresponding regions in the contralateral hemisphere. CC size changes and atrophy occur in AD patients and correlate with progression of dementia severity.9, 10, 11 This, coupled with the fact that stem cells migrate through large white matter tracts,12, 13, 14 makes the CC a potentially effective target. We therefore hypothesize that the CC is an ideal treatment target for cellular therapies for AD. Here, we assessed optimal immunosuppression and the feasibility of CC targeting with a modified human neural stem cell (NSC) line, which we previously characterized and targeted to the fimbria fornix of AD mice.15
Materials and Methods
Surgical transplantation of NSCs to the CC
All procedures were approved by the University of Michigan (U‐M) Institutional Animal Care and Use Committee. Male Tg‐AD B6C3‐Tg(APPswe/PSEN1ΔE9)85Dbo/J (APP/PS1) mice were obtained from the Jackson Laboratory (Bar Harbor, ME). Research grade human NSCs, NSI‐HK532‐IGF‐I, a modified line we previously characterized,15 were provided by Neuralstem, Inc. (Germantown, MD). NSCs were administered to 12‐week‐old mice by three injections into the CC bilaterally at three sites (total six injections), represented by the following atlas coordinates from the bregma (posterior, lateral, ventral): −0.82/(±)0.6/1.9, −1.82/(±)0.6/1.5, −2.3/(±)0.6/1.5 mm, respectively. Each 1 μL injection was administered over 60 sec, with a 60‐sec delay prior to needle withdrawal, at a cell concentration of 30,000 cells/μL, delivering a total of 180,000 cells per animal.
Immunosuppression
Tacrolimus (FK‐506, Prograf®, Astellas Pharmas US Inc., Northbrook, IL) was administered subcutaneously to all animals at 3 mg/kg starting 2 days prior to surgery and daily until postoperative day #6. On the day of the procedure and daily for 6 days later (seven doses total), 30 mg/kg of mycophenolate mofetil (CellCept; Genentech USA Inc., South San Francisco, CA) was administered subcutaneously in conjunction with the tacrolimus. On postoperative day #7, CellCept was discontinued and animals were randomly assigned to three groups (n = 5 per group) to assess three tacrolimus doses: 0.3 mg/kg, 1.5 mg/kg, and 3 mg/kg.
Immunohistochemical analyses
The following primary antibodies were applied overnight at 4°C (Table S1) and detected with appropriate Alexa Fluor‐conjugated secondary antibodies (Thermo Fisher Scientific, Hampton, NH): human‐specific anti‐human nuclei (HuNu; Millipore, Billerica, MA), glial fibrillary acidic protein (GFAP; Dako, Carpinteria, CA), and oligodendrocyte‐specific protein (OSP; Abcam, Cambridge, UK). DAPI and Nissl stains (Thermo Fisher Scientific) were used to visualize nuclei and neurons, respectively. Fluorescent images were captured by an investigator blinded to treatment groups using Leica SP2 confocal (Leica Microsystems, Buffalo Grove, IL) and Nikon Microphot‐FXA (Nikon Instruments, Chiyoda, Japan) microscopes. Engrafted HuNu‐positive NSCs were counted in 10–15 coronal sections per animal (n = 2 per group), for average counts over ~140 μm within the injection coordinates. Statistical significance was determined by one‐way analysis of variance followed by Tukey's multiple comparison test using GraphPad Prism (GraphPad Software Inc., La Jolla, CA) Data S1.
