Abstract
Numerous studies have employed repeated social defeat stress (RSDS) to study the neurobiological mechanisms of depression in rodents. An important limitation of RSDS studies to date is that they have been conducted exclusively in male mice due to the difficulty of initiating attack behavior directed toward female mice. Here, we establish a female mouse model of RSDS by inducing male aggression toward females through chemogenetic activation of the ventrolateral subdivision of the ventromedial hypothalamus (VMHvl). We demonstrate that females susceptible to RSDS display social avoidance, anxiety-like behavior, reduction of body weight, and elevated levels of circulating interleukin 6. In contrast, a subset of mice we term resilient only display anxiety-like behaviors after RSDS. This model allows for investigation of sex differences in the neurobiological mechanisms of defeat‒induced depression‒like behaviors. A robust female social defeat model is a critical first step in the identification and development of novel therapeutic compounds to treat depression and anxiety disorders in women.
Introduction
Violence against women is a major public health problem—an estimated 35% of women worldwide have experienced physical and/or sexual violence at some point in their lives1. Women who are victims of violence are more likely to develop mental illnesses including mood disorders and attempt suicide at higher rates2,3. Mood disorders are one of the most common mental health disorders, and women are more than twice as likely as men to develop depression and anxiety disorders4–7. In addition to being at increased risk for mood disorders, women exhibit different symptomatic profiles and comorbidities than men. While women are more likely to experience comorbid anxiety, men show higher comorbidity with substance abuse8. There is also increasing evidence of sex differences in the underlying biology of stress-related disorders in men and women9–11. Despite these important issues, the majority of preclinical studies of stress-related disorders focus heavily on male rodents.
The RSDS model has been employed extensively in male rodents to study neurobiological mechanisms of depression, as the model has strong ethological significance and face validity to human mood disorders, distinguishing it from other preclinical models of depression12–18. It is well established in humans that repeated experience of social stress, such as bullying or physical abuse, increases risk of developing depression19. Much like humans, socially defeated intruder male mice develop several depressive-like phenotypes characterized by social avoidance, reduction of sexual motivation, anhedonia, behavioral despair, reduction of body weight, risk for atherosclerosis, changes in hormonal and cardiovascular responses and enhanced pro-inflammatory responses12,17,18,20–25. RSDS also has pharmacological validity as chronic, but not acute, treatment of mice with standard antidepressants normalizes the behavioral effects of defeat17,26. Of further utility, RSDS yields a pool of rodents that exhibit large individual differences in response to defeat, with 50-60% of mice displaying stress susceptibility and about 30% of mice displaying stress resilience17,18, allowing for the study of biological mechanisms underlying susceptibility and resilience to social stress27,28. Identification of active biological coping mechanisms in resilient mice has spurred investigation of novel therapeutics to promote natural resilience mechanisms as an alternative to traditional anti-depressant drug development29,30.
Nevertheless, an important caveat of the RSDS model is its reliance on a male’s innate territorial aggression toward other males that intrude into his territory. Because aggression toward sexually matured female mice (Mus musculus) were rare or low compared to inter-male aggression, RSDS studies to date have been conducted exclusively in male mice. In the present study, we aimed to establish a female RSDS model by inducing male aggression toward adult female mice through a chemogenetic approach. The hypothalamic attack area is a locus tightly linked to attack behavior31,32. Previous studies have shown that activation of the ventrolateral subdivision of the ventromedial hypothalamus (VMHvl) in male mice induces atypical attack behavior in which male mice attacked female intruders as well as inanimate objects33,34. Here, we used designer receptors exclusively activated by designer drugs (DREADDs) to activate the VMHvl of male mice, promoting aggression toward adult females and enabling successful RSDS outcomes in female mice. Our results describe a reliable and reproducible method of RSDS to induce depression-like behavioral responses in both female and male mice.
Results
Establishment of aggressive males that attack female mice
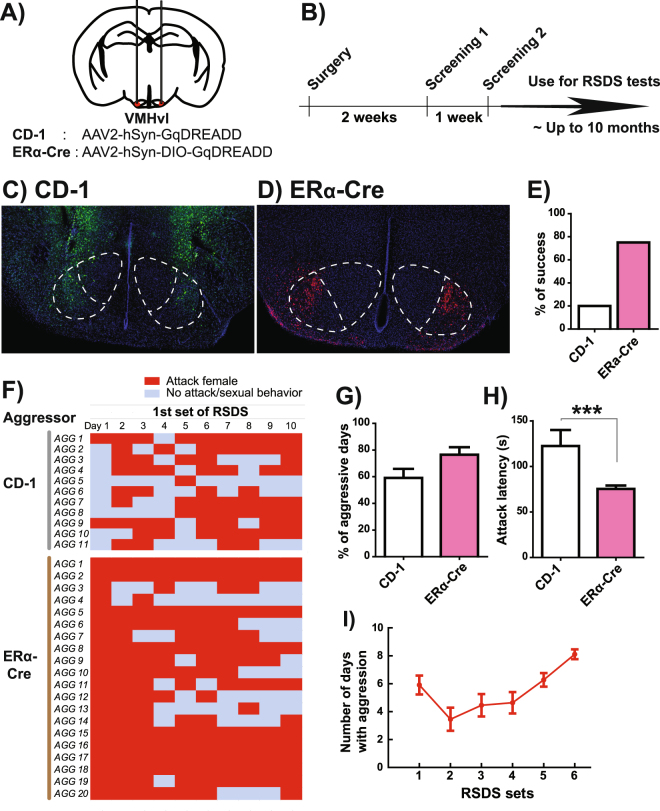
To establish male aggression toward adult, sexually mature female mice, we first injected Gq-DREADD expressing AAV bilaterally into the VMHvl of CD-1 male mice (Fig. 1A,B). We screened these animals for aggression toward female mice both two and three weeks post-surgery. For screening, CD-1 males were given intraperitoneal injections of 1.0 mg/kg clozapine-N-oxide (CNO). Thirty-five minutes later, a C57BL/6J female was introduced into the homecage of a CD-1 male to screen his aggressive behavior. Under these conditions, approximately 20% of CD-1 males (21 out of 105 males) showed consistent aggressive behavior toward females during the screening and were used for the RSDS procedure (Fig. 1E). Histological examination indicated that animals lacking attack behavior had low or no infection of virus in the VMHvl area as only 20% of mice showing aggression had expression of AAV-Gq-DREADD within the VMHvl (Fig. 1C).
Figure 1.
Generation of male aggressors to defeat female mice. (A) Schematics of Gq-DREADD expressing AAV injection bilaterally into VMHvl. AAV2-hSyn-GqDREADD-IRES-mCitrine and AAV2-hSyn-DIO-GqDREADD-mCherry were injected into male wild-type CD-1 and ERα-Cre mice, respectively. (B) Time course of surgery and screening of aggressive behavior. (C) Representative picture of CD-1 male with Gq-DREADD injections into the VMHvl. mCitrine expression (green) is not restricted to the VMHvl but is widely spread around the VMH area. (D) Representative picture of ERα-Cre F1 male with Gq-DREADD injections into the VMHvl. mCherry expression (red) is localized in the VMHvl area. (E) Percentage of males that showed aggressive behavior toward female C57BL/6J intruders during the aggression screening. (F) Daily monitoring of aggressive behavior during the first 10-day RSDS procedure in each aggressor male. (G) Number of days that males showed aggressive behaviors and (H) average attack latency of aggressor males during 10-day RSDS. (I) The same CD-1 aggressor males were used for several iterations of RSDS without reducing aggressive behavior. Data represent mean ± SEM, ***p < 0.001 in unpaired t-test.
