Abstract
Statement of the Problem:
Squamous cell carcinoma (SCC) is the most frequent oral cancer whose 5-year survival rate is 80% for early-detected lesions and nearly 30-50% for advanced lesions. Early detection of oral cancers and precancerous lesions can improve the patient’s survival and decrease the morbidity.
Purpose:
This study aimed to evaluate and compare the Ki-67 and MCM3 expression in cytologic smear of oral SCC (OSCC).
Materials and Method:
We examined 48 oral brush biopsies including 28 OSCC and 20 healthy non-smoking samples. Immunocytochemistry staining was performed for Ki-67 and MCM3 by using an EnVision-labeled peroxidase system, and labeling index (LI) was calculated.
Results:
Out of 28 OSCC cases, 27(96.4%) cases contained MCM3 positive cells and 22(78%) cases contained Ki-67 positive cells. All normal mucosa were Ki-67 and MCM3 negative. MCM3 and Ki-67 LI were significantly higher in OSCC than normal mucosa (p< 0.001). MCM3 LI was significantly higher than Ki-67 LI in OSCC group (p< 0.001).
Conclusion:
Immunocytologic evaluation of Ki-67 and MCM3 can be used for early detection of OSCC. Furthermore, MCM3 may be a more sensitive cytologic biomarker than Ki-67 in SCC patients.
Keywords: Squamous Cell Carcinoma , Cytology , Biopsy , Ki-67 antigen , Minichromosome Maintenance Complex Component 3
Introduction
Oral cancer is a wide term that includes various forms of malignancy presenting in the oral cavity.[1] It is one of the sixth most common cancers in Asia (3% of malignancy) and affects nearly 300,000 patients each year.[2] Squamous cell carcinoma (SCC) is the most frequent oral cancer with different tissue presentations.[3] The 5-year survival rate of early-detected oral cancer is 80%, although for advanced lesions, this number decreases to nearly 30-50%.[4] Moreover, treatment modalities often influence the speech, nutrition, and swallowing.[5]
Patients’ fear, asymptomatic primary lesions, and misdiagnosis result in delayed diagnosis.[6] Oral premalignant lesion such as leukoplakia, erythroplakia and oral lichen planus (OLP) may convert into oral cancer.[7] OLP is a chronic inflammatory disease of the oral mucosa, which seems to be associated with the process of cell-mediated immunity.[8] Prevalence of oral lesions is reported to be 0.5-2.2 percent.[9] The risk of malignant transformation is low and the incidence is nearly 0.2% per year.[3] Early detection of oral cancer and precancerous lesions can significantly improve the patient’s survival and decrease the morbidity.[6] Definitive diagnosis of potentially malignant and malignant lesions is through tissue biopsy and histopathology. However, some approaches such as oral brush biopsy, vital tissue staining, and light technology are suggested as diagnostic aids. Cytology was used to evaluate the morphology of epithelial cells,[10] but the primary results were not satisfactory because the cotton-tipped tool could only separate the surface cells. Subsequently, the brush cytology was introduced that can separate all the three layers of epithelium,[2] and allows assessment of both morphology and keratinization. This method is 95% accurate, with a painless procedure that requires no anesthesia.[11] Different studies have used the oral brush biopsy for the diagnosis of malignant lesions.[2,12-13] The sensitivity of this method was reported to be in the range of 71% to 97.2%.[14-15] Consequently, to increase the diagnostic accuracy of cytology, various methods were used such as bio-molecular techniques and determining the mutation.[16-19] Ki-67 is a protein that is detected on phases G1, S, G2, M cell cycle (not on G0 phase).[20] It is known as a cell proliferation factor. In premalignant lesions, expression of this factor was reported to increase with severity of the dysplasia.[9] Main chromosome maintenance (MCM) is another protein that plays a key role in the initiation and continuation of DNA replication[21] and includes six sub-groups (MCM 2 to MCM 7).[22] Mcm3 protein is acetylated in mammalian cells by a protein called MCM3AP, which was originally isolated in a two-hybrid screen for Mcm3 interactors. Peculiarly, MCM3AP inhibits DNA replication in a cell-free system, suggesting it as a negative regulator. However, it clearly functions positively by promoting the nuclear localization and chromatin binding in MCM3. This paradox has yet to be resolved. MCM3AP is a splice variant of a much larger protein called GANP that is found in B cells. The association of GANP with DNA primase activity suggests its involvement in the regulation of B-cell proliferation.[21-22]
According to the basic role of this protein in the regulation of DNA replication and cell proliferation, it seems that the MCM is one of the important markers of cell cycle.[22] Some studies have also reported MCM3 as a diagnostic marker in human tumors.[23] Nonetheless, only one study has evaluated expression of Ki-67 markers and no study had assessed MCM3 on cytology samples. Therefore, the aim of this study was to evaluate and compare the ki-67 and MCM3 expression in cytologic smear of OSCC samples.
