Abstract
We have developed a highly regioselective homogeneous gold(I)-catalyzed anti-hydrochlorination of unactivated alkynes at room temperature. We have overcome the incompatibility between conventional cationic gold catalysts and chloride by using a hydrogen-bonding activation of the Au–Cl bond. This approach is scalable, exhibits excellent functional group tolerance, and can be conducted in open air.
Keywords: gold catalysis, hydrochlorination, chloride-tolerant, alkyne, HCl/DMPU
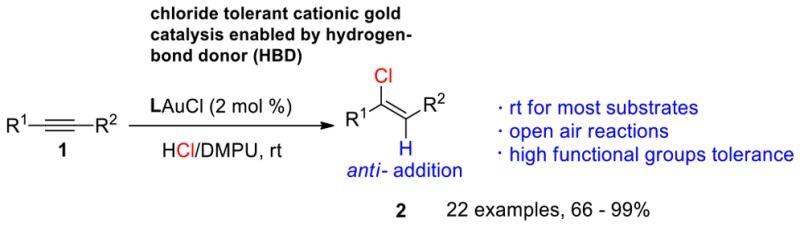
Chlorination is one of the most important transformations in organic synthesis because of the biological activities and synthetic value of the chlorinated products.1 More specifically, vinyl chloride is one of the most important group of chlorine-containing compounds. Vinyl chlorides are widely found in natural products, pharmaceuticals and agrochemicals,1,2 and also are valuable coupling partners in coupling reactions such as Buchwald–Hartwig amination3 and Suzuki–Miyaura coupling.4 Compared to the traditional syntheses of vinyl chlorides, such as halogenations of carbonyl compounds5 and electrophilic chlorination of alkynes,6 the direct hydrochlorination of alkynes from HCl is a straightforward and atom-economic method. However, given that HCl itself is a dangerous gas and handling is problematic, synthetic chemists have focused on indirect hydrochlorination strategies for activated alkynes,7 using RCOCl, TMSCl, or metal chlorides as chlorine sources (Scheme 1a).8 One notable example is the recent work by Engle and co-workers on the palladium-catalyzed anti-hydrochlorination of alkynes using a directing group (Scheme 1b).9 The direct hydrochlorination of unactivated alkynes using HCl has been rarely reported. Dai and co-workers found that gaseous HCl could hydrochlorinate electron-rich phenylacetylenes but the use of gaseous HCl and hydration side products present major drawbacks.10 Derien and co-workers developed a ruthenium-catalyzed hydrochlorination of alkynes that gave good yields (Scheme 1c).11 Although this method works well for terminal alkynes, higher temperature was needed and low stereoselectivity was observed for internal alkynes (Scheme 1c). Moreover, a restrictive Schlenk environment was essential. The heterogeneous gold-catalyzed hydrochlorination of acetylene for manufacture of vinyl chloride monomer (VCM) is a well-studied process12 and has been industrialized recently (Scheme 1d).13 Recently, and independently, the Corma group14 and our group15 reported a heterogeneous gold (TiO2/Au)-catalyzed hydrochlorination reaction of alkynes. Although good syn-addition selectivity was observed, high temperatures were needed (Scheme 1d).
Scheme 1. Major Synthetic Methods for Chlorination of Alkynes to Synthesize Vinylchlorides.