Results
Survival, migration, and differentiation of NSCs targeted to the CC
There were no animal deaths or morbidity postoperatively, and all 15 mice were healthy until study end at 4 weeks posttransplantation. The CC was successfully targeted, as injection tracts were located in all animals at the correct anatomical location (data not shown) and further demonstrated by the presence of HuNu‐positive engrafted cells within the CC of animals that received adequate immunosuppression of 3 mg/kg tacrolimus (Fig. 1). In this group, extensive graft survival and migration was observed, with HuNu‐positive NSCs present throughout and adjacent to the CC. NSCs were located throughout the entire ~1.7 mm injection area, on average migrating 1 mm beyond the injection site (data not shown). The largest distribution in one animal spanned a total of 2.2 mm, indicating a migration distance of ~0.5 mm (Fig. 1A–D). This shows that the CC can be successfully, accurately, and safely targeted in 12‐week‐old Tg‐AD mice, and that transplanted NSCs survive and migrate in this brain structure 4 weeks postoperatively when immunosuppression levels are adequate. We also performed phenotypic analyses of transplanted NSCs in the 3 mg/kg group (Fig. 2). Evidence of neuronal and astrocytic differentiation was noted, defined by Nissl and GFAP co‐localization with HuNu, respectively. We did not observe oligodendrocyte differentiation, with no evident HuNu and OSP co‐localization.
Figure 1.
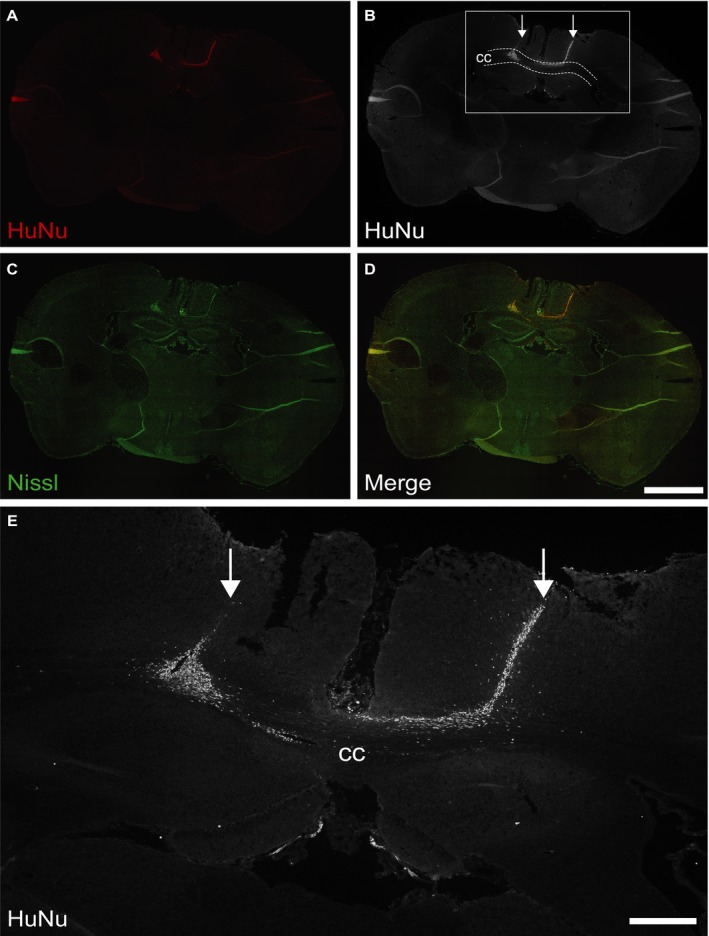
Transplanted NSCs survive and migrate in the CC of AD mice. Accurate targeting of NSCs to the CC of the Tg‐AD mouse brain (3.0 mg/kg tacrolimus). Representative confocal imaging of transplanted NSC survival and migration along the white matter tract structure (A–D). High magnification of the grafted area showing clear migration of NSCs beyond the injection points (E). NSCs labeled with HuNu (red; also shown in black and white); neurons labeled with Nissl (green). CC, corpus callosum; Arrows indicate targeted sites of injection. A–D: scale bar 1.5 mm; E: scale bar 300 μm.
Figure 2.
