Abstract
Immunoglobulin gamma (IgG) heavy chain genes are associated with susceptibility to multiple sclerosis (MS) and IgG levels in the cerebrospinal fluid (CSF). However, how these variants are implicated in disease mechanisms remains unknown. Here, we show that proliferating plasmablasts expressing the G1m1 allotype of IgG1 are selectively enriched in CSF of G1m1/G1m3 heterozygous MS patients, whereas plasmablasts expressing either G1m1 or G1m3 are evenly distributed in blood. Moreover, there was a preferential intrathecal synthesis of oligoclonal IgG1 of the G1m1 allotype in heterozygous patients, whereas controls with Lyme neuroborreliosis displayed oligoclonal IgG1 of both allotypes. This points to a disease‐specific mechanism involved in B‐cell establishment within the central nervous system in MS.
Introduction
The strong therapeutic effect of B‐cell depletion shows that these cells play an essential role in multiple sclerosis (MS).1 B cells may contribute to disease in several ways, including antigen presentation, cytokine secretion, and antibody production.2 Intrathecal production of immunoglobulin G (IgG), which was demonstrated for more than half a century ago,3 is considered a hallmark of the disease and can be visualized as oligoclonal bands (OCBs) by electrophoresis or isoelectric focusing of cerebrospinal fluid (CSF) in most patients.4
Genetic markers of the IgG heavy chain genes known to be determinants of Gm allotype polymorphisms have recently been shown to be associated with intrathecal levels of IgG,5, 6, 7 age at onset,6 and disease risk.7 The intrathecal humoral immune response in MS is dominated by the IgG1 subclass,8 and an early study showed an increased G1m1 to G1m3 ratio of IgG1 in the CSF of G1m1/G1m3 heterozygous MS patients.9 However, it remains unknown whether this observation is due to a preferential synthesis of IgG1 of the G1m1 allotype, or if it can be explained by an increased turnover of IgG1 of the G1m3 allotype under inflammatory conditions.
Here, we demonstrate that proliferating plasmablasts expressing the G1m1 allotype of IgG1 are selectively enriched in the CSF of G1m1/G1m3 heterozygous MS patients. Furthermore, a vast majority of MS patients displayed IgG1 OCBs of the G1m1 allotype only, whereas OCBs of both allotypes were present in heterozygous controls with Lyme neuroborreliosis. These results suggest that the G1m1 allotype is involved in establishment of B‐cell clones within the central nervous system (CNS) of MS patients, and opens up for more selective B‐cell depletion in a subgroup of patients, where B cells carrying G1m1 are removed while leaving B cells carrying all other isotypes and allotypes intact.
Methods
Patients
For flow cytometry, patients with relapsing remitting MS (n = 25) or primary progressive MS (n = 4) were recruited at the Departments of Neurology at Akershus University Hospital and Oslo University Hospital. Nine patients had not received immunomodulatory treatment at inclusion, and the remaining received different treatments (glatiramer acetate, teriflunomide, or dimethyl fumarate). For isoelectric focusing, we analyzed paired serum and CSF samples from patients with MS from the Norwegian MS Registry and Biobank at Haukeland University Hospital, and from patients with Lyme neuroborreliosis from diagnostic biobanks at the Departments of Microbiology at Oslo University Hospital, Haukeland University Hospital, and Sørlandet Hospital, Norway. Only G1m1/G1m3 heterozygous individuals displaying IgG1 OCBs were included. MS patients fulfilled the 2010 McDonald criteria, and neuroborreliosis patients met the criteria for definite Lyme neuroborreliosis. The Regional Ethical Committee South East approved the study (2009/23 S‐04143a), and the Regional Ethical Committee West approved the research biobank at Haukeland University Hospital (046.03/2010.1821). Written informed consent was obtained from all participants before inclusion.