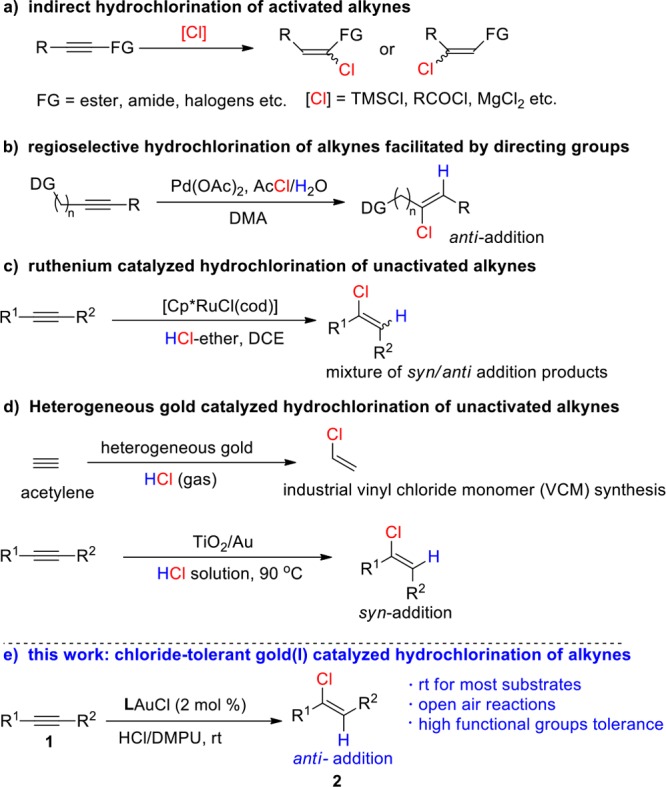
Although homogeneous cationic gold catalysts are considered as the most powerful catalysts for the electrophilic activation of alkynes,16 the cationic gold-catalyzed hydrochlorination of alkynes has not been reported because of the high affinity between cationic gold and chloride.17 Indeed, commonly used gold precatalysts, such as PPh3AuCl, usually need silver salts to break the strong Au–Cl bond and release an active cationic gold species and chloride is known to easily poison cationic gold species (Scheme 2a).18 We are now pleased to report a homogeneous gold-catalyzed alkyne hydrochlorination with exclusive anti-selectivity that overcomes the incompatibility between cationic gold catalysts and chloride (Scheme 1e).
Scheme 2. Conventional Cationic Gold Catalysis and Chloride-Tolerant Homogenous Gold Catalysis.
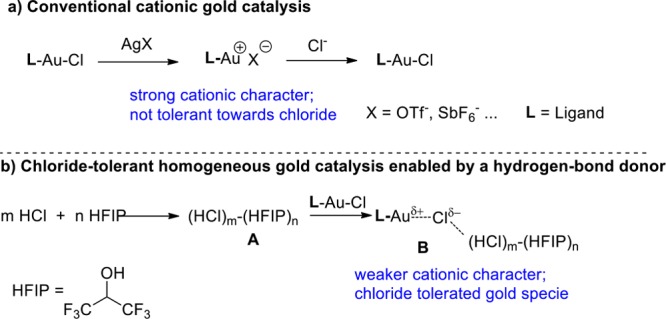
We proposed that a combination of strong hydrogen bond donor solvents such as hexafluoroisopropanol (HFIP) and HCl would generate a strong hydrogen-bonding donor network A, and that the hydrogen bonding between chlorine in L–Au-Cl and A might weaken the Au–Cl bond (Scheme 2b). This would generate a partially cationic gold species B via hydrogen-bonding interaction. This strategy would avoid the use of a silver salt to activate the gold precatalyst, a step that is known to exert negative effects in gold catalysis.19
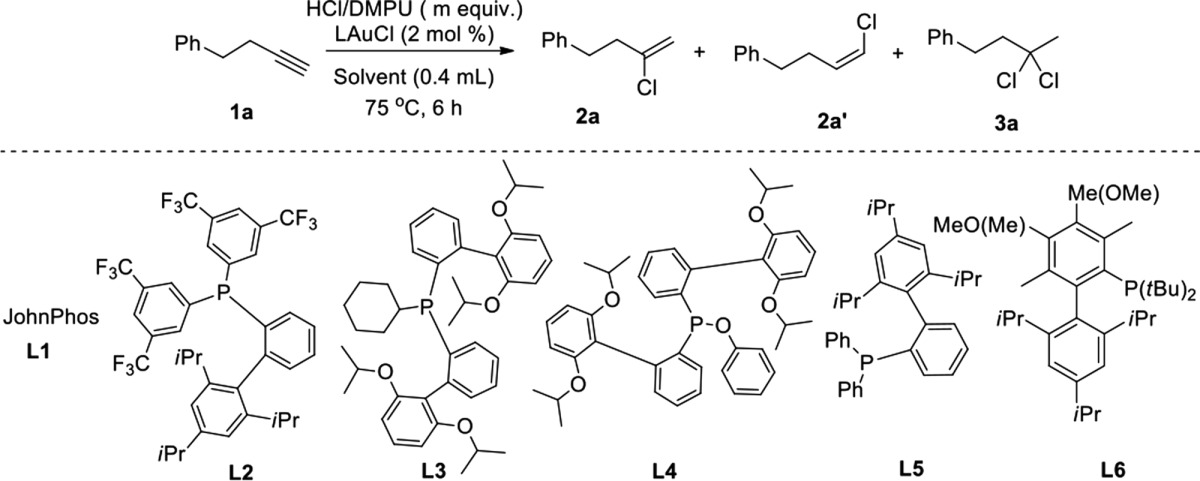
We used 4-phenylbutyne 1a as the model substrate for reaction optimization. We screened various ligands, HCl sources, and solvents (Table 1).