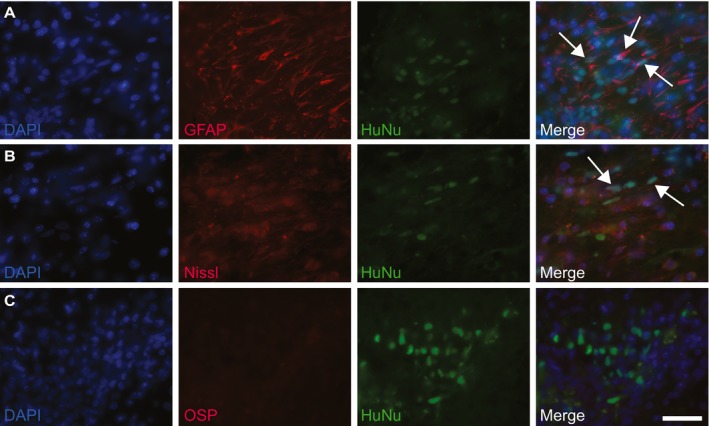
Differentiation profile of transplanted NSCs. Representative phenotypic analyses of transplanted NSCs (3 mg/kg tacrolimus). Differentiation into neuronal and astrocytic lineages indicated by GFAP and Nissl co‐localization with HuNu; examples are marked with arrows (A–B). No oligodendrocyte differentiation was observed (C). NSCs labeled with HuNu (green); GFAP, Nissl, and OSP indicate differentiation (red). Scale bar 50 μm.
Minimum dose of immunosuppression for transplanted NSC survival in the CC
Minimizing immunosuppression dosages is desirable in order to mitigate drug‐related side‐effects that may affect health and subsequent behavior. We assessed the effect of three levels of tacrolimus immunosuppression on NSC survival. In animals treated with 0.3 mg/kg, no HuNu‐positive cells were detectable 4 weeks postoperatively (Fig. 3A). With 1.5 mg/kg, NSCs were identified in four of five mice. HuNu‐positive NSCs were present in all five mice that received 3 mg/kg tacrolimus (Fig. 3B–C). Qualitative cell counts show that 3.0 mg/kg tacrolimus significantly increased NSC survival compared to the other doses tested (Fig. 3D). These data indicate that the minimum dose of tacrolimus required to support survival of this human NSC line after transplantation to the CC is 3 mg/kg daily.
Figure 3.
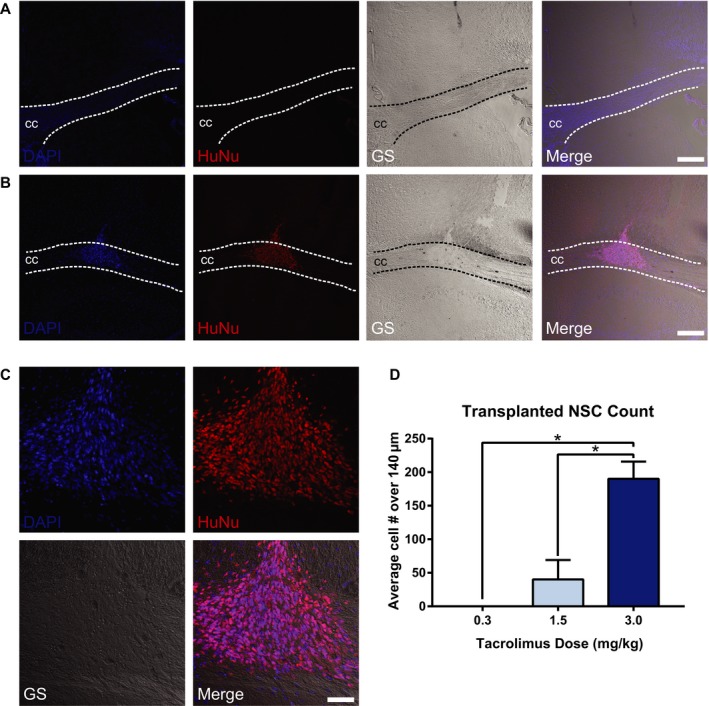
Immunosuppression requirement for CC‐targeted NSC survival in AD mice. Representative confocal imaging of NSC 4 weeks after CC targeting in Tg‐AD mice immunosuppressed with tacrolimus at 0.3 mg/kg (A) and 3.0 mg/kg (B). Immunosuppression with 0.3 mg/kg shows no evidence of surviving NSCs in the targeted area (A). HuNu‐positive NSCs are visible in the CC at the 3.0 mg/kg dose, with extensive NSC survival and migration along the white matter tract structure (B–C). NSCs are labeled with HuNu (red); nuclei are labeled with DAPI (blue); CC, corpus callosum; GS, grayscale. (A–C) scale bar 200 μm; D: scale bar 100 μm. Cell counts expressed as average numbers of HuNu‐positive cells over a distance of 140 μm within the injection coordinates (−0.8 to −1.6 mm from bregma) show significantly increased NSCs with 3.0 mg/kg tacrolimus versus other doses tested (D). (*P < 0.05).