Flow cytometry
Mononuclear cells were isolated from CSF and blood as previously described.10 The following fluorochrome‐conjugated mouse anti‐human antibodies from BD Biosciences were used: CD3‐BV510 (HIT3a), CD14‐BV510 (MφP9), CD19‐BV650 (SJ25C1), CD27‐PE‐CF594 (M‐T271), CD38‐PerCP‐Cy5.5 (HIT2), CD138‐APC (MI15), HLADR‐APC‐H7 (G46‐6), IgG‐PE‐Cy7 (G18‐145), and Ki‐67‐PE (B56). In addition, we used antibodies against IgG1 (4.0 μg/mL, HP6070, Thermo Fisher Scientific), G1m1 (0.32 μg/mL, 10H1, Sanquin), and G1m3 (0.30 μg/mL, SG‐16, Sigma‐Aldrich) conjugated with Alexa Fluor 680, Alexa Fluor 488, and Pacific Blue, respectively, using antibody‐labeling kits (Invitrogen, Molecular Probes). Data were collected on an LSRFortessa cell analyzer (BD Biosciences) equipped with 488‐, 561‐, 405‐, and 640‐nm lasers, and analyzed using FlowJo version 10.2.
Isoelectric focusing and immunoblot
One of us (A.L.) randomized the patients and controls and prepared paired samples of serum and CSF in new relabeled tubes. Researchers blinded to the diagnoses and the group sizes performed the isoelectric focusing and immunoblotting (A.TB.), and the interpretation of the immunoblots (A.TB. and F.V.). All samples were diluted in Milli‐Q water to 25 mg/L, and the isoelectric focusing and transferring to nitrocellulose membranes were performed as previously described.11 After blocking in 2% milk, the membrane was incubated overnight in mouse anti‐human antibodies against G1m3 (0.20 μg/mL, HP6027, Abingdon Health), G1m1 (31 ng/mL, 10H1), or IgG1 (1.0 μg/mL, HP6069, Invitrogen, Molecular Probes). After washing, the membrane was incubated in a goat anti‐mouse IgG antibody (Southern Biotec) at a 1:20,000 dilution for 1 h, and visualized using SuperSignal West Pico (Thermo Fisher Scientific) in a G:BOX digital camera system (Syngene). Between the staining with anti‐G1m3 and anti‐G1m1 antibodies, the membranes were stripped in Restore Western Blot Stripping Buffer (Thermo Fisher Scientific).
Determining G1m allotypes using ELISA
The plates (Nunc, 436014; Thermo Fisher Scientific) were coated with primary antibodies against G1m1 (1.5 μg/mL, 10H1) or G1m3 (1.0 μg/mL, HP6027) at 4°C. After washing, serum diluted 1:5,000 was incubated in duplicate wells for 1 h. Finally, the plates were washed again and incubated with alkaline phosphatase‐conjugated goat anti‐human IgG (Sigma‐Aldrich) for 1 h. The signal was visualized with phosphatase substrate (Sigma‐Aldrich) reactivity measured at 405 nm.
Statistics
All statistical tests are given in the figure legends. The level of significance was set at 5%, and all tests were two‐sided. The analyses were performed in JMP Pro 12 and GraphPad Prism 6 for Mac OS X.
Results
In order to characterize the phenotypes and allotypes of antibody‐secreting cells (ASCs) in MS, we performed flow cytometry on mononuclear cells from blood and CSF from 29 patients (Fig. 1A). The frequencies of B cells were lower in CSF compared to blood, while the CSF B‐cell compartment was highly enriched for CD19dimCD27hiCD38+ ASCs (median 14% [interquartile range [IQR] 0–39%] in CSF vs. 1.6% [0.71–3.1%] in blood; Fig. 1B). Furthermore, a vast majority of the ASCs in the CSF expressed the IgG1 isotype (94% [79–100%]; Fig. 1B).
Figure 1.
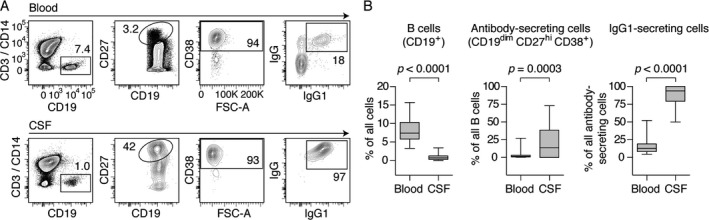
The majority of the antibody‐secreting cells in the cerebrospinal fluid (CSF) of MS patients express the IgG1 isotype. (A) After excluding debris and doublets (not shown), mononuclear cells in blood and CSF were gated for the expression of CD19, C27, and CD38, and assayed for the expression of IgG and IgG1. (B) The frequencies of B cells, of antibody‐secreting cells among B cells, and of antibody‐secreting cells expressing IgG1 in blood and CSF are shown (n = 29, Wilcoxon signed‐rank test). Results are presented as box plot diagrams with median, upper and lower quartile, and minimum/maximum values.