Table 1. Screening of Homogeneous Gold Catalyzed Hydrochlorination of Alkynes.
| entry | L | m | HCl | solvent | yield (%)a | ratio of 2a:2a′:3a |
|---|---|---|---|---|---|---|
| 1 | L1 | 1.5 | HCl/DMPU | HFIP | 80 | 75:8:17 |
| 2 | L2 | 1.5 | HCl/DMPU | HFIP | 55 | 76:9:15 |
| 3 | L3 | 1.5 | HCl/DMPU | HFIP | 65 | 90:8:2 |
| 4 | L4 | 1.5 | HCl/DMPU | HFIP | 38 | 82:9:9 |
| 5 | L5 | 1.5 | HCl/DMPU | HFIP | 95 | 93:4:3 |
| 6 | L6 | 1.5 | HCl/DMPU | HFIP | 96 | 90:7:3 |
| 7 | L5 | 1.2 | HCl/DMPU | HFIP | 95 | 95:4:1 |
| 8b | L5 | 1.2 | HCl/DMPU | HFIP | 98 | 97:3: - |
| 9b | L6 | 1.2 | HCl/DMPU | HFIP | 95 | 97:3: - |
| 10 | - | 2.4 | HCl/DMPU | HFIP | 34 | 59:16:25 |
| 11b | L5 | 1.2 | HCl/iPrOH | HFIP | 72 | 88:12: - |
| 12b | L5 | 1.2 | HCl/Et2O | HFIP | <5 | n. a. |
| 13b,c | L6 | 1.2 | HCl/DMPU | tBuOH | 85 | 51: -: - |
| 14b,c | L6 | 1.2 | HCl/DMPU | THF | 78 | 82:8: - |
| 15b,d | L6 | 1.2 | HCl/DMPU | HFIP/MeNO2 | 97 | 99:1: - |
| 16b,d | L6 | 1.2 | HCl/DMPU | HFIP/DMF | 93 | 98:2: - |
Yields were determined by GC-MS using dodecane as internal standard.
Reaction run at rt for 24 h.
Ketone product formed (accounts for the remaining ratio of product).
Solvent mixture is 1:1 by volume.
Due to its high hydrogen bond basicity but low Brønsted basicity,20,21 DMPU is able to form a stable and highly concentrated complex with hydrogen chloride (HCl).15 Using 1.5 equiv of HCl/DMPU and 2 mol % of gold catalyst and various ligands (L1–L6) led to high yields of product (Table 1, entries 1–6). However, the anti-Markovnikov and dichlorinated products were also obtained in varying amounts. The use of L5 improved the selectivity of the produced vinyl chloride to 95% yield, needing only 1.2 equiv of HCl/DMPU (Table 1, entry 7). Formation of the dichlorinated byproduct was avoided by running the reaction at ambient temperature (Table 1, entry 8–9), with both L5 and L6 working equally well. A poor yield was obtained in the absence of gold (Table 1, entry 10). Notably, lower selectivity and poor yields were observed when commercially available HCl sources (HCl/iPrOH and HCl/ether) were used (Table 1, entries 11 and 12). We screened other solvents to further optimize the regioselectivity. We found that a 1:1 HFIP: CH3NO2 mixture provided optimal conditions. It is worth noting that tert-butanol and THF gave substantial amounts to the corresponding ketone. A 1:1 HFIP: DMF combination worked well also (Table 1, entry 16).