To increase the efficiency of targeting the VMHvl, we used estrogen receptor alpha (ERα)-Cre mice in which Cre expression is highly localized to the VMHvl and absent in adjacent structures34. Previous work has implicated this ERα-positive neuronal population in aggressive behaviors34,35. To ensure consistent aggression, we crossed ERα-Cre mice with CD-1 outbred mice used in standard male RSDS protocols to obtain F1 offspring with the ERα-Cre allele36. We then injected Cre-dependent DIO-Gq-DREADD-expressing AAV into bilateral VMHvl in F1 ERα-Cre males and subsequently screened aggression toward a female intruder mouse 2 and 3 weeks after viral injection (Fig. 1A,B). Remarkably, 75% of these mice (30 out of 40 males) exhibited CNO-induced aggressive behavior toward females during the screening test (Fig. 1D,E).
Quantitatively, we observed greater aggression toward females in Gq-DREADD-injected ERα-Cre mice compared to Gq-DREADD-injected CD-1 mice. CD-1 male mice exhibited aggressive behavior toward females for an average of 59.1% of each defeat session (ranging from 10 to 90%) whereas ERα-Cre mice exhibited aggressive behavior for an average of 76.5% of each defeat session (Fig. 1F,G). Latency to attack initiation was shorter in ERα-Cre compared to CD-1 aggressors (Fig. 1H). Aggressive behavioral patterns in both CD-1 and ERα-Cre aggressor males were similar to inter-male aggressive behavior (Supplemental Videos 1 and 2).
Gq-DREADD-injected aggressors can be used repeatedly in the RSDS paradigm for up to 10 months and for as many as 6 separate RSDS studies with consistent levels of CNO-induced aggression (Fig. 1I, CD-1 aggressors). Thus, chronic Gq-DREADD activation of VMHvl neurons produces reliable, stable aggression toward intruder female mice.
Characterization of a conventional RSDS model in females
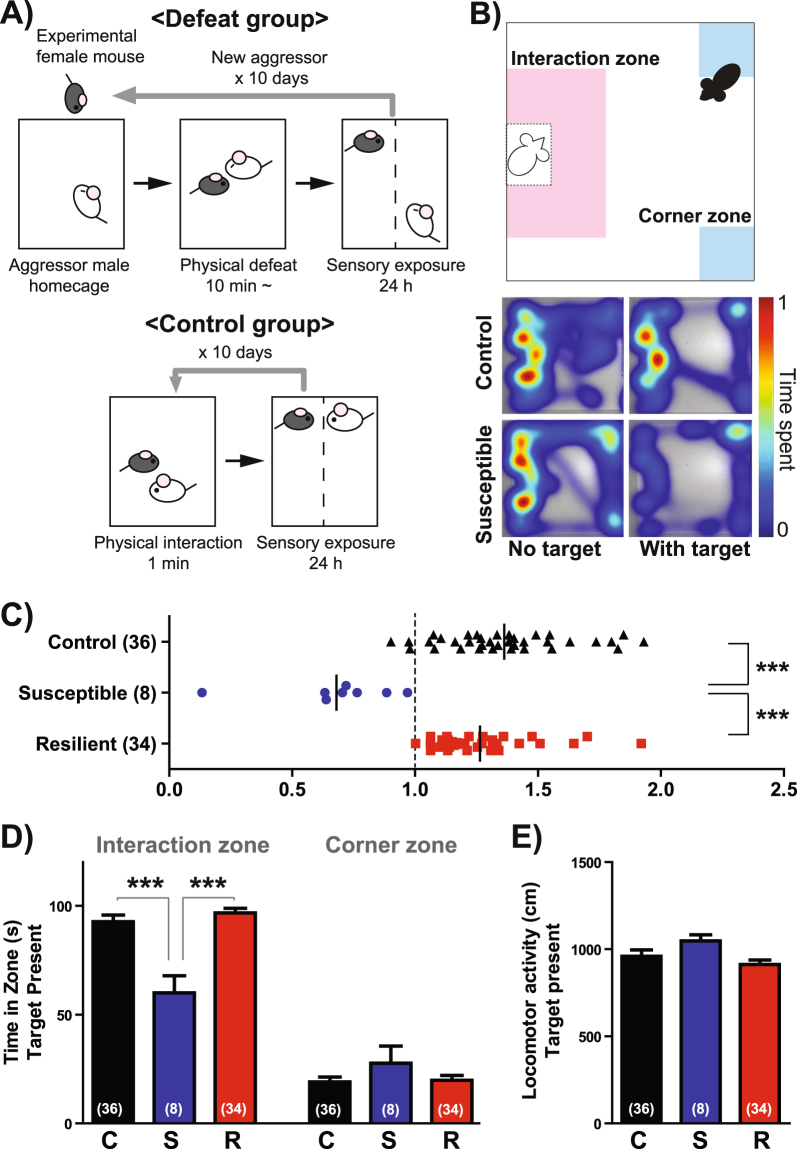
We first tested a conventional RSDS protocol based upon our previous studies in male C57BL/6J mice12. Female C57BL/6J mice were introduced into the homecage of a CNO-injected aggressor for ~10 min following an initial attack. Under these circumstances, we observed inconsistent CNO-induced attack behavior, finding that females were defeated on about 5.9 days (ranging from 2 to 10 days) or 9.6 days (ranging from 9 to 10 days) of the 10-day RSDS protocol in CD-1 or ERα-Cre aggressors, respectively. After each 10-minute defeat bout, the female mouse was placed on the opposite side of a Plexiglas divider for 24 hours until the next physical defeat bout, allowing for persistent sensory contact with the male aggressor as a form of psychological stress (Fig. 2A). Twenty-four hours after the last defeat bout, we assayed female social interaction behavior by performing a social interaction (SI) test as previously described12. During this 5-minute SI test, we record an animal’s exploratory behavior in an open-field arena containing a small wire enclosure that is empty (first 2.5 minutes) or houses a novel CD-1 male (second 2.5 minutes) (Fig. 2B). The ratio of time spent in the immediate vicinity of the wire enclosure (i.e., the interaction zone) when the target mouse is present over the time spent near the enclosure when the target mouse is absent serves as an index of social avoidance/approach and is termed SI ratio. Similar to the findings of studies employing male RSDS17,18, we found a large distribution of SI ratio in defeated females compared to controls (Fig. 2C). The distribution of SI ratios in defeated females was not normal (KS normality test: p = 0.0106), and thus we split the defeated population into stress-resilient (SI ratio > 1.0) and stress susceptible groups (SI ratio < 1.0). Interestingly, less than 20% of defeated females showed social avoidance (susceptible) in this test using either CD-1 (19%, Fig. 2C) or ERα-Cre F1 aggressors (10%, Supplemental Fig. 1). This percentage is quite low compared to inter-male RSDS, in which approximately 60% of males become susceptible18. Despite this low level of susceptibility, one-way ANOVA indicated a significant group differences for SI ratio (Fig. 2C; F(2,77) = 28.31, p < 0.0001) and time spent in interaction zone (Fig. 2D; F(2,66) = 16.32, p < 0.0001), whereby susceptible females exhibited lower SI ratios and time spent in interaction zone compared to both control and resilient females. In contrast, there were no differences between groups in time spent in the corner zone or in locomotor activity. The number of defeated days was not different between resilient and susceptible females (Supplemental Fig. 2a), and there was no correlation between SI ratio and the number of defeated days (Supplemental Fig. 2b) or the number of attack bouts received during 10-day RSDS (Supplemental Fig. 2c). Also, we did not observe any effect of estrous cycle stage on SI ratio in control or defeated female mice (Supplemental Fig. 2d,e).