Remarkably, in G1m1/G1m3 heterozygous patients, nearly all IgG1 ASCs in the CSF displayed the G1m1 allotype (median 99% [IQR 94–100%]; Fig. 2A and B). Further characterization of the IgG1 ASCs showed that the great majority expressed CD138 (90% [84–96%]) and HLA‐DR (82% [65–88%]), and virtually all expressed high levels of the proliferation marker Ki‐67 (100% [99–100%]; Fig. 2C and D). This phenotype is compatible with that of recently activated plasmablasts.12 By utilizing an alternative gating strategy, we could not find any CD19−CD27+CD38+ IgG ASCs in the CSF (data not shown). In contrast to the findings in the CSF, the G1m1 and G1m3 allotypes were evenly distributed among IgG1 ASCs B cells in the blood of G1m1/G1m3 heterozygous patients, as expected from random allelic exclusion (Fig. 2B).
Figure 2.
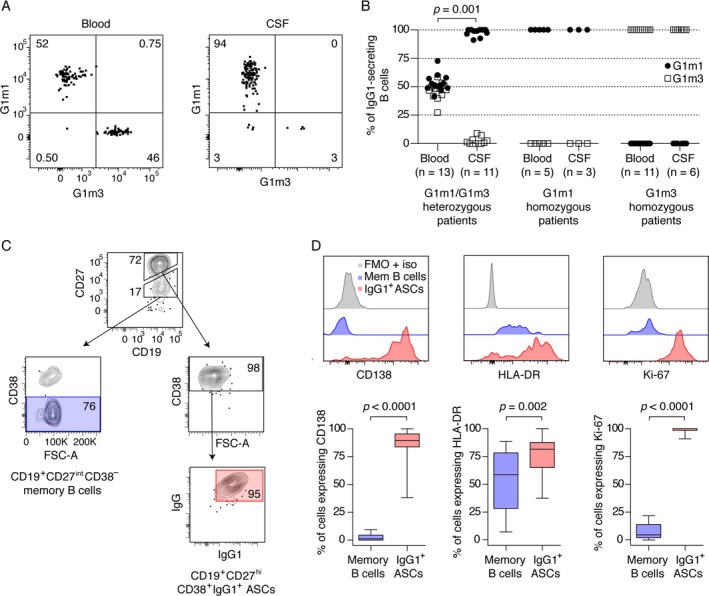
Antibody‐secreting cells of the G1m1 allotype are selectively enriched in the cerebrospinal fluid (CSF) of G1m1/G1m3 heterozygous MS patients, and the cells have a phenotype compatible with highly proliferating plasmablasts. (A) A representative flow cytometry experiment from a G1m1/G1m3 heterozygous MS patient. The cells were gated for expression of IgG1 as shown in Fig. 1, and assayed for the expression of G1m1 and G1m3. (B) The frequencies of G1m1 and G1m3 antibody‐secreting cells in the CSF of G1m1/G1m3 heterozygous patients (left panel), G1m1 homozygous patients (middle panel) and G1m3 homozygous (right panel) are shown. Double negative cells (in CSF median [range] 0% [0–3.8%] and in blood 2.7% [1.4–7.2%]) were excluded when estimating the frequencies. Two G1m1/G1m3 heterozygous patients, two G1m1 homozygous patients, and five G1m3 homozygous patients had no detectable IgG1‐secreting B cells in the CSF. Frequencies of G1m1‐ and G1m3‐expressing cells were compared between CSF and blood for G1m1/G1m3 heterozygous patients (n = 11 paired samples, Wilcoxon signed‐rank test). (C) Antibody‐secreting cells (ASCs) were gated for the expression of CD19 and high levels of CD27, in addition to the expression of CD38, IgG, and IgG1, while memory B cells were gated for the expression of CD19, intermediate levels of CD27, and absence of CD38. (D) The frequencies of IgG1 ASCs and memory B cells expressing CD138, HLA‐DR, and the proliferation marker Ki‐67 (n = 20, Wilcoxon signed‐rank test). Positive gates were set using fluorescence minus one with isotype controls (FMO + iso). Results are presented as box plot diagrams with median, upper and lower quartile, and minimum/maximum values.