With optimized conditions in hand, we investigated the substrate scope (Table 2). We first examined the scope of aliphatic alkynes. A wide variety of terminal alkynes reacted smoothly to give the corresponding vinyl chlorides in good to excellent yields.
Table 2. Substrate Scope of Homogeneous Gold-Catalyzed Hydrochlorination of Alkynesa.
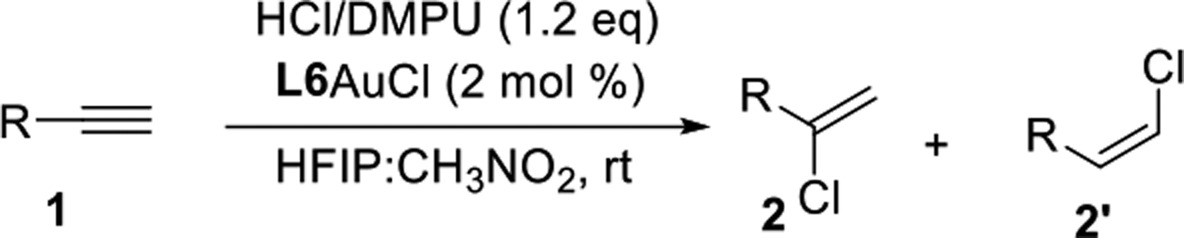
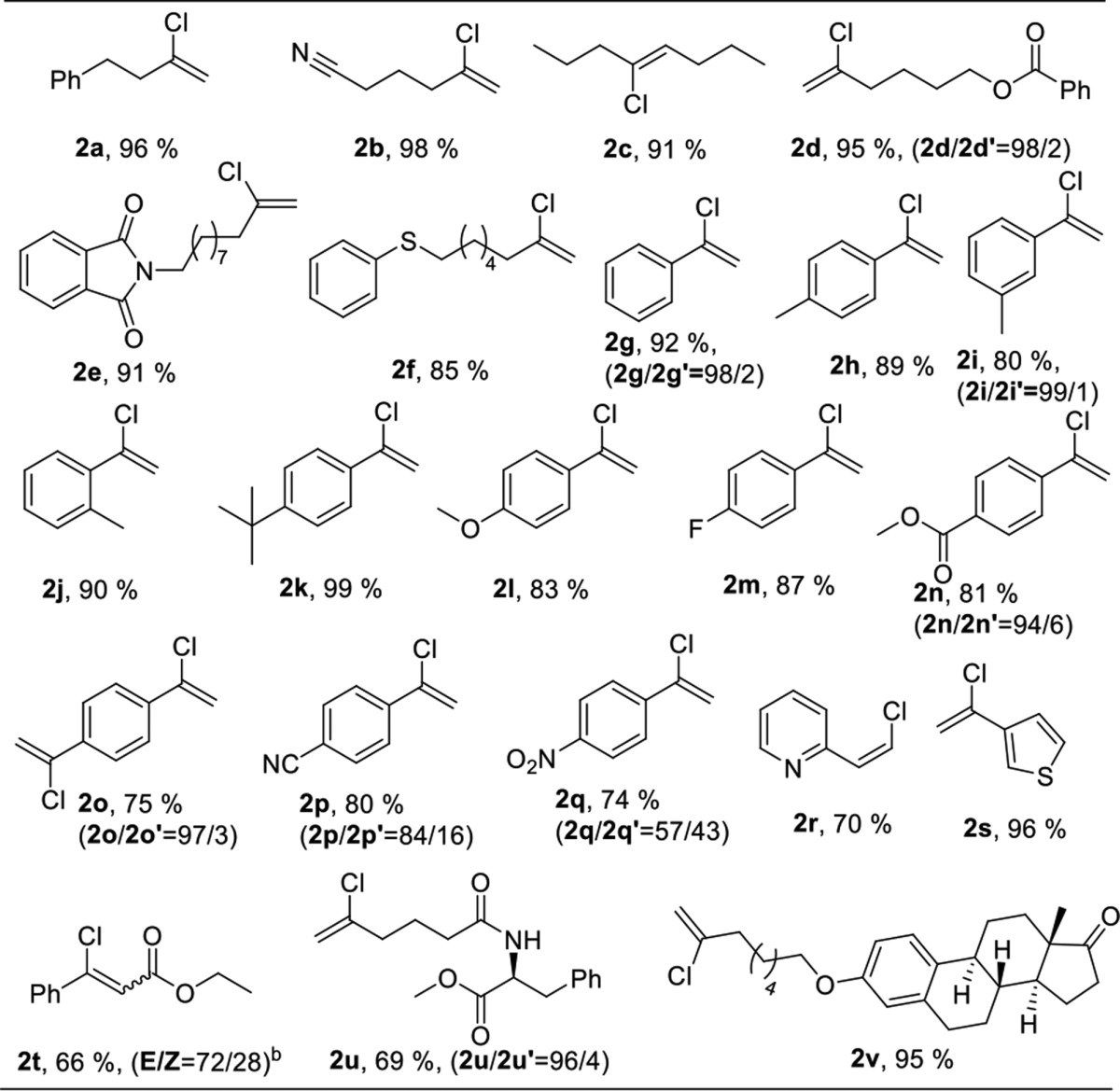
Reaction conditions: alkyne 1 (0.5 mmol), HCl/DMPU (1.2 equiv), L6AuCl (2 mol %), HFIP:CH3NO2 (0.125 mL) at rt for 16–36 h. Isolated yields.
75 °C was used.
Nitrile (2b), ester (2d), imide (2e), sulfide (2f), amide (2u), and ketone ether (2v) functional groups were well-tolerated under the standard conditions. The reaction with derivatized amino acid (2u) and the natural product estrone (2v) led to the corresponding products in 69% and 95% yield, respectively. The internal alkyne 4-octyne (2c) also worked well, although higher temperature and longer reaction time were needed. When we explored the scope of arylacetylenes, we found good to excellent yields using electron-rich and electron-deficient arylacetylenes. Para (1h), meta (1i), and ortho (1j) tolylacetylenes furnished the corresponding vinyl chlorides in excellent yields. It is worth noting that electron-deficient arylacetylenes gave increasing amounts of the anti-Markovnikov products (2n, 2o, 2p, and 2q). A remarkable example was the heteroaromatic alkyne 2-ethynylpyridine (1r), which was expected to quench the reactivity of homogeneous cationic gold due to its basic nature, but under our conditions, 1r furnished the anti-Markovnikov vinyl chloride (2r) in 70% yield.