Materials and Method
Sampling
By using toothbrush, oral brush biopsies were obtained from the patients referring to the ENT Department of Khalili Hospital and Oral Medicine Department of Shiraz Dental School from January to November 2015. Forty-eight oral brush biopsies were examined, including 28 OSCC and 20 healthy, non-smoking individuals as control. Those suffering from any systemic disease, having a history of radiotherapy, chemotherapy, or other cancers were excluded. Each selected patient was asked to rinse his/her mouth with water. Having determined the site of biopsy, without using local anesthesia, the brush was placed on the selected area and rotated 5-10 times with mild pressure until pinpoint bleeding area appeared. The cells collected with the brush were spread uniformly on three clean glass slides. They were fixed in 96% ethanol for 30 minutes, and then delivered to Oral Pathology Department. The scalpel biopsy was also done immediately in the site of pinpoint bleeding and the tissue was sent for pathologic examination.
Immunocytochemistry (ICC)
ICC staining was performed by use of an EnVision-labeled peroxidase system (Dako; Carpinteria, CA, USA). Antigen retrieval was performed by using Dako estimation and a target retrieval solution for 20 minutes. The endogenous peroxidase was quenched by 3% H2O2. The brush biopsies were incubated with both pre-diluted Ki-67 rabbit antihuman antibody and anti-MCM3 polyclonal antibody (Rabbit; Abcam Corporation, abq7282, UK) at 1\100 dilution, each for 60 minutes. Omission of the primary antibody was employed as a negative control; while, positive controls were the sections of oral squamous cell carcinoma which were previously found to be positive for Ki-67 and MCM3. Brown nuclear staining was considered as positive. Samples with adequate cellularity were enrolled. All of the samples were examined blindly by two pathologists who were unaware of the clinicopathologic diagnosis. The positive cells were counted at 400x magnification and the labeling index (LI) was determined by assessing the percentage of positive stained cells out of the total epithelial cells counted. At least 500 cells were counted per slide. All the results were analyzed by using SPSS software, version 12.0 (Statistical Package for Social Sciences; Chicago, IL, USA) and were subjected to exact test. Non-parametric Kruskal-Wallis, Mann-Whitney, Dunn test and Wilcoxon signed-rank tests were used as appropriated. p Value< 0.05 was considered significant.
Results
Out of 48 samples, 25 cases were male and 23 were female with the mean age of 47.25 (Table 1).
Table 1.
Demographic data of study and control group
| Groups | N | Sex [male/female%] | Mean Age |
|---|---|---|---|
| Healthy | 27.39 | 9/11[45-55%] | 38.9 |
| SCC | 38.35 | 14/14[50-50%] | 55.6 |
Out of the 28 brush biopsies taken from OSCC cases, 27 (96.4%) contained MCM3 positive cells and 22 (78%) contained Ki-67 positive cells (Figures 1a and 1b). All samples of normal mucosa were Ki-67 and MCM3 negative. In OSCC specimen, the mean Ki-67 LI was 0.15 (0-0.3) and MCM3 LI was 0.53 (0-0.8), which was significantly higher than LI of normal mucosa (p< 0.001). The expression of MCM3 was significantly higher than that of Ki-67 in OSCC group (p< 0.001) (Table 2).
Figure1.
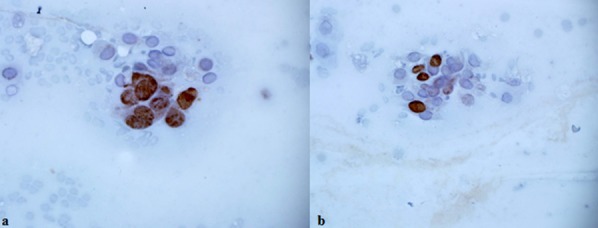
a: Nuclear MCM3 expression in OSCC patients [×400], b: Nuclear Ki-67 expression in OSCC patients [×400]
Table 2.