We reasoned that the strong dominance of G1m1 positive B cells in CSF found by flow cytometry would also be reflected in intrathecally synthesized IgG1. In order to examine whether this phenomenon could also be observed in a disease where the target antigen of the intrathecal immune response has been defined, we determined the G1m allotypes in additional cohorts of patients with MS and controls with Lyme neuroborreliosis by serological typing using ELISA. We subsequently compared paired samples of CSF and serum from G1m1/G1m3 heterozygous patients with MS (n = 28) and controls with Lyme neuroborreliosis (n = 16) by isoelectric focusing and immunoblotting (Fig. 3A and B). A vast majority of the MS patients displayed OCBs of the G1m1 allotype only (Fig. 3B). In contrast, close to all controls had an intrathecal synthesis also of the G1m3 allotype (Fig. 3B). The difference between the proportions of patients and controls with an intrathecal synthesis of only G1m1, only G1m3, or both allotypes was highly statistically significant (P < 0.0001).
Figure 3.
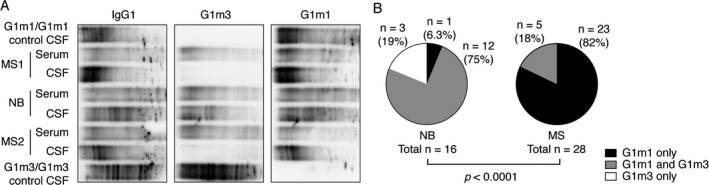
The intrathecal IgG1 synthesis is dominated by the G1m1 allotype in G1m1/G1m3 heterozygous MS patients, but not in controls with Lyme neuroborreliosis (NB). (A) A representative isoelectric focusing experiment with paired serum and cerebrospinal fluid (CSF) samples from two G1m1/G1m3 heterozygous MS patients and a heterozygous control with NB. CSF samples from a G1m1 homozygous and a G1m3 homozygous MS patient were included as control samples. Intrathecal synthesis was determined as the presence of two or more bands exclusive to CSF or at least two times stronger than corresponding bands in serum. (B) Isoelectric focusing with immunoblotting was performed on G1m1/G1m3 heterozygous patients with MS (n = 28) and controls with NB (n = 16). The numbers of patients versus controls with IgG1 OCBs using only G1m1, only G1m3 or both were compared using Fischer's exact test for a 2 × 3 table.
Finally, to investigate whether the G1m1 allotype is associated with a more severe disease course, we serologically determined the allotypes of an additional cohort of MS patients and calculated the MS Severity Score (MSSS) in our total patient population (n = 124). There were no significant differences in MSSS between patients stratified according to their G1m allele status (Fig. S1, P = 0.15).
Discussion
This is the first observation of a B‐cell response in any human or animal disease that is restricted to a certain allotype variant. Importantly, the finding that G1m1 and G1m3 are evenly distributed among OCBs in the CSF of patients with neuroborreliosis suggests that the strong selective enrichment of G1m1 positive B cells is uniquely related to MS.
The mechanisms driving the B‐cell response in MS are not known. We and others have shown that the IgG‐producing B cells in CSF are somatically hypermutated and have identical or clonally related counterparts in blood,10, 13 and it was recently shown that the maturation of CNS B cells could be initiated in cervical lymph nodes.14 Although it is possible that the selection of B cells carrying the G1m1 allotype occurs during the initiation of the B‐cell response in the periphery, the lack of any allotype restriction in the blood could indicate a selection at the blood–brain barrier or within the intrathecal compartment. Moreover, the lack of association between the G1m1 allotype and disease course, and the finding of allotype restriction in patients with different disease duration and MS subtype (progressive and relapsing remitting), suggest that the mechanism operates throughout the disease course and is not directly linked to disease progression. Further characterization of the IgG1 ASCs in the CSF of MS patients showed that these cells express CD38, CD138, and HLA‐DR, together with dim levels of CD19 and high levels of CD27. This is in line with the results of a previous study characterizing intrathecal B cells,15 but contrasts another study that demonstrated increased numbers of CD19–CD138+ plasma cells.16 Importantly, we show for the first time that the intrathecal IgG1 ASCs express high levels of Ki‐67, suggesting that the cells are newly derived and proliferate intrathecally.