Figure 2.
Conventional RSDS produces social avoidance in a small fraction of female mice. (A) Experimental schematics of conventional RSDS applied to female C57BL/6J mice. (B) Social interaction (SI) testing apparatus depicting the interaction zone (pink) and corner zone (blue) (top). Representative traces of the exploratory behavior of control and susceptible female mice in the presence and absence of a novel male mouse (bottom). (C) Distribution of SI ratio in control and defeated females. Defeated females that showed social avoidance (SI ratio <1) were categorized as susceptible, and females that showed social preference (SI ratio ≥1) were categorized as resilient. (D) Time spent in the interaction zone and corner zone and (E) locomotor activity when the social target was present in the arena. C: control, S: susceptible, R: resilient. Data represent mean ± SEM. Number of animals is indicated in parentheses. *p < 0.05; **p < 0.01; ***p < 0.001 in Tukey’s post hoc test after One-way ANOVA.
A possible explanation for the surprisingly high rate of resilience in females exposed to conventional RSDS is the confound of sexual motivation. When we initiated female RSDS according to the conventional model, we noted that females, unlike males because of their larger body size, were able to climb over the Plexiglas divider and enter the opposite side of the defeat cage. Interestingly, many of the defeated females moved to the male’s compartment after the pro-aggression effect of CNO had subsided, suggesting that, unlike male C57BL/6J mice exposed to RSDS, defeated females do not find the 24-hour psychosensory exposure period aversive. It is well known that sensory exposure to male pheromones alters the behavior and sexual receptivity of female mice. Male mice also emit ultrasonic vocalizations that can attract females37. To examine the effect of sensory contact itself on RSDS behavioral outcome, we prepared two types of control groups: a female mouse co-housed with another female or co-housed with a male for 10 days (Supplemental Fig. 3a). Similar to the RSDS procedure, we co-housed these animals using a perforated divider to provide sensory, but not physical, contact. We found that, compared to females co-housed with females, control females co-housed with males exhibited higher body weight gain and water intake (Supplemental Fig. 3b,c), as well as reduced total arm entry in the elevated plus maze (Supplemental Fig. 3f) and reduced immobility in forced swim test (Supplemental Fig. 3i). There was no effect of co-housing on social interaction behavior in SI test, % of open arm entry in the elevated plus maze, and sucrose preference (Supplemental Fig. 3d,e,g,h).
Characterization of an adapted RSDS model without extended sensory contact
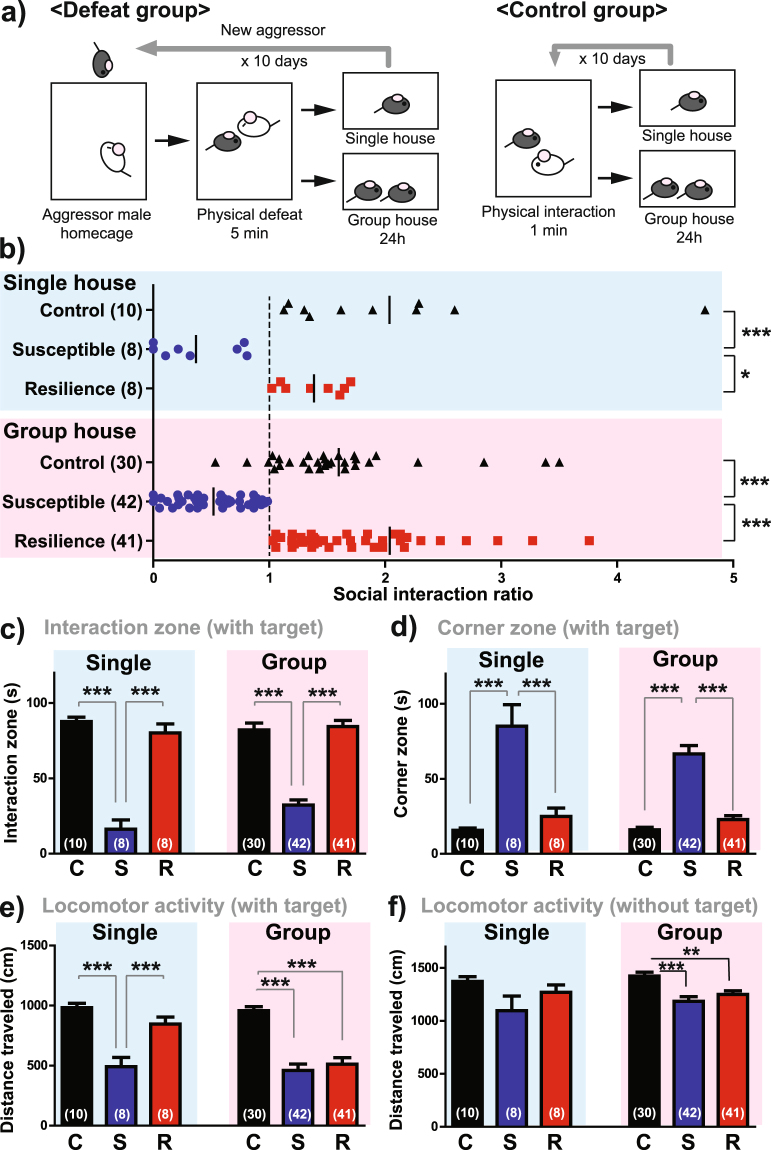
To minimize the confounding effect of exposing females to mating-associated sensory signals following physical defeat, we performed RSDS without any post-defeat sensory contact. We conducted all aggressive encounters in a standard, static mouse cage with a clear Plexiglas top. Because defeat in these smaller cages tended to be more intense, we shortened the duration of defeat to 5 minutes in order to prevent significant injury. Following each defeat bout, we housed females either alone or together with another female (without divider) in a separate mouse cage until the next defeat episode (Fig. 3a). In both single-housed and group-housed condition, we found that about 50% of defeated females showed strong social avoidance (Fig. 3b). One-way ANOVA indicated a significant main effect of group on SI ratio for both single-housed (F(2,25) = 11.87, p = 0.0003) and group-housed conditions (F(2,112) = 28.82, p < 0.0001), in which susceptible females exhibited lower SI ratios than both control and resilient females. When the social target was present, susceptible females showed a strong reduction of time spent in the interaction zone (Single: F(2,25) = 61.09, p < 0.0001, Group: F(2,112) = 58.67, p < 0.0001; Fig. 3c), increased duration in corner zones (Single: F(2,25) = 20.65, p < 0.0001, Group: F(2,112) = 48.95, p < 0.0001; Fig. 3d), and reduced locomotor activity (Single: F(2,25) = 19.77, p < 0.0001, Group: F(2,112) = 25.72, p < 0.0001; Fig. 3e). In group-housed condition, both resilient and susceptible animals showed reduced locomotor activity compared to control females regardless of existence of target (Fig. 3e,f). In the absence of a social target, we observed no differences between groups in most behavioral indices, with the exception of a slight reduction in interaction zone duration in single-housed susceptible mice compared to resilient mice, but not control mice (F(2,25) = 3.575, p = 0.0445).