Discussion
With hippocampal‐targeted stem cell therapies improving AD‐associated cognitive deficits in preclinical models, comes an opportunity to identify novel targets that could be equally or increasingly efficacious in disease treatment. The CC is a prime target, given that it is the largest white matter tract affected early in AD.11 Moreover, it connects numerous areas of the brain, including the nucleus basalis of Meynert, a cholinergic structure affected in AD.9 Targeting the CC can also theoretically facilitate increased migration of transplanted cells, providing additional trophic support and a source of neuroglial tissue to larger areas, including the orbital and prefrontal regions, thereby having a greater impact on AD pathology. This study demonstrates the feasibility of targeting the CC in a relevant preclinical model of AD and identifies an effective tacrolimus‐based immunosuppression regimen for survival and engraftment of a novel NSC line, which we are currently exploring as a potential candidate for therapeutic development.15
As shown in Figure 1, we safely and accurately targeted the CC, with no complications postoperatively throughout the study period. Interestingly, transplanted NSCs migrated along the tracts of the CC, traversing distances up to 0.5 mm outside of injection points. This is in line with many reports of stem cell migration via white matter tracts.12, 13, 14 Given that stem cell efficacy is improved when coupled with trophic factor delivery,2, 16, 17, 18 facilitating increased NSC migration could provide enhanced and widespread neurotrophic support to local brain areas. Moreover, targeting the CC provides the largest migratory area for transplanted stem cells, likely more than any other intracranial target, enabling further enrichment throughout the diseased brain environment. We also assessed the differentiation profile of transplanted NSCs. In our previous studies, this NSC line differentiated into neuronal and glial lineages in vitro and in vivo in nude rats (data not shown). Although we observed similar results, with low levels of neuronal and astrocytic differentiation, there was no evidence of oligodendrocyte differentiation in this study. Of course, diverse differentiation profiles are expected depending upon transplantation location, and with this short 4‐week study duration, it is possible that many transplanted NSCs remained in an undifferentiated or progenitor state.1, 19 Comprehensive graft characterization with early and terminal differentiation markers will be required in future long‐term efficacy studies to disclose possible therapeutic mechanisms of cell transplantation.
With regard to immunosuppression, previous studies in our laboratory utilized 3 mg/kg tacrolimus, which is largely based on other rodent species.20 As further dose reduction could potentially mitigate the toxic side‐effects of prolonged immunosuppression, we assessed three dosing regimens. At 1.5 mg/kg tacrolimus, transplanted NSCs were observed in only four of the five animals and HuNu‐positive cell counts were significantly reduced at this dose compared to 3 mg/kg. Given the reliability of cell targeting and preserved cell viability after the injection procedure, the 1.5 mg/kg dose is likely close to the threshold where the immune system could still reject the transplanted human cells, even in the presence of tacrolimus. Based on these data, we report that 3 mg/kg tacrolimus administered subcutaneously is the minimum dosage necessary for reliable human NSC engraftment in this Tg‐AD mouse model. However, it is possible that only a minority of Tg‐AD mice will reject cells at the 1.5 mg/kg dose of tacrolimus and that we observed an enrichment of this phenomenon due to our small sample size. Repeating both intermediate and high doses of tacrolimus in additional animals and for a longer duration is necessary and should be addressed in future interventional studies targeting the CC.