The change of regioselectivity in 2r could have been caused by the pyridinyl nitrogen acting as a directing group and coordinating with HCl or gold. 3-Ethynylthiophene (1s) gave an excellent yield of the corresponding chloro product. The electron-deficient internal alkyne ethylphenylpropiolate (1t) also worked well, albeit a higher temperature (75 °C) was needed, and the yield was modest (66%). In this case, a mixture of E/Z isomers was obtained, possibly due to the isomerization caused by the elevated temperature.
It should be noted that our homogeneous gold catalyzed reaction required milder conditions (room temperature) than the recently reported heterogeneous gold catalyzed process, and that the stereochemistry of the addition was different.14 Due to the milder conditions of our reaction, we expected better functional group tolerance. Indeed, the peptide (2u) and derivatized estrone (2v) were tolerated under our conditions. The homogeneous gold process gave the anti-addition pattern (Table 2, 2c and 2r), which is consistent with typical homogeneous gold catalysis where cationic gold activates alkyne substrates. On the other hand, the nanogold catalyzed process gave syn-addition products, which suggests that nanogold initially activates HCl rather than the alkyne.14
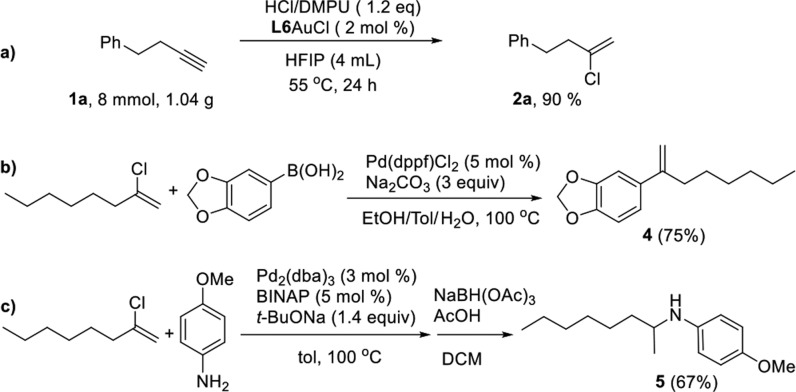
Our strategy for the hydrochlorination of unactivated alkynes was easily expanded to large-scale synthesis, without affecting yield and regioselectivity (Scheme 3a). To examine the synthetic value of the synthesized vinyl chlorides, we conducted two classic reactions that use vinyl chlorides as building blocks, namely the Suzuki coupling and the Buchwald–Hartwig amination. In both cases, the desired products were isolated in good yields (Scheme 3b,c).