Figure 3.
Adapted RSDS model without extended sensory contact produces robust social avoidance in a subset of female mice. (a) Experimental schematic of the RSDS model for female C57BL/6J mice without extended sensory contact. (b) Distribution of SI ratios in control and defeated females in single housed (top) and group housed (bottom) conditions. Time spent in the interaction zone (c), time spent in the corner zone (d) in the presence of novel male social target. Locomotor activity in the presence (e) and absence (f) of a social target. Data from the single-housed condition (blue) and group-housed condition (pink) are presented separately. C: control, S: susceptible, R: resilient. Data represent mean ± SEM. Number of animals is indicated in parentheses. *p < 0.05; **p < 0.01; ***p < 0.001 in Tukey’s post hoc test after One-way ANOVA.
We found no difference in the amount of aggression experienced by susceptible and resilient females (Supplemental Fig. 4a). We also observed no effect of defeat on estrous cycle stage in susceptible or resilient females compared to controls (Supplemental Fig. 4b). Furthermore, 2-way ANOVA revealed no effect of estrous cycle stage on SI ratio or time spent in the interaction zone in control, susceptible and resilient females (Supplemental Fig. 4c‒e). However, there was a significant interaction of estrous cycle stage and defeat on time spent in the corner zone (F(2,55) = 7.358, p = 0.0015) and a trend toward an interaction of estrous cycle stage and defeat on time spent in the interaction zone (F(2,55) = 2.705, p = 0.0757), whereby, only within the susceptible group, diestrus females spent less time in the interaction zone and more time in the corner zones compared to estrus females (Supplemental Fig. 4d,e). There was no effect of estrous cycle stage on these indices in control or resilient groups. Also, there was no difference in the degree of wounding between resilient and susceptible animals (Supplemental Fig. 5).
In order to compare males and females using our RSDS paradigm, it is essential that our modified procedure for females is also effective in males. Accordingly, we tested male C57BL/6J mice with the same, sensory contact-lacking RSDS protocol using wild type CD-1 males as aggressors (Supplemental Fig. 6a). In contrast to females, the proportion of susceptible males was greater in the group-housed condition (36%) than single-housed condition (20%). Importantly there were no differences in the proportion of susceptible group-housed males (36%) versus susceptible group-housed females (36%) using this protocol. Susceptible males in both housing conditions exhibited lower SI ratios (Single: F(2,29) = 7.127, p = 0.0033, Group: F(2,27) = 7.523, p = 0.0028), spent less time in the interaction zone when the target was present (Single: F(2,29) = 9.070, p = 0.0010, Group: F(2,27) = 15.43, p < 0.0001) and spent more time in the corner zone when the target was present (Single: F(2,29) = 3.165, p = 0.0582, Group: F(2,27) = 5.975, p = 0.0076) compared to control and resilient males (Supplemental Fig. 6b‒d). We found no difference between groups in locomotor activity or body weight gain (Supplemental Fig. 6e,f).
In order to utilize this model to study sex differences in depression-like behavior, one must be sure that there are no differences in aggressive behaviors towards male versus female intruders. As shown in Supplemental Fig. 7, we do see a slight non-significant trend toward an increase in number of attack bouts and attack duration in aggressive interactions of DREAAD-injected ERα-Cre F1 aggressors toward males than toward females. Compared to CD-1 aggressors in traditional male aggressive behavior, DREAAD-injected ERα-Cre F1 aggressors attack with low latencies in both males and females (less than 10 seconds). Duration and frequency of attack bouts of DREAAD-injected ERα-Cre mouse toward female was close to the level of aggressive behavior of CD-1 aggressors toward male. In order to study sex differences, experimenters should be aware of this potential difference in selecting DREAAD-injected ERα-Cre F1 aggressors during the screening process. Experimenters should try to select aggressors that attack males and females equally to avoid using aggressors that exhibit a male attack bias.
Behavioral and physiological profile of females exposed to adapted RSDS
To further characterize the effect of RSDS on female group-housed mice, we measured body weight during the adapted RSDS procedure, performed the elevated plus maze test to examine anxiety-like behaviors, and assayed blood levels of interleukin 6 (IL-6) previously implicated in male susceptibility to RSDS22 (Fig. 4A). We observed a significant main effect of defeat on body weight gain (F(2,64) = 7.642, p = 0.0011; Fig. 4B), with susceptible females exhibiting a reduction of body weight compared to control and resilient females. We also observed a significant main effect of stress on total arm entries (F(2,57) = 8.045, p = 0.0009; Fig. 4C) and duration in the open-arm in the elevated plus maze (F(2,57) = 4.508, p = 0.0154; Fig. 4D) in defeated mice compared to controls; however, there were no differences between susceptible and resilient mice in any of these measurements. This pattern of reduced exploratory behavior was also evident in the SI test when a social target was absent from the wire enclosure (essentially an open-field test) in both susceptible and resilient females compared to controls (Fig. 3f). These behavioral findings are consistent with those previously reported in male C57BL/6J mice following standard RSDS18.
Figure 4.
Behavioral and physiological profile of defeated females. (A) Time line of this experiment. EPM: elevated plus-maze. (B) Change of body weight from Day 1 (before defeat) to Day 10 of RSDS. (C) Total arm entry and (D) time spent in the open arm in the EPM. (E) Serum IL-6 level 24 hours after the SI test. Data from single-housed condition (blue) and group-housed condition (pink) were presented separately. C: control, S: susceptible, R: resilient. Data represented mean ± SEM. Number of animals is indicated in parentheses. *p < 0.05; **p < 0.01; ***p < 0.001 in Tukey’s post hoc test after One-way ANOVA.
In contrast, there was no effect of social defeat in single-housed females on any of the above measures. This finding was largely due to a pro-depressant and anxiogenic effect of social isolation in control females that caused a ceiling effect (Fig. 4B‒D).
We next tested whether RSDS affects peripheral inflammatory responses in females as we have shown such responses to be an important determinant of stress susceptibility in male mice and in humans22,38. We measured blood IL-6 levels of females in both the group-housed and single-housed conditions 48 hours after the last defeat bout. As in males, we found that RSDS strongly increased IL-6 in the blood of group-housed susceptible mice, whereas resilient and control mice showed low to undetectable levels of IL-6 (F(2,39) = 12.13, p < 0.0001; Fig. 4E). However, in the single-housed condition, both susceptible and resilient females showed elevated blood levels of IL-6 (F(2,25) = 5.681, p = 0.0099) after RSDS, which again suggests that single housing adds a significant stressor to the female RSDS protocol. Overall, our data indicate that RSDS in the group-housed condition most consistently reproduces the behavioral and physiological endpoints observed in male mice following standard RSDS.