The percentage of positive cases for both markers, as well as the mean of MCM3 and Ki-67 staining for positive cases
| Groups | Type of marker | Mean MCM3 and Ki-67 | Positive Cases (%) |
|---|---|---|---|
| Healthy | MCM3 | 0 | 0 |
| Ki-67 | 0 | 0 | |
| SCC | MCM3 | 0.53 [0-0.83] | 96.4% |
| Ki-67 | 0.15 [0-0.3] | 78% |
Discussion
Various types of mucosal lesions frequently develop in oral cavity, a small number of which exhibit the potential to become malignant tumors.[24] Oral cancers are the most prevalent malignancies in the head and neck region, especially in developing countries where many individuals are affected by irritants and carcinogenic agents including tobacco smoke and betel nut extract.[25]
Identification of small malignant or premalignant lesion plays an imperative role in improving the diagnosis and treatment of cancers. Surgical biopsy is the best and most accurate technique for diagnosis of oral cavity lesions; however, it cannot be applied for some patients.[24]
Exfoliative cytology technique is a simple non-invasive method used to investigate the mucosal surface epithelial cells; it is referred to as the conventional cytologic smear technique. Initially, it was introduced to detect the cervical cancers at early stages. Nonetheless, it has limited applications in oral medicine[26] because contrary to the cervix uteri, the oral mucosa does not exhibit a transformation zone for the squamous and glandular epithelia to meet. Therefore, the atypical cells can be detectable in the deeper layer or at the surface of the lesions in later stages.[24] Consequently, the reliability of brush cytology procedures should be improved by employing supportive techniques such as DNA cytometry, automated image analysis, immunocytochemistry, and molecular biological tests.[19]
Epithelial dysplasia and malignancy exhibit ectopic cell cycles as an important feature[27] that can be evaluated by proliferation markers. The present study focused on the proliferation of MCM3 and Ki-67 in cytologic brush biopsies of OSCC and OLP patients. The result showed that MCM3 and Ki-67 positive epithelial cells were present in 96% and 78% of OSCC patients, respectively; but absent in normal mucosa. So, over-expression of Ki-67 and MCM3 was seen in the cytology of OSCC. It indicates that some isolated tumor cells in brush biopsies exhibited the biological properties of a malignant tumor. This finding emphasizes the fact that brush biopsy is a new and non-invasive technique for detecting oral cancers. Sharma et al.[28] evaluated the Ki-67 labeling index in OSCC patients and found that Ki-67 positive cells were detected in 23.3% of samples whereas in the current study, the Ki-67 expression was seen in 60% of the patients. This contrast might be due to the different sampling technique. Sharma et al. used wooden spatula that could not accurately separate the deeper cells; while, our sampling with brush yielded better results. We noted that MCM3 expression was higher than Ki-67 in OSCC group, which was in accordance with what was found by Lamerica et al. who evaluate these two markers in tissue specimen.
To the best of our knowledge, there was no similar study evaluating the MCM3 in brush biopsy. Our result reported the MCM3 biomarker to be more sensitive than Ki-67 for cytological evaluation of OSCC. Differences between the expression of MCM3 and Ki-67 might be attributed to the differences in the expression of these markers in the cell cycle; i.e. MCM3 was expressed later in the cycle. Expression of MCM3 is reported in the early G1 phase and in the entire cell cycle; while, the expression of Ki-67 is reported in the late G1 to M phases.[23]
The limitation of the present study was the contamination of the specimen with blood, bacteria, necrotic or proteinaceous debris that can be eliminated by employing liquid-based technique.
Conclusion
The present study found that Ki-67 and MCM3 immunocytology could be used for early detection of OSCC. The results also revealed that MCM3 is a more sensitive cytologic biomarker than Ki-67.
Acknowledgement
The authors would like to thank the Vice-chancellery of Shiraz University of Medical Sciences for supporting this research (Grant#8634). This article was based on the thesis by Dr. Rahele Ebrahimi from Dental School of Shiraz University of Medical Sciences. The authors would also appreciate Dr. Vosough from the Dental Research Development Center of the Dental School for helping with the statistical analysis.
Conflict of Interest:The authors disclose no potential conflicts of interest.