In summary, the CC is affected early in the course of AD, and this pilot study assessed the feasibility of targeting this white matter structure, as this has not yet been determined in preclinical Tg‐AD mouse models. Here, for the first time, we demonstrate that the APP/PS1 mouse CC can be accurately and safely targeted for stem cell transplantation. We also define an effective immunosuppression regimen and report that subcutaneous injection of 3 mg/kg tacrolimus is the minimum dose necessary for survival of this human NSC line. Together, these data demonstrate the feasibility of CC targeting and support further evaluation of CC‐targeted stem cell therapies in AD.
Author Contributions
L.M.M. and O.N.K. designed the study, and collected, analyzed, and interpreted data, and wrote the manuscript. K.S.C., J.M.H., and C.B. collected, analyzed, and interpreted data and edited the manuscript. E.S.B., S.F., and B.N.K. collected and analyzed data and edited the manuscript. K.J. designed the study and provided study materials. E.L.F. designed and directed the study, contributed to discussion, edited and approved the manuscript, and provided financial support.
Conflict of Interest
K.J. is the chief scientific officer of Neuralstem, Inc., but was not involved in data acquisition or analysis. E.L.F. is an unpaid consultant to Neuralstem, Inc. All other authors have no conflicts of interest to disclose.
Supporting information
Data S1. Materials and Methods
Table S1. List of primary antibodies and stains used for immunohistochemical analyses including manufacturer, catalog number, dilution factor, and applied secondary antibody.
Acknowledgments
The authors thank Holly Wagner and Stacey A. Sakowski, Ph.D. (University of Michigan) for administrative and editorial support. Funding was provided by the A. Alfred Taubman Medical Research Institute, the Program for Neurology Research & Discovery, and the Robert E. Nederlander Sr. Program for Alzheimer's Research. K.S.C. and O.N.K. were supported by the University of Michigan Clinician Scientist Training Programs (Grants NINDS R25NS089450 and NIH T32NS07222).
Funding Statement
This work was funded by A. Alfred Taubman Medical Research Institute grant ; Program for Neurology Research & Discovery grant ; Robert E. Nederlander Sr. Program for Alzheimer's Research grant ; University of Michigan Clinician Scientist Training Programs grants NINDS R25NS089450 and NIH T32NS07222.