Discussion
Despite higher lifetime prevalence rates of depression and anxiety disorders in women, the majority of rodent studies examining the mechanisms of mood disorders have been conducted exclusively in males. In this study, we established a female model of RSDS by using DREADD-based activation of the hypothalamic attack locus VMHvl to induce aggression in male resident mice toward female intruders. CNO-induced aggression toward female mice altered key behavioral and physiological properties of defeated females, consistent with previously validated results in male mice. In addition, as observed in males17,18, we found subpopulations of defeated females to be stress-susceptible or stress-resilient following RSDS. Susceptible females showed social avoidance, reduced body weight, and increased IL-6 compared to control and resilient females. By contrast, susceptible and resilient females both showed increased anxiety-like behaviors compared to control females, however, resilient females did not exhibit social avoidance or weight loss. These patterns of behavioral abnormalities closely resemble susceptibility and resilience in male mice after RSDS12,18.
Aside from inducing aggressive behavior with DREADDs, we made several additional modifications to the RSDS paradigm to adapt the model to female mice. During our initial experiments, we found that overnight housing across a Plexglas partition from a formerly aggressive male had a buffering effect on the development of depression- and anxiety-like behaviors in females. We therefore tested two housing conditions (group housed with other females or single housed) to mitigate these buffering effects. However, we found that social isolation by itself in females caused increased expression of depression- and anxiety-like phenotypes in the absence of RSDS compared to group-housed controls. This finding is consistent with work showing that female mice are more sensitive to social isolation than males during the preadolescent period39. In addition, male rats, which tend to be more sensitive to social isolation than male mice, exhibit enhanced expression of anxiety-like behaviors and hypothalamic-pituitary-adrenal (HPA) activity following social defeat40.
In our current study, we found that the group-housed condition is most appropriate for investigation of individual differences in stress susceptibility, as susceptible females in this condition exhibited behavioral and physiological outcomes consistent with those reported in males exposed to RSDS18,22—strong social avoidance, significant reduction of body weight and elevated peripheral IL-6. Unlike in females, the single-housed condition did not seem to compound the stressful effect of RSDS in C57BL/6J male mice as social isolation during the course of 10-day defeat did not increase the percentage of susceptible male mice. Thus, our data show that female mice are more vulnerable than males to brief periods of social isolation whereas sensory contact enhances the defeat phenotype in male mice.
It is generally thought that female mice are more sensitive to stress than males. However, this is highly dependent upon the type of stress and the behavioral indices and species being investigated. For example, following repeated variable stress, which is a combination of mild footshock, tail suspension and restraint stress, female mice develop depressive- and anxiety-like behaviors more rapidly than males11,41. In contrast, in the learned helplessness model, female rats42 and C57BL/6 mice (but not 129SvEv mice)43 are less sensitive to uncontrollable stress and show active avoidance behaviors even after repeated uncontrollable footshock stress.
In social defeat stress models, there are some species where both females and males exhibit aggression toward a same-sex intruder, including the Syrian hamster (Mesocricetus auratus) and California mouse (Peromyscus californicus)44,45. A single social defeat experience from same sex conspecifics induces learned submissive behaviors in all male Syrian hamsters, while only a subset of females exhibit similar behaviors46,47. Conversely, female, but not male, California mice display social avoidance behavior following social defeat by a same sex conspecific48. Postpartum female rats exhibit aggression toward female intruders, and such maternal aggression induces several depression-like behaviors including reduced body weight and disruption of estrous cycle49,50 (but see51).
Other models have lesioned the mediobasal hypothalamus of female rats to induce female aggressive behavior, however, these models do not induce the change in body weight or corticosterone that is observed in male rats52. While all of the above mentioned models have value for better understanding female stress responses, the model described here allows us to exploit key transgenic and knockout technologies to test mechanistic hypotheses related to stress-induced depression- and anxiety-like behavior. In our female defeat model, it is important to note that use of a male aggressor to socially defeat a female intruder (opposite sex), may be qualitatively different from male social defeat stress (inter-male). However, because male-female aggression has a major impact on women’s mental health, we feel that this model fills a critical gap in our ability to understand biological mechanisms of susceptibility and resilience to social stress in female rodents.
In summary, we have established a female social defeat model in C57BL/6J mice that, consistent with the standard RSDS model in males, produces individual differences in behavioral susceptibility to social stress, also reflected in peripheral IL-6 levels, showing at least some overlap in biological mechanisms between the sexes. This new female social defeat model will thus allow us to expand upon previously published reports in males through the study of sex differences in depression- and anxiety-related biological pathways. Such studies may ultimately enable us to identify sex-related mechanisms of stress susceptibility and antidepressant efficacy, and to better formulate clinical studies for sex-specific therapeutic approaches.
Methods
Animals
7 week old C57BL/6J female mice (The Jackson Laboratory, Bar Harbor, Maine) were group housed (5 mice per cage) in standard mouse cages (28.5 cm (w) × 18.5 cm (d) × 12.5 cm (h)) for a week before the social defeat procedure. Body weights were taken one day before the beginning of repeated social defeat to assign defeat and control groups to have similar average starting body weight.
For aggressors, we used CD-1 male mice and Esr1 (ERα)-Cre mice. CD-1 male mice were sexually experienced breeders at least 4 months of age (Charles River Laboratories). ERα-Cre mice with a C57BL/6J background34 were purchased from the Jackson laboratory and crossed with CD-1 females in the breeding facility at the Icahn School of Medicine at Mount Sinai (ISMMS) to obtain F1 offspring. F1 offspring were group housed with their siblings until the VMHvl surgery, which was conducted when the mice were about 8 weeks old. After the surgery, all males were housed individually throughout the experiment. Mouse procedures were performed in accordance with the National Institute of Health Guide for Care and Use of Laboratory animals and all procedures were approved by the ISMMS Animal Care and Use Committee (approval number LA10-00266).
DREADD expression in the VMHvl of aggressor males
AAV vectors encoding a non-conditional Gq-DREADD53,54 (AAV2-hSyn-HA-hM3D(Gq)-IRES-mCitrine; University of North Carolina, Chapel Hill, NC) or a conditional AAV vector (rAAV2/hSyn-DIO-hM3D(Gq)-mcherry; University of North Carolina, Chapel Hill, NC) were used in wild type CD-1 or ERα-Cre F1 mice, respectively, to target the VMHvl. Male mice were anesthetized with a cocktail of ketamine HCl (100 mg/kg) and xylazine (10 mg/kg) injected intraperitoneally (i.p.). A 33 gauge needle attached to a glass Hamilton syringe was stereotaxically inserted into the VMHvl (AP, −1.5; ML, ± 0.7; DV, −5.7 mm from bregma) bilaterally as calculated from the mouse brain atlas (Paxinos and Franklin). The AAV vector was infused in a volume of 0.3 ul/side over 3 min, and the needle was left in place for 5 min after the injection. Two-weeks after the AAV injection, these mice were screened for aggressive behavior toward females. Thirty-five min before the screening, each male mouse was intraperitoneally injected with CNO (1 mg/kg). Following this incubation period, a C57BL/6J female was introduced into the male’s homecage for 5 min. This screening was repeated one week later, and male mice that showed aggressive behavior toward female in both screenings were used for the subsequent social defeat procedure as aggressors. If the male showed aggressive behavior only once during these initial screenings, third screening session was performed to select males that attacked during at least 2 out of 3 sessions.