References
- 1.Greenberg MS, Glick M, Ship JA. Burket's oral medicine: 12th ed. BC Decker Inc: Hamilton; 2015. pp. 173–200. [Google Scholar]
- 2.Koch FP, Kunkel M, Biesterfeld S, Wagner W. Diagnostic efficiency of differentiating small cancerous and precancerous lesions using mucosal brush smears of the oral cavity--a prospective and blinded study. Clin Oral Investig. 2011; 15: 763–769. doi: 10.1007/s00784-010-0434-6. [DOI] [PubMed] [Google Scholar]
- 3.Little JW, Falace D, Miller C, Rhodus NL. Dental Management of the Medically Compromised Patient. 8th ed. St Louis: Mosby; 2013. pp. 460–480. [Google Scholar]
- 4.Silverman S Jr. Demographics and occurrence of oral and pharyngeal cancers. The outcomes, the trends, the challenge. J Am Dent Assoc. 2001; 132 Suppl: 7S–11S. doi: 10.14219/jada.archive.2001.0382. [DOI] [PubMed] [Google Scholar]
- 5.Meyer TK, Kuhn JC, Campbell BH, Marbella AM, Myers KB, Layde PM. Speech intelligibility and quality of life in head and neck cancer survivors. Laryngoscope. 2004; 114: 1977–1981. doi: 10.1097/01.mlg.0000147932.36885.9e. [DOI] [PubMed] [Google Scholar]
- 6.Gupta S, Shah JS, Parikh S, Limbdiwala P, Goel S. Clinical correlative study on early detection of oral cancer and precancerous lesions by modified oral brush biopsy and cytology followed by histopathology. J Cancer Res Ther. 2014; 10: 232–238. doi: 10.4103/0973-1482.136539. [DOI] [PubMed] [Google Scholar]
- 7.Delavarian Z, Mohtasham N, Mosannen-Mozafari P, Pakfetrat A, Shakeri MT, Ghafoorian-Maddah R. Evaluation of the diagnostic value of a Modified Liquid-BasedCytology using OralCDx Brush in early detection of oral potentiallymalignant lesions and oral cancer. Med Oral Patol Oral Cir Bucal. 2010; 15: e671–e676. doi: 10.4317/medoral.15.e671. [DOI] [PubMed] [Google Scholar]
- 8.Hirota M, Ito T, Okudela K, Kawabe R, Yazawa T, Hayashi H, et al. Cell proliferation activity and the expression of cell cycle regulatory proteins in oral lichen planus. J Oral Pathol Med. 2002; 31: 204–212. doi: 10.1034/j.1600-0714.2002.310403.x. [DOI] [PubMed] [Google Scholar]
- 9.Acay RR, Felizzola CR, de Araújo N, de Sousa SO. Evaluation of proliferative potential in oral lichen planus and oral lichenoid lesions using immunohistochemical expression of p53 and Ki67. Oral Oncol. 2006; 42: 475–480. doi: 10.1016/j.oraloncology.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 10.Gardner AF. An investigation of the use of exfoliative cytology in the diagnosis of malignant lesions of the oral cavity. The cytologic diagnosis of oral carcinoma. Acta Cytol. 1964; 8: 436–445. [PubMed] [Google Scholar]
- 11.Loss R, Sandrin R, França BH, de Azevedo-Alanis LR, Grégio AM, Machado MÂ, et al. Cytological analysis of the epithelial cells in patients with oral candidiasis. Mycoses. 2011; 54: e130–e135. doi: 10.1111/j.1439-0507.2009.01857.x. [DOI] [PubMed] [Google Scholar]
- 12.Pérez-Sayáns M, Reboiras-López MD, Somoza-Martín JM, Barros-Angueira F, Diz PG, Rey JM, et al. Measurement of ATP6V1C1 expression in brush cytology samples as a diagnostic and prognostic marker in oral squamous cell carcinoma. Cancer Biol Ther. 2010; 9: 1057–1064. doi: 10.4161/cbt.9.12.11880. [DOI] [PubMed] [Google Scholar]
- 13.Remmerbach TW, Meyer-Ebrecht D, Aach T, Würflinger T, Bell AA, Schneider TE, et al. Toward a multimodal cell analysis of brush biopsies for the early detection of oral cancer. Cancer. 2009; 117: 228–235. doi: 10.1002/cncy.20028. [DOI] [PubMed] [Google Scholar]