Scheme 3. Gram-Scale Synthesis and the Synthetic Utility of Vinylchlorides.
In conclusion, we have reported the first efficient regioselective homogeneous gold-catalyzed hydrochlorination of unactivated alkynes. We have overcome the traditional incompatibility between conventional cationic gold catalysts and chloride. This method can be easily scaled up, and the reactions can be conducted in the open air.
Acknowledgments
We are grateful to the National Institutes of Health for financial support (1R01GM121660-01). B.X. is grateful to the National Science Foundation of China for financial support (NSFC-21472018).
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acscatal.7b02567.
Experimental procedures and characterization data of products (PDF)
Author Contributions
§ (R.E., S.L.) These authors contributed equally to this work.
The authors declare no competing financial interest.
Supplementary Material
References
- Petrone D. A.; Ye J.; Lautens M. Chem. Rev. 2016, 116, 8003–8104. 10.1021/acs.chemrev.6b00089. [DOI] [PubMed] [Google Scholar]
- a Engvild K. C. Phytochemistry 1986, 25, 781–791. 10.1016/0031-9422(86)80002-4. [DOI] [Google Scholar]; b Gribble G. W. Acc. Chem. Res. 1998, 31, 141–152. 10.1021/ar9701777. [DOI] [Google Scholar]; c Hernandes M. Z.; Cavalcanti S. M. T.; Moreira D. R. M.; de Azevedo Junior W.; Leite A. C. Curr. Drug Targets 2010, 11, 303–314. 10.2174/138945010790711996. [DOI] [PubMed] [Google Scholar]; d Jeschke P. Pest Manage. Sci. 2010, 66, 10–27. 10.1002/ps.1829. [DOI] [PubMed] [Google Scholar]
- a Hartwig J. F. Acc. Chem. Res. 2008, 41, 1534–1544. 10.1021/ar800098p. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Ruiz-Castillo P.; Buchwald S. L. Chem. Rev. 2016, 116, 12564–12649. 10.1021/acs.chemrev.6b00512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Martin R.; Buchwald S. L. Acc. Chem. Res. 2008, 41, 1461–1473. 10.1021/ar800036s. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Miyaura N.; Suzuki A. Chem. Rev. 1995, 95, 2457–2483. 10.1021/cr00039a007. [DOI] [Google Scholar]; c Han F.-S. Chem. Soc. Rev. 2013, 42, 5270–5298. 10.1039/c3cs35521g. [DOI] [PubMed] [Google Scholar]
- a Su W.; Jin C. Org. Lett. 2007, 9, 993–996. 10.1021/ol062991c. [DOI] [PubMed] [Google Scholar]; b Kamei K.; Maeda N.; Tatsuoka T. Tetrahedron Lett. 2005, 46, 229–232. 10.1016/j.tetlet.2004.11.075. [DOI] [Google Scholar]; c Spaggiari A.; Vaccari D.; Davoli P.; Torre G.; Prati F. J. Org. Chem. 2007, 72, 2216–2219. 10.1021/jo061346g. [DOI] [PubMed] [Google Scholar]
- a Brown H.; Hamaoka T.; Ravindran N.; Subrahmanyam C.; Somayaji V.; Bhat N. G. J. Org. Chem. 1989, 54, 6075–6079. 10.1021/jo00287a019. [DOI] [Google Scholar]; b Gu Y.; Snider B. B. Org. Lett. 2003, 5, 4385–4388. 10.1021/ol0356789. [DOI] [PubMed] [Google Scholar]; c Heathcock C. H.; Mahaim C.; Schlecht M. F.; Utawanit T. J. Org. Chem. 1984, 49, 3264–3274. 10.1021/jo00192a004. [DOI] [Google Scholar]