The G1m1 and the G1m17 allotypes are in near perfect linkage disequilibrium in Caucasians and are thus inherited together on the same chromosome. Accordingly, the mechanisms responsible for the selection of G1m1 plasmablasts could be connected to the G1m1 and/or the G1m17 allotypes. It is possible that the allotypes interfere with the Igα/Igβ heterodimer of the B‐cell receptor (BCR) and influence receptor clustering and signaling, or that they modulate the intracellular transport of the antigen–antibody complexes and the subsequent antigen‐presentation on HLA class II molecules. Another possibility is that yet unidentified MS‐associated polymorphisms in the variable heavy chain (VH) region are inherited together with the G1m polymorphisms. Alternatively, the G1m1 and/or G1m17 polymorphisms could indirectly cause allosteric changes in the VH region influencing the binding affinity of the IgG1 BCR.17 Finally, it has been shown that the G1m allotypes could affect the binding affinities of soluble IgG1 for the neonatal Fc receptor (FcRn).18 The FcRn is indeed expressed at the blood–brain barrier where it contributes to the efflux of IgG from the CNS.19, 20
A limitation of the present study is the lack of controls with other inflammatory neurological diseases in the flow cytometry experiments. However, such diseases are rare, and only approximately 40% of them would be G1m1/G1m3 heterozygous, out of whom only a proportion would be expected to have a sufficient number of plasmablasts in the CSF. Therefore, in order to include a homogenous control group of a sufficient size, we performed immunoblotting of isoelectric focused paired samples of CSF and serum from patients with Lyme neuroborreliosis determined to be G1m1/G1m3 heterozygous and positive for oligoclonal bands in the CSF.
In conclusion, our study indicates that the G1m allotypes may have a fundamental influence on the immune response in MS, and that the G1m1 allotype could represent a new therapeutic target in a subset of patients.
Author Contributions
Study concept and design: A.L., T.H., C.A.V., Å.R.L., I.C., F.V.; data acquisition and analysis: A.L., A.TB., T.H., C.A.V., Å.R.L., E.R., I.C., F.V.; drafting the manuscript and/or images: A.L., A.TB., F.V.
Conflicts of Interest
Nothing to report.
Supporting information
Figure S1: G1m allotypes are not associated with the disease severity in MS. The MS severity score (MSSS) was estimated for G1m3 homozygous patients (n = 50), G1m1/G1m3 heterozygous patients (n = 58), and G1m1 homozygous patients (n = 18). Bars depict mean ± SD, and differences between the three groups were tested using Kruskal–Wallis test.
Acknowledgment
The study was supported by grant 2016079 from the South‐Eastern Norway Regional Health Authority. We thank Prof. Halvor Rollag, Helvi Holm Samdal, and Tone Berge at the Department of Microbiology, Oslo University Hospital, and Liesbeth Kroondijk at the Norwegian MS Registry and Biobank at Haukeland University Hospital for providing samples used for the isoelectric focusing experiments.
Funding Statement
This work was funded by South‐Eastern Norway Regional Health Authority grant 2016079.