- 14.Poate TW, Buchanan JA, Hodgson TA, Speight PM, Barrett AW, Moles DR, et al. An audit of the efficacy of the oral brush biopsy technique in a specialist Oral Medicine unit. Oral Oncol. 2004; 40: 829–834. doi: 10.1016/j.oraloncology.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 15.Remmerbach TW, Mathes SN, Weidenbach H, Hemprich A, Böcking A. Noninvasive brush biopsy as an innovative tool for early detection of oral carcinomas. Mund Kiefer Gesichtschir. 2004; 8: 229–236. doi: 10.1007/s10006-004-0542-z. [DOI] [PubMed] [Google Scholar]
- 16.Zhang L, Rosin MP. Loss of heterozygosity: a potential tool in management of oral premalignant lesions? . J Oral Pathol Med. 2001;30:513–520. doi: 10.1034/j.1600-0714.2001.300901.x. [DOI] [PubMed] [Google Scholar]
- 17.Scheifele C, Schlechte H, Bethke G, Reichart PA. Detection of TP53-mutations in brush biopsies from oral leukoplakias. Mund Kiefer Gesichtschir. 2002; 6: 410–414. doi: 10.1007/s10006-002-0425-0. [DOI] [PubMed] [Google Scholar]
- 18.Driemel O, Dahse R, Berndt A, Pistner H, Hakim SG, Zardi L, et al. High-molecular tenascin-C as an indicator of atypical cells in oral brush biopsies. Clin Oral Investig. 2007; 11: 93–99. doi: 10.1007/s00784-006-0086-8. [DOI] [PubMed] [Google Scholar]
- 19.Driemel O, Dahse R, Hakim SG, Tsioutsias T, Pistner H, Reichert TE, et al. Laminin-5 immunocytochemistry: a new tool for identifying dysplastic cells in oral brush biopsies. Cytopathology. 2007; 18: 348–355. doi: 10.1111/j.1365-2303.2006.00401.x. [DOI] [PubMed] [Google Scholar]
- 20.Schlüter C, Duchrow M, Wohlenberg C, Becker MH, Key G, Flad HD, Gerdes J. The cell proliferation-associated antigen of antibody Ki-67: a very large, ubiquitous nuclear protein with numerous repeated elements, representing a new kind of cell cycle-maintaining proteins. J Cell Biol. 1993; 123: 513–522. doi: 10.1083/jcb.123.3.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ashkavandi ZJ, Najvani AD, Tadbir AA, Pardis S, Ranjbar MA, Ashraf MJ. MCM3 as a novel diagnostic marker in benign and malignant salivary gland tumors. Asian Pac J Cancer Prev. 2013; 14: 3479–3482. doi: 10.7314/apjcp.2013.14.6.3479. [DOI] [PubMed] [Google Scholar]
- 22.Forsburg SL. Eukaryotic MCM proteins: beyond replication initiation. Microbiol Mol Biol Rev. 2004; 68: 109–131. doi: 10.1128/MMBR.68.1.109-131.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giaginis C, Vgenopoulou S, Vielh P, Theocharis S. MCM proteins as diagnostic and prognostic tumor markers in the clinical setting. Histol Histopathol. 2010; 25: 351–370. doi: 10.14670/HH-25.351. [DOI] [PubMed] [Google Scholar]
- 24.Driemel O, Kosmehl H, Rosenhahn J, Berndt A, Reichert TE, Zardi L, et al. Expression analysis of extracellular matrix components in brush biopsies of oral lesions. Anticancer Res. 2007; 27: 1565–1570. [PubMed] [Google Scholar]
- 25.Weiss S, Enzinger GJ. Weiss’s soft tissue tumors. 6th ed. St Louis, MO: Mosby; 2001. pp. 100–132. [Google Scholar]
- 26.Hayama FH, Motta AC, Silva Ade P, Migliari DA. Liquid-based preparations versus conventional cytology: specimen adequacy and diagnostic agreement in oral lesions. Med Oral Patol Oral Cir Bucal. 2005; 10: 115–122. [PubMed] [Google Scholar]
- 27.Scott IS, Odell E, Chatrath P, Morris LS, Davies RJ, Vowler SL, et al. A minimally invasive immunocytochemical approach to early detection of oral squamous cell carcinoma and dysplasia. Br J Cancer. 2006; 94: 1170–1175. doi: 10.1038/sj.bjc.6603066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sharma P, Kumar N, Bahadur AK, Mandal AK. Ki-67 expression in cytologic scrapes from oral squamous cell carcinoma before and after 24 gray radiotherapy--a study on 43 patients. Med Oral Patol Oral Cir Bucal. 2005; 10 Suppl 1: E15–E17. [PubMed] [Google Scholar]