- a Ma S.; Lu X.; Li Z. J. Org. Chem. 1992, 57, 709–713. 10.1021/jo00028a055. [DOI] [Google Scholar]; b Sun A.; Huang X. Synthesis 2000, 2000, 1819–1821. 10.1055/s-2000-8233. [DOI] [Google Scholar]; c Yu W.; Jin Z. J. Am. Chem. Soc. 2000, 122, 9840–9841. 10.1021/ja000903s. [DOI] [Google Scholar]; d Mulder J. A.; Kurtz K. C. M.; Hsung R. P.; Coverdale H.; Frederick M. O.; Shen L.; Zificsak C. A. Org. Lett. 2003, 5, 1547–1550. 10.1021/ol0300266. [DOI] [PubMed] [Google Scholar]; e Zhu G.; Chen D.; Wang Y.; Zheng R. Chem. Commun. 2012, 48, 5796–5798. 10.1039/c2cc31553j. [DOI] [PubMed] [Google Scholar]
- a Wang Z.; Lu X. Chem. Commun. 1996, 535–536. 10.1039/CC9960000535. [DOI] [Google Scholar]; b Hua R.; Shimada S.; Tanaka M. J. Am. Chem. Soc. 1998, 120, 12365–12366. 10.1021/ja9822299. [DOI] [Google Scholar]; c Wang B.; Wang S.; Li P.; Wang L. Chem. Commun. 2010, 46, 5891–5893. 10.1039/c0cc01182g. [DOI] [PubMed] [Google Scholar]; d Wada T.; Iwasaki M.; Kondoh A.; Yorimitsu H.; Oshima K. Chem. - Eur. J. 2010, 16, 10671–10674. 10.1002/chem.201000865. [DOI] [PubMed] [Google Scholar]; e Lu Z.; Kong W.; Yuan Z.; Zhao X.; Zhu G. J. Org. Chem. 2011, 76, 8524–8529. 10.1021/jo2015278. [DOI] [PubMed] [Google Scholar]; f Kokubo K.; Matsumasa K.; Miura M.; Nomura M. J. Org. Chem. 1996, 61, 6941–6946. 10.1021/jo960915p. [DOI] [PubMed] [Google Scholar]; g Iwai T.; Fujihara T.; Terao J.; Tsuji Y. J. Am. Chem. Soc. 2009, 131, 6668–6669. 10.1021/ja901778y. [DOI] [PubMed] [Google Scholar]; h Iwai T.; Fujihara T.; Terao J.; Tsuji Y. J. Am. Chem. Soc. 2012, 134, 1268–1274. 10.1021/ja209679c. [DOI] [PubMed] [Google Scholar]; i Li J.-H.; Tang S.; Xie Y.-X. J. Org. Chem. 2005, 70, 477–479. 10.1021/jo048358r. [DOI] [PubMed] [Google Scholar]; j Kashiwabara T.; Fuse K.; Hua R.; Tanaka M. Org. Lett. 2008, 10, 5469–5472. 10.1021/ol802260w. [DOI] [PubMed] [Google Scholar]; k Hua R.; Onozawa S.-y.; Tanaka M. Chem. - Eur. J. 2005, 11, 3621–3630. 10.1002/chem.200401279. [DOI] [PubMed] [Google Scholar]
- Derosa J.; Cantu A. L.; Boulous M. N.; O’Duill M. L.; Turnbull J. L.; Liu Z.; De La Torre D. M.; Engle K. M. J. Am. Chem. Soc. 2017, 139, 5183–5193. 10.1021/jacs.7b00892. [DOI] [PubMed] [Google Scholar]
- Xu C.-X.; Ma C.-H.; Xiao F.-R.; Chen H.-W.; Dai B. Chin. Chem. Lett. 2016, 27, 1683–1685. 10.1016/j.cclet.2016.04.019. [DOI] [Google Scholar]
- Dérien S.; Klein H.; Bruneau C. Angew. Chem., Int. Ed. 2015, 54, 12112–12115. 10.1002/anie.201505144. [DOI] [PubMed] [Google Scholar]