Conventional repeated social defeat stress (RSDS) procedure
The RSDS protocol was similar to previously published protocols in males with the following modifications12. A standard hamster cage (26.7 cm (w) × 48.3 cm (d) × 15.2 cm (h)) containing hard woodchip bedding was divided into two compartments by two clear perforated Plexiglas dividers (0.6 cm (thickness) × 45.7 cm (d) × 15.2 cm (h)) inserted in the middle of the cage. Two dividers between the aggressor and females were necessary because females tended to cross a single divider to the aggressor male compartment during the evening when CNO-induced aggression subsided. The hamster cage was covered by a steel-wire top, and food and water were supplied ad libitum to both compartments. One day before the first social defeat, aggressor CD-1 males were housed in one side of the hamster cage for habituation.
On the first day of RSDS, aggressors were injected with CNO (1 mg/kg, i.p.) 35 min before the encounter. A C57BL/6J female was confined to the aggressor’s side for a total of 10 min following an initial attack. Then, females were transferred to the opposite compartment of the same cage. If aggressor males exhibited any type of sexual behaviors such as mounting behavior or thrusting, we immediately removed the female and placed her in another aggressor’s cage. If the second aggressor also did not show any aggressive behavior, we ended the encounter for the day. Following successful aggressive interaction with a male, the female was housed on the opposite side of the hamster cage from the aggressor for the remainder of the 24 hour period. This replicates the psychosensory exposure period in the male RSDS protocol12, where the defeated mouse can see, smell and hear the aggressor in the absence of physical contact, and is thought to produce psychological stress. The following day, aggressors were again injected with CNO (1 mg/kg, i.p.) 35 min before the encounter, and the procedure was repeated for 10 days total. Animals were then individually housed in standard mouse cage after the last physical defeat session. Mouse body weight was measured every day before the physical defeat stress.
Control mice were placed in a standard mouse cage containing hard woodchip bedding divided into two compartments by a clear perforated Plexiglas divider. Due to the possibility that exposure to male pheromones could change the behavior of females (Supplemental Fig. 3), we housed a control C57BL/6J female with a CD-1 male to be as consistent as possible with the defeat group. The female was allowed to interact with the CD-1 male (without CNO injection) by removing divider for 1 min every day in their homecage. The control interaction was restricted to 1 min to prevent the initiation of sexual behavior. After the 10 days of RSDS, both defeated and control females were housed individually in new standard mouse cage.
Adapted RSDS without extended sensory contact
Male aggressors were housed individually in standard mouse cages (28.5 cm (w) × 18.5 cm (d) × 12.5 cm (h)) with hard woodchip bedding. Test females were housed either individually or paired with another female (defeated female pair-housed with defeated female) in the standard mouse cage without divider. Similar to the conventional RSDS method, aggressors were injected with CNO (1 mg/kg, i.p.) 35 min before the encounter. To better observe aggressors’ behavior, the wire mesh cage top was removed and replaced with a clear Plexiglas top just prior to the aggressive encounter. A C57BL/6J female was introduced into the aggressor’s cage for 5 min after the first attack. Because defeat in these smaller cages tended to be more intense, we shortened the duration of defeat from 10 min to 5 min in order to reduce significant injury. If females were not physically attacked by an aggressor, they were removed to another aggressor’s cage for 1 more session. After the physical defeat, the female was returned to her homecage. On the following day, the female was introduced to a novel aggressor’s cage to experience 5 min of physical defeat stress and this procedure was repeated for a total of 10 days. Body weight was measured every day throughout the physical defeat stress. Control females were housed either individually or two per cage together in a standard mouse cage without divider. Each control female was placed in the homecage of an ERα-Cre F1 control male (without CNO injection) for a 1 min period of interaction on each of the ten days of RSDS. After the final RSDS or control interaction session, both defeated and control females were housed individually in new standard mouse cages.
Social interaction (SI) test
The SI test was conducted one day after the last defeat session under exactly the same test conditions described for males in previous studies12. A wire-mesh enclosure (10 cm (w) × 6.5 cm (d) × 42 cm (h); Nationwide Plastics) was placed at one end of the white plastic open field (42 cm (w) × 42 cm (d) × 42 cm (h); Nationwide Plastics). A CCD camera was placed above the open field, and the video data was sent to a computer and analyzed by video-tracking software (Ethovision 3.0; Noldus Information Technology). Animals were acclimated to the test suite for 1 hour before the test. The test consisted of two 150-sec phases; a “target absent” phase and a “target present” phase. During the “target absent” phase (phase 1), no social stimulus was placed in the wire-mesh enclosure. During the “target present” phase (phase 2), a novel aggressor male was placed in the wire-mesh enclosure. Male mice were chosen such that they were novel but had the same genetic background as aggressors used for the RSDS procedure. Social target mice with or without CNO injection 30 min before the test were used. Social avoidance did not differ in groups exposed to aggressors with and without CNO prior to SI. In each phase, a C57BL/6J female was introduced into the side of the open field opposite the wire-mesh enclosure, and her exploratory behavior was automatically tracked for 150 sec by video-tracking software. Between phase 1 and 2, the C57BL/6J female mouse was returned to her homecage and for an approximately 30 second interval. Within the Ethovision program, an interaction zone (an 8 cm wide corridor surrounding the wire-mesh enclosure) and a corner zone (two 9 × 9 cm squares in the corners of the field opposite the wire-mesh enclosure) were defined within the open field, and the time spent by the test animal in those zones, as well as the total distance traveled in the open field were calculated. At the end of each test, both the C57BL/6J test female and the social target male mouse were returned to their homecages, and the test apparatus was cleaned with a quatricide solution. All behavioral tests were conducted under red light conditions.
Estrous cycle monitoring
Vaginal cellular samples were collected immediately after the SI test by gently pipetting 10 µL of sterile saline into the vagina and dispensing the vaginal sample onto a glass slide. Samples were air dried and stained with Toludine blue11. Cycle stage was determined by visual inspection under a light microscope.
Serum sampling and IL-6 measurement
Twenty-four hours after the SI test, blood samples were collected from the submandibular vein into protein Lobind tubes (Eppendorf). Whole blood was kept at room temperature for 1 hour prior to centrifugation for 15 min at 956 × g at 4 °C. Serum was collected and stored at −80 °C until the protein assay. Serum IL-6 levels in control and defeated animals were measured by Enzyme Linked Immunosorbent Assay (ELISA, Mouse IL-6 ELISA Kit, Cat#55950, BD OptEIATM, BD Biosciences, USA) according to the manufacturer’s instructions. All samples were measured in duplicate.