References
- 1. Hauser SL, Waubant E, Arnold DL, et al. B‐cell depletion with rituximab in relapsing‐remitting multiple sclerosis. N Engl J Med 2008;358:676–688. [DOI] [PubMed] [Google Scholar]
- 2. Michel L, Touil H, Pikor NB, et al. B cells in the multiple sclerosis central nervous system: trafficking and Contribution to CNS‐Compartmentalized Inflammation. Front Immunol 2015;6:636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kabat EA, Moore DH, Landow H. An electrophoretic study of the protein components in cerebrospinal fluid and their relationship to the serum proteins. J Clin Invest 1942;21:571–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dobson R, Ramagopalan S, Davis A, Giovannoni G. Cerebrospinal fluid oligoclonal bands in multiple sclerosis and clinically isolated syndromes: a meta‐analysis of prevalence, prognosis and effect of latitude. J Neurol Neurosurg Psychiatry 2013;84:909–914. [DOI] [PubMed] [Google Scholar]
- 5. Buck D, Albrecht E, Aslam M, et al. Genetic variants in the immunoglobulin heavy chain locus are associated with the IgG index in multiple sclerosis. Ann Neurol 2013;73:86–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Goris A, Pauwels I, Gustavsen MW, et al. Genetic variants are major determinants of CSF antibody levels in multiple sclerosis. Brain 2015;138:632–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Delgado‐Garcia M, Matesanz F, Alcina A, et al. A new risk variant for multiple sclerosis at the immunoglobulin heavy chain locus associates with intrathecal IgG, IgM index and oligoclonal bands. Mult Scler 2015;21:1104–1111. [DOI] [PubMed] [Google Scholar]
- 8. Vartdal F, Vandvik B. Multiple sclerosis: subclasses of intrathecally synthesized IgG and measles and varicella zoster virus IgG antibodies. Clin Exp Immunol 1983;54:641–647. [PMC free article] [PubMed] [Google Scholar]
- 9. Salier JP, Goust JM, Pandey JP, Fudenberg HH. Preferential synthesis of the G1m(1) allotype of IgG1 in the central nervous system of multiple sclerosis patients. Science 1981;213:1400–1402. [DOI] [PubMed] [Google Scholar]
- 10. Johansen JN, Vartdal F, Desmarais C, et al. Intrathecal BCR transcriptome in multiple sclerosis versus other neuroinflammation: equally diverse and compartmentalized, but more mutated, biased and overlapping with the proteome. Clin Immunol 2015;160:211–225. [DOI] [PubMed] [Google Scholar]
- 11. Virtanen JO, Wohler J, Fenton K, et al. Oligoclonal bands in multiple sclerosis reactive against two herpesviruses and association with magnetic resonance imaging findings. Mult Scler 2014;20:27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nutt SL, Hodgkin PD, Tarlinton DM, Corcoran LM. The generation of antibody‐secreting plasma cells. Nat Rev Immunol 2015;15:160–171. [DOI] [PubMed] [Google Scholar]
- 13. von Budingen HC, Kuo TC, Sirota M, et al. B cell exchange across the blood‐brain barrier in multiple sclerosis. J Clin Invest 2012;122:4533–4543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stern JN, Yaari G, Vander Heiden JA, et al. B cells populating the multiple sclerosis brain mature in the draining cervical lymph nodes. Sci Transl Med 2014;6:248ra107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cepok S, Rosche B, Grummel V, et al. Short‐lived plasma blasts are the main B cell effector subset during the course of multiple sclerosis. Brain 2005;128:1667–1676. [DOI] [PubMed] [Google Scholar]
- 16. Corcione A, Casazza S, Ferretti E, et al. Recapitulation of B cell differentiation in the central nervous system of patients with multiple sclerosis. Proc Natl Acad Sci U S A 2004;101:11064–11069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Torres M, Casadevall A. The immunoglobulin constant region contributes to affinity and specificity. Trends Immunol 2008;29:91–97. [DOI] [PubMed] [Google Scholar]
- 18. Ternant D, Arnoult C, Pugniere M, et al. IgG1 Allotypes Influence the pharmacokinetics of therapeutic monoclonal antibodies through FcRn binding. J Immunol 2016;196:607–613. [DOI] [PubMed] [Google Scholar]
- 19. Schlachetzki F, Zhu C, Pardridge WM. Expression of the neonatal Fc receptor (FcRn) at the blood‐brain barrier. J Neurochem 2002;81:203–206. [DOI] [PubMed] [Google Scholar]
- 20. Cooper PR, Ciambrone GJ, Kliwinski CM, et al. Efflux of monoclonal antibodies from rat brain by neonatal Fc receptor. FcRn. Brain Res 2013;1534:13–21. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: G1m allotypes are not associated with the disease severity in MS. The MS severity score (MSSS) was estimated for G1m3 homozygous patients (n = 50), G1m1/G1m3 heterozygous patients (n = 58), and G1m1 homozygous patients (n = 18). Bars depict mean ± SD, and differences between the three groups were tested using Kruskal–Wallis test.