- a Dong Y.; Li W.; Yan Z.; Zhang J. Catal. Sci. Technol. 2016, 6, 7946–7955. 10.1039/C6CY01241H. [DOI] [Google Scholar]; b Liu X.; Conte M.; Elias D.; Lu L.; Morgan D. J.; Freakley S. J.; Johnston P.; Kiely C. J.; Hutchings G. J. Catal. Sci. Technol. 2016, 6, 5144–5153. 10.1039/C6CY00090H. [DOI] [Google Scholar]; c Johnston P.; Carthey N.; Hutchings G. J. J. Am. Chem. Soc. 2015, 137, 14548–14557. 10.1021/jacs.5b07752. [DOI] [PubMed] [Google Scholar]; d Hutchings G. J. Top. Catal. 2008, 48, 55–59. 10.1007/s11244-008-9048-5. [DOI] [Google Scholar]; e Zhang J.; Liu N.; Li W.; Dai B. Front. Chem. Sci. Eng. 2011, 5, 514–520. 10.1007/s11705-011-1114-z. [DOI] [Google Scholar]
- Ciriminna R.; Falletta E.; Della Pina C.; Teles J. H.; Pagliaro M. Angew. Chem., Int. Ed. 2016, 55, 14210–14217. 10.1002/anie.201604656. [DOI] [PubMed] [Google Scholar]
- Oliver-Meseguer J.; Doménech-Carbó A.; Boronat M.; Leyva-Pérez A.; Corma A. Angew. Chem., Int. Ed. 2017, 56, 6435–6439. 10.1002/anie.201700282. [DOI] [PubMed] [Google Scholar]
- Liang S.; Ebule R.; Hammond G. B.; Xu B. Org. Lett. 2017, 19, 4524. 10.1021/acs.orglett.7b02101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Hashmi A. S. K. Chem. Rev. 2007, 107, 3180–3211. 10.1021/cr000436x. [DOI] [PubMed] [Google Scholar]; b Gorin D. J.; Sherry B. D.; Toste F. D. Chem. Rev. 2008, 108, 3351–3378. 10.1021/cr068430g. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Arcadi A. Chem. Rev. 2008, 108, 3266–3325. 10.1021/cr068435d. [DOI] [PubMed] [Google Scholar]
- a Evans C. J.; Lesarri A.; Gerry M. C. L. J. Am. Chem. Soc. 2000, 122, 6100–6105. 10.1021/ja000874l. [DOI] [Google Scholar]; b Brown J. R.; Schwerdtfeger P.; Schröder D.; Schwarz H. J. Am. Soc. Mass Spectrom. 2002, 13, 485–492. 10.1016/S1044-0305(02)00370-7. [DOI] [PubMed] [Google Scholar]
- Kumar M.; Hammond G. B.; Xu B. Org. Lett. 2014, 16, 3452–3455. 10.1021/ol501663f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z.; Han J.; Hammond G. B.; Xu B. Org. Lett. 2015, 17, 4534–4537. 10.1021/acs.orglett.5b02224. [DOI] [PubMed] [Google Scholar]
- Our group had previously reported a novel nucleophilic fluorinating reagent HF/DMPU:; a Okoromoba O. E.; Li Z.; Robertson N.; Mashuta M. S.; Couto U. R.; Tormena C. F.; Xu B.; Hammond G. B. Chem. Commun. 2016, 52, 13353–13356. 10.1039/C6CC07855A. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Okoromoba O. E.; Hammond G. B.; Xu B. Org. Lett. 2015, 17, 3975–3977. 10.1021/acs.orglett.5b01919. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Okoromoba O. E.; Han J.; Hammond G. B.; Xu B. J. Am. Chem. Soc. 2014, 136, 14381–14384. 10.1021/ja508369z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurence C.; Brameld K. A.; Graton J.; Le Questel J.-Y.; Renault E. J. Med. Chem. 2009, 52, 4073–4086. 10.1021/jm801331y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.