Elevated plus maze
Female mice were acclimated to the testing room for 1 hour before testing. The elevated plus maze apparatus consisted of black Plexiglas with two open arms (12 cm (w) × 50 cm (d) × 0.5 cm (h)) and two closed arms (12 cm (w) × 50 cm (d) × 40 cm (h)) placed 1 m above the floor. A CCD camera was placed on the ceiling of the testing room, and the video data was sent to a computer and analyzed by video-tracking software (Ethovision 3.0; Noldus Information Technology). The animal was placed in the center of the elevated plus maze, and her exploratory behavior was analyzed for 5 min. From the tracking data, total time spent in the open arm, total time spent in the closed arm, and total distance traveled were calculated.
Statistical analysis
GraphPad Prism 5 software was used for statistical analysis. Unpaired t-tests were used to compare two groups, and one-way ANOVA was conducted to compare resilient, susceptible, and control females followed by Tukey’s multiple comparison post hoc tests. Two-way ANOVA was conducted to examine the effect of estrous cycle in susceptible, resilient, and control females followed by Tukey’s multiple comparison post hoc test. To analyze whether the distribution of SI ratio in defeated females followed a normal distribution, we used the Kolmogorov-Smirnov Goodness (KS) Test.
Availability of materials and data
The datasets generated during the current study are available from the corresponding author on reasonable request.
Electronic supplementary material
Acknowledgements
This research was supported by US National Institutes of Health grants R01 MH104559, R01 MH090264, P50 MH096890 and P50 AT008661 (S.J.R.), R21 MH112081 (M.H.H), NARSAD (M.H.H.), T32 MH087004 (M.L.P., and M.F.), T32 MH096678 (M.L.P.), F31 MH105217 (M.L.P.). We thank Dr. Koichi Hashikawa and Dr. Dayu Lin (New York University) for advice on generation of aggressors.
Author Contributions
A.T. and S.J.R. wrote the manuscript, A.T., S.Z., H.Z., Y.G., D.D., G.E.H., B.S.M., E.J.N., M.H.H., S.J.R. designed the experiment, A.T., J.C., S.Z., H.Z., Y.G., H.A., M.E.F, M.L.P., C.M. performed surgery, behavioral experiments and analyzed data. All authors read and approved the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-017-12811-8.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.WHO, Department of Reproductive Health and Research, London School of Hygiene and Tropical Medicine & South African Medical Research Council. Global and regional estimates of violence against women. WHO (World Health Organization, 2013).
- 2.Devries K, et al. Violence against women is strongly associated with suicide attempts: evidence from the WHO multi-country study on women’s health and domestic violence against women. Soc. Sci. Med. 2011;73:79–86. doi: 10.1016/j.socscimed.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 3.Ellsberg M, et al. Intimate partner violence and women’s physical and mental health in the WHO multi-country study on women’s health and domestic violence: an observational study. Lancet (London, England) 2008;371:1165–72. doi: 10.1016/S0140-6736(08)60522-X. [DOI] [PubMed] [Google Scholar]
- 4.Kessler RC. The effects of stressful life events on depression. Annu. Rev. Psychol. 1997;48:191–214. doi: 10.1146/annurev.psych.48.1.191. [DOI] [PubMed] [Google Scholar]
- 5.Ford DE, Erlinger TP. Depression and C-Reactive Protein in US Adults. Arch. Intern. Med. 2004;164:1010. doi: 10.1001/archinte.164.9.1010. [DOI] [PubMed] [Google Scholar]
- 6.McLean CP, Asnaani A, Litz BT, Hofmann SG. Gender differences in anxiety disorders: Prevalence, course of illness, comorbidity and burden of illness. J. Psychiatr. Res. 2011;45:1027–1035. doi: 10.1016/j.jpsychires.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kornstein SG, et al. Gender differences in chronic major and double depression. J. Affect. Disord. 2000;60:1–11. doi: 10.1016/S0165-0327(99)00158-5. [DOI] [PubMed] [Google Scholar]
- 8.Najt P, Fusar-Poli P, Brambilla P. Co-occurring mental and substance abuse disorders: A review on the potential predictors and clinical outcomes. Psychiatry Res. 2011;186:159–164. doi: 10.1016/j.psychres.2010.07.042. [DOI] [PubMed] [Google Scholar]
- 9.Seney ML, Sibille E. Sex differences in mood disorders: perspectives from humans and rodent models. Biol. Sex Differ. 2014;5:17. doi: 10.1186/s13293-014-0017-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kendler KS, Gardner CO, Neale MC, Prescott CA. Genetic risk factors for major depression in men and women: similar or different heritabilities and same or partly distinct genes? Psychol. Med. 2001;31:605–16. doi: 10.1017/s0033291701003907. [DOI] [PubMed] [Google Scholar]
- 11.Hodes GE, et al. Sex Differences in Nucleus Accumbens Transcriptome Profiles Associated with Susceptibility versus Resilience to Subchronic Variable Stress. J. Neurosci. 2015;35:16362–16376. doi: 10.1523/JNEUROSCI.1392-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Golden SA, Covington HE, Berton O, Russo SJ. A standardized protocol for repeated social defeat stress in mice. Nat. Protoc. 2011;6:1183–91. doi: 10.1038/nprot.2011.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bartolomucci A, et al. Chronic psychosocial stress persistently alters autonomic function and physical activity in mice. Physiol. Behav. 2003;80:57–67. doi: 10.1016/S0031-9384(03)00209-9. [DOI] [PubMed] [Google Scholar]
- 14.Kudryavtseva NN, Bakshtanovskaya IV, Koryakina LA. Social model of depression in mice of C57BL/6J strain. Pharmacol. Biochem. Behav. 1991;38:315–20. doi: 10.1016/0091-3057(91)90284-9. [DOI] [PubMed] [Google Scholar]
- 15.Miczek KA, Yap JJ, Covington HE. Social stress, therapeutics and drug abuse: preclinical models of escalated and depressed intake. Pharmacol. Ther. 2008;120:102–28. doi: 10.1016/j.pharmthera.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koolhaas JM, De Boer SF, De Rutter AJ, Meerlo P, Sgoifo A. Social stress in rats and mice. Acta Physiol. Scand. Suppl. 1997;640:69–72. [PubMed] [Google Scholar]
- 17.Berton O, et al. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311:864–8. doi: 10.1126/science.1120972. [DOI] [PubMed] [Google Scholar]
- 18.Krishnan V, et al. Molecular Adaptations Underlying Susceptibility and Resistance to Social Defeat in Brain Reward Regions. Cell. 2007;131:391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 19.Huhman KL. Social conflict models: Can they inform us about human psychopathology? Horm. Behav. 2006;50:640–646. doi: 10.1016/j.yhbeh.2006.06.022. [DOI] [PubMed] [Google Scholar]
- 20.Kahn MW. The effect of socially learned aggression or submission on the mating behavior of C57 mice. J. Genet. Psychol. 1961;98:211–7. doi: 10.1080/00221325.1961.10534371. [DOI] [PubMed] [Google Scholar]
- 21.Blanchard RJ, McKittrick CR, Blanchard DC. Animal models of social stress: effects on behavior and brain neurochemical systems. Physiol. Behav. 2001;73:261–71. doi: 10.1016/S0031-9384(01)00449-8. [DOI] [PubMed] [Google Scholar]