References
- 1. Ager RR, Davis JL, Agazaryan A, et al. Human neural stem cells improve cognition and promote synaptic growth in two complementary transgenic models of Alzheimer's disease and neuronal loss. Hippocampus. 2015;25:813–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Blurton‐Jones M, Kitazawa M, Martinez‐Coria H, et al. Neural stem cells improve cognition via BDNF in a transgenic model of Alzheimer disease. Proc Natl Acad Sci USA 2009;106:13594–13599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gu G, Zhang W, Li M, et al. Transplantation of NSC‐derived cholinergic neuron‐like cells improves cognitive function in APP/PS1 transgenic mice. Neuroscience 2015;291:81–92. [DOI] [PubMed] [Google Scholar]
- 4. Hampton DW, Webber DJ, Bilican B, et al. Cell‐mediated neuroprotection in a mouse model of human tauopathy. J Neurosci 2010;30:9973–9983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lee IS, Jung K, Kim IS, et al. Human neural stem cells alleviate Alzheimer‐like pathology in a mouse model. Mol Neurodegener 2015;10:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhang W, Wang PJ, Sha HY, et al. Neural stem cell transplants improve cognitive function without altering amyloid pathology in an APP/PS1 double transgenic model of Alzheimer's Disease. Mol Neurobiol 2014;50:423–437. [DOI] [PubMed] [Google Scholar]
- 7. Lee HJ, Lee JK, Lee H, et al. Human umbilical cord blood‐derived mesenchymal stem cells improve neuropathology and cognitive impairment in an Alzheimer's disease mouse model through modulation of neuroinflammation. Neurobiol Aging 2012;33:588–602. [DOI] [PubMed] [Google Scholar]
- 8. Lee JK, Jin HK, Bae JS. Bone marrow‐derived mesenchymal stem cells reduce brain amyloid‐beta deposition and accelerate the activation of microglia in an acutely induced Alzheimer's disease mouse model. Neurosci Lett 2009;450:136–141. [DOI] [PubMed] [Google Scholar]
- 9. Hong JH, Jang SH. Neural pathway from nucleus basalis of Meynert passing through the cingulum in the human brain. Brain Res 2010;1346:190–194. [DOI] [PubMed] [Google Scholar]
- 10. Teipel SJ, Bayer W, Alexander GE, et al. Progression of corpus callosum atrophy in Alzheimer disease. Arch Neurol 2002;59:243–248. [DOI] [PubMed] [Google Scholar]
- 11. Di Paola M, Spalletta G, Caltagirone C. In vivo structural neuroanatomy of corpus callosum in Alzheimer's disease and mild cognitive impairment using different MRI techniques: a review. J. Alzheimer's Dis. 2010;20:67–95. [DOI] [PubMed] [Google Scholar]
- 12. Chen CC, Hsu YH, Jayaseema DM, et al. White matter tracts for the trafficking of neural progenitor cells characterized by cellular MRI and immunohistology: the role of CXCL12/CXCR4 signaling. Brain Struct Funct 2015;220:2073–2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Suzuki SO, Goldman JE. Multiple cell populations in the early postnatal subventricular zone take distinct migratory pathways: a dynamic study of glial and neuronal progenitor migration. J Neurosci 2003;23:4240–4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yang J, Liu J, Niu G, et al. In vivo MRI of endogenous stem/progenitor cell migration from subventricular zone in normal and injured developing brains. NeuroImage 2009;48:319–328. [DOI] [PubMed] [Google Scholar]
- 15. McGinley LM, Sims E, Lunn JS, et al. Human cortical neural stem cells expressing insulin‐like growth factor‐I: a novel cellular therapy for Alzheimer's Disease. Stem Cells Transl Med 2016;5:379–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Blurton‐Jones M, Spencer B, Michael S, et al. Neural stem cells genetically‐modified to express neprilysin reduce pathology in Alzheimer transgenic models. Stem Cell Res Ther 2014;5:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Garcia KO, Ornellas FL, Martin PK, et al. Therapeutic effects of the transplantation of VEGF overexpressing bone marrow mesenchymal stem cells in the hippocampus of murine model of Alzheimer's disease. Front Aging Neurosci 2014;6:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Martinez‐Serrano A, Bjorklund A. Protection of the neostriatum against excitotoxic damage by neurotrophin‐producing, genetically modified neural stem cells. J Neurosci 1996;16:4604–4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Naimi S, Jeny R, Hantraye P, et al. Ontogeny of human striatal DARPP‐32 neurons in fetuses and following xenografting to the adult rat brain. Exp Neurol 1996;137:15–25. [DOI] [PubMed] [Google Scholar]
- 20. Hefferan MP, Johe K, Hazel T, et al. Optimization of immunosuppressive therapy for spinal grafting of human spinal stem cells in a rat model of ALS. Cell Transplant 2011;20:1153–1161. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Materials and Methods
Table S1. List of primary antibodies and stains used for immunohistochemical analyses including manufacturer, catalog number, dilution factor, and applied secondary antibody.