- 22.Hodes GE, et al. Individual differences in the peripheral immune system promote resilience versus susceptibility to social stress. Proc. Natl. Acad. Sci. 2014;111:16136–16141. doi: 10.1073/pnas.1415191111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Avitsur R, Padgett DA, Sheridan JF. Social Interactions, Stress, and Immunity. Neurol. Clin. 2006;24:483–491. doi: 10.1016/j.ncl.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 24.Chuang J-C, et al. A β3-Adrenergic-Leptin-Melanocortin Circuit Regulates Behavioral and Metabolic Changes Induced by Chronic Stress. Biol. Psychiatry. 2010;67:1075–1082. doi: 10.1016/j.biopsych.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giannarelli C, et al. Susceptibility to chronic social stress increases plaque progression, vulnerability and platelet activation. Thromb. Haemost. 2017;117:816–818. doi: 10.1160/TH16-10-0817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsankova NM, et al. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat. Neurosci. 2006;9:519–525. doi: 10.1038/nn1659. [DOI] [PubMed] [Google Scholar]
- 27.Nestler EJ, Hyman SE. Animal models of neuropsychiatric disorders. Nat. Neurosci. 2010;13:1161–1169. doi: 10.1038/nn.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Russo SJ, Nestler EJ. The brain reward circuitry in mood disorders. Nat. Rev. Neurosci. 2013;14:609–625. doi: 10.1038/nrn3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Friedman AK, et al. KCNQ channel openers reverse depressive symptoms via an active resilience mechanism. Nat. Commun. 2016;7:11671. doi: 10.1038/ncomms11671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Friedman AK, et al. Enhancing Depression Mechanisms in Midbrain Dopamine Neurons Achieves Homeostatic Resilience. Science. 2014;344:313–319. doi: 10.1126/science.1249240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Siegel A, Roeling TA, Gregg TR, Kruk MR. Neuropharmacology of brain-stimulation-evoked aggression. Neurosci. Biobehav. Rev. 1999;23:359–89. doi: 10.1016/S0149-7634(98)00040-2. [DOI] [PubMed] [Google Scholar]
- 32.Kruk MR. Current topics in behavioral neurosciences. 2014;17:143–188. doi: 10.1007/7854_2014_313. [DOI] [PubMed] [Google Scholar]
- 33.Lin D, et al. Functional identification of an aggression locus in the mouse hypothalamus. Nature. 2011;470:221–226. doi: 10.1038/nature09736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee H, et al. Scalable control of mounting and attack by Esr1+ neurons in the ventromedial hypothalamus. Nature. 2014;509:627–632. doi: 10.1038/nature13169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sano K, Tsuda MC, Musatov S, Sakamoto T, Ogawa S. Differential effects of site-specific knockdown of estrogen receptor α in the medial amygdala, medial pre-optic area, and ventromedial nucleus of the hypothalamus on sexual and aggressive behavior of male mice. Eur. J. Neurosci. 2013;37:1308–1319. doi: 10.1111/ejn.12131. [DOI] [PubMed] [Google Scholar]
- 36.Golden SA, et al. Persistent conditioned place preference to aggression experience in adult male sexually-experienced CD-1 mice. Genes Brain Behav. 2016;16:44–55. doi: 10.1111/gbb.12310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Asaba A, Hattori T, Mogi K, Kikusui T. Sexual attractiveness of male chemicals and vocalizations in mice. Front. Neurosci. 2014;8:231. doi: 10.3389/fnins.2014.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hodes GE, Ménard C, Russo SJ. Integrating Interleukin-6 into depression diagnosis and treatment. Neurobiol. Stress. 2016;4:15–22. doi: 10.1016/j.ynstr.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Senst, L., Baimoukhametova, D., Sterley, T.-L. & Bains, J. S. Sexually dimorphic neuronal responses to social isolation. Elife5 (2016). [DOI] [PMC free article] [PubMed]
- 40.Ruis MA, et al. Housing familiar male wildtype rats together reduces the long-term adverse behavioural and physiological effects of social defeat. Psychoneuroendocrinology. 1999;24:285–300. doi: 10.1016/S0306-4530(98)00050-X. [DOI] [PubMed] [Google Scholar]
- 41.LaPlant Q, et al. Role of nuclear factor kappaB in ovarian hormone-mediated stress hypersensitivity in female mice. Biol. Psychiatry. 2009;65:874–80. doi: 10.1016/j.biopsych.2009.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dalla C, et al. Sex differences in the effects of two stress paradigms on dopaminergic neurotransmission. Physiol. Behav. 2008;93:595–605. doi: 10.1016/j.physbeh.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 43.Chourbaji S, et al. The suitability of 129SvEv mice for studying depressive-like behaviour: both males and females develop learned helplessness. Behav. Brain Res. 2010;211:105–10. doi: 10.1016/j.bbr.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 44.Payne AP, Swanson HH. Agonistic behaviour between pairs of hamsters of the same and opposite sex in a neutral observation area. Behaviour. 1970;36:260–9. doi: 10.1163/156853970X00402. [DOI] [PubMed] [Google Scholar]
- 45.Davis ES, Marler CA. c-fos Changes following an aggressive encounter in female California mice: a synthesis of behavior, hormone changes and neural activity. Neuroscience. 2004;127:611–24. doi: 10.1016/j.neuroscience.2004.05.034. [DOI] [PubMed] [Google Scholar]
- 46.Huhman KL, et al. Conditioned defeat in male and female Syrian hamsters. Horm. Behav. 2003;44:293–9. doi: 10.1016/j.yhbeh.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 47.Solomon MB, Karom MC, Huhman KL. Sex and estrous cycle differences in the display of conditioned defeat in Syrian hamsters. Horm. Behav. 2007;52:211–219. doi: 10.1016/j.yhbeh.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 48.Trainor BC, et al. Sex differences in social interaction behavior following social defeat stress in the monogamous California mouse (Peromyscus californicus) PLoS One. 2011;6:e17405. doi: 10.1371/journal.pone.0017405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shimamoto, A., Debold, J. F., Holly, E. N. & Miczek, K. A. Blunted accumbal dopamine response to cocaine following chronic social stress in female rats: exploring a link between depression and drug abuse. Psychopharmacology (Berl). (2011). [DOI] [PMC free article] [PubMed]
- 50.Bourke CH, Neigh GN. Exposure to repeated maternal aggression induces depressive-like behavior and increases startle in adult female rats. Behav. Brain Res. 2012;227:270–5. doi: 10.1016/j.bbr.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ver Hoeve ES, Kelly G, Luz S, Ghanshani S, Bhatnagar S. Short-term and long-term effects of repeated social defeat during adolescence or adulthood in female rats. Neuroscience. 2013;249:63–73. doi: 10.1016/j.neuroscience.2013.01.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Haller J, Fuchs E, Halász J, Makara GB. Defeat is a major stressor in males while social instability is stressful mainly in females: towards the development of a social stress model in female rats. Brain Res. Bull. 1999;50:33–9. doi: 10.1016/S0361-9230(99)00087-8. [DOI] [PubMed] [Google Scholar]
- 53.Armbruster BN, Li X, Pausch MH, Herlitze S, Roth BL. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc. Natl. Acad. Sci. 2007;104:5163–5168. doi: 10.1073/pnas.0700293104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Roth BL. DREADDs for Neuroscientists. Neuron. 2016;89:683–694. doi: 10.1016/j.neuron.2016.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.